Abstract
Purpose of Review
This review summarizes recent developments highlighting the clinical utility of diastolic stress testing along the heart failure continuum.
Recent Findings
Invasive hemodynamic assessment of cardiac filling pressures during physiological stress is the gold-standard technique for unmasking diastolic dysfunction. Non-invasive surrogate techniques, such as Doppler ultrasound, have shown excellent agreement with invasive approaches and are now recommended by the American Society of Echocardiography and the European Association of Cardiovascular Imaging. While cycle exercise is often advocated, recent evidence supports the use of isometric handgrip as a viable alternative stressor.
Summary
Diastolic stress testing is a powerful tool to enhance detection of diastolic dysfunction, is able to differentiate between cardiac and non-cardiac pathology, and should be incorporated into routine clinical assessment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Normal left ventricular (LV) diastole requires the coordination of several physiological processes which allow the heart to fill sufficiently under low filling pressures. As systole ends, LV elastic recoil and active relaxation gives rise to an abrupt decline in LV pressure until the mitral valve opens, and blood flows along a pressure gradient toward the apex. Upon pressure equilibration between the left atrium and the LV (i.e., diastasis), the final component of ventricular filling occurs when the atrium contracts and systole resumes. Impairment of any one of these processes can result in a rise in LV filling pressure that is transmitted to the left atrium and pulmonary veins, and can be associated with pulmonary edema and dyspnea [1]. Progression along the American College of Cardiology/American Heart Association (ACC/AHA) heart failure continuum from stage A (presence of cardiovascular risk factors with no structural adaptations) to stage C (structural adaptation and symptoms of heart failure) is associated with graded levels of diastolic dysfunction.
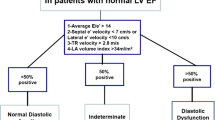
Conventional resting measures of diastolic function, particularly Doppler derived mitral inflow and annular tissue velocities, are both prognostic and predictive of events in overt heart disease (e.g., stage C) [2,3,4]. However, when disease is less advanced (e.g., stage A) and/or when the diagnosis remains equivocal, diastolic stress testing may be indicated to differentiate cardiac vs. non-cardiac pathology. Indeed, over the past decade, assessment of diastolic function during physiological stress, termed “diastolic stress testing,” has emerged as a powerful tool to enhance detection of diastolic dysfunction as the etiological feature of exertional dyspnea [5]. As a result, diastolic stress testing is now recommended by both the American Society of Echocardiography and the European Association of Cardiovascular Imaging [2, 3, 6].
This article reviews the evolution of diastolic stress testing, current practices, and procedures, and discusses the potential for diastolic stress testing across the heart failure continuum.
Pathophysiology of Diastolic Dysfunction
Diastole is a complex process governed by multiple factors that regulate active LV pressure decay and passive LV diastolic stiffness (Fig. 1). Determinants of active LV pressure decay include oxygen delivery and intracellular calcium handling. Indeed, diastole is a highly energy-dependent process, requiring sufficient delivery of oxygen for the generation of adenosine triphosphate (ATP). Unlike systole, which only requires ATP for the removal of troponin-C from actin, diastole requires ATP for the (1) reuptake of calcium into the sarcoplasmic reticulum via the sarco-endoplasmic reticulum calcium ATP-ase (SERCA), (2) dissociation of actin and myosin, and (3) uncoupling of calcium from troponin-C [7]. In addition to these direct consequences, oxygen deprivation also contributes to diastolic dysfunction by shifting substrate utilization away from fatty acid metabolism toward glucose metabolism [8,9,10].
Key pathological mechanisms involved in the development of diastolic dysfunction and the role of diastolic stress testing in exacerbating each mechanistic pathway. Clockwise from top-left: functional myocardial ischemia caused by an increased myocardial oxygen (O2) demand can lead to insufficient production of myocardial adenosine triphosphate (ATP); impaired calcium (Ca2+) handling due to elevated intracellular Ca2+ concentrations can lead to prolonged and delayed myocardial relaxation; pericardial constraint can be exacerbated by increases in preload and lead to an increased right ventricular pressure, causing a leftward shift of the interventricular septum, and ultimately leading to increased left ventricular end-diastolic pressures (LVEDP); increased diffuse fibrosis and impaired titin function can be exacerbated by increases in preload and lead to a shift in the end-diastolic pressure volume relationship upwards and to the left. SERCA2a sarco/endoplasmic reticulum Ca2+ ATP-ase; RyR ryanodine receptor; NCX sodium-Ca2+ exchanger
Impaired intracellular calcium handling has also been implicated as a primary mechanism driving diastolic dysfunction. For example, excess calcium entry through L-type calcium channels, over activity of calcium-release-activated calcium channels (such as Orai-1), impaired sodium-calcium exchanger pumps, calcium reuptake and leaky ryanodine receptors have each been implicated in a variety of conditions associated with diastolic dysfunction, including heart failure with preserved ejection fraction (HFpEF) [11,12,13,14,15]. These detrimental molecular processes ultimately impair actin-myosin cross-bridge cycling and lead to a stiff ventricle.
