Abstract
In the present study, the potential role of hydrogen peroxide (H2O2) and nitric oxide (NO) has been well recorded in the induction of cadmium (Cd) stress tolerance in cyanobacteria. In this regard, H2O2 and SNP (sodium nitroprusside, NO donor), were applied to Nostoc muscorum and Anabaena sp. exposed to Cd (6 µM) stress, to analyze different physiological and biochemical parameters. Results revealed that treatment of Cd reduced the growth, pigment contents, photosynthetic oxygen yield and performance of PS II photochemistry (decreased chlorophyll a fluorescence parameters, i.e., ФPo, Ψo, ФEo, PIABS along with Fv/Fo and increased the energy flux parameters, i.e., ABS/RC, TRo/RC, ETo/RC, DIo/RC along with Fo/Fv. Similarly, uptake of nitrate (NO3−) and nitrite (NO2−), as well as the activities of nitrate and ammonia assimilating enzymes along with carbohydrate content, were severely affected by Cd toxicity and notwithstanding this, glutamate dehydrogenase (GDH) activity exhibited reverse trend. Exogenous application of a very low dose (1 µM) of H2O2 (only for 3 h) and NO (SNP; 10 µM) notably counteracted Cd-induced toxicity. Nevertheless, the positive impact of H2O2 got reversed under the treatment of PTIO (NO scavenger) and LNAME (inhibitor of nitric oxide synthase; NOS) while NO could work efficiently even in the presence of NAC (H2O2 scavenger) and DPI (inhibitor of NADPH oxidase); hence indicated towards the H2O2 mediated NO signaling in averting Cd induced toxicity in test cyanobacteria. In conclusion, current finding demonstrated a positive cross-talk between H2O2 and NO for providing tolerance to cyanobacteria against Cd stress.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The presence of heterocystous, photosynthetic cyanobacteria in paddy fields serves as biofertilizers by directly fixing the atmospheric nitrogen into ammonia, hence support the paddy crops. Among them, Nostoc muscorum and Anabaena sp. are the most valuable and natural inhabitants of paddy fields (Singh et al. 2016; Tiwari and Prasad 2020). They can fix atmospheric nitrogen at an average of about 20–25 kg/ha/season, hence considered one of the necessary components for sustainable agricultural development. Besides this, they can enhance the aeration capacity of the soil; release some plant growth-promoting hormones along with numerous beneficial extracellular products (Prasanna et al. 2013; Chittora et al. 2020). They can also provide resistivity to our staple crops against various diseases and abiotic stresses (Saadatnia and Riahi 2009; Chittora et al. 2020). Thus, the luxuriant growth of cyanobacteria in rice fields is extremely important for the sustainability of agriculture thereby enhanced yield of rice may fulfill the food demand of our fast growing population.
In recent years, as a result of improper waste disposal management practices, the effluents of industries containing toxic metals are being discharged directly to the water of life saving rivers which are frequently used by farmers to irrigate soils. The metal-contaminated water severely affects the health of such beneficial microflora as well as crop plants by altering their metabolisms, and this leads to a considerable loss in crop productivity. Among the heavy metals, cadmium is considered as most toxic one. The study of Rizwan et al. (2016) reported that 100 mg Cd in kg−1 soil caused chlorosis and necrosis resulting in greater loss in rice yield and biomass. Overall, rice production as well as quality of rice are highly affected by Cd contamination. Besides this, the microflora of rice fields are also found to be affected by excess Cd, thereby the sustainable production of rice is compromised causing a serious issue for our agricultural economy. The studies have demonstrated that a proper input of nitrogen fertilizers (containing N of 2.2–2.4% w/v) can alleviate Cd toxicity in rice seedlings (Jalloh et al. 2009; Lin et al. 2011; Rizwan et al. 2016). So considering this fact we hypothesized that heterocystous cyanobacteria Nostoc muscorum and Anabaena sp. might play an important role in the alleviation of Cd toxicity in rice crops by providing proper N2-supply. Nevertheless, excess level of Cd in soil interrupts the heath and metabolic processes of cyanobacteria (Verma and Prasad 2021). Ahad and Syiem (2018a) have reported the presence of P-type Cd2+ATPases (e.g. cadA1 and cadA2) in Synechocystis sp. Cadmium can easily enter into the cells of these organisms through P-type Cd2+ATPases, which leads to excessive ROS production that finally degrades the membrane integrity and antioxidant machinery (Ahad and Syiem 2019). Such type of damaging impacts of Cd on protein has also been demonstrated by Mehta et al. (2014) where FutA2, a periplasmic Fe binding protein decreased under Cd exposure in Synechocystis. In recent years, for sustainable growth of cyanobacteria that are exposed to metal or other abiotic stresses several medications were suggested by recent studies. Exogenous application of growth-promoting hormones such as kinetin (KN) (Tiwari et al. 2018; Tiwari and Prasad 2020) and indole acetic acid (IAA) (Tiwari et al. 2020a), Ca2+ as a second messenger (Ahad and Syiem 2019), and NO as a signaling molecule (Verma and Prasad 2021; Tiwari et al. 2019) are the important medications suggested. Among these modulators, the application of signaling molecules might be cost-effective and one of the reliable techniques found to reduce the negative effects of various abiotic stresses because a very low dose of these signaling molecules is effective and sufficient to alter various physiological and biochemical processes.
Among various signaling molecules, NO and H2O2 are potentially active and multitasking signaling molecules. In the recent past, nitric oxide (NO) has conquered much attention due to its wider roles in plants at every developmental stage, from seed germination to plant death (Kushwaha et al. 2019; Sharma et al. 2020; Verma et al. 2020). Moreover, several studies have shown the positive implication of NO in the regulation of abiotic stress including heavy metals in plants (Corpas and Barroso 2017; Romero-Puertas et al. 2019) as well as in cyanobacterium Anabaena sp. PCC 7120 (Tiwari et al. 2019). It has been demonstrated that the pretreatment of H2O2 enhanced the cellular defense against oxidative stress induced by Cd in rice and Brassica napus seedlings (Hu et al. 2009; Hasanuzzaman et al. 2017), aluminium (Al) and salinity in wheat seedlings (Xu et al. 2010; Li et al. 2011). Moreover, interactive responses of H2O2 and NO towards the induction of thermo-tolerance in maize seedlings (Li et al. 2015), salinity tolerance in strawberry plants (Christou et al. 2014) as well as in basil seedlings (Gohari et al. 2019) have also been recorded. But, the implication of H2O2 and NO and their relative coordination chemistry in mitigation of Cd stress is still not studied in cyanobacteria. Till date the application of H2O2 and NO in the alleviation of abiotic stress is confined only to higher plants. The positive assessments of H2O2 and NO on overall PS II photochemistry and N2-metabolism of cyanobacteria under Cd stress are also the uniqueness of the present study. Thus the present study followed this unique concept to achieve a reliable and most significant method to mitigate Cd toxicity in cyanobacterial cells and it is hypothesized that H2O2 might have a role in NO-mediated management of Cd toxicity in cyanobacteria.
