Abstract
Background
Previous meta-analyses have shown an ethnic dependency of the C677T and the A1298C methylenetetrahydrofolate reductase (MTHFR) polymorphisms, with no focus on the Latino population. For Latinos, many studies have examined these polymorphisms and breast cancer susceptibility, yielding no concise result. Therefore, we undertook this meta-analysis to determine the effect these polymorphisms have on breast cancer risk for Latinos.
Methods
PubMed, EBSCO, LILACS, Scopus, and Latin American-specific databases were searched for studies exploring the association between the MTHFR polymorphisms and breast cancer susceptibility in Latinos until January 2019. Genotype distributions were extracted and, depending on the level heterogeneity determined by the ψ2-based Q test and the I2 test, fixed-effects or random-effects models were used to calculate pooled odds ratios (ORs) with 95% confidence intervals (95% CIs) for the heterozygous, homozygous, dominant, recessive, and allelic genetic models. No publication bias was detected by the Begg–Mazumdar’s test and Egger’s test.
Results
Of the 280 retrieved publications, 9 studies were included: 9 for the C677T polymorphism and 5 for the A1298C polymorphism. For the C677T polymorphism, there was an elevated risk for the homozygous (OR 1.42, 95% CI 1.05–1.92), the dominant (OR 1.16, 95% CI 1.02–1.31), the recessive (OR 1.33, 95% CI 1.01–1.75), and the allelic model (OR 1.17, 95% CI 1.03–1.33, p < 0.01). No association between the A1298C polymorphism and the risk to develop breast cancer was determined.
Conclusion
The results indicated that, for Latinos, the C677T polymorphism is associated with a significant risk for developing breast cancer, whereas the A1289C polymorphism does not.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cancer is one of the leading causes of death worldwide. In Latin America, breast cancer is the most frequently diagnosed cancer, with over 150,000 new cases annually [1]. Numerous reports have demonstrated the association between developing breast cancer and a patient’s exposure to ultraviolet and ionizing radiation, chemical and biological carcinogens, as well as diet [2]. One key enzyme associated with the dietary influences on breast cancer development is methylenetetrahydrofolate reductase (MTHFR) [3]. MTHFR, which is encoded by the mthfr gene on Chromosome 1, plays an important role for the reduction of 5,10-methylenetetrahydrofolate to 5-methyltetrahydrofolate, a key step in the one-carbon metabolism pathway. 5-methyltetrahydrofolate is utilized by homocysteine to make methionine. Afterwards, methionine transfers a methyl group to SAM, which is eventually used for DNA methylation, promoting the expression of certain oncogenes and suppression of tumor suppressor genes [4, 5]. Thus, MTHFR provides a key link between folate consumption and DNA methylation, which is associated with cancer development. However, the exact mechanism remains unclear. Some have posited that, during cell division, low folate availability results in increased production and incorporation of uracil into the DNA, due to the limited availability of thymidine [5]. In support of this, Gao et al. and Shrubsole et al. both demonstrated that low folate consumption was associated with an increased risk of developing breast cancer [6, 7]. For a comprehensive review about MTHFR and cancer, please refer Crider et al. 2012 [5].
Two single nucleotide polymorphisms have been shown to effect MTHFR enzymatic activity. The first is the C677T polymorphism (Ala222Val, rs1801133), located in exon 4. This polymorphism was shown to decrease enzymatic activity by 75% for the homozygote genotype [8]. The other polymorphism is A1298C (Glu429Ala, rs1801131), located in exon 7. This polymorphism was also shown to decrease enzymatic activity but to a lesser degree [8]. Carriers of these MTHFR polymorphisms were shown to be associated with breast cancer risk. When considering the C677T genotypes, compared to high folate consumption with CC genotype, Gao et al. and Shrubsole et al. both demonstrate that the risk was significantly augmented for the CC, CT and TT genotypes with low folate consumption [6, 7]. Interestingly, these polymorphisms appear to have an ethnic dependency. For example, for the Asian population, the C677T polymorphism was associated with an increased risk for developing breast cancer [9, 10]. However, the results for the A1298C polymorphism are conflicting, with one study determining a benefit for the heterozygous genetic model [11] and others demonstrating no effect at all [12, 13]. Whereas, for Caucasians, the A1298C polymorphism was associated with an increased risk of developing breast cancer [12] and not the C677T polymorphism [9, 10, 13, 14]. Some meta-analyses have tried to elucidate the effect, either determining detrimental effects or no effect at all, with no specific analysis aimed towards Latinos. Nonetheless, there is no definitive assessment among the Latin American population.