In addition to the contributions from active LV pressure decay, diastolic dysfunction is also associated with increased passive LV stiffness. Expansion of the extracellular matrix (increased myocardial fibrosis), left ventricular hypertrophy, and dysregulation of structural proteins like titin can each reduce passive ventricular compliance both independently and in concert [16,17,18,19,20]. Moreover, factors external to the myocardium can also negatively affect passive LV stiffness. For example, pericardial fat deposition, in combination with pericardial constraint, has recently been implicated as a major source of diastolic dysfunction [21,22,23]. Under this paradigm, with the LV constrained by the pericardium, right ventricular filling causes a leftward septal shift and an elevation in LV end-diastolic pressure [23].
While in extreme cases, each of the above mechanisms can independently contribute to overt diastolic dysfunction, less extreme cases often fail to present under resting conditions. Diastolic stress testing is therefore necessary to exacerbate these underlying mechanisms and unmask diastolic dysfunction (Fig. 1). For example, increasing myocardial oxygen demand can disrupt myocardial energetics and the processes governing calcium handling [24,25,26, 27••, 28•]. Increasing cardiac afterload floods the myocardium with calcium to support increased force production, but places greater stress on the processes governing intracellular calcium homeostasis. Finally, increasing cardiac preload (e.g., saline infusion, leg lifts, dynamic exercise) can exacerbate LV passive stiffness, augment LV/RV interaction, and adversely increase cardiac filling pressures [23].
Diastolic Stress Testing Along the Heart Failure Continuum
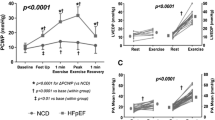
The term “diastolic stress testing” was first coined by Ha and colleagues [29], describing an abnormal rise in left ventricular filling pressures during exercise. However, in practice, “diastolic stress testing” has been utilized for several decades [30, 31]. For example, more than 60 years ago, Lewis and colleagues demonstrated an abnormal rise in pulmonary capillary wedge pressure (PCWP) in response to recumbent cycle exercise in some, but not all, patients with cardiovascular disease despite normal PCWP at rest [30]. More recently, Levine and coworkers have used acute volume loading/unloading to characterize LV compliance in health and disease [24, 31,32,33,34,35,36,37,38]. Today, diastolic stress testing is recognized as the most robust method for discriminating between cardiac and non-cardiac involvement in exercise-induced dyspnea [39,40,41,42]. Some of the most compelling and influential examples of this have recently come from Borlaug and colleagues at the Mayo Clinic in Rochester, MN, using invasive assessment of left ventricular filling pressures during submaximal cycle exercise to differentiate patients with HFpEF from those without cardiac involvement [5, 10, 25, 39,40,41, 43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62, 63••]. Importantly, while the majority of these studies have focused on direct, gold-standard, invasive measures of LV filling pressure, Borlaug and colleagues have also shown strong agreement between PCWP and its non-invasive, Doppler-derived surrogate (early mitral inflow velocity to early annual tissue velocity ratio, E/e’) [63••]. Indeed, this helps further validate the non-invasive work of Ha and colleagues, who have consistently used E/e’ during cycle exercise to differentiate cardiac from non-cardiac pathology [29, 64]. Specifically, in 2005, this group was able to identify individuals who had seemingly normal resting diastolic function (i.e., normal E/e’), but upon exercise, shared an exaggerated E/e’ response (i.e., abnormal rise in cardiac filling pressure) [29]. Across each of these studies, the common threshold defining an abnormal rise in cardiac filling pressure was a change in E/e’ > 1.5.
Cycle echocardiography is susceptible to several limitations, however, including respiratory and movement artifacts that are exaggerated in clinical populations at risk for diastolic dysfunction (obese, elderly, etc.). Moreover, while Borlaug and colleagues have convincingly demonstrated that only a mild-level of exercise is needed to elicit an abnormal diastolic response (~ 20–40 W), this approach hinges upon the ability of patients to perform dynamic leg exercise. In an effort to overcome these limitations, our group has advocated replacing cycle exercise with isometric handgrip [27••, 28•]. Indeed, isometric handgrip causes a robust, and highly reproducible pressor-mediated increase in heart rate and blood pressure [65], without causing dramatic increases in respiration or chest wall movement. Importantly, isometric handgrip also elicits marked increases in invasively measured LV filling pressures [42, 66, 67, 68•]. In our hands, isometric handgrip echocardiography is capable of differentiating normal from abnormal diastolic function (defined as a rise in E/e’ > 1.5) [28•], with comparable hemodynamic changes to conventional cycle exercise [27••].
While isometric handgrip produces a similar hemodynamic challenge compared to low-level cycle exercise [27••], these two stressors likely exacerbate diastolic dysfunction through somewhat different mechanisms. Independent of increased myocardial oxygen demand, the primary mechanism driving diastolic dysfunction during cycle exercise is likely related to the demand for increased cardiac output and reduced LV relaxation time. In this scenario, a stiff ventricle combined with increased venous return leads to an increase in LV filling pressure. In contrast, isometric handgrip uniquely increases LV afterload secondary to a neurally mediated exercise pressor reflex [69,70,71,72,73]. To support the ejection of blood during systole, this increase in afterload is met by a concomitant increase in intracellular calcium, which must either be sequestrated back in to the sarcoplasmic reticulum or extruded from the myocyte during ventricular relaxation [74,75,76]. Dysregulation of this processes will lead to prolonged actin-myosin cross-bridge formation and impaired active relaxation [77, 78] and thus increased LV stiffness and elevated LV filling pressure [24]. The potential for varying mechanistic pathways ought to be considered when designing diastolic stress tests and warrants future investigation.