Materials and methods
Test organisms and culture conditions
The homogenous, filamentous, and heterocystous cyanobacteria Nostoc muscorum ATCC 27893 and Anabaena sp. PCC 7120 were cultured in BG-11 medium (pH 7.5) in a temperature- controlled room having 25 ± 2 °C under 75 μmol photons m−2 s−1photosynthetically active radiation (PAR, 400–700 nm) provided by white fluorescent tubes (Osram L 40 W/25–1) with a 14:10 h regime of light:dark. The experiments were carried out by using the cultures of the exponential phase.
Experimental design and culture treatment
For all the experiments, exponential phase cultures of both the tested cyanobacteria were collected by centrifuging them at 3,000 g for 15 min and cells were washed twice with sterile distilled water. Henceforth, cyanobacterial cells were suspended in a growth medium containing Cd (6 µM), 10 µM SNP (sodium nitroprusside; a donor of NO), 20 µM PTIO (2-phenyl-4,4,5,5-tetramethylimidazoline-1-oxyl 3-oxide; scavenger of NO), 100 µM LNAME (Nω-Nitro-L-arginine methyl ester hydrochloride; NOS enzyme’s inhibitor), 1 µM H2O2, 1 mM NAC (N-acetyl-L-cysteine; scavenger of H2O2), 1 µM DPI (diphenyleneiodonium chloride; an inhibitor of NADPH oxidase enzyme) wherever required. Accordingly, the experimental combinations were: Control, Cd, Cd + H2O2, Cd + NAC, Cd + DPI, Cd + SNP, Cd + PTIO, Cd + LNAME, Cd + H2O2 + PTIO, Cd + H2O2 + LNAME, Cd + SNP + NAC, Cd + SNP + DPI, Cd + H2O2 + SNP + PTIO + LNAME, Cd + H2O2 + SNP + NAC + DPI.
Hydrogen peroxide (H2O2), a short-lived photodegradable molecule has been used for 24 h of the experiment to treat the cyanobacterial cultures grown for 14:10 dark and light conditions. So for the present experiment the required concentration of H2O2 was prepared very carefully in dark and poured into the medium at the end of addition of different components in each experimental setup, and then the cultures of all the setups were incubated for 3 h in dark prior to the exposure of cultures under 14:10 h light/dark period at 25 ± 2 °C. Each parameter was analyzed just after 24 h of experiment.
Measurement of growth
The growth of both the test organisms was analyzed in terms of optical density (OD) for that 3 ml of treated and untreated cells of both the cultures were harvested and thereafter they were well homogenized. Then optical density of the culture of each set was recorded at 750 nm by using a double beam UV–visible spectrophotometer (Shimadzu, Japan).
Histochemical analysis for cadmium accumulation
Histochemical analysis for Cd accumulation inside the cells was observed in the form of red patches that appeared due to the complex formation between dithiozone and Cd. This analysis was carried out by using the method of Seregin and Kozhevnikova (2011) with some minor changes. To detect the intracellular Cd accumulation; 3 ml of treated and untreated cells were collected by centrifugation after 24 h of the treatment. Thereafter, collected cells were gently washed twice or thrice with distilled water (DW) to remove excess Cd. The stock solution of dithiozone was prepared by dissolving 6 g of dithiozone in acetone and then DW was added in the ratio of 3:1. Further, 1–2 drops of acetic acid glacial were added to solution. To the cells of each set up 1 ml of dithiozone solution was added and after 4 h of incubation cells were observed under the microscope (Leica, model-DM 2500).
Estimation of carbohydrate content
The content of carbohydrate was determined according to Dubois et al. (1956). The 100 ml cultures from each set were centrifuged (10,000 g) for 10 min, the pellets were suspended in 10 ml DW and 1 ml of cell suspension was added to the reagent containing 1 ml of 5% phenol and 5 ml H2SO4. The absorbance of respective treatments was recorded at 490 nm with the help of spectrophotometer and content was calculated by using a standard curve prepared with pure glucose.
Estimation of the photosynthetic pigments
The contents of photosynthetic pigments i.e. chlorophyll a (Chl a) and carotenoids (Car) in test organisms were estimated by following the methods of Porra et al. (1989) and Goodwin (1954), respectively, and read the absorbance by using spectrophotometer (Double beam UV–Visible, Shimadzu, Japan) at 665 nm and 450 nm, respectively. The amounts of phycobiliproteins i.e. phycoerythrin (PE), allophycocyanin (APC) and phycocyanin (PC) were determined by recording the absorbance at 615, 652 and 562 nm by adopting the method of Bennett and Bogorad, (1973). The following equations were used to calculate the contents of phycobiliproteins:
Measurement of whole cell O2 evolution and respiration
The rate of photosynthesis as whole-cell oxygen evolution in treated and untreated cells of the test organisms was measured by using Clark type O2 electrode (Digital oxygen system model-10, Rank Brothers, U.K) under the saturating light intensity of 360 μmol photons m−2 s−1 PAR at temperature 25 °C. Respiratory activity of the same sample as oxygen consumption was measured under darkness under similar conditions.
Measurement of PS II photochemistry (JIP-Test)
The parameters of Chl a fluorescence kinetics were measured by using handheld fluorometer (AquaPen AP 100, Photon System Instruments, Czech Republic) as per the method of Strasser et al. (2000) in the dark-adapted (to make the reaction centers open) cyanobacterial samples. The analyzed parameters i.e. Fv/Fo (size and number of active reaction centers); Fo/Fv (efficiency of water splitting complex); PIABS (Overall performance index); Phi_Poor ФPo (quantum yield of primary photochemistry); Psi_o or Ψo (yield of electron transport per trapped exciton); Phi_Eo or ФEo (quantum yield of electron transport); Phi_Do or ФDo (probability of dissipation of an absorbed photon); the specific energy fluxes i.e. ABS/RC (absorption flux per reaction center); TRo/RC (trapping flux per reaction center); ETo/RC (electron transport per reaction center); DIo/RC (dissipation energy per reaction center) represent the overall status of PS II photochemistry under tested conditions.
Uptake of inorganic nitrogen
Nitrate uptake
Nitrate (NO3−) uptake was estimated spectrophotometrically at 210 nm by measuring the depletion of NO3− from the medium of untreated and treated cultures as per the method of Cawse (1967).
Nitrite uptake
Nitrite (NO2−) uptake in each sample was estimated as per the method of Snell and Snell (1949), Depletion of NO2− from the medium was determined by recording the absorption at 540 nm.
Assay of nitrogen assimilating enzymes
Nitrate reductase (NR; EC 1.6.6.1) and nitrite reductase (NiR; EC 1.7.7.1) activities
Methods proposed by Herrero et al. (1981, 1984) and Herrero and Guerrero (1986) were adopted to analyze the activities of nitrate reductase (NR; EC 1.6.6.1) and nitrite reductase (NiR; EC 1.7.7.1), respectively. The experiments were performed with dithionite-reduced methyl viologen as a reductant in cyanobacterial cells which were permeabilzed by adding alkyltrimethyl ammonium bromide (MTA). The ions of NO2– in cell-free reaction mixture were estimated according to the method of Snell and Snell (1949). One unit (U) NR activity is defined as 1 nmol NO2– formed min–1 whereas one unit (U) NiR activity is defined as 1 nmol NO2– consumed min–1.
