Abstract
The present study examined the regulatory mechanism of hydrogen sulfide (H2S) and nitric oxide (NO) in nickel (Ni) stressed cyanobacteria viz., Nostoc muscorum and Anabaena sp. by analyzing growth, photosynthetic pigments, biochemical components (protein and carbohydrate), exopolysaccharides (EPS), inorganic nitrogen content, and activity of enzymes comprised in nitrogen metabolism and Ni accumulation. The 1 µM Ni substantially diminished growth by 18% and 22% in N. muscorum and Anabaena sp. respectively, along with declining the pigment contents (Chl a/Car ratio and phycobiliproteins), and biochemical components. It also exerted negative impacts on inorganic uptake of nitrate and nitrite contents; nitrate reductase and nitrite reductase; and ammonium assimilating enzymes (glutamine synthetase, glutamate synthase, and glutamate dehydrogenase exhibited a reverse trend) activities. Nonetheless, the adverse impact of Ni can be mitigated through the exogenous supplementation of NaHS [sodium hydrosulfide (8 µM); H2S donor] and SNP [sodium nitroprusside (10 µM); NO donor] which showed substantial improvement on growth, pigments, nitrogen metabolism, and EPS layer and noticeably occurred as a consequence of a substantial reduction in Ni accumulation content which minimized the toxicity effects. The accumulation of Ni on both the cyanobacterial cell surface (EPS layer) are confirmed by the SEM–EDX analysis. Further, the addition of NO scavenger (PTIO; 20 µM) and inhibitor of NO (L-NAME; 100 µM); and H2S scavenger (HT; 20 µM) and H2S inhibitor (PAG; 50 µM) reversed the positive responses of H2S and NO and damages were more prominent under Ni stress thereby, suggesting the downstream signaling of H2S on NO-mediated alleviation. Thus, this study concludes the crosstalk mechanism of H2S and NO in the mitigation of Ni-induced toxicity in rice field cyanobacteria.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The ongoing expansion of industrialization driven by increased human involvement has led to the emergence of diverse organic and inorganic pollutants that directly or indirectly contaminate the aquatic ecosystem (Chittora et al. 2020). Through inappropriate waste disposal management exercises, toxic metal-containing industrial sludges are directly discharged into water bodies, which are the prime source of irrigation of agricultural fields and thereby primarily affect the soil fertility and crop yield along with their associated valuable microflora (Singh et al. 2022). Nickel (Ni) is a requisite trace element, required at concentration 0.01–5 µg g dry weight−1 by plants and promotes plant growth by coupling several enzymes, e.g., glyoxalase, ureases, hydrogenase, superoxide dismutases, methyl-Coenzyme M reductase and peptide deformylases (Khan et al. 2020; Soliman et al. 2019). But the use of Ni in various industries like Ni–Cd battery manufacturing, mining, smelting, electroplating, stainless steel, and food industries has led to the discharge of Ni-contaminated water into aquatic bodies, which reach into the soil and negatively hampered the photosynthesis, mineral absorption, plant water relation, and enzymatic activity of plant and microalgae (Rizwan et al. 2019; Verma et al. 2022). The previous findings showed Ni-induced toxicity in Cucurbita pepo (Valivand and Amooaghaie 2021), Scenedesmus quadricauda, (Strejckova et al. 2019), Nostoc muscorum (Verma et al. 2021), and Microcystis aeruginosa (Martínez-Ruiz and Martínez-Jerónimo 2016). The Ni stress enhances the generation of reactive oxygen species, disrupts large biomolecules (such as proteins, lipids and carbohydrates), and affects exopolysaccharides. Further, it leads to electrolytic leakage and alters various aspects, including growth, photosynthetic pigments, oxygen evolution rate and nitrogen metabolism in studied organisms.
Cyanobacteria, as pioneer photosynthetic oxygen-evolving microbes, are recognized for their significant potential in contributing to sustainable agricultural development, particularly in rice (paddy) fields (Tiwari and Prasad 2020). Nostoc muscorum and Anabaena sp. are the major natural inhabitants of rice fields. They have unique heterocyte cells (also known as heterocyst) that fix atmospheric nitrogen (20–25 kg ha−1 season−1) into ammonia (Tiwari et al. 2020; Verma and Prasad 2021). Some Asian countries such as China, India, Sri Lanka, Bangladesh, Philippines, Thailand etc., used cyanobacteria as a significant biofertilizer in rice (staple food) fields to increase crop productivity to fulfill the food demand and security for the growing populations (Singh et al. 2022). Moreover, cyanobacteria are a significant source of lipids, carbohydrates, vitamins, amino acids, sugars, and phenolic compounds (Pandey et al. 2022; Patel et al. 2020). An excessive amount of Ni substantially declined the photosynthetic pigments, mineral absorption, nitrogen metabolism and uptake of inorganic nitrogen in Zea mays (Tipu et al. 2021), Brassica juncea (Allah et al. 2019; Khan et al. 2020), Brassica napus (Hannan et al. 2021), and N. muscorum (Verma et al. 2021). Nitrogen (N) is a pivotal large nutrient and important constituent of amino acids (protein), nucleotides, photosynthetic pigments, vitamins and enzymes, and it promotes the growth of cyanobacteria (Verma et al. 2022). Hence, Ni toxicity significantly reduces inorganic nitrogen uptake and, as a result, decreases nitrogen and ammonia assimilating enzymes, i.e., NR (nitrate reductase), NiR (nitrite reductase), GS (glutamine synthetase) and GOGAT (glutamate synthase) apart from GDH (glutamate dehydrogenase) (performed ammonia assimilation by an alternative pathway), along with reduced the protein, lipid and carbohydrate content (Martínez-Ruiz and Martínez-Jerónimo 2016; Verma et al. 2021). Besides this, exopolysaccharides (EPS) are a polymeric form of carbohydrate that acts as a defensive layer against metal toxicity and were also found to diminish under Cd stress (Shen et al. 2021).
To overcome the adverse impact of various stresses such as heavy metals, salinity, and pesticides in plants and cyanobacteria, the application of signaling molecules, phytohormones and management of numerous nutrients is gaining immense importance (Raju and Prasad 2021; Tiwari and Prasad 2020; Verma and Prasad 2021). Hydrogen sulfide (H2S) and nitric oxide (NO) are two bioactive multitasking signaling molecules, which have shown alleviation against different stress responses (Singh et al. 2022). H2S has been proposed to be the third endogenous gaseous transmitter after carbon monoxide and nitric oxide. It has a significant role in growth and development processes like root development, seed germination, stomatal movement, photosynthesis, pigmentation, nodulation and nitrogen fixation in plants (Mukherjee and Corpas 2020; Raju and Prasad 2021). The previous studies reported that exogenous supplementation of H2S showed amelioration against different stress responses such as salinity in Solanum lycopersicum and Solanum melongena (Raju and Prasad 2021), Cr toxicity in black bean and mung bean seedlings (Husain et al. 2022), and allelopathic damage in Chlamydomonas reinhardtii (Chen et al. 2020). Similarly, nitric oxide (NO) is also involved in various physiological and biochemical processes in plants and cyanobacteria (Iqbal et al. 2021; Pandey et al. 2022). Exogenous exposure of NO increases stress tolerance under stress conditions such as in N. muscorum and Anabaena sp. (Tiwari et al. 2019; Verma and Prasad 2021), and showed amelioration under Cd and Al stress and in Triticum aestivum (Iqbal et al. 2021; Kaya et al. 2019) under heat stress and Cd stress. Moreover, there are other studies that described the individual and combined alleviating mechanism of H2S and NO on plants under stress (Iqbal et al. 2021; Kaya et al. 2019). However, their cross-talk function remains unclear in cyanobacteria. Hence, the present work focused on the possible role and synergistic regulation of H2S and NO under Ni stress on growth, photosynthetic pigments, biochemical constituents and nitrogen metabolism of cyanobacteria i.e., Nostoc muscorum ATCC 27893 and Anabaena sp. PCC 7120.