Regardless of the mechanism driving diastolic dysfunction during physiological stress, or the method by which diastolic dysfunction is measured, there is increasing body of literature supporting the use of diastolic stress testing across the heart failure continuum (Table 1). Indeed, diastolic stress testing can successfully unmask diastolic dysfunction in asymptomatic patients with hypertension with no structural remodeling (i.e., stage A, [10, 79, 80]), asymptomatic patients with mild aortic stenosis (i.e., stage B, [81, 82]), and compensated HFpEF patients (i.e., stage C, [10, 39, 42, 49, 50, 63••, 68•, 83,84,85,86,87,88]. While cycle exercise has been the predominant method of diastolic stress testing [10, 39, 86], isometric handgrip echocardiography has been shown to be a robust alternative [27••, 28•, 42, 68•], with comparable end-results [27••].
The Future of Diastolic Stress Testing
The demonstration of clinical benefit for early diagnosis and management of diastolic dysfunction advocates for the widespread clinical adoption of diastolic stress testing. Indeed, cardiac stress testing (particularly recumbent cycle exercise) is already integrated and practiced in echocardiography laboratories worldwide. Inclusion of simple Doppler-derived estimates of LV filling pressures can be easily added to standard of care measures, providing relevant diagnostic and prognostic information [2,3,4, 63••]. That non-invasive diastolic stress testing can also be done by simply performing handgrip exercise [27••, 28•] holds even greater promise for widespread clinical adoption. In an ideal world, every echocardiography machine would come equipped with a stress ball or handgrip dynamometer so that diastolic stress testing may be included as part of every routine cardiac scan. While diastolic stress testing has strong prognostic and diagnostic utility in patients with unexplained dyspnea and/or heart failure symptoms, it remains unclear what the predictive capacity is for asymptomatic patients (e.g., ACC/AHA stage A). Longitudinal studies are therefore needed to define the predictive value of diastolic stress testing across the heart failure continuum.
Conclusions
Diastolic stress testing provides diagnostic and prognostic value in those at risk for heart failure and those with symptoms of unexplained dyspnea. The ability of non-invasive diastolic stress testing to successfully discriminate between cardiac and non-cardiac limitation to exercise and unmask diastolic dysfunction in both clinical and sub-clinical patients highlights its potential application in the cardiology clinic. That non-invasive diastolic stress testing, either with cycle echocardiography or isometric handgrip echocardiography, is both simple and relatively low cost, holds great promise. Future work is needed using this approach to better understand specific pathophysiological mechanisms and the predictive capacity of these novel diastolic stress tests.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Borlaug BA, Paulus WJ. Heart failure with preserved ejection fraction: pathophysiology, diagnosis, and treatment. Eur Heart J. 2011;32(6):670–9. https://doi.org/10.1093/eurheartj/ehq426.
Nagueh SF, Smiseth OA, Appleton CP, Byrd BF 3rd, Dokainish H, Edvardsen T, et al. Recommendations for the evaluation of left ventricular diastolic function by echocardiography: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging. 2016;17(12):1321–60. https://doi.org/10.1093/ehjci/jew082.
Nagueh SF, Smiseth OA, Appleton CP, Byrd BF 3rd, Dokainish H, Edvardsen T, et al. Recommendations for the evaluation of left ventricular diastolic function by echocardiography: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2016;29(4):277–314. https://doi.org/10.1016/j.echo.2016.01.011.
Burgess MI, Jenkins C, Sharman JE, Marwick TH. Diastolic stress echocardiography: hemodynamic validation and clinical significance of estimation of ventricular filling pressure with exercise. J Am Coll Cardiol. 2006;47(9):1891–900. https://doi.org/10.1016/j.jacc.2006.02.042.
Borlaug BA. Exercise haemodynamics and outcome in patients with dyspnoea. Eur Heart J. 2014;35(44):3085–7. https://doi.org/10.1093/eurheartj/ehu350.
Mitter SS, Shah SJ, Thomas JD. A test in context: E/A and E/e’ to assess diastolic dysfunction and LV filling pressure. J Am Coll Cardiol. 2017;69(11):1451–64. https://doi.org/10.1016/j.jacc.2016.12.037.
Gorski PA, Ceholski DK, Hajjar RJ. Altered myocardial calcium cycling and energetics in heart failure--a rational approach for disease treatment. Cell Metab. 2015;21(2):183–94. https://doi.org/10.1016/j.cmet.2015.01.005.
Lopaschuk GD, Stanley WC. Glucose metabolism in the ischemic heart. Circulation. 1997;95(2):313–5.
