Abstract
Ischemic stroke is a serious condition associated with severe functional disability and high mortality, however; effective therapy remains elusive. Empagliflozin, a sodium-glucose cotransporter 2 inhibitor, has been shown to exert additional non-glycemic benefits including anti-apoptotic effects in different disease settings. Thereby, this study was designed to investigate the ameliorative effect of empagliflozin on the neuronal apoptosis exhibited in cerebral ischemia/reperfusion (I/R) in a rat model targeting HIF-1α/VEGF signaling which is involved in this insult. Global cerebral I/R injury was induced in male Wistar rats through occlusion of the bilateral common carotid arteries for 30 min followed by one-hour reperfusion. Empagliflozin doses of 1 and 10 mg/kg were administered 1 and 24 h after reperfusion. In I/R-injured rats, empagliflozin treatments significantly reduced infarct size and enhanced neurobehavioral functions in a dose-dependent manner. The drug alleviated neuronal death and cerebral injury inflicted by global ischemia as it suppressed neuronal caspase-3 protein expression. In parallel, protein expressions of HIF-1α and its downstream mediator VEGF were upregulated in the ischemic brain following empagliflozin treatment. The results indicated that empagliflozin attenuates cerebral I/R-induced neuronal death via the HIF-1α/VEGF cascade.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ischemic stroke is one of the main causes of adult mortality as well as long-term disability worldwide (Katan and Luft 2018). Blood vessel occlusion during global cerebral ischemia causes inadequate oxygen supply to various brain regions, which is associated with ischemia-evoked pathophysiologic changes (Radak et al. 2017; Sanganalmath et al. 2017). In response to oxygen lack, many intrinsic protective and transcriptional factors are rapidly upregulated in multiple brain regions, including hypoxia-induced factor-1α (HIF-1α) (Jin et al. 2000a). HIF-1α is a master regulator of cellular adaptation to hypoxia as it activates tissue survival pathways by regulating numbers of downstream genes involved in cell metabolism (i.e., glucose transporters), angiogenesis (i.e., vascular endothelial growth factor (VEGF)), and cell survival/death (i.e., caspase-3) (Déry et al. 2005; Dong et al. 2013).
VEGF is a HIF-1α-transcriptionally regulated angiogenic factor that was found to be upregulated following ischemic stroke (Jin et al. 2000a; Mu et al. 2003). VEGF is engaged in both vasculogenesis and angiogenesis (Ho and Fong 2015), which help tissue restore oxygen supply, exert direct neurotrophic signaling, and promote adult neurogenesis (Theis and Theiss 2018). Beyond its angiogenic and neurotrophic actions, the neuroprotective effect of VEGF is suggested to be mediated via regulating pro-and anti-apoptotic factors expressions in ischemic brains (Plate et al. 1999; Lee et al. 2007). Apoptosis-related proteins, including caspase-3, are highly implicated in neuronal death following cerebral ischemia/reperfusion (I/R) injury in rats (Liu et al. 2013). Studies showed that inhibition of caspase-3 reduces neuronal loss and brain edema after brain ischemic injuries (Simard et al. 2009; Dong et al. 2014). Hence, we hypothesized that therapies suppressing proapoptotic-targeted genes, including HIF-1α and VEGF, might have a significant role in the amelioration of neuronal apoptosis associated with cerebral I/R injury.
Empagliflozin, a novel antidiabetic therapy, inhibits sodium/glucose cotransporter 2 (SGLT2), which results in increased renal glucose excretion (Barnett et al. 2014). Apart from kidneys, SGLTs have been detected in many other organs of the body, including brain tissues, which suggests their potential role in the regulation of neuronal activity. Pioneering studies have established SGLT1 expression in many areas of the brain, including neuron cell bodies, axons, and the dentate gyrus hippocampal subfields (Poppe et al. 1997; Yu et al. 2012). While more recent studies reported significant cerebral SGLT2 expressions in response to neurological insults (Kepe et al. 2018; Oerter et al. 2019). Indeed, recent studies have shown the neuroprotective role of different SGLT2 inhibitors in the modulation of different neuropathological conditions including epilepsy as well as memory and cognitive impairment (Arafa et al. 2017; Erdogan et al. 2018; Wang and Fan 2019). On the other hand, the effect of SGLT2 inhibitors on cerebral I/R injury has not been conclusively investigated yet and their role is still unclear in modulating cerebral ischemia.