Material and methods
Growth conditions and experimental design
The experimental organisms Nostoc muscorum ATCC 27893 and Anabaena sp. PCC 7120 were grown at pH 7.5 containing BG-11 medium at 25 ± 2 °C in the culture room with the regime of light: dark at 14:10 h under 75 μmol photons (PAR) m−2 s−1. The pure inoculum of both tested cyanobacteria were initially obtained from the laboratory of Prof L. C. Rai, Department of Botany, Banaras Hindu University, Varanasi, India. The cultures of Nostoc muscorum ATCC 27893 and Anabaena sp. PCC 7120 were maintained in the Ranjan plant physiology and biochemistry laboratory under aseptic conditions. Unialgal cultures were obtained through serial dilution, and the cells were examined frequently under a light microscope. The homogenous cultures were collected at the exponential phase, and cells were washed with double sterile distilled water, and centrifugation was performed for 15 min at 3,000 g for each experiment. Initially, nickel as NiCl2·6H2O was used at 0.25–3.0 µM concentrations for screening experiments, and finally 1 µM Ni that symbolizes the reduction of 18% on growth of N. muscorum and of 22% on growth of Anabaena sp. respectively, was chosen for the present study. Similarly, screening experiments for hydrogen sulfide as NaHS (sodium hydrosulfide; H2S donor), at different concentrations such as 1–12 µM and for nitric oxide as SNP (sodium nitroprusside; NO donor) at varied concentrations (5–20 µM) were performed. Finally, 8 µM NaHS and 10 µM SNP were selected for further study. Furthermore, doses of hypotaurine (HT, 20 µM; scavenger of H2S), and propargylglycine (PAG, 50 µM; biosynthetic inhibitor of H2S) were considered from our earlier findings (Husain et al. 2021; Raju and Prasad 2021). Likewise, PTIO (2-phenyl-4,4,5,5-tetramethylimidazoline- 1-oxyl 3-oxide, 20 µM; scavenger of NO) and Nѡ-Nitro-L-arginine methyl ester hydrochloride (L-NAME, 100 µM; biosynthetic inhibitor of NO) doses were taken in accordance with the study of Verma and Prasad (2021). The treatment setup consisted of combinations, i.e., Control, Ni, Ni + NaHS, Ni + SNP, Ni + NaHS + SNP, Ni + NaHS + PTIO, Ni + NaHS + L-NAME, Ni + SNP + HT, Ni + SNP + PAG, Ni + NaHS + SNP + PTIO + L-NAME and Ni + NaHS + SNP + HT + PAG and after 72 h of experiment all the parameters were examined.
Estimation of growth
In both tested cyanobacteria, growth was assessed using culture absorbance (optical density, OD). For this purpose, 3 mL of both treated and untreated cells from each culture were harvested and subsequently homogenized. The absorbance of the culture was quantified at 750 nm after 72 h of treatment by applying UV–Visible Spectrophotometer (Double beam-1700; Shimadzu, Japan).
Measurement of cellular accumulation of nickel (Ni)
For intracellular accumulation of Ni, 80 mL of treated cyanobacterial cultures were centrifuged, and the pellets were washed with 1 mM EDTA. Subsequently, they were re-suspended in chilled phosphate buffer for 15 min to remove apoplastic Ni. The pellets were oven-dried at 70–80 °C for 48 h until completely dry. After that, a tri-acid mixture (5 mL) comprising a 5:1:1 ratio of HNO3, H2SO4 and HClO4 respectively, was added to digest the dried samples at 80 °C until obtaining a clear solution. The Ni was measured by applying atomic absorption spectrophotometer (AAS; Model-iCE 3300 series, Thermo Scientific, USA). Through the standard solution of Ni, the instrument was calibrated.
Measurement of photosynthetic pigment contents
The chlorophyll a (Chl a) and carotenoids (Car) contents were estimated according to the method of Porra et al. (1989) and Goodwin (1954) accordingly. The cyanobacterial cultures (10 mL) were centrifuged at 4,000 g for 10 min. Chl a and Car contents were extracted in 2 mL chilled pure methanol (100%) and incubated overnight at 4 °C. Further, absorbance was taken at 665 nm and 470 nm, respectively (UV–Visible Spectrophotometer; Double beam-1700; Shimadzu, Japan). The amounts of chlorophyll a and carotenoids were calculated by using formula i.e., 12.5 × A665 and 5 × A450, respectively. Similarly, measurements were taken for phycobiliproteins (PBPs) viz., PC (phycocyanin), APC (allophycocyanin), and PE (phycoerythrin), samples treated with toluene (0.5 mL) and kept at 4 °C overnight. After that, PBPs contents were extracted with 2.5 mM potassium phosphate buffer having pH 7.0 and the sample absorbance was recorded at 615, 652 and 562 nm according to the method of Bennett and Bogorad (1973). The following equations were used to calculate the contents of phycobiliproteins:
Measurement of inorganic nitrogen contents: nitrate (NO3 −) and nitrite (NO2 −) uptake
To measure the uptake rate of NO3− and NO2−, cells were pre-incubated in 100 µM solution of KNO3 and KNO2 respectively, for 24 h. Subsequently, cells were collected and the amount of uptake of NO3− was measured at 210 nm according to the method of Cawse (1967), and similarly the amount of NO2− uptake was quantified at 540 nm by applying the method of Snell and Snell (1949). After centrifugation of the cultures at 4,000 g for 10 min, the pellets were subjected to washing with a 25 mM of Tricine-NaOH buffer (pH 7.0) and subsequently resuspended in the same buffer. The experimental procedure commenced by introducing 100 μM of KNO3 for NO3− uptake and KNO2 for NO2− uptake to the cell suspension at time zero. After an incubation period of 4 h, cultures were retrieved, subjected to centrifugation, and the resulting cell-free supernatants were examined for residual NO3− and NO2− contents.
Estimation of nitrate assimilating enzymes: assay of nitrate reductase (NR) and nitrite reductase (NiR)
According to the method of Herrero et al. (1981, 1984) and Herrero and Guerrero (1986), accordingly, the NR (EC 1.6.6.1) and NiR (EC 1.7.7.1) activity were estimated. The cyanobacterial cells treated with dithionite reduced methyl-viologen (reductant) and permeabilized by adding alkyl trimethyl ammonium bromide (MTA) to the reaction mixture. After that, the reaction mixture was incubated at 25 °C for 5 min, and NO2− was quantified in correlating cell-free media following the method of Snell and Snell (1949). One unit (U) activity of NR is described as 1 nmol NO2− formed min−1 while one unit (U) activity of NiR is described as 1 nmol NO2− consumed min−1.
Estimation of ammonium-assimilating enzymes activity
Glutamine synthetase (GS) activity
The activity of glutamine synthetase (GS; EC 6.3.1.2)) was quantified by using the method of Mérida et al. (1991). By method of sonication (Model VCX-130 PB, Sonics Vibra Cell, USA), cyanobacterial cells were disrupted and centrifuged at 15,000 g at 4 °C for 20 min (Remi, Model CPR-30, India), and by nitrogen-free media cells were washed and resuspended in HEPES–NaOH buffer containing pH 7, and the enzyme activity was estimated as the generation of γ-glutamylhydroxamate. The reaction mixture, comprised of 50 μL of cell extract, 60 μM of HEPES–NaOH buffer (pH 7.0), 40 μM of l-glutamine, 4 μM of MnCl2, 60 μM of hydroxylamine, 1 μM of ADP, and 20 μM of sodium arsenate, was initiated by the addition of sodium arsenate. The quantity of γ-glutamylhydroxamate generated after 10 min incubation at 28 °C was determined by measuring the absorbance at 500 nm. One unit (U) of GS activity is determined as 1 nmol γ-glutamylhydroxamate formed min−1.
Glutamate synthase (GOGAT) activity
The GOGAT (glutamine 2-oxoglutarate aminotransferase; EC 1.4.1.14) activity was estimated according to the method of Meers et al. (1970) and Navarro et al. (1995) accordingly, for NADH-GOGAT in N. muscorum and Fd-GOGAT in Anabaena sp. Samples were centrifuged and resuspended in buffer (Tris–HCl) solution (pH 7.6), and cells were cleaved by sonication and again performed centrifugation for 15 min at 15,000 g (4 °C). The obtained enzyme extract was utilized to measure the activity of GOGAT by taking sample absorbance at 340 nm to quantify the NADH oxidation for N. muscorum and glutamate formation for Anabaena sp. One unit (U) activity of GOGAT is described as 1 nmol NADH oxidized min−1 for N. muscorum and 1 nmol glutamate formed min−1 for Anabaena sp.
Glutamate dehydrogenase (NADH-GDH) activity
According to the method of Chávez and Candau (1991), the GDH (EC 1.4.1.2) activity was estimated. Treated cells were harvested and centrifuged for 10 min at 4,000 g and pellets were collected and crushed in buffer (HEPES–NaOH) solution (pH 7.0). The initiation of the reaction occurred through the addition of NH4Cl. The obtained enzyme extract was utilized to measure the NADH oxidation activity at 340 nm. One unit (U) activity of GDH is described as 1 nmol NADH oxidized min−1.
Biochemical components
Measurement of protein content
The estimation of protein content was done according to the method of Bradford (1976). Cells were subjected to centrifugation at 4,000 g and homogenized using a 50 mM potassium phosphate buffer (PPB) with a pH of 7.8, which included 1 mM EDTA and 2% polyvinyl pyrrolidone at 4 °C. Subsequently, 0.1 mL of the resulting homogenate was combined with 2.5 mL of Coomassie brilliant blue G-250 reagent. The mixture was then incubated for 2 min in the dark at 25 °C, and the absorbance was measured at 595 nm.
Measurement of carbohydrate content
The carbohydrate content was measured as per the given method by Dubois et al. (1956) and dried samples (10 mg) were extracted in 2.5 N HCl, and centrifugation as performed for 10 min at 10,000 g. The 1.0 mL of supernatant was mixed with 1 mL phenol (5%) and 5 mL H2SO4 and the sample absorbance was taken at 490 nm. Each sample of carbohydrate content was estimated by applying a standard curve prepared with a glucose graded solution.