Zile MR, Baicu CF, Gaasch WH. Diastolic heart failure--abnormalities in active relaxation and passive stiffness of the left ventricle. N Engl J Med. 2004;350(19):1953–9. https://doi.org/10.1056/NEJMoa032566.
van Empel VP, Mariani J, Borlaug BA, Kaye DM. Impaired myocardial oxygen availability contributes to abnormal exercise hemodynamics in heart failure with preserved ejection fraction. J Am Heart Assoc. 2014;3(6):e001293. https://doi.org/10.1161/JAHA.114.001293.
Schroder F, Handrock R, Beuckelmann DJ, Hirt S, Hullin R, Priebe L, et al. Increased availability and open probability of single L-type calcium channels from failing compared with nonfailing human ventricle. Circulation. 1998;98(10):969–76.
Luo X, Hojayev B, Jiang N, Wang ZV, Tandan S, Rakalin A, et al. STIM1-dependent store-operated Ca(2)(+) entry is required for pathological cardiac hypertrophy. J Mol Cell Cardiol. 2012;52(1):136–47. https://doi.org/10.1016/j.yjmcc.2011.11.003.
Goonasekera SA, Hammer K, Auger-Messier M, Bodi I, Chen X, Zhang H, et al. Decreased cardiac L-type Ca(2)(+) channel activity induces hypertrophy and heart failure in mice. J Clin Invest. 2012;122(1):280–90. https://doi.org/10.1172/JCI58227.
Hasenfuss G, Reinecke H, Studer R, Meyer M, Pieske B, Holtz J, et al. Relation between myocardial function and expression of sarcoplasmic reticulum Ca(2+)-ATPase in failing and nonfailing human myocardium. Circ Res. 1994;75(3):434–42.
Sipido KR, Volders PG, Vos MA, Verdonck F. Altered Na/Ca exchange activity in cardiac hypertrophy and heart failure: a new target for therapy? Cardiovasc Res. 2002;53(4):782–805.
Ahmed SH, Clark LL, Pennington WR, Webb CS, Bonnema DD, Leonardi AH, et al. Matrix metalloproteinases/tissue inhibitors of metalloproteinases: relationship between changes in proteolytic determinants of matrix composition and structural, functional, and clinical manifestations of hypertensive heart disease. Circulation. 2006;113(17):2089–96. https://doi.org/10.1161/CIRCULATIONAHA.105.573865.
Gonzalez A, Lopez B, Querejeta R, Zubillaga E, Echeverria T, Diez J. Filling pressures and collagen metabolism in hypertensive patients with heart failure and normal ejection fraction. Hypertension. 2010;55(6):1418–24. https://doi.org/10.1161/HYPERTENSIONAHA.109.149112.
Mohammed SF, Hussain S, Mirzoyev SA, Edwards WD, Maleszewski JJ, Redfield MM. Coronary microvascular rarefaction and myocardial fibrosis in heart failure with preserved ejection fraction. Circulation. 2015;131(6):550–9. https://doi.org/10.1161/CIRCULATIONAHA.114.009625.
Hidalgo C, Hudson B, Bogomolovas J, Zhu Y, Anderson B, Greaser M, et al. PKC phosphorylation of titin’s PEVK element: a novel and conserved pathway for modulating myocardial stiffness. Circ Res. 2009;105(7):631–8, 17 p following 8. https://doi.org/10.1161/CIRCRESAHA.109.198465.
Hidalgo C, Granzier H. Tuning the molecular giant titin through phosphorylation: role in health and disease. Trends Cardiovasc Med. 2013;23(5):165–71. https://doi.org/10.1016/j.tcm.2012.10.005.
Motoki H, Alraies MC, Dahiya A, Saraiva RM, Hanna M, Marwick TH, et al. Changes in left atrial mechanics following pericardiectomy for pericardial constriction. J Am Soc Echocardiogr. 2013;26(6):640–8. https://doi.org/10.1016/j.echo.2013.02.014.
Yamamoto K, Masuyama T, Tanouchi J, Uematsu M, Doi Y, Naito J, et al. Decreased and abnormal left ventricular filling in acute heart failure: role of pericardial constraint and its mechanism. J Am Soc Echocardiogr. 1992;5(5):504–14.
Borlaug BA, Carter RE, Melenovsky V, DeSimone CV, Gaba P, Killu A, et al. Percutaneous pericardial resection: a novel potential treatment for heart failure with preserved ejection fraction. Circ Heart Fail. 2017;10(4):e003612. https://doi.org/10.1161/CIRCHEARTFAILURE.116.003612.
Arbab-Zadeh A, Dijk E, Prasad A, Fu Q, Torres P, Zhang R, et al. Effect of aging and physical activity on left ventricular compliance. Circulation. 2004;110(13):1799–805. https://doi.org/10.1161/01.CIR.0000142863.71285.74.
van Empel VP, Kaye DM, Borlaug BA. Effects of healthy aging on the cardiopulmonary hemodynamic response to exercise. Am J Cardiol. 2014;114(1):131–5. https://doi.org/10.1016/j.amjcard.2014.04.011.