Glutamine synthetase (GS; EC 6.3.1.2) activity
Method of Mérida et al. (1991) was adopted for the determination of glutamine synthetase (GS) enzyme activity considering the production of γ–glutamylhydroxamate. Treated and untreated cells were collected, centrifuged and washed with nitrogen-free medium and resuspended in HEPES–NaOH buffer (pH 7.0). Cells were disrupted by sonication (by using Vibra-sonicator, Sonics and materials) and homogenate was centrifuged at 15,000 g for 20 min at 4 °C. The 50 µl of cell extract added to the reaction mixture containing 60 µM of HEPES–NaOH buffer (pH 7.0), 40 µM of L–glutamine, 4 µM of MnCl2, 60 µM of hydroxylamine, 1 µM of ADP, 20 µM of sodium arsenate. The reaction was started by adding sodium arsenate and the amount of γ–glutamylhydroxamate formed after 10 min of incubation at 28 °C was determined by recording the absorbance at 500 nm. One unit (U) GS activity is defined as 1 nmol γ-glutamylhydroxamate formed min–1.
Glutamate synthase (GOGAT; EC 1.4.1.14) activity
GOGAT activity was measured by following the methods of Meers et al. (1970) and Navarro et al. (1995), respectively for NADH-GOGAT in Nostoc muscorum and Fd-GOGAT in Anabaena sp. Enzyme activity was estimated by recording the absorbance at 340 nm to measure the oxidation of NADH for Nostoc muscorum and formation of glutamate for Anabaena sp. One unit of GOGAT activity is defined as 1 nmol NADH oxidized min−1 for Nostoc muscorum and 1 nmol glutamate formed min−1 for Anabaena sp.
Glutamate dehydrogenase (NADH-GDH; EC 1.4.1.2) activity
Glutamate dehydrogenase activity was determined by following the method of Chávez and Candau (1991). Treated and untreated cells were collected, centrifuged and crushed in HEPES–NaOH buffer (pH 7.0) and supernatant obtained was used as the enzyme extract. The reaction was started by the addition NH4Cl. The activity was analyzed spectrophotometrically at 340 nm by measuring the oxidation of NADH. One unit (U) of GDH activity is defined as 1 nmol NADH oxidized min–1.
Statistical analysis
Analysis of variance (ANOVA) was used for the statistical analysis of results with the help of SPSS 16.0. Tukey test was used for mean separation for significant differences among the treatments at P < 0.05 significance levels. Presented results are the means ± standard error of three replicates (n = 3).
Results and discussion
H2O2 promotes NO to enhance the growth of Cd stressed cyanobacteria
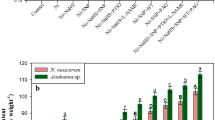
The impact of exogenous H2O2 and NO on the growth of Cd stressed Nostoc muscorum ATCC 27893 and Anabaena sp. PCC 7120 was analyzed by recording optical density (OD750nm) and the results have been displayed in Fig. 1a, b. The 6 µM of Cd significantly (P < 0.05) declined the growth as it was diminished by 28% in N. muscorum and 30% in Anabaena sp., as compared with respective controls. The H2O2 and NO, two physiologically and biologically important signaling molecules are reported to be essential for the overall growth and development of plants (Qiao et al. 2014; Saxena et al. 2016). The H2O2 can enter into the cell directly via the cell wall and regulates the antioxidant defence system. Once signaling is initiated inside the cell, the cyanobacteria get empowered to cope up the stress. In the present study, exogenous H2O2 or NO (SNP) markedly reduced the negative effect of Cd on growth (the effect of NO was found to be more prominent than H2O2), and the reduction in growth was remained only 9 and 7%, in N. muscorum and 11 and 7%, in Anabaena sp. respectively in comparison to control. The alleviation in Cd toxicity on growth due to exogenous H2O2 and NO (SNP) could be correlated with (i) enhanced photosynthase carbohydrate content (Fig. 1c) (ii) reduced intracellular accumulation of Cd (Fig. 2) (iii) improved photosynthetic pigments (Fig. 3) and N-metabolism (Tables 1 and 2) and (iv) appreciable recovery in PS II performance (Figs. 5, 6, 7). Similar to current finding, Christou et al. (2014) also noticed positive role of H2O2 and SNP in salt stress alleviation in strawberry plants. Further, the scavengers (NAC for H2O2 and PTIO for NO) and inhibitors (DPI for H2O2 biosynthetic enzyme NADPH-oxidase and LNAME for NO biosynthetic enzyme NOS-synthase) of H2O2 and NO were applied exogenously and a crucial decrease in growth was noticed thereby suggesting the role of NOS and NADPH oxidase enzymes in maintenance of endogenous basal levels of NO and H2O2 in Cd stress alleviation (Fig. 1b). Similar positive response of NO and H2O2 was also noticed in soybean plants under arsenate stress (Singh et al. 2020). Furthermore, to understand the interaction between H2O2 and NO in Cd toxicity alleviation, scavengers and inhibitors were also applied exogenously together with the contrasting signaling molecules (Fig. 1a). Thus, the results revealed that H2O2 alone was not able to alleviate the Cd toxicity in the presence PTIO or LNAME hence indicated that H2O2 might be acting as upstream-regulator of NO in Cd stress alleviation. On the other hand, NO (SNP) could work independently even in the presence of NAC or DPI, however its action may be initiated by H2O2 to release Cd stress in test cyanobacterial strains. Our study is in congruence with other findings where cross-talk between H2O2 and NO was also observed during equipping the copper stress tolerance in alga Ulva compressa (Gonzalez et al. 2012), thermotolerance in maize seedlings (Li et al. 2015) and salt stress tolerance in Oscimum basilicum (Gohari et al. 2019).