Many Latin American countries have examined the risk associated with these MTHFR polymorphisms and breast cancer; however, conflicting results were obtained [15,16,17,18]. With so many studies focusing on the C677T and the A1298C polymorphisms and breast cancer susceptibility in Latinos yielding no concise result, we undertook this meta-analysis to determine the effect these MTHFR polymorphisms have on breast cancer risk for Latinos.
Materials and methods
Publication search strategy
PubMed, EBSCO, LILACS, and Scopus databases were searched for published studies that examined the association between the MTHFR polymorphisms and breast cancer susceptibility in Latinos until January 21, 2019. The search strategy included keywords and any of their derivation for MTHFR (methylenetetrahydrofolate reductase, MTHFR), polymorphism (polymorphism, SNP, mutation, C677T, rs1801133, A1298C, rs1801131), cancer (cancer, cáncer, carcinoma), and Latin Americans (Latino, Hispanic, etc.) as well for Latin American countries. Furthermore, we manually reviewed the reference lists for more relevant studies. We increased our coverage by also including other Latino databases (DOAJ, Periodica, Bibliat, Latin index, Imbiomed). No language limitation was applied in the literature search.
Inclusion and Exclusion Criteria
Two authors independently examined each publication for inclusion. If the two authors disagreed about a publication, a third author assessed the publication and made a final decision. The inclusion criteria were: (1) evaluated the association between the C677T and/or the A1298C polymorphisms and breast cancer susceptibility; (2) case–control studies; (3) genotype data were available for cases and controls; (4) data based on the Latino population. The exclusion criteria were: (1) no detailed information of genotype data; (2) duplicate of a previously published study; (3) no Latino population or was mixed with another ethnicity; (4) cancer was not confirmed by histological and laboratorial methods. When duplicate data appeared in different publications, this meta-analysis only adopted the most recent study or the study with the most complete information.
Data extraction
To guarantee the accuracy of the extracted information, two authors individually reviewed each publication. The following data were collected: author’s name, year of publication, country, polymorphism(s), type of breast cancer, source of the controls (hospital or population based), number of cases and controls, and genotype frequency. Two authors independently assessed the quality of the studies using the Newcastle–Ottawa Quality Assessment Scale [19]. The following aspects of each study were appraised: selection of cases and controls, comparability, and outcome or exposure. For analysis, the quality scores ranged from 0 to 9 (Electronic supplementary material 1 and Electronic supplementary material 2). Studies that scored more than six stars were considered as a high-quality study.
Statistical analysis
The relationship between the C677T and the A1289C polymorphisms and breast cancer susceptibility was analyzed using five genetic models: the dominant model (12 + 22 v. 11), the recessive model (22 v. 12 + 11), the homozygous model (22 v. 11), the heterozygous model (12 v. 11), and the allele model (2 v. 1), where 1 is the wild-type and 2 is the mutation. Hardy–Weinberg Equilibrium (HWE) was tested by the ψ2-test for the control groups of each study. The level of heterogeneity was determined by the Cochran Q test and the Inconsistency index (I2). Crude odds ratios (ORs) with 95% confidence intervals (95% CIs) were used to evaluate the strength of association between the C677T and the A1298C polymorphisms and breast cancer susceptibility. Based on the level of heterogeneity, either the Fixed-effect model (Mantel–Haenszel method, p < 0.10 and I2 < 40%) [20] or the Random-effects model (Dersimonian–Laird method) was selected [21]. To examined publication bias, Egger’s test, Begg–Mazumdar’s test, and examining the funnel plots were performed. Sensitivity analysis was conducted by removing one study at a time and recalculating the pooled ORs to observe the stability of the crude pooled OR. All the statistical analyses were conducted using Review Manager Software (RevMan V5.3, Copenhagen, Denmark) and StatsDirect Statistical Software (StatsDirect V2.8, Cheshire, United Kingdom). Unless noted otherwise, p values < 0.05 (two-tailed) were considered statistically significant. This study was conducted in accordance with the PRISMA guidelines, see Electronic supplementary material 7.