Measurement of exopolysaccharide (EPS) content
Exopolysaccharide content was quantified as per the given method by Sharma et al. (2008). The 100 mL cyanobacterial culture was taken and each treated sample was centrifuged for 20 min at 3,000 g to determine the EPS extraction. The obtained supernatant comprising soluble EPS was collected and concentrated to tenfold by evaporation at 40 °C and the precipitate was washed three times with isopropanol and again dried at 37 °C and acid hydrolyzed (HCl, 2 M) at 90 °C. Further, the hydrolysate was examined for glucose (Dubois et al. 1956) and EPS content was estimated as per the standard curve obtained for glucose.
Scanning electron micrography (SEM) and energy-dispersive X-ray (EDX)
The surface morphology of the dry absorbent (exopolysaccharide) of both tested cyanobacteria, before (control samples) and after exposing to Ni stress (treated samples) was studied by using a SEM and EDX analysis. The cyanobacterial cultures were centrifuged, and the obtained pellets were washed with double sterile distilled water and ethanol to eliminate any impurities. The resulting samples were then oven-dried. Subsequently, the dried samples were mounted on a holder with a two-side carbon tab attached. Finally, before EDX analysis in the machine, the samples were coated with gold to prevent charging under the electron beam, and then samples were analyzed. The SEM images were taken by using a field emission microscope (Carl Zeiss, Merlin Gemini II, Germany). The analysis of elemental mapping and energy-dispersive X-ray (EDX) were completed by using a scanning electron microscope (Carl Zeiss, ZEISS EVO 60, Germany) equipped with an energy dispersive X-ray spectroscopy (EDS) system (Oxford INCA Energy 400; Oxford instruments, UK).
Statistical analysis
The presented results are the means ± standard error of three replicates (n = 3). By using analysis of variance (one-way ANOVA) the results were statistically confirmed. The DMRT (Duncan multiple range test) was applied for mean separation to exhibit substantial variations among treatments at P < 0.05 significance level. Principal component analysis (PCA) plot was also performed by applying SPSS 16.0 software to describe the correlation between growth and other experimental parameters with an alleviatory set of treatments. Furthermore, the correlation matrix test was performed on experimental parameters viz., photosynthetic pigments (Chl a/Car, PC, APC, PE), intracellular Ni content, biochemical constituents, inorganic nitrogen (nitrate and nitrite) content, and enzymatic actions of GS, GOGAT and GDH that are required in nitrogen metabolism to recognize the effect of all treatment sets on examined parameters.
Results
Growth
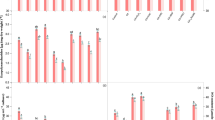
The growth of both cyanobacteria were estimated in terms of absorbance (optical density) at 750 nm, presented in Fig. 1a. In the present study, 1 µM nickel (Ni) significantly diminished (P < 0.05) the growth by 18% in Nostoc muscorum and by 22% in Anabaena sp., respectively. Nonetheless, the exogenous application of NaHS (as H2S donor; 8 µM) and SNP (as NO donor; 10 µM) along with Ni stress showed inhibition in growth by only 8% and 8% in N. muscorum and by 12% and 12% in Anabaena sp. accordingly, while combined exposure i.e., Ni + NaHS + SNP, showed maximum alleviation and inhibition was noticed by 5% in N. muscorum and by 7% in Anabaena sp. To know the regulatory role of endogenous H2S and NO in toxicity amelioration, the scavengers (PTIO and HT) and inhibitors (L-NAME and PAG) of NO and H2S, respectively, were added on to the growth medium with Ni stress (Ni + NaHS + PTIO, Ni + NaHS + L-NAME, Ni + SNP + HT and Ni + SNP + PAG) and the results showed a significant reduction in growth i.e., 26%, 30%, 33% and 37% in N. muscorum and by 30%, 33%, 37% and 39% in Anabaena sp. accordingly. Furthermore, we observed in combination with Ni + NaHS + SNP + PTIO + L-NAME and Ni + NaHS + SNP + HT + PAG, the damaging effect on growth was more pronounced and inhibition in growth was noticed by 38% and 41% in N. muscorum and by 43% and 46% in Anabaena sp. accordingly, even in the presence of both contrasting signaling molecules.
Effect of exogenous hydrogen sulfide and nitric oxide on (a) growth (absorbance) and (b) intracellular Ni accumulation of cyanobacteria N. muscorum and Anabaena sp. exposed to Ni stress after 72 h of treatment. Data presented as means ± standard error of three replicates (n = 3). Bars followed by different letters show significant difference at P < 0.05 significance level according to the Duncan’s multiple range test (DMRT). NaHS (sodium hydrosulfide as donor of hydrogen sulfide); SNP (sodium nitroprusside as donor of nitric oxide)
Nickel accumulation
The result regarding the accumulation of intracellular Ni has been illustrated in Fig. 1b. The cellular accumulation of Ni was recorded as 78.50 ± 1.35 μg Ni g dry weight−1 in N. muscorum and 86.35 ± 1.49 μg Ni g dry weight−1 in Anabaena sp. upon exposure of 1 µM. Nonetheless, the exogenous application of NaHS and SNP (Ni + NaHS, Ni + SNP and Ni + NaHS + SNP) significantly declined the intracellular Ni concentration by 69.78 ± 1.20 μg Ni g dry weight−1, 70.15 ± 1.21 μg Ni g dry weight−1 and 63.80 ± 1.10 μg Ni g dry weight−1 in N. muscorum and by 76.70 ± 1.32 μg Ni g dry weight−1, 77.16 ± 1.33 μg Ni g dry weight−1 and 70.18 ± 1.21 μg Ni g dry weight−1 in Anabaena sp. accordingly. Furthermore, excessive Ni accumulation was noticed on supplementation of PTIO, L-NAME, HT and PAG (Ni + NaHS + PTIO, Ni + NaHS + L-NAME, Ni + SNP + HT and Ni + SNP + PAG) by 82.50 ± 1.42 μg Ni g dry weight−1, 86.77 ± 1.50 μg Ni g dry weight−1, 91.36 ± 1.58 μg Ni g dry weight−1 and 94.70 ± 1.63 μg Ni g dry weight−1 in N. muscorum and by 90.75 ± 1.57 μg Ni g dry weight−1, 95.45 ± 1.65 μg Ni g dry weight−1, 100.49 ± 1.74 μg Ni g dry weight−1 and 104.16 ± 1.80 μg Ni g dry weight−1 in Anabaena sp. respectively. Moreover, the combined treatment of both signaling molecules along with their respective scavenger and inhibitor (Ni + NaHS + SNP + PTIO + L-NAME and Ni + NaHS + SNP + HT + PAG) showed the crucial accumulation of cellular Ni content i.e., by 96.96 ± 1.67 μg Ni g dry weight−1 and 102.91 ± 1.78 μg Ni g dry weight−1 in N. muscorum and by 106.66 ± 1.84 μg Ni g dry weight−1 and 113.20 ± 1.96 μg Ni g dry weight−1 in Anabaena sp. respectively.
Photosynthetic pigments
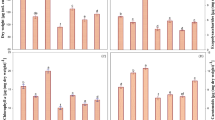
Results regarding the photosynthetic pigment contents, i.e., chlorophyll a (Chl a) to carotenoids (Car) ratio and phycobiliproteins (PBPs) contents in N. muscorum and Anabaena sp. exposed to Ni stress have been illustrated in Table 1. Substantial reduction (P < 0.05) in the ratio of Chl a/Car by only 4% in N. muscorum and by 5% in Anabaena sp. was observed under 1 µM Ni stress. Further, substantial improvement was noticed under the exogenous supplementation of NaHS and SNP as an individual and in combination i.e., (Ni + NaHS, Ni + SNP and Ni + NaHS + SNP) and inhibition was noticed by 2%, 2% and 1% in N. muscorum and by 3%, 3% and 2% in Anabaena sp. accordingly under Ni stress. Notwithstanding, amelioration responses of H2S and NO on Chl a/Car ratio were reversed upon the exogenous exposure of PTIO, L-NAME, HT and PAG and showed critical reduction under Ni stress.
Similarly, the main light-harvesting pigments [phycobiliproteins (PBPs); phycocyanin (PC), allophycocyanin (APC), and phycoerythrin (PE)] of cyanobacteria were also observed to be severely influenced under Ni stress (Table 1). The contents of PBPs; PC, APE and PE contents were majorly affected and significantly reduced (P < 0.05) by 23%, 22% and 26% in N. muscorum and by 25%, 24% and 28% in Anabaena sp. accordingly, exposed with Ni (1 µM) stress. Further, exogenous supplementation of NaHS and SNP (Ni + NaHS, Ni + SNP and Ni + NaHS + SNP) caused substantial improvement and inhibition was noticed only 13%, 14% and 7% in PC, by 13%, 13% and 6% in APC and 16%, 16% and 10% in PE contents of N. muscorum while in Anabaena sp. by 16%, 16% and 10% in PC, by 15%, 15% and 9% in APC and 18%, 18% and 13% in PE contents respectively. Although, under similar stress, on the exposure of PTIO, L-NAME, HT and PAG along with NaHS and SNP (Ni + NaHS + PTIO, Ni + NaHS + L-NAME, Ni + SNP + HT and Ni + SNP + PAG), more inhibition was noticed i.e., 26%, 28%, 30% and 32% in PC content, by 25%, 27%, 29% and 32% in APC content and by 31%, 33%, 37% and 39% in PE content in N. muscorum respectively, and in Anabaena sp. by 30%, 32%, 34% and 36% in PC content, by 29%, 32%, 34% and 37% in APC content and by 34%, 37%, 41% and 44% in PE content respectively. Moreover, combined exogenous supplementation of NaHS and SNP along with their respective scavenger and inhibitor under Ni stress exhibited a critical reduction in the PBPs contents.