Although the SGLT2 inhibitor, empagliflozin, has been shown to exert non-glycemic benefits for direct reno/cardioprotective functions including attenuation of apoptosis and proapoptotic markers (Ojima et al. 2015; Li et al. 2019), its precise role in regulating neuronal apoptosis is also not properly identified. Therefore, the present study was designed to evaluate whether empagliflozin has a protective effect against global cerebral I/R injury-induced neuronal apoptosis. Additionally, this study aims to investigate the mechanisms undelaying this postulated protective effect including HIF-1α/VEGF regulation.
Materials and methods
Animals and induction of cerebral I/R injury
Adult male Wistar rats (Animal Care Center, Nahda University, Egypt), weighing from 250 to 300 g each, were housed under controlled environmental conditions (temperature 27 ± 2 °C and alternating 12 h light and dark cycles). They were fed standard pellet chew and permitted free access to tap water ad libitum.
Animals were left at least one week for accommodation before testing. All procedures used in this study were carried out according to the guidelines of the NIH Guide for the Care and Use.
of Laboratory Animals and approved by the Animal Care Community, Minia University, Egypt (Permit Number: MPH-08-018).
A procedure of bilateral common carotid arteries occlusion was performed to induce cerebral ischemia in rats. Briefly, anesthesia was induced with an intraperitoneal cocktail injection of 50 mg/kg ketamine and 10 mg/kg xylazine and rectal temperature was maintained at 37 °C throughout the procedures. Both common carotid arteries were exposed, carefully separated, and freed from their adventitial sheath and vagus nerve. Arteries were occluded for 30 min with aneurysm clips to induce global cerebral ischemia. Afterward, the clips were removed to allow a 24 h reperfusion, then rats were subjected to behavior and neurological tests. At the end of the experiment, animals were sacrificed by decapitation under light ether anesthesia. Sham-operated rats underwent the same surgical procedure, except for common carotid artery ligation.
Study design and drug treatments
Rats were allocated randomly into five groups of eight animals each. Group 1 served as a sham-operated group, which received a vehicle only. Group 2 is vehicle-treated cerebral I/R rats. Groups 3 and 4 were cerebral I/R rats administered empagliflozin (Jardiance®) in two different doses (1 and 10 mg/kg, p.o., respectively). Empagliflozin treatments were administered twice, after 1 and 24 h of reperfusion. Animal decapitation and subsequent testing were conducted in one hour after the last dose of empagliflozin.
Assessment of behavioral and neurological functions
At the end of the experiment just before decapitation, animals were subjected to both open field and Garcia neurobehavioral tests to measure behavioral and neurological functions. In open field test, the frequency of rats’ ambulation was measured by placing each rat in the center of a square wooden box (72 × 72 × 36 cm) whose bottom was divided into 16 equal squares. Using a video camera, the rats’ ambulation was calculated by counting the number of squares crossed in three minutes. Additionally, the rats’ neurological defects were also measured via Garcia neurobehavioral test, in which each animal was scored for six individual tests. Garcia’s neurobehavioral test includes evaluation of spontaneous activity, symmetry in the movement of the four limbs, forepaw outstretching, climbing, body proprioception, and response to vibrissae touch (Garcia et al. 1995). Neurological functions were graded on a scale of 5 to 18. Behavioral analysis and scoring were carried out by an observer, who was blinded to the experimental procedures.