Results
Selection of eligible studies
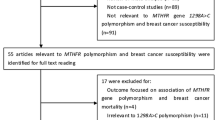
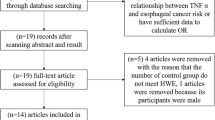
A total of 280 publications were retrieved from searching the multiple databases and from reviewing the publications’ bibliographies (Fig. 1). 252 publications were exclude because they were not an original research articles, focused on animals or cell lines, did not focus on breast cancer, or did not examine for MTHFR or its polymorphisms. The remaining 28 publications were extensively evaluated. 16 publications did not focus solely on the Latino population, 1 publication lacked sufficient information, and 2 publications used previously published data. This resulted in 9 publications that were included in this meta-analysis: 9 studies for the C677T polymorphism (case = 2136 and controls = 2436) [15,16,17,18, 22,23,24,25,26] and 5 studies for the A1298C polymorphism (cases = 1226 and controls = 1739) [22,23,24,25,26]. Most studies determine the presence of either polymorphism using the restriction fragment length polymorphism method (RFLP; n = 6), with the remaining three using other technologies (Table 1). We determined that the Calderon-Garcidueñas study [16] contained a high level of bias (Electronic supplementary material 1 and Electronic supplementary material 2). The genotype distributions of the controls were consistent with HWE, except for the Batschahuer study [15] and the Lopez-Cortes study [24]. The publication years of the involved studies ranged from 2001 to 2017. The characteristics of the included studies are shown in Table 1. For the C677T polymorphism, four different countries are represented in this meta-analysis, with the most representative population being Brazil with 5 studies [15, 18, 22, 23, 25], followed by Mexico with 2 studies [16, 17], 1 study from USA, which focused on the Latino population [23], and 1 study from Ecuador [24]. For the A1289C polymorphism, the most representative country was Brazil with 3 studies [22, 25, 26], followed by USA [23] and Ecuador [24] with 1 study each.
MTHFR C677T polymorphism increased the risk of breast cancer for Latinos
With respect to the C677T polymorphism, the homozygous, recessive, and allelic genetic models presented with significant heterogeneity (p < 0.10, I2 > 30%). Using the Random Effects model for these genetic models, we determined that for the homozygous (OR 1.42, 95% CI 1.05–1.92, p = 0.02), the recessive (OR 1.33, 95% CI 1.01–1.85, p = 0.04), and the allelic models (OR 1.17, 95% CI 1.03–1.33, p = 0.01, Fig. 2), the polymorphism was significantly associated with an increased risk of developing breast cancer. With the remaining models, using the fixed effects model, we determined that the dominant model was associated with an increased risk of developing breast cancer (OR 1.16, 95% CI 1.02–1.31, p = 0.02), whereas the heterozygous model did not show any association (OR 1.09, 95% CI 0.96–1.24, p = 0.20). However, the results are similar independent of the effects models used.