Nitrate (NO3 −) and nitrite (NO2 −) uptake
Results regarding the uptake of NO3− and NO2− in test cyanobacteria have been presented in Fig. 2a, b. Ni (1 µM) caused a substantial reduction (P < 0.05) in the uptake of NO3− by 23% in N. muscorum and by 27% in Anabaena sp. respectively, and the corresponding decrease in uptake of NO2− by 18% in N. muscorum and by 23% in Anabaena sp. accordingly. However, Ni- stressed cyanobacteria were subjected to exogenous NaHS and SNP as individual and in combination i.e., Ni + NaHS, Ni + SNP and Ni + NaHS + SNP; the amount of uptake of NO3− and NO2− were observed to be significantly improved and inhibition was noticed for NO3− uptake only 9%, 9% and 3% in N. muscorum and by 13%, 14% and 5% in Anabaena sp. accordingly, while for NO2− uptake by 10%, 10% and 4% in N. muscorum and by 13%, 14% and 8% in Anabaena sp. accordingly. Further, upon supplementation of PTIO, L-NAME HT and PAG along with NaHS and SNP under Ni stress severely diminished the amount of NO3− and NO2− uptake in both test cyanobacteria while in the combination treatment set i.e., Ni + NaHS + SNP + PTIO + L-NAME and Ni + NaHS + SNP + HT + PAG showed a more pronounced deduction in uptake level and inhibition was observed for NO3− uptake by 42% and 45% in N. muscorum and by 43% and 46% in Anabaena sp. respectively while for NO2− uptake by 38% and 43% in N. muscorum and by 39% and 45% in Anabaena sp. accordingly.
Effect of exogenous hydrogen sulfide and nitric oxide on (a) nitrate (NO3−) uptake rate and (b) nitrite (NO2−) uptake rate of cyanobacteria N. muscorum and Anabaena sp. exposed to Ni stress after 72 h of treatment. Data presented as means ± standard error of three replicates (n = 3). Bars followed by different letters show significant difference at P < 0.05 significance level according to the Duncan’s multiple range test (DMRT). NaHS (sodium hydrosulfide as donor of hydrogen sulfide); SNP (sodium nitroprusside as donor of nitric oxide)
Nitrate assimilating enzymes: activity of nitrate reductase (NR) and nitrite reductase (NiR)
The results related to the activities of NR and NiR in both cyanobacteria have been depicted in Fig. 3a, b. The Ni (1 µM) stress substantially declined (P < 0.05) the activities of NR and NiR by 33% and 25% in N. muscorum and by 36% and 28% in Anabaena sp. accordingly. Furthermore, upon exposure of NaHS and SNP, the adverse impacts on activities of NR and NiR caused by Ni stress were mitigated and reduction was noticed by 19% and 19%, and by 18% and 18% in N. muscorum accordingly, and by 21% and 21% and by 20% and 20% in Anabaena sp. respectively, and with NaHS + SNP treatment to Ni stressed both cyanobacteria appeared to be more pronounced in improving the adverse impact on activities of NR and NiR by 9% and 11% in N. muscorum and by 10% and 13% in Anabaena sp. accordingly. However, exogenous treatment of PTIO, L-NAME, HT and PAG along with NaHS and SNP under similar stress conditions diminished the activities of NR and NiR by 38%, 44%, 48% and 52% and by 34%, 38%, 43% and 51% in N. muscorum respectively and by 41%, 47%, 53% and 57% and by 35%, 41%, 44% and 53% in Anabaena sp. respectively. Whereas, we observed in combination (Ni + NaHS + SNP + PTIO + L-NAME and Ni + NaHS + SNP + HT + PAG), a critical reduction on NR and NiR activities by 56% and 60% and by 60% and 67% in N. muscorum and by 60% and 65% and by 62% and 68% in Anabaena sp. respectively.
Effect of exogenous hydrogen sulfide and nitric oxide on (a) nitrate reductase (NR) and (b) nitrite reductase (NiR) of cyanobacteria N. muscorum and Anabaena sp. exposed to Ni stress after 72 h of treatment. Data presented as means ± standard error of three replicates (n = 3). Bars followed by different letters show significant difference at P < 0.05 significance level according to the Duncan’s multiple range test (DMRT). NaHS (sodium hydrosulfide as donor of hydrogen sulfide); SNP (sodium nitroprusside as donor of nitric oxide)
Ammonium-assimilating enzymes activity
Glutamine synthetase (GS; EC 6.3.1.2) activity, glutamate synthase (GOGAT; EC 1.4.1.14) activity and glutamate dehydrogenase (NADH-GDH; EC 1.4.1.2) activity
The results regarding to GS, GOGAT and GDH activities of both cyanobacteria are shown in Fig. 4a–c. Nickel (1 µM) repressed the enzymatic action of GS and GOGAT by 25% and 24% in N. muscorum and by 27% and 26% in Anabaena sp. accordingly. Exogenous application of NaHS and SNP as individual and in combination (NaHS + SNP) to Ni-stressed cyanobacterial cells caused substantial recovery in the GS and GOGAT activities, and a more pronounced impact was observed under NaHS + SNP treatment. Nonetheless, application with PTIO, L-NAME, HT and PAG along with NaHS and SNP under the similar stress condition reduced the activities of GS and GOGAT by 32%, 36%, 39% and 41% and by 32%, 35%, 39% and 41% in N. muscorum and by 35%, 39%, 43% and 46% and by 39%, 43%, 46% and 48% in Anabaena sp. respectively. Further, cells were subjected to the combined set of treatments along with NaHS and SNP showed a critical reduction in GS and GOGAT activity with Ni stress in N. muscorum and Anabaena sp. accordingly. Whereas, a reversed response was observed as GDH activity with Ni stress depicted substantial enhancement exhibiting an increase of 23% in N. muscorum and 34% in Anabaena sp. accordingly and it was further accelerated under PTIO, L-NAME, HT and PAG supplementation as it was raised by 27%, 34%, 40% and 44% in N. muscorum and by 37%, 39%, 46% and 49% in Anabaena sp. accordingly. Furthermore, upon exposure of NaHS and SNP or NaHS + SNP under Ni stress a diminishing pattern was noticed.
Effect of exogenous hydrogen sulfide and nitric oxide on (a) glutamine synthetase (GS), (b) glutamate synthase (GOGAT) and (c) glutamate dehydrogenase (GDH) of cyanobacteria N. muscorum and Anabaena sp. exposed to Ni stress after 72 h of treatment. Data presented as means ± standard error of three replicates (n = 3). Bars followed by different letters show significant difference at P < 0.05 significance level according to the Duncan’s multiple range test (DMRT). NaHS (sodium hydrosulfide as donor of hydrogen sulfide); SNP (sodium nitroprusside as donor of nitric oxide)
Biochemical components
Protein content
Results related to the impact of NaHS and SNP application on protein content in the Ni-stressed N. muscorum and Anabaena sp. have been presented in Fig. 5a. The Ni at 1 µM dose substantially reduced the content of protein by 22% in N. muscorum and by 25% in Anabaena sp. accordingly. Nevertheless, upon exogenous exposure of NaHS and SNP to Ni stressed cyanobacteria, noticeably decreased the negative impact of Ni on the content of protein. On the other hand, the ameliorative response of NaHS and SNP on protein content was diminished on the supplementation of PTIO, L-NAME, HT and PAG under similar stress condition.
Effect of exogenous hydrogen sulfide and nitric oxide on (a) protein (b) carbohydrate and (c) exopolysaccharides (EPS) of cyanobacteria N. muscorum and Anabaena sp. exposed to Ni stress after 72 h of treatment. Data presented as means ± standard error of three replicates (n = 3). Bars followed by different letters show significant difference at P < 0.05 significance level according to the Duncan’s multiple range test (DMRT). NaHS (sodium hydrosulfide as donor of hydrogen sulfide); SNP (sodium nitroprusside as donor of nitric oxide)
Carbohydrate content
The content of carbohydrate in test cyanobacteria was decreased at 1 µM dose of Ni by 23% in N. muscorum and by 27% in Anabaena sp. accordingly (Fig. 5b). The exogenous exposure of NaHS and SNP to Ni treated cells showed a significant recovery in carbohydrate content in the test cyanobacteria, but the reverse impact was observed in the presence of PTIO, L-NAME, HT, and PAG in the appearance of both signaling molecules. Further, a drastic decline in carbohydrate content was observed in the combined set of treatments i.e., Ni + NaHS + SNP + PTIO + L-NAME and Ni + NaHS + SNP + HT + PAG and the reduction was noticed by 41% and 45% in N. muscorum and by 44% and 48% in Anabaena sp. respectively with the same stress.