Impact of exogenous H2O2 and NO on growth (optical density) (a, b) and carbohydrate content (c) of Nostoc muscorum ATCC 27893 and Anabaena sp. PCC 7120 exposed to Cd after 24 h of experiments. Data represent the mean value ± standard error of three replicates (n = 3). Bars followed by different letters show significant difference at P < 0.05 significance level according to Tukey test
In vivo analysis of Cd accumulation (red patches indicated by red arrows) inside the cells of Nostoc muscorum ATCC 27893 and Anabaena sp. PCC 7120 exposed to Cd stress. Where lane I: Control, lane II: Cd, lane III: Cd + H2O2, lane IV: Cd + SNP, lane V: Cd + H2O2 + PTIO, lane VI: Cd + H2O2 + LNAME, lane VII: Cd + SNP + NAC, lane VIII: Cd + SNP + DPI, lane IX: Cd + H2O2 + SNP + PTIO + LNAME, lane X: Cd + H2O2 + SNP + NAC + DPI
Impact of exogenous H2O2 and NO on photosynthetic pigments contents: chlorophyll a (a), carotenoids (b), phycoerythrin (c), phycocyanin (d) and allophycocyanin (e) of Nostoc muscorum ATCC 27893 and Anabaena sp. PCC 7120 exposed to Cd after 24 h of experiments. Data represent the mean value ± standard error of three replicates (n = 3). Bars followed by different letters show significant difference at P < 0.05 significance level according to Tukey test
H2O2 up regulates NO to enhance carbohydrate content in Cd stressed cyanobacteria
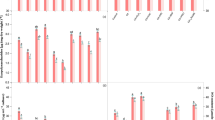
The data pertaining to the carbohydrate content in both the test cyanobacteria have been portrayed in Fig. 1c. Carbohydrates are the principle components of cyanobacterial exopolysaccharides (EPS) which act as a defensive layer around the cell environment and are important for their survival in stressed conditions (Chakraborty and Pal 2014). A simplified lipopolysaccharide contains about 31–80% carbohydrates (Bhatnagar and Bhatnagar 2019). Despite this, carbohydrates are also reserved as food products inside the cyanobacterial cells which are directly utilized by them as the energy source for survival under stressful environments, hence directly linked with the growth of cyanobacteria. Results revealed that Cd at the elevated concentration (6 µM) declined the content of carbohydrate by 31% in N. muscorum and 32% in Anabaena sp. with respect to controls (Fig. 1c). Substantial reduction in carbohydrate content in both the cyanobacteria under Cd stress might have led to the formation of a weak defensive layer of exopolysaccharides subsequently allowed more Cd to enter inside the cell as evident by the histochemical analysis (Fig. 2). The in vivo analysis of intracellular Cd accumulation has been displayed by the appearance of intense red patches in the cells of both the test cyanobacteria. Excessive Cd accumulation caused more damages to the cell thereby disturbing the cellular metabolisms i.e. photosynthesis and nitrogen metabolism of test organisms. Nonetheless, exogenous application of H2O2 or NO (SNP) significantly (P < 0.05) recovered the cell from the negative impact of Cd on carbohydrates content which was significantly increased by 9 and 12% in N. muscorum and 8 and 10% in Anabaena sp., respectively with the more pronounced effect of NO. Improved carbohydrate content (Fig. 1c) probably strengthened the exopolysacchride defensive layer, hence reduced Cd accumulation (Fig. 2) as shown in our results (less red patches) when cells were exposed to Cd + H2O2 and Cd + NO (SNP). The previous study of Ahad and Syiem (2018a) also reported that Cd reduced the carbohydrate content but exogenously applied calcium significantly improved the carbohydrate content in Cd challenged cyanobacterium Nostoc muscorum Meg 1. Similarly, Weifeng et al. (2018) reported that exogenous SNP potentially reduced the Cd content in leaves of perennial ryegrass. A recent study by Liu et al. (2020) has experimentally demonstrated that Cd uptake was significantly reduced in maize grain by exogenous NO supplementation. Furthermore, H2O2 alone (with the addition of PTIO or LNAME) did not show significant recovery in carbohydrate content under Cd stress, while NO effectively regulated the negative effect as considerable improvement in carbohydrate content was recorded even in presence of NAC or DPI. The PTIO and LNAME addition to cells worsened the Cd toxicity due to increased accumulation of Cd. Thus, when cells of both the organisms were subjected to Cd + PTIO + H2O2 and Cd + LNAME + H2O2, intense red patches were found while the intensity became considerably low under the treatment of Cd + SNP(NO) + NAC and Cd + SNP(NO) + DPI combinations (Figs. 1c, 2). The results indicated that the NO appears to be prime player in regulating the accumulation of Cd inside the cells in both the cyanobacteria (Verma and Prasad 2021).
H2O2 indorses NO to enhance photosynthetic pigments in Cd stressed cyanobacteria
Being crucial constituents of photosynthesis, photosynthetic pigments (Chl a and Car) and prime light-harvesting antenna complexes (PE, APC and PC) of PS II were also evaluated in both the test cyanobacteria exposed to Cd stress together with different modulators (Fig. 3). Results revealed that 6 µM Cd caused a significant (P < 0.05) decrease in the contents of Chl a and Car by 29 and 31% in N. muscorum and 32 and 33% in Anabaena sp., respectively in comparison to control. Hence, these results are in corroboration with the previous study of Ahad and Syiem (2018b). This damaging impact induced by Cd might be due to the decrease in pigment biosynthesis as reported by Prasad et al. (2015) in Azolla pinnata under cypermethrin stress or degradation of precursors related to chlorophyll biosynthesis (Mishra et al. 2016). Furthermore, the reason behind the more reduction in phycobilisomes contents might be due to their presence at the outward direction of the thylakoid membrane which gets directly and simply accessible to stress agents such as heavy metals. Reverse to this, the application of H2O2 effectively reduced the negative impact of Cd on Chl a and Car contents. However, exogenous NO (SNP) more efficiently minimized the reduction in contents of Chl a and Car showing only 5 and 7% reduction in N. muscorum and 8 and 9% reduction in Anabaena sp., respectively (Fig. 3a, b). Phycobilisomes contents (PE, PC and APC) also showed a similar response in the presence of Cd alone as well as in combination with H2O2 or NO (SNP) (Fig. 3c–e). The exogenous H2O2 and NO (SNP) alleviated the damaging impact of Cd possibly by promoting the biosynthesis of these pigments. Similarly, Christou et al. (2014), recorded that pretreatment of H2O2 and SNP significantly enhanced the contents of Chl a, b and Car in salt challenged strawberry plants. Further, in the present study, it was noticed that exogenous PTIO or LNAME were maximally abolished the positive influence of H2O2, whereas NAC or DPI application could not hinder the alleviatory mechanism of NO in both the tested cyanobacteria suggesting the importance of NO in H2O2 signaling mechanism.
H2O2 mediates NO to enhance the rate of photosynthesis in Cd stressed cyanobacteria
Our findings revealed that the most important growth-promoting biological process, photosynthesis is adversely affected by Cd stress. Exposure of Cd significantly (P < 0.05) declined the photosynthetic oxygen evolution rate by 25% in N. muscorum and 28% in Anabaena sp. over the respective control values (Fig. 4a). Similar to this, Ahad and Syiem (2018b) also reported that Cd reduced the rate of photosynthesis in N. muscorum Meg 1. The possible causes behind the loss of photosynthetic efficiency could be the damage in light harvesting system or the direct interference of Cd with light reaction that occurred in the thylakoid membrane as suggested by Tiwari et al. (2020b) in cypermethrin stressed N. muscorum. Notwithstanding to this, in the present study under Cd stress H2O2 and NO (SNP) up regulated the rate of photosynthesis by improving the levels of photosynthetic pigments and by bringing down ROS level under the limit (data not shown). However, the effect was found more pronounced with NO, which showed a 14 and 12% increase the in rate of photosynthesis in N. muscorum and Anabaena sp., respectively (Fig. 4a). But PTIO and LNAME reversed the positive effects of exogenous H2O2 and thus critical damage to photosynthesis occurred. However, NO (SNP) efficiently improved the rate of O2 evolution even in the presence of NAC or DPI, hence suggests towards the importance of NO in the regulation of photosynthesis under Cd stress even in absence of H2O2.