Forest plots of breast cancer risk associated with the MTHFR C677T polymorphism for the heterozygous (a), homozygous (b), dominant (c), recessive (d), and allelic (e) genetic models. The squares and horizontal lines correspond to the study-specific odds ratio (OR) and 95% confidence interval (95% CI), respectively. The area of the squares reflects the study-specific weight. The diamond represents the pooled OR and 95% CI, determined using either the fixed effects or random effects, depending on the level of heterogeneity. Plots were generated using Review Manager Software (RevMan V5.3, Copenhagen, Denmark)
When the sensitivity of the results was determined by removing one study and recalculating the ORs, the heterozygous and allelic models were resistant (Electronic supplementary material 3). However, for the homozygous model, the results were sensitive to the Lopez-Cortes study (OR 1.34, 95% CI 0.99–1.80), the Ramos-Silva study (OR 1.24, 95% CI 0.96–1.60), and the Zara-Lopes study (OR 1.35, 95% CI 0.99–1.85). For the dominant model, the results were sensitive to the Lopez-Cortes study (OR 1.12, 95% CI 0.99–1.27) and the Ramos-Silva study (OR 1.13, 95% CI 0.99–1.30). Lastly, for the recessive model, the results were sensitive to the Calderon-Garcidueñas study (OR 1.33, 95% CI 0.97–1.82), the Le Marchand study (OR 1.37, 95% CI 0.99–1.90), the Lopez-Cortes study (OR 1.28, 95% CI 0.96–1.72), the Ramos-Silva study (OR 1.15, 95% CI 0.94–1.40), the Rezende study (OR 1.35, 95% CI 0.99–1.85), and the Zara-Lopes study (OR 1.28, 95% CI 0.96–1.70).
Publication bias was assessed by examining the funnel plot for each of the genetic model. The funnel plots demonstrated no significant asymmetry and the shape of the funnel plots suggested no evidence of publication bias (Electronic supplementary material 4). However, a significant publication bias was determined by the Begg–Mazumdar’s test and Egger’s test only for the heterozygous model (Fig. 2). No correlation was determined by the Begg–Mazumdar’s test or bias by Egger’s test for the remaining models.
MTHFR A1289C polymorphism not increased the risk of breast cancer for Latinos
With respect to the A1298C polymorphism, none of the genetic models presented with significant heterogeneity (p < 0.10, I2 0%). Using either the fixed-effects or random-effect models, no association between the A1298C polymorphism and the risk to develop breast cancer was observed (Fig. 3). Removing one study had no effect on pooled ORs for any of the genetic models (Electronic supplementary material 5). When publication bias was assessed, no significant asymmetry was determined by examining the funnel plots (Electronic supplementary material 6); furthermore, this was confirmed by the Begg–Mazumdar’s test and Egger’s test (Fig. 3).
Forest plots of breast cancer risk associated with the MTHFR A1298C polymorphism for the heterozygous (a), homozygous (b), dominant (c), recessive (d), and allelic (e) genetic models. The squares and horizontal lines correspond to the study-specific odds ratio (OR) and 95% confidence interval (95% CI), respectively. The area of the squares reflects the study-specific weight. The diamond represents the pooled OR and 95% CI, determined using either the fixed effects or random effects, depending on the level of heterogeneity. Plots were generated using Review Manager Software (RevMan V5.3, Copenhagen, Denmark)
Discussion
Folate deficiency can influence the genetic stability of DNA, which increases the risk of cancer development [4, 27]. Moreover, dysregulation of this pathway is associated with many pathological outcomes, ranging from atherothrombosis to the risk of ischemic stroke to various forms of cancer [14, 28]. Although numerous case–control studies and meta-analyses have investigated the association between the C677T and the A1298C polymorphisms and the risk for developing breast cancer, there is no definite conclusion or a sole focus for the Latin American population. Here, we determined that the C677T polymorphism was associated with an increased risk of developing breast cancer, whereas the A1298C polymorphism had no effect.
For the C677T polymorphism, there was a significant presence of heterogeneity among the studies included in this meta-analysis. The diagnosis of breast cancer was confirmed by histology or by biochemical/molecular techniques (Table 1). Thus, it is possible that the level of heterogeneity is due to the different forms and proportions of breast cancer cases. The method used to determine the polymorphism could increase the heterogeneity of the sample; however, most studies used a similar technique—RFLP. Another cause of heterogeneity could be due to the populations themselves. For example, in Mexico the genetic composition of Mexicans—which is composed of Native American, Europeans, and Africans—varies from region to region [29], this is also seen among different regions of Brazil [30]. These differences do lead to various development rates and pathologies of similar diseases.