Exopolysaccharides (EPS) content
The impact of exogenous supplementation of NaHS and SNP on the secretion of EPS in both cyanobacteria has been depicted in Fig. 5c. In test cyanobacteria N. muscorum and Anabaena sp., EPS secretion was substantially decreased (P < 0.05) by 15% and 17% accordingly under Ni stress. Under the treatment of Ni + NaHS, Ni + SNP and Ni + NaHS + SNP, the secretion of EPS was increased substantially (P < 0.05) by 13%, 14% and 19% in N. muscorum and by 11%, 11% and 16% in Anabaena sp. accordingly. While, under similar stress, the deterioration in a layer of EPS secretion appeared more pronounced under the treatment of PTIO, L-NAME, HT and PAG along with NaHS and SNP and the reduction was noticed about 28%, 33%, 39% and 44% in N. muscorum and by 32%, 37%, 47% and 49% in Anabaena sp. accordingly. Further, combined treatment of NaHS and SNP with their respective inhibitor and scavenger under Ni stress showed a crucial decline in EPS content in both the experimental organisms.
Scanning electron microscopy (SEM) and energy-dispersive X-ray (EDX) analysis
The surface morphology of both test cyanobacteria N. muscorum and Anabaena sp. on Ni accumulation was observed by using SEM (Fig. 6a–c). After the Ni accumulation, SEM images exhibited the occurrence of groves and depressions on the cyanobacterial cell surface, lessening the size of cell and showing cell contraction under Ni stress. Over the aperture, the white crusts appearance is observable in the SEM images that are suggested to be bindings of metal ions as shown by the red arrow. However, exogenous supplementation of NaHS and SNP caused significant alleviation and exhibited a lesser amount of Ni accumulation on the cell surface of test cyanobacteria which was confirmed by EDX analysis (Fig. 6a–c). The other elements such as carbon (C), nitrogen (N), sulfur (S), and oxygen (O) are also detected by the EDX analysis (Fig. 6b, c). In control treatment C, N, O and S are present normally in both the test cyanobacteria. The 1 µM Ni caused a significant reduction in weight and atomic percentage (%) of C, N, S and O content. In alleviatory sets i.e., Ni + NaHS, Ni + SNP, and Ni + NaHS + SNP exhibited enhanced weight % and atomic % in C, N, O, and S of both cyanobacteria under Ni stress (Fig. 6b, c).
a Experimental setups showing effect of exogenous hydrogen sulfide and nitric oxide in Ni stressed N. muscorum and Anabaena sp. at 72 h of treatment. Scanning electron micrograph (SEM) and energy-dispersive X-ray (EDX) analysis of dry algal absorbent of b N. muscorum and c Anabaena sp. treated with Ni upon exogenous supplementation of hydrogen sulfide and nitric oxide. NaHS (sodium hydrosulfide as donor of hydrogen sulfide); SNP (sodium nitroprusside as donor of nitric oxide)
Principal component analysis (PCA) and correlation matrix
To study and appraise the association between the impact of NaHS and SNP on growth (optical density), photosynthetic pigments [Chl a/Car, phycobiliproteins; PC, APC and PE], Ni accumulation, biochemical components (protein, carbohydrate and EPS), nitrate, nitrite, NR, NiR and GS, GOGAT and GDH (enzymes involved in ammonia assimilation) activities of both cyanobacteria, PCA plots have been prepared (Fig. 7a, b). Among all the studied experimental parameters, most of them were largely influenced by NaHS and SNP illustrating its key function in the amelioration of Ni stress. The activity of enzymes i.e., NR, NiR, GS and GOGAT are positively associated with NaHS and SNP exhibiting their significance in the regulation of Ni-induced toxicity. Cellular Ni accumulation and GDH activity are nearly correlated with Ni stress suggesting their improvement in the occurrence of stress condition. The PCA plot suggests that growth, pigments and biochemical components are firmly associated with NaHS and SNP depicting their ameliorative effect on both cyanobacteria N. muscorum and Anabaena sp. respectively (Fig. 7a, b).
Image of the correlation matrix (Fig. 8a, b) exhibited clear interactions among various examined experimental parameters. The growth is observed to be positively correlated with photosynthetic pigments, biochemical components and all the nitrate and nitrite assimilating enzymes, whereas GDH and intracellular Ni accumulation showed negative correlation thereby, indicating the amelioration of NaHS and SNP on augmentation of growth and deduction in the level of Ni stress in both test cyanobacteria (Fig. 8a, b).
Image of correlation matrix to show the interaction among different parameters in a N. muscorum and b Anabaena sp. [(− ve) shows negative correlation and (+ ve) shows positive correlation between the parameters]. NaHS (sodium hydrosulfide as donor of hydrogen sulfide); SNP (sodium nitroprusside as donor of nitric oxide)
Discussion
The current study emphasizes the positive impact of two signaling molecules, hydrogen sulfide (H2S) and nitric oxide (NO) on growth, photosynthetic pigments, biochemical components and nitrogen metabolism of two cyanobacteria Nostoc muscorum and Anabaena sp. accordingly under nickel (Ni) stress. Ni at tested dose (1 µM) showed a substantial inhibition in growth (Fig. 1a) in both test organisms and this might be because of (i) enhancement in cellular Ni accumulation inside the cell (Fig. 1b), (ii) decline in the photosynthetic pigment contents (Table 1), (iii) inhibition in nitrate and nitrite uptake, and activity of nitrogen assimilatory enzymes (nitrate and NH4+ assimilation) besides GDH (Figs. 2, 3, 4), and subsequently deleterious impacts on biochemical components (protein, carbohydrate and exopolysaccharides) (Fig. 5a–c). Similar outcomes were also described by Rizwan et al. (2019) in rice seedlings and by Verma et al. (2021) in N. muscorum exposed to Ni stress. Moreover, heavy metal stress also reduces the endogenous level of H2S and NO that also might be associated with growth inhibition (Fig. 1a) (Husain et al. 2022; Verma and Prasad 2021). Further, the exogenous supplementation of NaHS (H2S donor) and SNP (NO donor), alone and together lowered the Ni-induced toxicity on analyzed parameters which perhaps associated with (i) reduction in accumulation of intracellular Ni content (Fig. 1b), (ii) substantial recovery in prime light harvesting pigments; PBPs (Table 1), (iii) significant recovery in inorganic nitrogen uptake and enzymes participating in nitrate and ammonium assimilation (Figs. 2, 3, 4) and appreciable enhancement in biochemical components (Fig. 5). Similar observations were also described by Chen et al. (2020) in Chlamydomonas reinhardtii under allelochemical treatment and by Verma and Prasad (2021) in N. muscorum and Anabaena sp. exposed to Cd stress. While, exogenous exposure of scavengers (PTIO for NO and HT for H2S) and inhibitors (L-NAME for NO and PAG for H2S) of NO and H2S respectively, showed significant reduction in growth and thereby suggested the endogenous role of H2S and NO under Ni stress alleviation (Fig. 1a). Similar results of H2S and NO were also described in wheat seedlings under heat stress (Iqbal et al. 2021). Moreover, to recognize the cross-talk role of H2S and NO under Ni stress alleviation, their respective scavenger and inhibitor were exogenously supplied along with NaHS and SNP. Hence, the results suggested that both H2S and NO might have worked in a synergistic manner and both molecules alone were not efficient to mitigate the Ni toxicity in the application of PTIO, L-NAME, HT and PAG also suggested that H2S might exhibit the downstream action on NO-mediated alleviation in Ni stress. The present study is in accordance with other observations where the interactive role of H2S and NO was presented in wheat seedlings exposed to Cd stress (Kaya et al. 2019), in soyabean under Al stress (Wang et al. 2019) and heat tolerance in Triticum aestivum (Iqbal et al. 2021). Upon exposure to H2S and NO, a significant reduction in cellular Ni in experimental organisms might have occurred due to the downregulation of the UreH nickel transporter protein. This protein is primarily responsible for the uptake of Ni within the cell, resulting in a considerable reduction in intracellular Ni accumulation (Fig. 1b). Similar findings have been also observed in some cyanobacteria, Synechococcus sp. and Prochlorochoccus (Huertas et al. 2014). While, the application of PTIO/L-NAME/HT and PAG further accelerates the amount of Ni content and intensified Ni toxicity interior to cells by declining the ameliorating function of H2S and NO (Fig. 1b).