After decapitation, brains from each group were rapidly dissected, washed, and divided into two sets. The first brain set was used for the assessment of cerebral infarction volume. While the forebrain regions of the second brain set were isolated and divided sagittally into two halves: The first subset of halves fixed in 4% paraformaldehyde for histopathological and immunohistochemical analyses. While the second subset of halves was stored at a − 80 °C for western blotting analysis.
Western blot analysis
HIF-1α and VEGF-A protein expressions were analyzed in the rats’ brains using western blotting analysis. In brief, after homogenization and centrifugation of brain tissues, aliquots containing 20 µg/lane total protein were denatured by heating at a 95 °C temperature in an SDS sample buffer and then loaded on a 10% polyacrylamide gel for electrophoresis. The gels were transferred to nitrocellulose membranes (Roth, Karlsruhe, Germany) which were incubated overnight with primary antibodies of either HIF-1α (Thermo Fischer Scientific, Waltham, MA, USA, dilution of 1:250) or VEGF-A (Thermo Fischer Scientific, Waltham, MA, USA, dilution of 1:500).
After being fully washed, the membranes were incubated with a secondary antibody conjugated to horseradish peroxidase (Amersham Life Sciences, NJ, USA) for one hour. The bands recognized by the primary antibody were visualized by a standard enhanced chemiluminescence method and detected using densitometrically measured using ImageJ software (freeware; rsbweb.nih.gov/ij). Densities of the immunoreactive protein bands were normalized to the corresponding density of the β-actin band from the same lane and presented as a ratio of the relative optical density (ROD). Each of the values was then normalized and calibrated as a fold-change value from the control.
Immunohistochemical analysis
According to a previously described method (Maae et al. 2011), brain tissues were fixed in formalin (24–72 h) and then embedded in paraffin blocks. Blocks were cut into 4 µm thick sections and mounted on positively charged glass slides. Sections then were deparaffinized by xylene, rehydrated through graded series of ethanol, and rinsed in water. Endogenous peroxidase activity nonspecific background staining was blocked with a 3% hydrogen peroxidase solution and Ultra V Block, respectively. Afterward, sections were incubated overnight in a humid chamber at a 4 °C temperature with the primary antibody of rabbit polyclonal anti-caspase-3 (Catalog # PA1-21796, dilution 1:250). The biotinylated secondary antibody was then applied for ten minutes followed by incubation with Diaminobenzidine (DAB) chromogen for 12 min for the development of the color reaction. The sections were washed, counterstained with Mayer’s hematoxylin, dehydrated, and cover slipped. Semi-quantitative analysis was accomplished by digitizing the images by using the Image-pro® image analysis system.
Cerebral infarct volume and histopathological analysis
Cerebral infarct volume assessment was done using a 2,3,5-triphenyl tetrazolium chloride (TTC) staining method which was described earlier (Bederson et al. 1986). The forebrains were carefully removed, washed, and frozen for one to two hours. Frozen tissues were then sliced into 2 mm thick serial coronal sections which were incubated for 20 min at a 37 °C temperature with a 2% TTC stain and then fixed by a 10% formaldehyde solution.
Each brain slice was scanned and calculated for the percentage of infarct volume using computerized image analysis (ImageJ, NIH, USA) (Heeba and El-Hanafy 2012). Image analysis was performed by an independent investigator who was unaware of the treatment condition.
For assessment of histopathological analysis, Brain tissues were fixed in a 4% formaldehyde solution for 24 h and then embedded in paraffin blocks. Paraffin embedded-brain tissues were sectioned at 4 µm thickness, deparaffinized, and stained with hematoxylin-eosin (H&E). The affected areas with degenerated neurons (deeply attained, shrunken cell bodies, and pyknotic nuclei) were analyzed. Sections of different experimental groups were evaluated for the percentage of degenerated neurons to the total number of neurons in ten non-overlapping fields at X400 using a light microscope (Olympus CX41). During analysis, samples were coded by numbers only and the investigators were not aware of the sample’s identity.