Other meta-analyses have determined that for Asians, the C677T polymorphism was shown to augment breast cancer risk [9, 10]. Due to the high level of influence of the European and African immigration, it was not expected that the C677T polymorphism would also increase the risk of developing breast cancer in Latin Americans. Interestingly, the C677T polymorphism was associated with an increased risk of developing breast cancer, except for the heterozygous model. The proposed oncogenic mechanism of the C677T polymorphism is highly correlated with low folate consumption [5]. Many Asian countries and Latin American countries are plagued with low folate consumption [31, 32], thus providing a link between the observed results here and the results for the Asian population. This does posit that folate consumption could attenuate the effects of the C677T polymorphism.
The A1298C polymorphism was not associated with an increased risk of developing breast cancer. Due to different degrees of potency the C677T and the A1298C polymorphisms have on MTHFR enzymatic activity, this does suggests that for breast cancer, the A1298C polymorphism does not inhibit MTHFR activity sufficiently enough to switch to an oncogenic promoting condition, as seen with the C677T polymorphism. Interestingly, others meta-analyses have determined a significant association between the risk of developing breast cancer and the A1298C polymorphism. Intriguingly, this effect is typically seen with the Caucasians [11, 12]. As mention above, Asians have minimal influence on the genetic make-up of Latinos, as do Europeans. Moreover, one study found that the A1298C polymorphism was associated with a decreased risk of developing breast cancer among the Asian population [11]. Taken together, these results suggest that the Latin American population represents a unique group, whose dietary intake must be taken into consideration.
Folate’s effect on DNA methylation is dependent on the level of serum folate [27], which comes from its dietary intake. Many women and children from Latin American countries suffer from low folate consumption and other members of the vitamin B family [32,33,34]. In Cuba, 80% of adults suffer from folate deficiency, whereas in Mexico, Venezuela, and Brazil, the prevalence of fotale deficiency is > 30%. Interestingly, using folate fortification procedures have reduced folate deficiency in selected populations to < 5% [33]. Nevertheless, the availability and compliance of these programs to the majority of the population, or food supplemented with folate, remain inadequate. Therefore, it is possible that for Latinos, which typically consume lower quantities of folate [30, 32, 34] their diets do not provide sufficient amount of folate to inhibit an oncogenic state when MTHFR activity is compromised. This does posit that augmented folate consumption could alter the risk of developing breast cancer.
Hormone changes that precede menopause have been as associated with decreased folic acid absorption, affecting DNA methylation [35]. Therefore, MTHFR polymorphisms are proposed to be a more heightening factor associated with breast cancer development during post-menopause. Indeed, with post-menopausal women, the C677T polymorphism does increase the risk of developing breast cancer [36, 37]; nonetheless, many reports have demonstrated that pre-menopausal status is associated with an increased risk [38, 39]. To add to the confusion, some reports have shown no effect [40]. To address this, Kumar and colleagues performed a meta-analysis for the C677T polymorphism. They determined that there were no associations with breast cancer and the C677T polymorphism for pre-menopausal women (9 studies) and post-menopausal women (9 studies) [10]. Two years later, Naushad and colleagues examined the affect menopausal status has on the association between the C677T polymorphism and breast cancer development [36]. With their meta-analysis, they determined that for post-menopausal women (11 studies), the C677T polymorphism was associated with breast cancer development and not for pre-menopausal women (8 studies). However, for both studies, there was no sub-analysis by ethnicity. For Latin Americans, this effect remains elusive. Here, four studies did examine if menopausal status affects MTHFR polymorphism’s effect on breast cancer development. For the Le Marchand study, the analysis was mixed with other ethnicities; thus, no clear conclusion can be made about Latin Americans [23]. For the Batschauer study, they did examine for this effect, determining that the C677T polymorphism had no effect [15]. Interestingly, only two studies, both from Brazil, provided sufficient information for an analysis—the Ma study and the Carvalho Barbosa study. For the Ma study, independent of the menopausal status, neither polymorphism affected the risk [25]. However, the Carvalho Barbosa study indicates that only for post-menopausal women, the CT genotype of C677T is associated with an increase risk [22]. For pre-menopausal women, no associations were found. With a lack of coverage of Latin America and few studies examining this affect, more studies are required.