Nickel stress caused a significant inhibition in the ratio of Chl a/Car and phycobiliproteins (PBPs) contents in both cyanobacteria that perform crucial functions in photosynthesis. Nickel reduced the photosynthetic pigment contents, also disrupting its precursor or declining the enzymatic action that is incorporated in the biosynthesis of pigment contents or because of the formation of ROS (reactive oxygen species) contents (Martínez-Ruiz and Martínez-Jerónimo 2016). As an accessory pigment carotenoid play a significant role in the photoinhibition process, and reduction in the Chl a/Car ratio exhibited Ni toxicity on photosynthetic pigment contents foremost to injuring results on performing function of the antenna complex. Moreover, a decline in phycobiliproteins (PBPs) content was observed under Ni stress (Table 1) and inhibition might be owing to modification of PBPs biosynthesis or undesirable impairment showed by Ni because of its direct availability for heavy metals as its outer localization on thylakoid membranes. Thus, these results align with the findings of Verma et al. (2022), who reported a reduction in photosynthetic pigment and growth in Anabaena sp. under Ni stress. On the contrary, exogenous supplementation of NaHS and SNP alone as well as in combination showed substantial improvement in the pigment content in both tested organisms under Ni stress, this could be ascribed to (i) enhancement in chlorophyll synthesis and (ii) stabilization of thylakoid membrane (Table 1) (Verma et al. 2021). However, all the positive responses of H2S and NO were reversed by exogenously supplied PTIO, L-NAME, HT and PAG on both the cyanobacteria exposed to Ni stress. Similar results also have been described in wheat seedlings under Cd stress (Kaya et al. 2019).
Nitrogen (N) is an essential macro-nutrient and is associated with various metabolic activities, and physiological processes inside the cyanobacterial cell that maintain the equilibrium between the uptake and nitrogen assimilation which directly influence on the growth of cyanobacteria (Verma and Prasad 2021). In cyanobacteria, heterocytes (also known as heterocysts) are specialized cells designed for nitrogen fixation in anaerobic conditions. This implies their ability to convert atmospheric nitrogen into bioavailable forms such as ammonia or nitrate, playing a crucial role in supporting the growth and survival of organisms, especially in nitrogen-deficient environment (Zulkefli and Hwang 2020). Cyanobacteria, the primary nitrogen-fixing photoautotrophs, utilize nitrate (NO3−) as an auspicious nitrogen source, converting it into NO2− (nitrite) and NH4+ (ammonia) via enzymatic activities i.e., NR (nitrate reductase) and NiR (nitrite reductase) (Verma and Prasad 2021). In this study, Ni stress caused the deduction in the rate of NO3− and NO2− uptake and enzymatic action of NR and NiR in both cyanobacteria primarily due to declined NO3− uptake (Figs. 2, 3). Nitrate (NO3−) uptake is an ATP-dependent process and actively mediated through ABC transporter protein and inhibition in the uptake mechanism might be due to the diminishing of electron transport chain (ETC) and possible inhibition in the supply of ATP under Ni stress in both test cyanobacteria. Similar findings were also observed in Nostoc muscorum and Phormidium foveolarum (Sheeba et al. 2011), Anabaena sp. PCC 7120 (Verma et al. 2022), and Chlamydomonas reinhardtii (Devriese et al. 2001) under pesticide, UV-B and heavy metal stress. Further, in the presence of NR enzyme NO3− is reduced into NO2− and after that NO2− is converted into NH4+ by catalyzing the NiR activity and Ni substantially diminished the NR and NiR activity, primarily due to enhanced intracellular accumulation of Ni (Fig. 1b). The present study is in concurrence with Rizwan et al. (2019), where Ni appreciably reduced the enzymes involved in nitrogen assimilation. Additionally, the decline in enzymatic action of NR and NiR might be attributed to (i) reduced fixation of carbon, (ii) less uptake of NO3− and (iii) impaired ETC which hinders reduced Fd as an electron donor to decline NO2− uptake (Verma et al. 2021). Furthermore, the exogenous supplementation of NaHS and SNP alone and in a combined manner showed significant alleviation under Ni stress on nutrient uptake and the substantial recovery was also noticed. Similarly, Raju and Prasad (2023) also reported the enhanced activity of NR and NiR upon exposure of NaHS in tomato and brinjal seedlings in salinity stress. Moreover, exogenous hydrogen sulfide (H2S) could act as an environmental signal inducing heavy metal tolerance in cyanobacterium Anabaena sp. The protective role of H2S on heavy metal stress such as Al stress could be related to its capacity to act as a mitigating agent and regulate the redox state of cells (Verma et al. 2023). Nonetheless, the exogenous addition of PTIO, L-NAME, HT and PAG along with NaHS and SNP under Ni stress showed critical inhibition in the inorganic uptake of nitrogen and activity of their assimilating enzymes (Figs. 2, 3) hence, the results suggested that coordinated function of H2S and NO in both cyanobacteria. These results are similar to the study of Rizwan et al. (2019) in rice seedling under Ni stress.
Ammonium ion (NH4+) is highly toxic and it must be removed from the cell or assimilated into other organic substances. In cyanobacteria, NH4+ assimilation primarily takes place by the GS-GOGAT pathway in the presence of ammonia-assimilating enzymes (Sanz-Luque et al. 2015). In the present study, the GS and GOGAT activities were noticed to decline substantially under Ni stress (Fig. 4a, b) which might be owing to (i) higher NH4+ ions accumulation (ii) impairment in ETC thereby resulting lowered amount of ATP synthesis, and (iii) alteration in osmotic balance and intracellular pH thereby reduced the growth and photosynthetic rate that directly repressed the activity of enzyme (Dai et al. 2008). Similar to the current study, Verma et al. (2021, 2022) have also found the declined activity of GS and GOGAT in Ni-stressed cyanobacteria N. muscorum and Anabaena sp. respectively. Under the stress condition, photoautotrophs overcome the toxicity of ammonium by another ammonium assimilating enzyme i.e., glutamate dehydrogenase (GDH) (Fig. 4c) that follow the alternative pathway thereby maintaining the glutamate levels including synthesis of proline and phytochelatin (non-enzymatic antioxidants). However, supplementation of NaHS and SNP alone and together with Ni stress caused appreciable improvement in the activity of GS and GOGAT while lowering the activity of GDH in both tested organisms (Fig. 4a–c), which could be elucidated due the lower availability of substate as NH4+ which is largely assimilated by GS-GOGAT pathway. The present study is in accordance with the study of Balotf et al. (2018) that described exogenous SNP accelerated the activity of GS and GOGAT (ammonium assimilating enzymes) and mitigated ammonium toxicity in wheat seedlings while similar to this, Raju and Prasad (2023) have demonstrated that the exogenously applied H2S can alleviate the salt stress and increased ammonium assimilating enzyme activities in tomato and brinjal seedlings. Furthermore, exogenous application of PTIO, L-NAME, HT and PAG along with NaHS and SNP under the same stress condition again lowered the activity of GS and GOGAT while negatively accelerates the GDH activity (Fig. 4a–c) and thereby suggesting the interlinked role of H2S and NO in removing ammonium toxicity from both tested cyanobacterial cells. Similar results were also noticed in N. muscorum with exogenous supplementation of SNP and calcium under Ni stress (Verma et al. 2021).
Cyanobacteria have enormous prospects to supply valuable food supplements like protein and carbohydrates which are precisely correlated with growth during stress conditions. The present study showed inhibition in protein content exposed to Ni stress (Fig. 5a). This inhibition might be due to the direct effect of Ni on protein biosynthesis, as reported by Verma and Prasad (2021) in N. muscorum and Anabaena sp., where Cd caused a reduction in protein content. However, the application of NaHS and SNP minimized the adverse effect of Ni on protein content which could be owing to overcome the response of ROS content interior to cell (Kaya et al. 2019). Moreover, the addition of PTIO/L-NAME/HT and PAG together with NaHS and SNP under similar stress condition reverse the positive response of H2S and NO thereby, critically damage protein content which is directly associated with the growth of both cyanobacteria. Our results are in congruence with the study of Raju and Prasad (2023) in tomato and brinjal seedlings under salinity and by Verma et al. (2021) in N. muscorum with Ni stress. Carbohydrate is the main reserve food in cyanobacteria which provide an energy source for the survival of cell under adverse conditions that are directly associated with growth. Apart from that, carbohydrate is the prime component of the extracellular polymeric substance of cyanobacteria that act as a defensive layer and protect the cell under stress condition (Patel et al. 2020). In the present study, a substantial reduction in carbohydrate content was noticed which might have appeared because of a decline in photosynthesis rate and inhibition in photosynthetic pigment contents (Fig. 5b). Similar results have been also observed in Chlorella vulgaris (Bajguz 2011), and in Spirulina platensis (Gupta et al. 2014) under lead and chromium toxicity. Nonetheless, exogenously applied NaHS and SNP substantially improved the carbohydrate content under Ni stress. Further, on exogenous supplementation of scavengers as well as inhibitors of H2S and NO exhibited a crucial decline in carbohydrate content exposed to Ni-induced toxicity in both test cyanobacteria thus, the results suggest the interdependent role of H2S and NO on each other’s function.