Statistical analysis
Data are represented as mean ± SEM of at least three independent experiments performed in triplicate. Differences in statistical significance were evaluated statistically using one-way Analysis of Variance (one-way ANOVA test) followed by the Tukey–Kramer post-analysis test for comparing groups. Statistical significance was presented at P < 0.05. The analysis was performed using GraphPad Prism® software (Version 5.0).
Results
Effect on behavior and neurological functions
In the open field test, rats subjected to I/R injury showed a significant decrease in ambulation frequency compared to sham-operated rats. The decreased ambulation rate observed in ischemic rats was greatly improved by empagliflozin treatment in both doses of 1 and 10 mg. However, ischemic rats that were administered the higher empagliflozin dose showed a significant elevation in the ambulation rate compared with those administered the lower dose. Similarly, for neurological assessment, cerebral I/R rats showed a significant (P < 0.05) greater neurological deficit compared with the sham-operated group. Empagliflozin, in both low and high dose levels, alleviated neurological defects in treated I/R rats compared with untreated ones. It is noteworthy that I/R rats received higher empagliflozin doses showed higher neurological evaluations more than those who received lower doses (Fig. 1).
Effect of Empagliflozin (1 and 10 mg/kg) treatments on ambulation frequency (a) as a part of open field test, and on Garcia score (b) as a part of Garcia neurobehavioral test in rats subjected to global cerebral I/R. Data are represented as mean ± S.E.M. *,†,‡Significant differences from sham, ischemic and empagliflozin (1 mg/kg) groups, respectively at P < 0.05 where n = 6–8
Effects on protein expression of cerebral HIF-1α
Compared with the sham-operated group, induction of cerebral I/R injury caused a significant elevation of HIF-1α protein expression in rats’ brain tissues. The HIF-1α levels in I/R-injured rats were significantly elevated by empagliflozin treatment in both doses of 1 and 10 mg/kg. Compared to I/R-injured rats treated with low empagliflozin doses, the level of HIF-1α protein expressions was significantly (P < 0.05) upregulated upon treatment with high empagliflozin dose (Fig. 2).
Representative Western blots analysis of cerebral HIF-1α protein expressions showing protein bands of each group (a) and graphs present their densitometric analysis (b). Data are represented as mean ± S.E.M. *,†,‡Significant differences from sham, ischemic and empagliflozin (1 mg/kg) groups, respectively at P < 0.05 where n = 3–4
Effects on cerebral VEGF-A protein expression
Figure 3 showed a significant (P < 0.05) increase in cerebral VEGF-A protein expression in the brain tissues of I/R-injured rats compared with sham-operated ones. Groups that received empagliflozin (in both doses 1 and 10 mg/kg) showed a significant increase in VEGF-A protein expression compared with vehicle-treated I/R rats. Additionally, an eminent increase in VEGF-A protein expression was observed in I/R rat group that received a high empagliflozin dose compared with those who received a low dose.
Representative Western blots analysis of cerebral VEGF protein expressions showing protein bands of each group (a) and graphs present their densitometric analysis (b). Data are represented as mean ± SEM. *,†,‡Significant differences from sham, ischemic and empagliflozin (1 mg/kg) groups, respectively at P < 0.05 where n = 3–4
Effects on cerebral caspase-3 immunohistochemical reactivity
Cerebral I/R caused a significant (P < 0.05) increment of caspase-3 expression compared with sham-operated rats. Empagliflozin treatment in a dose of 10 mg reverted these elevated levels to their normal levels. Notably, caspases-3 was significantly reduced by almost 87% in ischemic rats treated by a high dose of empagliflozin compared with those treated with a lower empagliflozin dose. Compared with vehicle-treated I/R rats, a trivial decrease in caspase-3 protein expression was noticed in the brain tissues of I/R rats treated with 1 mg empagliflozin (Fig. 4).