Our study has a few limitations. First, only five countries are represented in this meta-analysis, which does suggest that parts of the Latin American community are underrepresented. Second, we calculate the crude ORs and they are unadjusted estimations. Third, we did not perform a sub-analysis on the types of breast cancer. As indicated by the elevated level of heterogeneity, the different forms could be possibly affecting the associations. However, even with the crude pooled ORs, we did demonstrate the association between the C677T polymorphism and breast cancer for Latinos.
Conclusion
Overall, the results indicated that, for Latinos, the C677T polymorphism is associated with a significant risk for developing breast cancer. On the other hand, the A1289C polymorphism does not show an association. In Latin American women with a history of breast cancer, examining for the C677T polymorphism could identify at risk subjects, who should consider folate monitoring and supplementation management.
References
Cancer Key Facts. Publisher WHO Geneva, Headquarters in Geneva. 2018. http://www.unesco.org/new/en/unesco/worldwide/latin-america-and-the-caribbean/. Accessed 11 May 2018.
Partanen T, Monge P, Wesseling C. Causes and prevention of occupational cancer. Acta Médica Costarricense. 2009;51(4):195–205.
Yan W, Zhang Y, Zhao E, Zhang S. Association between the MTHFR C677T polymorphism and breast cancer risk: a meta-analysis of 23 case–control studies. Breast J. 2016;22(5):593–4.
Kim Y-I. Nutritional epigenetics: impact of folate deficiency on DNA methylation and colon cancer susceptibility. J Nutr. 2005;135(11):2703–9.
Crider KS, Yang TP, Berry RJ, Bailey LB. Folate and DNA methylation: a review of molecular mechanisms and the evidence for folate’s role. Adv Nutr. 2012;3(1):21–38.
Shrubsole MJ, Gao YT, Cai Q, Shu XO, Dai Q, Hebert JR, et al. MTHFR polymorphisms, dietary folate intake, and breast cancer risk: results from the Shanghai Breast Cancer Study. Cancer Epidemiol Biomark Prev. 2004;13(2):190–6.
Gao CM, Tang JH, Cao HX, Ding JH, Wu JZ, Wang J, et al. MTHFR polymorphisms, dietary folate intake and breast cancer risk in Chinese women. J Hum Genet. 2009;54(7):414–8.
van der Put NM, Gabreels F, Stevens EM, Smeitink JA, Trijbels FJ, Eskes TK, et al. A second common mutation in the methylenetetrahydrofolate reductase gene: an additional risk factor for neural-tube defects? Am J Hum Genet. 1998;62(5):1044–51.
He L, Shen Y. MTHFR C677T polymorphism and breast, ovarian cancer risk: a meta-analysis of 19,260 patients and 26,364 controls. OncoTargets Ther. 2017;10:227.
Kumar P, Yadav U, Rai V. Methylenetetrahydrofolate reductase gene C677T polymorphism and breast cancer risk: Evidence for genetic susceptibility. Meta Gene. 2015;6:72–84.
Zhu X-L, Liu Z-Z, Yan S-X, Wang W, Chang R-X, Zhang C-Y, et al. Association between the MTHFR A1298C polymorphism and risk of cancer: evidence from 265 case–control studies. Mol Genet Genom. 2016;291(1):51–63.
Liu W, Li Y, Li R, Han X, Ma Y, Liu B, et al. Association of MTHFR A1298C polymorphism with breast cancer and/or ovarian cancer risk: an updated meta-analysis. Afr J Tradit Complement Altern Med. 2016;13(5):72–86.