Exopolysaccharides (EPS) are high molecular weight polymeric substances and act as a physical barrier (defensive layer) that is secreted by cyanobacteria to suppress the negative impacts of heavy metal toxicity (Planchon et al. 2013). In the current study, the 1 μM Ni caused a notable reduction in EPS content in both cyanobacteria (Fig. 5c). This reduction is associated with increased Ni accumulation within the cells, as shown in Fig. 1b. A similar result regarding to decrease in EPS content was also observed in Nostoc muscorum and Anabaena sp. (Patel et al. 2020), Anabaena sp. PCC 7120 (Tiwari et al. 2019), and Nostoc muscorum (Verma et al. 2021) under As, Al and Ni stress. Furthermore, exposure to NaHS and SNP resulted in a significant increase in EPS content, which correlated with a raised amount of carbohydrate levels, and a notable decrease in the intracellular accumulation of Ni. This outcome suggests that both NaHS and SNP contribute to an enhanced formation of EPS, aiming to impede intracellular Ni accumulation. However, supplementation with PTIO/L-NAME/HT and PAG considerably increased the Ni content inside the cell, thereby reducing the positive impacts of H2S and NO (Fig. 5c). Similarly, Tiwari et al. (2019) demonstrated that the use of PTIO and L-NAME in Anabaena sp. PCC 7120 led to a decrease in EPS content and an increase in Al accumulation within the cells. The enhancement in EPS contents suggests the accumulation of Ni on the cell surface of cyanobacteria. This phenomenon may be attributed to Ni aggregation, surrounded by an outer thick layer of EPS secreted by cyanobacterial cells. The results of EPS secretion are illustrated by applying SEM analysis (Fig. 6b, c), along with exogenous supplementation of NaHS and SNP, which exhibited substantial improvement under Ni stress (Fig. 6a–c). In this study, EDX analysis confirmed that Ni accumulated on the cell surface of both cyanobacteria. Moreover, other elements such as C, N, O and S contents are also presented by weight and atomic percentage in Fig. 6b, c. Similarly, Ghoneim et al. (2014) examined the surface morphology of Ulva lactuca before and after Cd adsorption using SEM and observed that Cd precipitated around the cell surface or extracellularly. To examine the overall response, the principal component analysis was performed (Fig. 7a, b), which exhibits a more pronounced amelioration of NaHS and SNP on growth, photosynthetic pigments, biochemical components, nitrate, nitrite, NR, NiR, GS and GOGAT activity. Therefore, H2S and NO have been shown to be better in alleviating Ni-induced toxicity in N. muscorum and Anabaena sp.
Conclusion
The current study highlighted the noticeable role of two signaling molecules i.e., hydrogen sulfide (H2S) and nitric oxide (NO) in decreasing the deleterious impacts induced by Ni toxicity in rice field cyanobacteria Nostoc muscorum and Anabaena sp. The tested dose of Ni caused detrimental consequences on growth, Chl a/Car ratio and phycobiliproteins (PBPs) contents and nitrogen metabolism. and also reduced biochemical components namely, protein, carbohydrate and EPS contents. Along with physiological and biochemical parameters, biochemical components namely protein, carbohydrate, and EPS contents were also negatively affected by Ni stress. The negative effect on EPS was also shown by SEM images in both the cyanobacteria. Further, the exogenous application of H2S and NO reduced Ni induced toxicity and caused substantial improvement in the above-mentioned parameters. Overall, this study signifies the interplay function of both signaling molecules by applying their respective scavengers and inhibitors (PTIO/L-NAME/HT and PAG) (Fig. 9). H2S (NaHS) and NO (SNP) may be used as more cost-effective and feasible molecules. They both paly crucial roles in easing the stress on both cyanobacteria exposed to Ni and enhancing their survival. This, in turn, increases nitrogen content (acting as biofertilizer) in rice fields, improving soil fertility and crop yield.
Data availability
The authors do not have permission to share data.
Abbreviations
- APC:
-
Allophycocyanin
- EPS:
-
Exopolysaccharides
- HT:
-
Hypotaurine
- L-NAME:
-
Nѡ-Nitro-L-arginine methyl ester hydrochloride
- NaHS:
-
Sodium hydrosulfide
- PAG:
-
Propargylglycine
- PC:
-
Phycocyanin
- PE:
-
Phycoerythrin
- PTIO:
-
2-Phenyl-4,4,5,5-tetramethylimidazoline-1-oxyl-3-oxide
- SNP:
-
Sodium nitroprusside
References
Allah EFA, Hashem A, Alam P, Ahmad P (2019) Silicon alleviates nickel-induced oxidative stress by regulating antioxidant defense and glyoxalase systems in mustard plants. J Plant Growth Regul 38:1260–1273
Bajguz A (2011) Suppression of Chlorella vulgaris growth by cadmium, lead and copper stress and its restoration by endogenous brassinolide. Arch Environ Contamin Toxicol 60:406–416
Balotf S, Islam S, Kavoosi G, Kholdebarin B, Juhasz A, Ma W (2018) How exogenous nitric oxide regulates nitrogen assimilation in wheat seedlings under different nitrogen sources and levels. PLoS ONE 13:0190269
Bennett A, Bogorad L (1973) Complementary chromatic adaptation in a filamentous blue-green alga. J Cell Biol 58:419–435
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein–dye binding. Anal Biochem 72:248–254
Cawse PA (1967) The determination of nitrate in soil solution by ultraviolet spectrophotometry. Analyst 92:311–315
Chávez S, Candau P (1991) An NAD-specific glutamate dehydrogenase from cyanobacteria identification and properties. FEBS Lett 285:35–38
Chen XD, Liu Y, Yang LM, Hu XY, Jia AQ (2020) Hydrogen sulfide signaling protects Chlamydomonas reinhardtii against allelopathic damage from cyanobacterial toxin microcystin-LR. Front Plant Sci 11:1105
Chittora D, Meena M, Barupal T, Swapnil P (2020) Cyanobacteria as a source of biofertilizers for sustainable agriculture. Biochem Biophys Rep 22:100737
Dai GZ, Deblois CP, Liu SW, Juneau P, Qiu BS (2008) Differential sensitivity of five cyanobacterial strains to ammonium toxicity and its inhibitory mechanism on the photosynthesis of rice-field cyanobacterium Ge-Xian-Mi Nostoc. Aquat Toxicol 89:113–121
Devriese M, Tsakaloudi V, Garbayo I, León R, Vílchez C, Vigara J (2001) Effect of heavy metals on nitrate assimilation in the eukaryotic microalga Chlamydomonas reinhardtii. Plant Physiol Biochem 39:443–448
Dubois M, Gilles KA, Hamilton JK, Rebers PA, Smith F (1956) Colorimetric method for determination of sugars and related substances. Anal Chem 28:350–356
Ghoneim MM, El-Desoky HS, El-Moselhy KM, Amer A, Abou El-Naga EH, Mohamedein LI, Al-Prol AE (2014) Removal of cadmium from aqueous solution using marine green algae, Ulva lactuca. The Egypt J Aquat Res 40:235–242
Goodwin TW (1954) Carotenoids. In: Paech K, Tracey MVE (eds) Handbook of plant analysis, vol 3. Springer, Berlin, pp 272–311
Gupta S, Sharma S, Singh S (2014) Hexavalent chromium toxicity to cyanobacterium Spirulina platensis. Int Res J Pharm 12:910–914
Hannan F, Huang Q, Farooq MA, Ayyaz A, Ma J, Zhang N, Ali B, Deyett E, Zhou W, Islam F (2021) Organic and inorganic amendments for the remediation of nickel contaminated soil and its improvement on Brassica napus growth and oxidative defense. J Hazard Mater 416:125921
Herrero A, Guerrero MG (1986) Regulation of nitrite reductase in the cyanobacterium Anacystis nidulans. J Gen Microbiol 132:2463–2468
Herrero A, Flores E, Guerrero MG (1981) Regulation of nitrate reductase levels in the cyanobacteria Anacystis nidulans, Anabaena sp. strain 7119, and Nostoc sp. strain 6719. J Bacteriol 145:175–180
Herrero A, Flores E, Guerrero MG (1984) Regulation of the nitrate reductase level in Anacystis nidulans, activity decay under nitrogen stress. Arch Biochem Biophys 234:454–459
Huertas MJ, López-Maury L, Giner-Lamia J, Sánchez-Riego AM, Florencio FJ (2014) Metals in cyanobacteria: analysis of the copper, nickel, cobalt and arsenic homeostasis mechanisms. Life 4:865–886
Husain T, Suhel M, Prasad SM, Singh VP (2022) Ethylene and hydrogen sulphide are essential for mitigating hexavalent chromium in two pulse crops. Plant Biol 24:652–659
Husain T, Suhel M, Prasad SM, Singh VP (2021) Ethylene needs endogenous hydrogen sulfide for alleviating hexavalent chromium stress in Vigna mungo L. and Vigna radiata L. Environ Pollut 290:117968