Representative photomicrographs of immunohistochemical analysis of cerebral caspase-3 protein expressions. a Sham group showing negative expression. b Ischemic group showing high expression is noticed in the neurons of the different cerebral cortical layers (arrows). c Empagloflozin (1 mg/kg) treated group showing few neurons with positive expression (arrow). d Empagloflozin (10 mg/kg) treated group showing scattered neurons with positive immunoreactivity (arrow). Insets showing a high magnification for the immuno-stained neurons.anti-Caspase-3 immuno-staining; ×100, insets ×400. e A semi-quantitative analysis of caspase-3 expression in rats’ cerebral cortex, Data are represented as mean ± SEM. *,†,‡Significant differences from sham, ischemic and empagliflozin (1 mg/kg) groups, respectively at P < 0.05 where n = 3–4
Effects on infarct volume measurements and histopathological changes
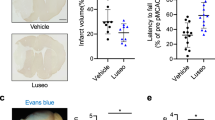
Figure 5 shows representative images of TTC staining in which, brain tissues of ischemic rats showed a significant (P < 0.05) increase in infarct volume compared with brains of sham-operated rats. Empagliflozin-treated rats with doses of 1 and 10 mg/kg exhibited a significant (P < 0.05) reduction in infarct volume compared with I/R vehicle-treated rats (7.42 ± 0.2, 2.35 ± 0.1 vs. 16.65 ± 0.5%, respectively). Results showed that the low dose of empagliflozin was less protective than the higher dose in the term of reduction of infarct size.
Representative coronal brain sections showed % of brain infarction in the sham group (a), ischemic group (b), empagliflozin (1 mg/kg) treated group (c), and empagliflozin (10 mg/kg) treated group (d). A dark colored region in the stained sections indicates non-ischemic and pale colored region that indicates an ischemic portion of the brain. The sections have scanned an area of infarction measured using ImageJ analysis software. (E) Quantitative changes in brain infarction are represented as % of infarct volume. Data are represented as mean ± SEM. *,†,‡Significant differences from sham, ischemic and empagliflozin (1 mg/kg) treated groups respectively at P < 0.05 where n = 3–4
These data are consistent with the results of histopathological findings (Figs. 6 and 7). I/R-injured brain tissues showed a significant (P < 0.05) increased number of degenerated neurons, with darkly stained shrunken cell bodies and pyknotic nuclei surrounded by pericellular halos, compared with brain tissues of sham-operated animals. In the cerebral cortex and hippocampus brain regions, high empagliflozin dosage reversed the neuron degenerations which were observed in vehicle-treated I/R rats. While low empagliflozin doses exhibited a non-significant improvement noted in brain tissues of ischemic rats.
Photomicrographs of sections of the cerebral cortex of a male albino rat of sham group (a), showing layers of cerebral cortex; molecular layer (I), outer granular layer (II), outer pyramidal layer (III), inner granular layer (IV), inner pyramidal layer (V) and polymorphic layer (VI). The neurons have vesicular nuclei with prominent nucleoli (inset). b Ischemic group, showing congested blood vessel (v), dilated perivascular space (*), large number of degenerated neurons within the external and internal granular pyramidal layers (arrows). c Empagliflozin (1 mg/kg) treated group showing many shrunken degenerated neurons noticed mainly in the external layers (arrow) and many congested blood vessel (v) with dilated perivascular space. d Empagliflozin (10 mg/kg) treated group showing some shrunken degenerated neurons noticed mainly in the external layers (arrow). Many blood capillaries are noticed with narrow perivascular space (*). a–d Insets have higher magnification showing the degenerated neurons with darkly stained shrunken cell bodies and pyknotic nuclei surrounded by pericellular halos. Pyramidal neurons (arrow) and granule cells (arrow head) (H&E ×100. Insets ×400). e A semi-quantitative analysis of degenerated neurons of rats’ cerebral cortex, Data are represented as mean ± SEM. *,†,‡Significant differences from sham, ischemic and empagliflozin (1 mg/kg) treated groups, respectively at P < 0.05 where n = 3–4