Impact of exogenous H2O2 and NO on photosynthetic O2 evolution (a) and respiratory rate (b) of Nostoc muscorum ATCC 27893 and Anabaena sp. PCC 7120 exposed to Cd after 24 h of experiments. Data represent the mean value ± standard error of three replicates (n = 3). Bars followed by different letters show significant difference at P < 0.05 significance level according to Tukey test
H2O2 mediates NO to maintain PS II photochemistry in Cd stressed cyanobacteria
To pinpoint the target sites of Cd in photosynthesis, photochemistry of PS II in the form of the fast signals of Chl a fluorescence were analyzed by JIP test and related results have been displayed in Fig. 5a–d in the form of a radar chart. In our findings, under Cd stress decreased kinetic parameters i.e. ФPo, Ψo, ФEo and PIABS along with the significant reduction in size and number of active reaction centers (Fv/Fo) were recorded in both the test cyanobacteria (Fig. 5). This indicates the hindrance in electron flow from PS II to PS I which might be due to the difficulty in the reoxidation of QA− which could be related to deprived dispersal of PQ throughout the thylakoid membrane (Magyar et al. 2018). However, these kinetic parameters were normalized in both the Cd stressed cyanobacteria when cells were subjected to exogenous H2O2 and NO (SNP). Similarly, Christou et al. (2014) recorded the maximum photochemical efficiency of PS II in salt challenged strawberry plants pretreated with NO and H2O2. The adverse impacts of Cd on PS II activity were reduced significantly by exogenous H2O2 and NO (SNP) application which could be due to: (i) considerable reduction in intracellular Cd accumulation that might have improved the overall functioning of thylakoid membrane and/or (ii) significant rise in the number of active reaction centers which was evident by the increased values of Fv/Fo, thereby reduction in specific energy fluxes i.e. ABS/RC, TRo/RC, ETo/RC, DIo/RC was observed. But the positive impacts of H2O2 and NO (SNP) on these parameters were diminished by PTIO and LNAME thereby pointing towards the importance of NO in sustaining the overall PS II photochemistry in Cd stressed cyanobacteria. Moreover, increased Fo/Fv values under Cd exposure in both the test organisms pointed towards the Cd induced damaging effects on OEC (oxygen-evolving complex) and increased dissipation (DIo/RC) of an absorbed photon which also get normalized under exogenous supplementation of H2O2 and NO (SNP). However, PTIO and LNAME addition to cultures weakened the positive influence of exogenously supplied H2O2 and NO (SNP).
The specific energy flux parameters, i.e., ABS/RC, TRo/RC, ETo/RC, DIo/RC were also represented by the phenomenological membrane pipeline model per reaction center (RS) and cell suspension pipeline modal in per ml cell- suspension or cross-section (CS) in both N. muscorum and Anabaena sp. (Figs. 6 and 7). The presented model has been constructed with the experimental data. In the figures, the width of each arrow signifies the intensity of the respective energy fluxes. In the cell suspension pipeline modal, each white small circle directs about active reaction centers having the capacity to reduce QA and each black small circle denotes inactive reaction centers or so-called silent reaction centers because of non-QA reducing ability. In the membrane model, the outer oval corresponds to all absorbing pigments per one active reaction center and the inner oval represents the absorbing pigments specially belonging to that particular active reaction center. The membrane model clearly showed that exposure of Cd enhanced the electron transport (ETo/RC) per active reaction center. However, the apparent activity, ETo/RC of the cells per cross-section was not as much increased because of the presence of less active reaction centers which were damaged by Cd toxicity. But the supplementation of H2O2 or NO (SNP), significantly detoxified the Cd stress resulted in an increased number of active reaction centers that’s why electron transport per RC gets normalized and at per CS condition, it was increased significantly. Further, the positive responses of H2O2 or NO (SNP) got hindered by PTIO or LNAME supplementation. Similar responses were observed in the case of trapping flux per RC (TRo/RC). On the other hand, dissipation energy per RC (DIo/RC) was found increased under Cd stressed condition because of the extra load on remaining active RCs, whereas in the entire cell means per CS condition dissipation energy comparatively not as much increased due to presence of less active RCs per cell. But DIo/RC had been normalized under the exposure of H2O2 and NO (SNP) and got aberrantly enhanced under PTIO or LNAME application. Enlarged width of energy fluxes in the membrane model showed the adverse impacts of Cd on energy fluxes, successively indicated towards the need for extra energy absorption, trapping of electrons and electron transport but due to damage caused by Cd inside the thylakoid membrane electrons were not utilized properly and dissipation of energy was also increased. But in the case of per CS condition energy fluxes were not found crucially increased due to the presence of a higher number of active reaction centers. Furthermore, this adverse condition created by Cd was normalized by supplementation of H2O2 and NO (SNP) and again suppressed under exposure of PTIO and LNAME which indicated the beneficial role of NO in balancing of energy flux parameters. Next to this, the color intensity in the cell suspension model represents the concentration of chlorophyll in the respective cell culture or ABS/RC that showed a similar response to other specific energy fluxes in both test cyanobacteria.
H2O2 stimulates NO to normalize the rate of respiration in test cyanobacteria under Cd stress
Reverse to photosynthesis, enhanced rate of respiration was recorded by 20% in N. muscorum and 25% in Anabaena sp. over the respective control values under Cd exposure (Fig. 4b). This probably occurred to maintain the ATP production needed for the basal metabolism and/or excessive consumption of oxygen to generate ROS under Cd stress. Similar results were also reported by Tiwari et al. (2019) in Al stressed Anabaena sp. Further, the abnormal high rate of respiration was brought to the normal level by the exogenous application of H2O2 and NO (SNP) (Fig. 4b) which again supports the previous study of Tiwari et al. (2019). However, PTIO and LNAME again abolished the effects of H2O2 and NO which indicated towards the fact that NO is more important for the normalization of abnormally increased rate of respiration caused by Cd toxicity.