Hollingsworth KG, Blamire AM, Keavney BD, Macgowan GA. Left ventricular torsion, energetics, and diastolic function in normal human aging. Am J Physiol Heart Circ Physiol. 2012;302(4):H885–92. https://doi.org/10.1152/ajpheart.00985.2011.
•• Samuel TJ, Beaudry R, Haykowsky MJ, Sarma S, Nelson MD. Diastolic stress testing: similarities and differences between isometric handgrip and cycle echocardiography. J Appl Physiol (1985). 2018; https://doi.org/10.1152/japplphysiol.00304.2018. This was the first study to demonstrate that isometric handgrip exercise has comparable utility at unmasking diastolic dysfunction measured non-invasively using echocardiography, compared to more commonly used cycle exercise.
• Samuel TJ, Beaudry R, Haykowsky MJ, Sarma S, Park S, Dombrowsky T, et al. Isometric handgrip echocardiography: a noninvasive stress test to assess left ventricular diastolic function. Clin Cardiol. 2017;40(12):1247–55. https://doi.org/10.1002/clc.22818. This was the first study to demonstrate that non-invasive isometric handgrip exercise can successfully differentiate between normal and abnormal diastolic function in a group of individuals at risk for heart failure (ACC/AHA stage A).
Ha JW, Oh JK, Pellikka PA, Ommen SR, Stussy VL, Bailey KR, et al. Diastolic stress echocardiography: a novel noninvasive diagnostic test for diastolic dysfunction using supine bicycle exercise Doppler echocardiography. J Am Soc Echocardiogr. 2005;18(1):63–8. https://doi.org/10.1016/j.echo.2004.08.033.
Lewis BM, Houssay HE, Haynes FW, Dexter L. The dynamics of both right and left ventricles at rest and during exercise in patients with heart failure. Circ Res. 1953;1(4):312–20.
Levine BD, Lane LD, Buckey JC, Friedman DB, Blomqvist CG. Left ventricular pressure-volume and Frank-Starling relations in endurance athletes. Implications for orthostatic tolerance and exercise performance. Circulation. 1991;84(3):1016–23.
Drazner MH, Prasad A, Ayers C, Markham DW, Hastings J, Bhella PS, et al. The relationship of right- and left-sided filling pressures in patients with heart failure and a preserved ejection fraction. Circ Heart Fail. 2010;3(2):202–6. https://doi.org/10.1161/CIRCHEARTFAILURE.108.876649.
Levine BD. Regulation of central blood volume and cardiac filling in endurance athletes: the Frank-Starling mechanism as a determinant of orthostatic tolerance. Med Sci Sports Exerc. 1993;25(6):727–32.
Popovic ZB, Prasad A, Garcia MJ, Arbab-Zadeh A, Borowski A, Dijk E, et al. Relationship among diastolic intraventricular pressure gradients, relaxation, and preload: impact of age and fitness. Am J Physiol Heart Circ Physiol. 2006;290(4):H1454–9. https://doi.org/10.1152/ajpheart.00902.2005.
Shibata S, Hastings JL, Prasad A, Fu Q, Bhella PS, Pacini E, et al. Congestive heart failure with preserved ejection fraction is associated with severely impaired dynamic Starling mechanism. J Appl Physiol (1985). 2011;110(4):964–71. https://doi.org/10.1152/japplphysiol.00826.2010.
Steppan J, Tran H, Benjo AM, Pellakuru L, Barodka V, Ryoo S, et al. Alagebrium in combination with exercise ameliorates age-associated ventricular and vascular stiffness. Exp Gerontol. 2012;47(8):565–72. https://doi.org/10.1016/j.exger.2012.04.006.
Fujimoto N, Borlaug BA, Lewis GD, Hastings JL, Shafer KM, Bhella PS, et al. Hemodynamic responses to rapid saline loading: the impact of age, sex, and heart failure. Circulation. 2013;127(1):55–62. https://doi.org/10.1161/CIRCULATIONAHA.112.111302.
Fujimoto N, Shibata S, Hastings JL, Carrick-Ranson G, Bhella PS, Palmer D, et al. Effects of pericardial constraint and ventricular interaction on left ventricular hemodynamics in the unloaded heart. Am J Physiol Heart Circ Physiol. 2011;300(5):H1688–95. https://doi.org/10.1152/ajpheart.01198.2010.
Borlaug BA, Nishimura RA, Sorajja P, Lam CS, Redfield MM. Exercise hemodynamics enhance diagnosis of early heart failure with preserved ejection fraction. Circ Heart Fail. 2010;3(5):588–95. https://doi.org/10.1161/CIRCHEARTFAILURE.109.930701.
Borlaug BA, Jaber WA, Ommen SR, Lam CS, Redfield MM, Nishimura RA. Diastolic relaxation and compliance reserve during dynamic exercise in heart failure with preserved ejection fraction. Heart. 2011;97(12):964–9. https://doi.org/10.1136/hrt.2010.212787.
Andersen MJ, Ersboll M, Bro-Jeppesen J, Gustafsson F, Hassager C, Kober L, et al. Exercise hemodynamics in patients with and without diastolic dysfunction and preserved ejection fraction after myocardial infarction. Circ Heart Fail. 2012;5(4):444–51. https://doi.org/10.1161/CIRCHEARTFAILURE.112.967919.