Photomicrographs of sections of the hippocampus of a male albino rat of sham group (a) showing stratum pyramidalis of cornu ammonis with its closely packed pyramidal neurons with vesicular nuclei (arrow). b Ischemic group, showing large number of degenerated darkly stained shrunken pyramidal neurons (arrow). Notice vacuolization of the neuropil (n). c Empagliflozin (1 mg/kg) treated group, showing some degenerated pyramidal neurons (arrows). The neuropil appear more or less normal (n). d Empagliflozin (10 mg/kg) treated group showing that most of the pyramidal hippocampal neurons appear normal with few degenerated neurons (arrow). The neuropil appear more or less normal (n). H&E ×100. e A semi-quantitative analysis of degenerated neurons of rats’ cerebral cortex, Data are represented as mean ± S.E.M. *,†,‡Significant differences from sham, ischemic and empagliflozin (1 mg/kg) treated groups, respectively at P < 0.05 where n = 3–4
Discussion
The present study provides insights into the neuroprotective effects of empagliflozin against global cerebral I/R injury. Empagliflozin, a SGLT2 inhibitor, showed great efficacy in glycemic control via the mediation of glucose uptake from the proximal tubules of the kidney (Kramer and Zinman 2019). Studies showed that SGLTs not only exist in kidneys but they are also normally expressed in different body tissues including the brain, suggesting the critical role of SGLTs in maintaining neuronal health (Wright et al. 2011; Yu et al. 2012). Hence, we hypothesized that modulation of SGLT2 via empagliflozin may reveal some neuroprotective effects in neuropathological conditions like ischemic strokes.
In this study, transient bilateral common carotid arteries occlusion in rats revealed an increase in cerebral infarct volume associated with significant neurological damage in different brain regions. Moreover, induction of cerebral I/R injury in rats was associated with reduced motor activity and neurological dysfunction in both the open field and Garcia tests, respectively. Empagliflozin treatment in low and high doses scenarios could reveal the motor dysfunction and neurological deficit observed in BCCAO-rats. The ameliorated neurological and functional deficits were consistent with the results of TTC staining for infarct volume and histopathological examination. Empagliflozin-treated groups presented smaller infarct volume and decreased neurological damage compared with the vehicle-treated group. However, it is crucial to point out that treatment with a large dose of empagliflozin provided a better neuroprotective effect than that of a lower dose. These observations went along previous studies showed that empagliflozin, dose-dependently could increase urinary glucose excretion and reduce plasma levels in healthy subjects and diabetic patients, respectively (Thomas et al. 2012; Seman et al. 2013). A dose-proportional increase in drug exposure exhibited by empagliflozin (Heise et al. 2013) might provide a plausible justification for the increased neuroprotective efficacy observed upon treatment with a larger empagliflozin dose.
HIF-1α is considered a key molecule for many intracellular responses mediated by hypoxia in many tissues including the brain (Khan et al. 2017; Robinson et al. 2017). In response to cerebral ischemia, HIF-1α is rapidly upregulated in response to the loss of the oxygen supply (Baranova et al. 2007; Chang et al. 2007). Results from the present study indicated that global cerebral ischemia activated a hypoxia-responsive signaling pathway in brain tissues, which was demonstrated by the concerted induction of HIF-1α. These results were in accordance with previous reports showed that HIF-1α is highly implicated in the ischemic brain (Bergeron et al. 1999; Jin et al. 2000a). Additionally, the accumulated evidence indicates that the upregulation of HIF-1α, as an ischemic tolerance mediator, may play a key role in neuroprotection against cerebral I/R injury (Ogle et al. 2012; Ryou et al. 2012). A consistent observation in this study revealed a significant upregulation of HIF-1α protein expression in cerebral I/R rats treated with different doses of empagliflozin. Current results are in harmony with a previous study of Zapata-Morales et al. (2014) who reported a clear reduction in renal SGLT2 during hypoxia mediated by HIF-1α.