Zhang J, Zhang L, Li G. Association between MTHFR gene 1298A> C polymorphism and breast cancer susceptibility: a meta-analysis based on 38 case–control studies with 40,985 subjects. World J Surg Oncol. 2016;14(1):230.
Xie S-Z, Liu Z-Z, Yu J-h, Liu L, Wang W, Xie D-L, et al. Association between the MTHFR C677T polymorphism and risk of cancer: evidence from 446 case–control studies. Tumor Biol. 2015;36(11):8953–72.
Batschauer AP, Cruz NG, Oliveira VC, Coelho FF, Santos IR, Alves MT, et al. HFE, MTHFR, and FGFR4 genes polymorphisms and breast cancer in Brazilian women. Mol Cell Biochem. 2011;357(1–2):247–53.
Calderón-Garcidueñas AL, Cerda-Flores RM, Lilia A. SNP C677T del gen metilentetrahidrofolato-reductasa y cáncer de mama en mujeres mexicanas. Rev Med Inst Mex Seguro Soc. 2017;55(6):720–4.
Ramos-Silva A, Figuera LE, Soto-Quintana OM, Puebla-Perez AM, Ramirez-Patino R, Gutierrez-Hurtado I, et al. Association of the C677T polymorphism in the methylenetetrahydrofolate reductase gene with breast cancer in a Mexican population. Genet Mol Res. 2015;14(2):4015–26.
Zara-Lopes T, Gimenez-Martins AP, Nascimento-Filho CH, Castanhole-Nunes MM, Galbiatti-Dias AL, Padovani-Junior JA, et al. Role of MTHFR C677T and MTR A2756G polymorphisms in thyroid and breast cancer development. Genet Mol Res. 2016;15(2):;gmr8222. https://doi.org/10.4238/gmr.15028222.
Stang A. Critical evaluation of the Newcastle–Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25(9):603–5.
Miller JJ. The inverse of the Freeman–Tukey double arcsine transformation. Am Stat. 1978;32(4):138-.
DerSimonian R, Laird N. Meta-analysis in clinical trials. Controlled Clin Trials. 1986;7(3):177–88.
Carvalho Barbosa RDC, Menezes DC, Freire TF, Sales DC, Alencar VH, Rabenhorst SH. Associations of polymorphisms of folate cycle enzymes and risk of breast cancer in a Brazilian population are age dependent. Mol Biol Rep. 2012;39(4):4899–907.
Le Marchand L, Haiman CA, Wilkens LR, Kolonel LN, Henderson BE. MTHFR polymorphisms, diet, HRT, and breast cancer risk: the multiethnic cohort study. Cancer Epidemiol Biomark Prev. 2004;13(12):2071–7.
Lopez-Cortes A, Echeverria C, Ona-Cisneros F, Sanchez ME, Herrera C, Cabrera-Andrade A, et al. Breast cancer risk associated with gene expression and genotype polymorphisms of the folate-metabolizing MTHFR gene: a case–control study in a high altitude Ecuadorian mestizo population. Tum Biol J Int Soc Oncodev Biol Med. 2015;36(8):6451–61.
Ma E, Iwasaki M, Junko I, Hamada GS, Nishimoto IN, Carvalho SM, et al. Dietary intake of folate, vitamin B6, and vitamin B12, genetic polymorphism of related enzymes, and risk of breast cancer: a case–control study in Brazilian women. BMC Cancer. 2009;9:122.
Rezende LM, Marson FAL, Lima CSP, Bertuzzo CS. Can. MTHFR C677T and a1298c polymorphisms alter the risk and severity of sporadic breast cancer in Brazilian women? Clin Breast Cancer. 2017;17(4):e199–208.
Duthie SJ, Narayanan S, Brand GM, Pirie L, Grant G. Impact of folate deficiency on DNA stability. J Nutr. 2002;132(8):2444S-9S.