Penicka M, Bartunek J, Trakalova H, Hrabakova H, Maruskova M, Karasek J, et al. Heart failure with preserved ejection fraction in outpatients with unexplained dyspnea: a pressure-volume loop analysis. J Am Coll Cardiol. 2010;55(16):1701–10. https://doi.org/10.1016/j.jacc.2009.11.076.
Abudiab MM, Redfield MM, Melenovsky V, Olson TP, Kass DA, Johnson BD, et al. Cardiac output response to exercise in relation to metabolic demand in heart failure with preserved ejection fraction. Eur J Heart Fail. 2013;15(7):776–85. https://doi.org/10.1093/eurjhf/hft026.
Andersen MJ, Borlaug BA. Invasive hemodynamic characterization of heart failure with preserved ejection fraction. Heart Fail Clin. 2014;10(3):435–44. https://doi.org/10.1016/j.hfc.2014.03.001.
Andersen MJ, Ersboll M, Bro-Jeppesen J, Moller JE, Hassager C, Kober L, et al. Relationships between biomarkers and left ventricular filling pressures at rest and during exercise in patients after myocardial infarction. J Card Fail. 2014;20(12):959–67. https://doi.org/10.1016/j.cardfail.2014.09.012.
Andersen MJ, Olson TP, Melenovsky V, Kane GC, Borlaug BA. Differential hemodynamic effects of exercise and volume expansion in people with and without heart failure. Circ Heart Fail. 2015;8(1):41–8. https://doi.org/10.1161/CIRCHEARTFAILURE.114.001731.
Borlaug BA. Mechanisms of exercise intolerance in heart failure with preserved ejection fraction. Circ J. 2014;78(1):20–32.
Borlaug BA, Kane GC, Melenovsky V, Olson TP. Abnormal right ventricular-pulmonary artery coupling with exercise in heart failure with preserved ejection fraction. Eur Heart J. 2016;37(43):3293–302. https://doi.org/10.1093/eurheartj/ehw241.
Borlaug BA, Koepp KE, Melenovsky V. Sodium nitrite improves exercise hemodynamics and ventricular performance in heart failure with preserved ejection fraction. J Am Coll Cardiol. 2015;66(15):1672–82. https://doi.org/10.1016/j.jacc.2015.07.067.
Borlaug BA, Melenovsky V, Koepp KE. Inhaled sodium nitrite improves rest and exercise hemodynamics in heart failure with preserved ejection fraction. Circ Res. 2016;119(7):880–6. https://doi.org/10.1161/CIRCRESAHA.116.309184.
Borlaug BA, Melenovsky V, Russell SD, Kessler K, Pacak K, Becker LC, et al. Impaired chronotropic and vasodilator reserves limit exercise capacity in patients with heart failure and a preserved ejection fraction. Circulation. 2006;114(20):2138–47. https://doi.org/10.1161/CIRCULATIONAHA.106.632745.
Borlaug BA, Reddy YN. Determinants and correlates of exercise capacity in heart failure. JACC Heart Fail. 2015;3(10):815–7. https://doi.org/10.1016/j.jchf.2015.07.005.
Gharacholou SM, Scott CG, Borlaug BA, Kane GC, McCully RB, Oh JK, et al. Relationship between diastolic function and heart rate recovery after symptom-limited exercise. J Card Fail. 2012;18(1):34–40. https://doi.org/10.1016/j.cardfail.2011.09.010.
Hussain I, Mohammed SF, Forfia PR, Lewis GD, Borlaug BA, Gallup DS, et al. Impaired right ventricular-pulmonary arterial coupling and effect of sildenafil in heart failure with preserved ejection fraction: an ancillary analysis from the phosphodiesterase-5 inhibition to improve clinical status and exercise capacity in diastolic heart failure (RELAX) trial. Circ Heart Fail. 2016;9(4):e002729. https://doi.org/10.1161/CIRCHEARTFAILURE.115.002729.
Kaye D, Shah SJ, Borlaug BA, Gustafsson F, Komtebedde J, Kubo S, et al. Effects of an interatrial shunt on rest and exercise hemodynamics: results of a computer simulation in heart failure. J Card Fail. 2014;20(3):212–21. https://doi.org/10.1016/j.cardfail.2014.01.005.
Lam CS, Grewal J, Borlaug BA, Ommen SR, Kane GC, McCully RB, et al. Size, shape, and stamina: the impact of left ventricular geometry on exercise capacity. Hypertension. 2010;55(5):1143–9. https://doi.org/10.1161/HYPERTENSIONAHA.109.146845.
Little WC, Borlaug BA. Exercise intolerance in heart failure with preserved ejection fraction: what does the heart have to do with it? Circ Heart Fail. 2015;8(2):233–5. https://doi.org/10.1161/CIRCHEARTFAILURE.114.001966.