Iqbal N, Umar S, Khan NA, Corpas FJ (2021) Nitric oxide and hydrogen sulfide coordinately reduce glucose sensitivity and decrease oxidative stress via ascorbate-glutathione cycle in heat-stressed wheat (Triticum aestivum L.) plants. Antioxidants 10:108
Kaya C, Ashraf M, Alyemeni MN, Ahmad P (2019) Responses of nitric oxide and hydrogen sulfide in regulating oxidative defence system in wheat plants grown under cadmium stress. Physiol Plant 168:345–360
Khan KMIR, Jahan B, Al Ajimi MF, Rehman MT, Khan NA (2020) Ethephon mitigates nickel stress by modulating antioxidant system, glyoxalase system and proline metabolism in Indian Mustard. Physiol Mol Biol Plants 26:1201–1213
Martínez-Ruiz EB, Martínez-Jerónimo F (2016) How do toxic metals affect harmful cyanobacteria? An integrative study with a toxigenic strain of Microcystis aeruginosa exposed to nickel stress. Ecotoxicol Environ Safe 133:36–46
Meers JL, Tempest DW, Brown CM (1970) Glutamine amide, 2- oxoglutarate amino transferase oxido-reductase NADP; an enzyme involved in the synthesis of glutamate by some bacteria. J Gen Microbiol 64:187–194
Mérida A, Candau P, Florencio FJ (1991) Regulation of glutamine synthetase activity in the unicellular cyanobacterium Synechocystis sp. strain PCC 6803 by the nitrogen source, effect of ammonium. J Bacteriol 173:4095–4100
Mukherjee S, Corpas FJ (2020) Crosstalk among hydrogen sulfide (H2S), nitric oxide (NO) and carbon monoxide (CO) in root-system development and its rhizosphere interactions: a gaseous interactome. Plant Physiol Biochem 155:800–814
Navarro F, Chävez S, Candau P, Florencio FJ (1995) Existence of two ferredoxin-glutamate synthases in the cyanobacterium Synechocystis sp. PCC 6803. Isolation and insertional inactivation of gltB and gltS genes. Plant Mol Biol 27:753–767
Pandey A, Kumar S, Singh G, Prasad SM (2022) Regulatory role of gamma-aminobutyric acid in cyanobacteria challenged with UV-B: the implication of nitric oxide. Plant Stress 5:100094
Patel A, Tiwari S, Prasad SM (2020) Effect of time interval on arsenic toxicity to paddy field cyanobacteria as evident by nitrogen metabolism, biochemical constituent, and exopolysaccharide content. Biol Trace Elem Res 199:2031–2046
Planchon M, Jittawuttipoka T, Cassier-Chauvat C, Guyot F, Gelabert A, Benedetti MF, Chauvat F, Spalla O (2013) Exopolysaccharides protect Synechocystis against the deleterious effects of titanium dioxide nanoparticles in natural and artificial waters. J Colloid Interface Sci 405:35–43
Porra RJ, ThompsonWA KPE (1989) Determination of accurate extinction coefficients and simultaneous equations for assaying chlorophylls a and b extracted with four different solvents, verification of the concentration of chlorophyll standards by atomic absorption spectroscopy. Biochim Biophys Acta Bioenerg 975:384–394
Raju AD, Prasad SM (2021) Hydrogen sulfide implications on easing NaCl induced toxicity in eggplant and tomato seedlings. Plant Physiol Biochem 164:173–184
Raju AD, Prasad SM (2023) Hydrogen sulfide regulates NaCl tolerance in brinjal and tomato seedlings by Na+/K+ homeostasis and nitrogen metabolism. Plant Stress 7:100129
Rizwan M, Mostofa MG, Ahmad MZ, Zhou Y, Adeel M, Mehmood S, Ahmad MA, Javed R, Imtiaz M, Aziz O, Ikram M, Tu S, Liu Y (2019) Hydrogen sulfide enhances rice tolerance to nickel through the prevention of chloroplast damage and the improvement of nitrogen metabolism under excessive nickel. Plant Physiol Biochem 138:100–111
Sanz-Luque E, Chamizo-Ampudia A, Llamas A, Galvan A, Fernandez E (2015) Understanding nitrate assimilation and its regulation in microalgae. Front Plant Sci 6:899
Sharma M, Kaushik A, Bala SK, Kamra A (2008) Sequestration of chromium by exopolysaccharides of Nostoc and Gleocapsa from dilute aqueous solution. J Hazard Mater 157:315–318
Sheeba SVP, Srivastava PK, Prasad SM (2011) Differential physiologicaland biochemical responses of two cyanobacteria Nostoc muscorum and Phormidium foveolarum against oxyfluorfenand UV-B radiation. Ecotoxicol Environ Saf 74:1981–1993
Shen L, Chen R, Wang J, Fan L, Cui L, Zhang Y, Cheng J, Wu X, Li J, Zeng W (2021) Biosorption behavior and mechanism of cadmium from aqueous solutions by Synechocystis sp. PCC 6803. RSC Adv 11:18637
Singh G, Patel A, Tiwari S, Gupta D, Prasad SM (2022) Signaling molecules hydrogen sulfide (H2S) and nitric oxide (NO): role in microalgae under adverse environmental conditions. Acta Physiol Plant 44:1–15
Snell FD, Snell CT (1949) Colorimetric methods of analysis, vol 3. Van Nostrand, New York, pp 804–805
Soliman M, Alhaithloul HA, Hakeem KR, Alharbi BM, El-Esawi M, Elkelish A (2019) Exogenous nitric oxide mitigates nickel-induced oxidative damage in eggplant by upregulating antioxidants, osmolyte metabolism, and glyoxalase systems. Plants 8:562
Strejckova A, Dvorak M, Klejdus B, Krystofova O, Hedbavny J, Adam V, Huska D (2019) The strong reaction of simple phenolic acids during oxidative stress caused by nickel, cadmium and copper in the microalga Scenedesmus quadricauda. New Biotechnol 48:66–75
Tipu MI, Ashraf MY, Sarwar N, Akhtar M, Shaheen MR, Ali S, Damalas CA (2021) Growth and physiology of maize (Zea mays L.) in a nickel-contaminated soil and phytoremediation efficiency using EDTA. J Plant Growth Regul 40:774–786
Tiwari S, Prasad SM (2020) Regulation of insecticide toxicity by kinetin in two paddy field cyanobacteria: physiological and biochemical assessment. Environ Pollut 259:113806
Tiwari S, Verma N, Singh VP, Prasad SM (2019) Nitric oxide ameliorates aluminium toxicity in Anabaena PCC 7120: regulation of aluminium accumulation, exopolysaccharides secretion, photosynthesis and oxidative stress markers. Environ Exp Bot 161:218–227
Valivand M, Amooaghaie R (2021) Calcium signaling confers nickel tolerance in Cucurbita pepo L. Int J Phytoremed 23:362–373
Verma N, Prasad SM (2021) Interplay of hydrogen peroxide and nitric oxide: systemic regulation of photosynthetic performance and nitrogen metabolism in cadmium challenged cyanobacteria. Physiol Mol Biol Plants 27:2181–2199
Verma N, Pandey A, Tiwari S, Prasad SM (2021) Calcium mediated nitric oxide responses: acquisition of nickel stress tolerance in cyanobacterium Nostoc muscorum ATCC 27893. Biochem Biophys Rep 26:100953
Verma N, Parihar P, Singh R, Prasad SM, Pandey A (2022) Salicylic acid induced signaling against nickel-induced toxicity: responses of growth, exopolysaccharides, photosystem II photochemistry, nitrogen metabolism status and antioxidant system of Anabaena sp. PCC 7120. S Afr J Bot 151:1–15
Verma N, Tiwari S, Singh VP, Prasad SM (2023) Exploring the potential of hydrogen sulfide in acquisition of aluminium stress tolerance in cyanobacterium Anabaena sp. PCC 7120. Vegetos. pp 1–13
Wang H, Ji F, Zhang Y, Hou J, Liu W, Huang J, Liang W (2019) Interactions between hydrogen sulphide and nitric oxide regulate two soybean citrate transporters during the alleviation of aluminium toxicity. Plant Cell Environ 42:2340–2356
Zulkefli NS, Hwang SJ (2020) Heterocyst development and diazotrophic growth of Anabaena variabilis under different nitrogen availability. Life 10:279
Acknowledgements
Garima Singh is thankful to the University grant commission (UGC) New Delhi, for getting financial assistance to the Department of Botany, University of Allahabad, Prayagraj, for conducting and succeed the present work. The authors are also thankful to Mr. Hemant Kumar Singh for assisting in the SEM-EDX images and AAS analysis (IIT Kharagpur, West Bengal).
Author information
Authors and Affiliations
Contributions
SMP hypothesized and designed the experiment. GS performed the experiment, finalized the data, figure, and table and drafted the original manuscript. SMP and GS finalized the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Singh, G., Prasad, S.M. Synergistic regulation of hydrogen sulfide and nitric oxide on biochemical components, exopolysaccharides, and nitrogen metabolism in nickel stressed rice field cyanobacteria. J Plant Res 137, 521–543 (2024). https://doi.org/10.1007/s10265-024-01530-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10265-024-01530-7