H2O2 mediates NO to enhance the inorganic nitrogen uptake and the activities of nitrate assimilating enzymes in Cd stressed test cyanobacteria
To maintain the nitrogen status inside the cells, cyanobacteria use nitrate (NO3−) as the most preferential source of nitrogen and then convert it to nitrite (NO2−) and ammonia (NH4+) by involving some specific enzymes i.e. NR and NiR. Results stated that Cd markedly reduced the uptake of NO3− and NO2− by 30 and 28% in N. muscorum and 32 and 30% in Anabaena sp., respectively over the control values (Tables 1 and 2) which could be mainly due to the damage in transporter proteins as a result of the overproduction of ROS that ultimately leads to the membrane damage (data not shown). Another possibility behind the less uptake of NO3− may be Cd mediated heavy damage in electron transport chain (ETC) of the cyanobacterial cells thereby possible reduction in ATP supply might have hindered the functioning of ABC-type transporter needed for NO3− transport and this view was substantiated by Sheeba et al. (2011) in UV-B stressed Nostoc muscorum and Phormedium foveolarum. On the other hand, results indicated that under Cd exposure activities of NR and NiR were also declined significantly (P < 0.05) by 35 and 32% in N. muscorum and 37 and 35% in Anabaena sp., respectively over the control values that might be due to the less uptake of NO3− and NO2−. Further, it may be assumed that Cd could decrease the activities of NR and NiR enzymes by affecting the biosynthesis of enzymes or by changing the structure of enzyme configuration as a result of excess ROS accumulation. Nevertheless, exogenous H2O2 and NO (SNP) reversed the negative impact of Cd on nutrient uptake and very less reduction was remained i.e. only by 6 and 7% for NO3− uptake and by 7 and 9% for NO2− uptake in N. muscorum and Anabaena sp., respectively. On the other hand, when Cd stressed cells were subjected to the H2O2 or NO (SNP), a significant recovery was noticed as the decrease was noticed only by 9 and 6% in NR and by 10 and 6% in NiR activity in N. muscorum and by 11 and 8% in NR and 12 and 9% in NiR activity in Anabaena sp., respectively (Tables 1 and 2). Similarly, Balotf et al. (2018) also noticed the increased NR and NiR activities under the treatment of SNP in wheat seedlings. However, uptake of inorganic nitrogen and the activities of nitrate assimilating enzymes were found critically reduced due to addition of PTIO and LNAME together with Cd (Tables 1 and 2), hence clearly points towards the involvement of NO in transcriptional and post-transcriptional regulation of nitrogen assimilation pathway enzymes as discussed in the previous study of Balotf et al. (2018).
H2O2 up-regulates NO to normalize the activities of ammonia assimilating enzymes in Cd stressed cyanobacteria
Ammonium ion (NH4+) is the end product of nitrate assimilation and is highly toxic to cells so it must be removed from the vicinity of cells quickly or be assimilated into other organic compounds. In cyanobacterial cells, assimilation of ammonia is performed by the GS-GOGAT pathway (Dai et al. 2008; Singh et al. 2012). In the present study, it was observed that under Cd stress GS and GOGAT activities were declined by 31 and 30% in N. muscorum and by 33 and 32% in Anabaena sp., respectively in comparison to control (Tables 1 and 2) which led to the over accumulation of NH4+ ions inside the cells hence that was responsible for reduced growth, cellular osmotic imbalance and reduction in the rate of photosynthesis (Bajguz 2011). Similar to our study, Ahad and Syiem (2018b) have also found reduced activity of GS in Cd stressed N. muscorum Meg 1. To release the cells from excessive ammonium toxicity another ammonium assimilating enzyme GDH is involved in an alternative pathway that was found more active under excessive ammonium accumulation. In the current study, GDH activity under Cd stress exhibited a significant (P < 0.05) increment showing a rise of 27% in N. muscorum and 28% Anabaena sp. However, under the exogenous supplementation of H2O2 and NO (SNP), the improved activities of GS and GOGAT were noticed that ultimately weakened the GDH activity (Tables 1 and 2) because of less availability of NH4+ as the substrate for GDH enzyme. Similar to this, the previous study of Balotf et al. (2018) also reported that exogenously provided SNP enhanced the activities of ammonia assimilating enzymes; GS and GOGAT and alleviated NH4+toxicity in wheat seedlings. Contrary to this, PTIO and LNAME along with H2O2 under similar stress, again diminished the activities of GS and GOGAT and critically enhanced GDH (Tables 1 and 2) activity was found that indicated towards the importance of NO signaling in releasing the ammonium toxicity from the cells of both tested cyanobacteria. The adverse impacts of PTIO and LNAME were found more crucial in Anabaena sp. indicated its sensitive behavior in comparison to N. muscorum.
Conclusions
In summary, our data showed that the signaling molecules H2O2 and NO have an important role in Cd stress removal from the cells of cyanobacteria N. muscorum ATCC 27893 and Anabaena sp. PCC 7120. Excessive Cd can cause the damaging impacts on vital cellular metabolism: PS II photochemistry as well as nitrogen metabolism, directly or/and indirectly by inducing excessive generation of ROS in cells thereby diminished the growth of the test cyanobacteria. Furthermore, exogenous application of H2O2 and NO regulated the cellular metabolism positively even under Cd stress and abolished the negative effects (Fig. 8). Hence, H2O2 and NO appeared to be primary acquisition for the tolerance of cyanobacteria N. muscorum and Anabaena sp. against heavy metal toxicity particularly Cd (Fig. 8). Furthermore, these extraordinary signaling molecules exhibit interdependency on each other, which defined that their signaling pathway is not so linear. In order to this, the present study clarifies that H2O2 regulates NO and further NO leads to govern the several physiological and biochemical processes as well as genetic modifications inside the stress challenged organisms that make them more capable to face environmental stress conditions.
The present study recommends the application of quite a cheap source of NO (SNP) as a growth regulator in paddy fields to grow efficient biofertilzers; cyanobacteria luxuriantly. The exogenous supplementation of a very low dose of NO (SNP) can provide full tolerance to these cyanobacteria to face Cd stress hence finally enhance the quality and productivity of rice crop.
References
Ahad RIA, Syiem MB (2018a) Ameliorating potential of Ca2+ on Cd2+ induced toxicity on carbon assimilation in the cyanobacterium Nostoc muscorum Meg 1. RJLBPCS 4:322–337
Ahad RIA, Syiem MB (2018b) Copper and cadmium-induced toxicity on the cyanobacterium Nostoc muscorum Meg 1: a comparative study. Eurasia J Biosci 12:333–345
Ahad RIA, Syiem MB (2019) Influence of calcium on cadmium to uptake and toxicity to the cyanobacterium Nostoc muscorum Meg1. Biotechnol Res Innov 3:231–241
Bajguz A (2011) Suppression of Chlorella vulgaris growth by cadmium, lead and copper stress and its restoration by endogenous brassinolide. Arch Environ Contamin Toxicol 60:406–416
Balotf S, Islam S, Kavoosi G, Kholdebarin B, Juhasz A, Ma W (2018) How exogenous nitric oxide regulates nitrogen assimilation in wheat seedlings under different nitrogen sources and levels. PLoS ONE 13:0190269. https://doi.org/10.1371/journal
Bennett A, Bogorad L (1973) Complementary chromatic adaptation in a filamentous blue-green alga. J Cell Biol 58:419–435
Bhatnagar M, Bhatnagar A (2019) Diversity of polysaccharides in cyanobacteria. In: Satyanarayana T, Johri BN, Das SK (eds) Microbial diversity in ecosystem sustainability and biotechnological applications, Vol. 1, Chapt. 10, pp 447–496. Springer, Singapore. doi: https://doi.org/10.1007/978-981-13-8315-1_15.