Mohammed SF, Borlaug BA, McNulty S, Lewis GD, Lin G, Zakeri R, et al. Resting ventricular-vascular function and exercise capacity in heart failure with preserved ejection fraction: a RELAX trial ancillary study. Circ Heart Fail. 2014;7(4):580–9. https://doi.org/10.1161/CIRCHEARTFAILURE.114.001192.
Redfield MM, Borlaug BA, Lewis GD, Mohammed SF, Semigran MJ, Lewinter MM, et al. PhosphdiesteRasE-5 inhibition to improve clinical status and exercise capacity in diastolic heart failure (RELAX) trial: rationale and design. Circ Heart Fail. 2012;5(5):653–9. https://doi.org/10.1161/CIRCHEARTFAILURE.112.969071.
Redfield MM, Chen HH, Borlaug BA, Semigran MJ, Lee KL, Lewis G, et al. Effect of phosphodiesterase-5 inhibition on exercise capacity and clinical status in heart failure with preserved ejection fraction: a randomized clinical trial. JAMA. 2013;309(12):1268–77. https://doi.org/10.1001/jama.2013.2024.
Wolsk E, Kaye D, Borlaug BA, Burkhoff D, Kitzman DW, Komtebedde J, et al. Resting and exercise haemodynamics in relation to six-minute walk test in patients with heart failure and preserved ejection fraction. Eur J Heart Fail. 2017;20:715–22. https://doi.org/10.1002/ejhf.976.
Zakeri R, Borlaug BA, McNulty SE, Mohammed SF, Lewis GD, Semigran MJ, et al. Impact of atrial fibrillation on exercise capacity in heart failure with preserved ejection fraction: a RELAX trial ancillary study. Circ Heart Fail. 2014;7(1):123–30. https://doi.org/10.1161/CIRCHEARTFAILURE.113.000568.
•• Obokata M, Kane GC, Reddy YN, Olson TP, Melenovsky V, Borlaug BA. The role of diastolic stress testing in the evaluation for HFpEF: a simultaneous invasive-echocardiographic study. Circulation. 2016; https://doi.org/10.1161/CIRCULATIONAHA.116.024822. This study confirmed the close relationship between invasively measured PCWP and non-invasive Doppler-derived E/e’ ratio in a large group of HFpEF patients ( n = 50). This study also demonstrated that low intensity cycle exercise (20W) was sufficient to elicit clinically significant increases in PCWP and E/e’ and was therefore formed a rationale for the use of low intensity exercise of many subsequent investigations.
Ha JW, Choi EY, Choi D, Park S, Shim CY, Lee JH, et al. Time course of recovery of left ventricular filling pressure after exercise in healthy subjects. Circ J. 2008;72(2):186–8.
Alam M, Smirk FH. Observations in man upon a blood pressure raising reflex arising from the voluntary muscles. J Physiol. 1937;89(4):372–83.
Westermann D, Kasner M, Steendijk P, Spillmann F, Riad A, Weitmann K, et al. Role of left ventricular stiffness in heart failure with normal ejection fraction. Circulation. 2008;117(16):2051–60. https://doi.org/10.1161/CIRCULATIONAHA.107.716886.
Kawaguchi M, Hay I, Fetics B, Kass DA. Combined ventricular systolic and arterial stiffening in patients with heart failure and preserved ejection fraction: implications for systolic and diastolic reserve limitations. Circulation. 2003;107(5):714–20.
• Rommel KP, von Roeder M, Oberueck C, Latuscynski K, Besler C, Blazek S, et al. Load-independent systolic and diastolic right ventricular function in heart failure with preserved ejection fraction as assessed by resting and handgrip exercise pressure-volume loops. Circ Heart Fail. 2018;11(2):e004121. https://doi.org/10.1161/CIRCHEARTFAILURE.117.004121. This study was one of the first invasive investigations to utilize isometric handgrip exercise to elicit significant upward and leftward shift in the left and right ventricular end-diastolic pressure volume relationship, a hallmark of diastolic dysfunction, in a population of HFpEF patients.
Mark AL, Victor RG, Nerhed C, Wallin BG. Microneurographic studies of the mechanisms of sympathetic nerve responses to static exercise in humans. Circ Res. 1985;57(3):461–9.
Victor RG, Secher NH, Lyson T, Mitchell JH. Central command increases muscle sympathetic nerve activity during intense intermittent isometric exercise in humans. Circ Res. 1995;76(1):127–31.
Victor RG, Vissing SF, Urias L, Scherrer U. Central motor command activates sympathetic outflow to skin during static exercise in humans. Clin Res. 1989;37(2):A524-A.
Delaney EP, Greaney JL, Edwards DG, Rose WC, Fadel PJ, Farquhar WB. Exaggerated sympathetic and pressor responses to handgrip exercise in older hypertensive humans: role of the muscle metaboreflex. Am J Physiol Heart Circ Physiol. 2010;299(5):H1318–27. https://doi.org/10.1152/ajpheart.00556.2010.
Ogoh S, Wasmund WL, Keller DM, O-Yurvati A, Gallagher KM, Mitchell JH, et al. Role of central command in carotid baroreflex resetting in humans during static exercise. J Physiol Lond. 2002;543(1):349–64. https://doi.org/10.1113/jphysiol.2002.019943.