Santilli F, Davì G, Patrono C. Homocysteine, methylenetetrahydrofolate reductase, folate status and atherothrombosis: a mechanistic and clinical perspective. Vasc Pharmacol. 2016;78:1–9.
Moreno-Estrada A, Gravel S, Zakharia F, McCauley JL, Byrnes JK, Gignoux CR, et al. Reconstructing the population genetic history of the Caribbean. PLoS Genet. 2013;9(11):e1003925.
Ramos BRDA, D’Elia MPB, Amador MAT, Santos NPC, Santos SEB, da Cruz Castelli E, et al. Neither self-reported ethnicity nor declared family origin are reliable indicators of genomic ancestry. Genetica. 2016;144(3):259–65.
McLean E, de Benoist B, Allen LH. Review of the magnitude of folate and vitamin B12 deficiencies worldwide. Food Nutr Bull. 2008;29(2_suppl1):38–51.
Marchetta CM, Hamner HC. Blood folate concentrations among women of childbearing age by race/ethnicity and acculturation, NHANES 2001–2010. Maternal Child Nutr. 2016;12(1):39–50.
Brito A, Mujica-Coopman MF, Olivares M, Lopez de Romana D, Cori H, Allen LH. Folate and vitamin B12 status in Latin America and the Caribbean: an update. Food Nutr Bull. 2015;36(2_suppl):109-S18.
Ramakrishnan U. Prevalence of micronutrient malnutrition worldwide. Nutr Rev. 2002;60(suppl_5):46–52.
Bae S, Ulrich CM, Bailey LB, Malysheva O, Brown EC, Maneval DR, et al. Impact of folic acid fortification on global DNA methylation and one-carbon biomarkers in the Women’s Health Initiative Observational Study cohort. Epigenetics. 2014;9(3):396–403.
Naushad SM, Divya C, Janaki Ramaiah M, Hussain T, Alrokayan SA, Kutala VK. Population-level diversity in the association of genetic polymorphisms of one-carbon metabolism with breast cancer risk. J Community Genet. 2016;7(4):279–90.
Ergul E, Sazci A, Utkan Z, Canturk NZ. Polymorphisms in the MTHFR gene are associated with breast cancer. Tum Biol J Int Soc Oncodev Biol Med. 2003;24(6):286–90.
Maruti SS, Ulrich CM, Jupe ER, White E. MTHFR C677T and postmenopausal breast cancer risk by intakes of one-carbon metabolism nutrients: a nested case–control study. Breast Cancer Res BCR. 2009;11(6):R91.
Suzuki T, Matsuo K, Hirose K, Hiraki A, Kawase T, Watanabe M, et al. One-carbon metabolism-related gene polymorphisms and risk of breast cancer. Carcinogenesis. 2008;29(2):356–62.
Platek ME, Shields PG, Marian C, McCann SE, Bonner MR, Nie J, et al. Alcohol consumption and genetic variation in methylenetetrahydrofolate reductase and 5-methyltetrahydrofolate-homocysteine methyltransferase in relation to breast cancer risk. Cancer Epidemiol Biomark Prev. 2009;18(9):2453–9.
Acknowledgements
The authors would like to express their gratitude to Mtro. Ricardo Villegas Tovar, Coordinator of Scientific Production and International Visibility, BUAP.
Funding
This study was supported by grants from Programa para el Desarrollo Profesional Docente (to CA-160 FACMED) and Vicerrectorıa de Investigacion, Benemerita Universidad Autonoma de Puebla, Mexico (to TORE-SAL18-G, PEFR-SAL18-G, and GOMM-SAL18-I).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
About this article
Cite this article
Meneses-Sanchez, P., Garcia-Hernandez, S.C., Porchia, L.M. et al. C677T and A1298C methylenetetrahydrofolate reductase polymorphisms and breast cancer susceptibility among Latinos: a meta-analysis. Breast Cancer 26, 602–611 (2019). https://doi.org/10.1007/s12282-019-00961-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12282-019-00961-8