Cawse PA (1967) The determination of nitrate in soil solution by ultraviolet spectrophotometry. Analyst 92:311–315
Chakraborty T, Pal R (2014) An overview of cyanobacterial exopolysaccharide: features, composition and effects of stress exposure. Int J Life Sci 8:1–9. https://doi.org/10.3126/ijls.v8i4.10891
Chávez S, Candau P (1991) A NAD-specific glutamate dehydrogenase from cyanobacteria identification and properties. FEBS Lett 285:35–38
Chittora D, Meena M, Barupal T, Swapnil P (2020) Cyanobacteria as a source of biofertilizers for sustainable agriculture. Biochem Biophys Rep 22:100737. https://doi.org/10.1016/j.bbrep.2020.100737
Christou A, Manganaris GA, Fotopouls V (2014) Systemic mitigation of salt stress by hydrogen peroxide and sodium nitroprusside in strawberry plants via transcriptional regulation of enzymatic and non-enzymatic antioxidants. Environ Exp Bot 107:46–54
Corpas FJ, Barroso JB (2017) Lead-induced stress, which triggers the production of nitric oxide (NO) and superoxide anion (O2-) in Arabidopsis peroxisomes, affects catalase activity. Nitric Oxide 68:103–110
Dai GZ, Deblois CP, Liu SW, Juneau P, Qiu BS (2008) Differential sensitivity of five cyanobacterial strains to ammonium toxicity and its inhibitory mechanism on the photosynthesis of rice-field cyanobacterium Ge–Xian–MiNostoc. Aquat Toxicol 89:113–121
Dubois M, Gilles KA, Hamilton JK, Rebers PA, Smith F (1956) Colorimetric method for determination of sugars and related substances. Anal Chem 28:350–356
Gohari G, Alavi Z, Esfandiari E, Panahirad S, Hajihoseinlou S, Fotopoulos V (2019) Interaction between hydrogen peroxide and sodium nitroprusside following chemical priming of Ocimum basilicum L. against salt stress. Physiol Plant 2:168
Gonzalez A, Cabrera MA, Henriquez MJ, Contreras RA, Morales B, Moenne A (2012) Cross talk among calcium, hydrogen peroxide and nitric oxide and activation of gene expression involving calmodulins and calcium-dependent protein kinases in Ulva compressa exposed to copper excess. Plant Physiol 158:1451–1462
Goodwin TW (1954) Carotenoids. In: Paech K, Tracey MVE (eds) Handbook of plant analysis, vol 3. Springer, Berlin, pp 272–311
Hasanuzzaman M, Nahar K, Gill SS, Alharby HF, Razafindrabe BHN, Fujita M (2017) Hydrogen peroxide pretreatment mitigates cadmium-induced oxidative stress in Brassica napus L.: an intrinsic study on antioxidant defense and glyoxalase systems. Front Plant Sci 8:115. https://doi.org/10.3389/fpls.2017.00115
Herrero A, Guerrero MG (1986) Regulation of nitrite reductase in the cyanobacterium Anacystis nidulans. J Gen Microbiol 32:2463–2468
Herrero A, Flores E, Guerrero MG (1981) Regulation of nitrate reductase levels in the cyanobacteria Anacystis nidulans, Anabaena sp. strain 7119 and Nostoc sp. strain 6719. J Bacteriol 145:175–180
Herrero A, Flores E, Guerrero MG (1984) Regulation of the nitrate reductase level in Anacystis nidulans, activity decay under nitrogen stress. Arch Biochem Biophys 234:454–459
Hu Y, Ge Y, Zhang C, Ju T, Cheng W (2009) Cadmium toxicity and translocation in rice seedlings are reduced by hydrogen peroxide pretreatment. Plant Growth Regul 59:51–61. https://doi.org/10.1007/s10725-009-9387-7
Jalloh MA, Chen J, Zhen F, Zhang G (2009) Effect of different N fertilizer forms on antioxidant capacity and grain yield of rice growing under Cd stress. J Hazard Mater 162:1081–1085
Kushwaha BK, Singh S, Tripathi DK, Sharma S, Prasad SM, Chauhan DK, Kumar V, Singh VP (2019) New adventitious root formation and primary root biomass accumulation are regulated by nitric oxide and reactive oxygen species in rice seedlings under arsenate stress. J Hazard Mater 361:134–140
Li JT, Qiu JB, Zhang XW, Wang LS (2011) Exogenous hydrogen peroxide can enhance tolerance of wheat seedlings to salt stress. Acta Physiol Plant 33:835–842. https://doi.org/10.1007/s11738-010-0608-5
Li ZG, Luo LJ, Sun YF (2015) Signal crosstalk between nitric oxide and hydrogen sulfide may be involved in hydrogen peroxide induced thermo tolerance in maize seedlings. Russ J Plant Physiol 62:507–514
Lin YL, Chao YY, Huang WD, Kao CH (2011) Effect of nitrogen deficiency on antioxidant status and Cd toxicity in rice seedlings. Plant Growth Regul 64:263–273
Liu X, Yin L, Deng X, Gong D, Du S, Wang S, Zhang Z (2020) Combined application of silicon and nitric oxide jointly alleviated cadmium accumulation and toxicity in maize. J Hazard Mater 395:122679
Magyar M, Sipka G, Kovács L, Ughy B, Zhu Q, Han G, Spunda V, Lambrev PH, Shen JR, Garab G (2018) Rate-limiting steps in the dark to light transition of Photosystem II: revealed by chlorophyll–a fluorescence induction. Sci Rep 8:2755. https://doi.org/10.1038/s41598018-21195-2
Meers JL, Tempest DW, Brown CM (1970) Glutamine amide, 2-oxoglutarate amino transferaseoxido-reductase NADP; an enzyme involved in the synthesis of glutamate by some bacteria. J Gen Microbiol 64:187–194
Mehta A, Lopez-Maury L, Florencio FJ (2014) Proteomic pattern alterations of the cyanobacterium Synechocystis sp. PCC 6803 in response to cadmium, nickel and cobalt. J Proteomics 102:98–112. https://doi.org/10.1016/j.jprot.2014.03.002
Mérida A, Candau P, Florencio FJ (1991) Regulation of glutamine synthetase activity in the unicellular cyanobacterium Synechocystis sp. Strain PCC 6803 by the nitrogen source, effect of ammonium. J Bacteriol 173:4095–4100
Mishra S, Alfeld M, Sobotka R, Andresen E, Falkenberg G, Küpper H (2016) Analysis of sublethal arsenic toxicity to Ceratophyllum demersum: Subcellular distribution of arsenic and inhibition of chlorophyll biosynthesis. J Exp Bot 67:4639–4646. https://doi.org/10.1093/jxb/erw238
Navarro F, Chävez S, Candau P, Florencio FJ (1995) Existence of two ferredoxin-glutamate synthases in the cyanobacterium Synechocystis sp. PCC 6803. Isolation and insertional inactivation of gltB and gltS genes. Plant Mol Biol 27:753–767
Porra RJ, Thompson WA, Kriedemann PE (1989) Determination of accurate extinction coefficients and simultaneous equations for assaying chlorophylls a and b extracted with four different solvents; verification of the concentration of chlorophyll standards by atomic absorption spectroscopy. Biochim Biophys Acta Bioenerg 975:384–394