Kawase Y, Ly HQ, Prunier F, Lebeche D, Shi Y, Jin H, et al. Reversal of cardiac dysfunction after long-term expression of SERCA2a by gene transfer in a pre-clinical model of heart failure. J Am Coll Cardiol. 2008;51(11):1112–9. https://doi.org/10.1016/j.jacc.2007.12.014.
Balderas-Villalobos J, Molina-Munoz T, Mailloux-Salinas P, Bravo G, Carvajal K, Gomez-Viquez NL. Oxidative stress in cardiomyocytes contributes to decreased SERCA2a activity in rats with metabolic syndrome. Am J Physiol Heart Circ Physiol. 2013;305(9):H1344–53. https://doi.org/10.1152/ajpheart.00211.2013.
Berridge MJ, Bootman MD, Roderick HL. Calcium signalling: dynamics, homeostasis and remodelling. Nat Rev Mol Cell Biol. 2003;4(7):517–29. https://doi.org/10.1038/nrm1155.
Gwathmey JK, Copelas L, MacKinnon R, Schoen FJ, Feldman MD, Grossman W, et al. Abnormal intracellular calcium handling in myocardium from patients with end-stage heart failure. Circ Res. 1987;61(1):70–6.
Hunter WC. Role of myofilaments and calcium handling in left ventricular relaxation. Cardiol Clin. 2000;18(3):443–57.
Shim CY, Park S, Choi EY, Hong GR, Choi D, Jang Y, et al. The relationship between ventricular-vascular uncoupling during exercise and impaired left ventricular longitudinal functional reserve in hypertensive patients. J Am Soc Hypertens. 2013;7(3):198–205. https://doi.org/10.1016/j.jash.2013.01.005.
Gibby C, Wiktor DM, Burgess M, Kusunose K, Marwick TH. Quantitation of the diastolic stress test: filling pressure vs. diastolic reserve. Eur Heart J Cardiovasc Imaging. 2013;14(3):223–7. https://doi.org/10.1093/ehjci/jes078.
Sonaglioni A, Lombardo M, Baravelli M, Trotta G, Sommese C, Anza C. Exercise stress echocardiography with tissue Doppler imaging in risk stratification of mild to moderate aortic stenosis. Int J Cardiovasc Imaging. 2015;31(8):1519–27. https://doi.org/10.1007/s10554-015-0724-9.
Christensen NL, Dahl JS, Carter-Storch R, Bakkestrom R, Jensen K, Steffensen FH, et al. Association between left atrial dilatation and invasive hemodynamics at rest and during exercise in asymptomatic aortic stenosis. Circ Cardiovasc Imaging. 2016;9(10) https://doi.org/10.1161/CIRCIMAGING.116.005156.
Obokata M, Nagata Y, Kado Y, Kurabayashi M, Otsuji Y, Takeuchi M. Ventricular-arterial coupling and exercise-induced pulmonary hypertension during low-level exercise in heart failure with preserved or reduced ejection fraction. J Card Fail. 2017;23(3):216–20. https://doi.org/10.1016/j.cardfail.2016.10.001.
Kosmala W, Przewlocka-Kosmala M, Rojek A, Marwick TH. Comparison of the diastolic stress test with a combined resting echocardiography and biomarker approach to patients with exertional dyspnea: diagnostic and prognostic implications. JACC Cardiovasc Imaging. 2018; https://doi.org/10.1016/j.jcmg.2017.10.008.
Gorter TM, Obokata M, Reddy YNV, Melenovsky V, Borlaug BA. Exercise unmasks distinct pathophysiologic features in heart failure with preserved ejection fraction and pulmonary vascular disease. Eur Heart J. 2018; https://doi.org/10.1093/eurheartj/ehy331.
Obokata M, Reddy YNV, Melenovsky V, Kane GC, Olson TP, Jarolim P, et al. Myocardial injury and cardiac reserve in patients with heart failure and preserved ejection fraction. J Am Coll Cardiol. 2018;72(1):29–40. https://doi.org/10.1016/j.jacc.2018.04.039.
Obokata M, Olson TP, Reddy YNV, Melenovsky V, Kane GC, Borlaug BA. Haemodynamics, dyspnoea, and pulmonary reserve in heart failure with preserved ejection fraction. Eur Heart J. 2018; https://doi.org/10.1093/eurheartj/ehy268.
Hieda M, Howden E, Shibata S, Tarumi T, Lawley J, Hearon C Jr, et al. Preload-corrected dynamic Starling mechanism in patients with heart failure with preserved ejection fraction. J Appl Physiol (1985). 2018;124(1):76–82. https://doi.org/10.1152/japplphysiol.00718.2017.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical Collection on Pathophysiology: Neuroendocrine, Vascular, and Metabolic Factors
Rights and permissions
About this article
Cite this article
Samuel, T.J., Beaudry, R., Sarma, S. et al. Diastolic Stress Testing Along the Heart Failure Continuum. Curr Heart Fail Rep 15, 332–339 (2018). https://doi.org/10.1007/s11897-018-0409-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11897-018-0409-5