Prasad SM, Singh A, Singh P (2015) Physiological, biochemical and growth responses of Azolla pinnata to chlorpyrifos and cypermethrin pesticides exposure: a comparative study. Chem Ecol 31:285–298
Prasanna R, Sharma E, Sharma P, Kumar A, Kumar R, Gupta V, Pal RK, Shivay YS, Nain L (2013) Soil fertility and establishment potential of inoculated cyanobacteria in rice crop grown under non-flooded conditions. Paddy Water Environ 11:175–183
Qiao W, Li C, Fan LM (2014) Cross-talk between nitric oxide and hydrogen peroxide in plant responses to abiotic stresses. Environ Exp Bot 100:84–93
Rizwan M, Ali S, Adrees M, Rizvi R, Rehman MZ, Hannan F, Qayyum MF, Hafeez F, Ok YS (2016) Cadmium stress in rice: toxic effects, tolerance mechanisms and management: a critical review. Environ Sci Pollut Res 23:17859–17879
Romero-Puertas MC, Terrón-Camero LC, Peláez-Vico MA, Olmedilla A, Sandalio LM (2019) Reactive oxygen and nitrogen species as key indicators of plant responses to Cd stress. Environ Exp Bot 161:107–119
Saadatnia H, Riahi H (2009) Cyanobacteria from paddy fields in Iran as a biofertilizer in rice plants. Plant Soil Environ 55:207–212
Saxena I, Srikanth S, Chen Z (2016) Cross talk between H2O2 and interacting signal molecules under plant stress response. Front Plant Sci 7:570. https://doi.org/10.3389/fpls.2016.00570
Seregin IV, Kozhevnikova AD (2011) Histochemical methods for detection of heavy metals and strontium in the tissues of higher plants. Russ J Plant Physiol 58:721–727
Sharma A, Soares C, Sousa B, Martins M, Kumar V, Shahzad B, Sidhu GPS, Bali AS, Asgher M, Bhardwaj R, Thukral AK, Fidalgo F, Zheng B (2020) Nitric oxide-mediated regulation of oxidative stress in plants under metal stress: a review on molecular and biochemical aspects. Physiol Plant 168:318–344
Sheeba SVP, Srivastava PK, Prasad SM (2011) Differential physiological and biochemical responses of two cyanobacteria Nostoc muscorum and Phormidium foveolarum against oxyfluorfen and UV-B radiation. Ecotoxicol Environ Saf 74:1981–1993. https://doi.org/10.1016/j.ecoenv.2011.07.006
Singh VP, Srivastava PK, Prasad SM (2012) UV-B induced differential effect on growth and nitrogen metabolism in two cyanobacteria under copper toxicity. Cell Mol Biol 58:85–95. https://doi.org/10.1170/T925
Singh JS, Kumar A, Rai AN, Singh DP (2016) Cyanobacteria, a precious bio-resource in agriculture, ecosystem and environmental sustainability. Front Microbiol 7:529
Singh S, Husain T, Kushwaha BK, Suhel M, Fatima A, Mishra V, Singh SK, Tripathi DK, Rai M, Prasad SM, Dubey NK, Chauhan DK, Bhatt JA, Fotopoulos V, Singh VP (2020) Regulation of ascorbate- glutathione cycle by exogenous nitric oxide and hydrogen peroxide in soybean roots under arsenate stress. J Hazard Mater. https://doi.org/10.1016/j.jhazmat.2020.123686
Snell FD, Snell CT (1949) Colorimetric methods of analysis, vol 3. Van Nostrand, New York, pp 804–805
Strasser RJ, Srivastava A, Tsimilli-Michael M (2000) The fluorescence transient as a tool to characterize and screen photosynthetic samples. Probing photo-synthesis: mechanisms, regulation and adaptation. Taylor and Francis, New York, London.
Tiwari S, Prasad SM (2020) Kinetin alleviates chromium toxicity on growth and PS II photochemistry in Nostoc muscorum by regulating antioxidant system. Environ Pollut 259:113806
Tiwari S, Patel A, Prasad SM (2018) Kinetin alleviates chromium toxicity on growth and PS II photochemistry in Nostoc muscorum by regulating antioxidant system. Ecotoxicol Environ Saf 161:296–304
Tiwari S, Verma N, Singh VP, Prasad SM (2019) Nitric oxide ameliorates aluminium toxicity in Anabaena PCC7120: regulation of aluminium accumulation, exopolysaccharides secretion, photosynthesis and oxidative stress markers. Environ Exp Bot 161:218–227
Tiwari S, Patel A, Prasad SM (2020a) Phytohormone up-regulates the biochemical constituent, exopolysaccharide and nitrogen metabolism in paddy-field cyanobacteria exposed to chromium stress. BMC Microbiol 20:206. https://doi.org/10.1186/s12866-020-01799-3
Tiwari S, Verma N, Prasad SM, Singh VP (2020b) Cytokinin alleviates cypermethrin toxicity in Nostoc muscorum by involving nitric oxide: regulation of exopolysaccharides secretion PS II photochemistry and reactive oxygen species homeostasis. Chemosphere 259:127356
Verma N, Prasad SM (2021) Regulation of redox homeostasis in cadmium stressed rice field cyanobacteria by exogenous hydrogen peroxide and nitric oxide. Sci Rep 11:2893
Verma N, Tiwari S, Singh VP, Prasad SM (2020) Nitric oxide in plants: an ancient molecule with new tasks. Plant Growth Regul 90:1–13
Weifeng C, Yuanjie D, Guoqin H, Xiaoying Y (2018) Effects of exogenous nitric oxide on cadmium toxicity and antioxidative system in perennial ryegrass. J Soil Sci Plant Nutri 18:129–143
Xu FJ, Jin CW, Liu WJ, Zhang YS, Lin XY (2010) Pretreatment with H2O2 alleviates aluminum-induced oxidative stress in wheat seedlings. J Integr Plant Biol 54:44–53. https://doi.org/10.1111/j.1744-7909.2010.01008.x
Acknowledgements
We are grateful to the Department of Botany, University of Allahabad for providing necessary lab facilities. Sheo Mohan Prasad and Nidhi Verma are also acknowledged to the SERB-DST (file No. EMR/2016/004745), New Delhi and UGC (as UGC-AU research scholar), respectively for providing financial assistant to carry out this work. Financial funding from Department of Biotechnology Government of India under RRFSP is gratefully acknowledged.
Author information
Authors and Affiliations
Contributions
Nidhi Verma: Writing- original draft. Sheo Mohan Prasad: Conceptualization, Writing- original draft.
Corresponding author
Ethics declarations
Conflict of interest
Authors declare that they have no any conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Verma, N., Prasad, S.M. Interplay of hydrogen peroxide and nitric oxide: systemic regulation of photosynthetic performance and nitrogen metabolism in cadmium challenged cyanobacteria. Physiol Mol Biol Plants 27, 2181–2199 (2021). https://doi.org/10.1007/s12298-021-01083-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12298-021-01083-2