One contradictory study of Bessho et al. (2019) demonstrated that luseogliflozin attenuated HIF-1α expression in renal epithelial tubular cells and cortical tubular cells of db/db mice. Although the study reported an attenuation of tubulointerstitial fibrosis upon SGLT2 inhibition, it showed conflicting data of suppressed VEGF that could aggregate fibrosis. Yet another study with similar methodologies showed a clear association between SGLT2 suppression under hypoxic conditions and the increase of expression of HIF-1α (Zapata-Morales et al. 2013). The later study is further supported by Chang et al. (2016) who showed that dapagliflozin induces HIF-1α in ischemic renal tissue and cultured ischemic tubular cells, as well as reduces apoptotic cell death in I/R injured kidneys.
Although this study did not address whether SGLT2 regulates HIF-1α directly or indirectly, it was previously suggested that SGLT2 inhibitor can increase HIF-1α expression in ischemic HK2 cells in the kidney directly, and not via low cellular glucose (Chang et al. 2016).
Furthermore, it has been previously reported that the protective effect of SGLT2 inhibitors could be mediated independently of SGLT2 activity. Direct inhibition of Na+/H+ exchanger (Baartscheer et al. 2017; Uthman et al. 2018) and activation of STAT3 antioxidant and anti-inflammatory properties (Andreadou et al. 2017) and AMPK Activation (Ye et al. 2018) are among of several mechanisms of action that are suggested to be implicated in SGLT2 inhibitors protective effect.
Under the conditions of hypoxia and post-hypoxia/reoxygenation, it has been shown that HIF-1α regulates a lot of genes including VEGF as one of the primary target genes (Ramakrishnan et al. 2014). VEGF is engaged in both vasculogenesis and angiogenesis, which leads to restoring the oxygen supply to tissues (Kim and Byzova 2014). Following an ischemic stroke, VEGF promotes neuro-restoration after ischemia whether directly as a neuroprotective agent or indirectly by inducing angiogenesis (Sun et al. 2003; Navaratna et al. 2009; Harms et al. 2010). Also, it has been postulated that VEGF protects the ischemic brain via direct neurotrophic effects (Jin et al. 2000b). Direct neuroprotection by VEGF may be related to the inhibition of cell death genes including caspase-3 (Jin et al. 2001; Wang et al. 2018).
Along with these findings, in the current study, we reported that VEGF protein expression was significantly going up in the I/R-injured rat group compared with the sham group. Empagliflozin treatment showed further cerebral VEGF upregulation in I/R-injured rats. Presumably, these incremental increases in VEGF protein expression may be linked to the activation of HIF-1α. We postulated that empagliflozin-induced cellular glucose deficiency may indirectly induce HIF-1α which in turn activates VEGF.
Supporting this assumption, several previous reports have demonstrated that VEGF could be induced by both hypoxia and hypoglycemia scenarios (Rosen 2002; Ferrara 2004). Additionally, a recent study of Zhang et al. (2018) showed that luseogliflozin, another SGLT2 inhibitor, prevented renal fibrosis after renal I/R injury through a VEGF-dependent pathway.
Inevitably, cerebral ischemia results in neuronal death along with the activation of apoptotic mechanisms and an increased level of the proapoptotic factor, caspase-3 (Radak et al. 2017). An upregulation in caspase-3 level has been detected in forebrain after cerebral I/R injury (Li et al. 2010; Zhou et al. 2003). Similarly, the current data showed a significant increase in caspase- 3 protein expression in the rat brain after I/R injury. Empagliflozin administration in two different doses significantly downregulated the expression of caspase-3 in brain tissues and inhibited the apoptotic cell death.
These data are consistent with the results of Sa-nguanmoo et al. (2017) who reported a decrease in brain apoptosis in HFD-induced obese rats along with SGLT2-inhibitor treatment. We claim that empagliflozin exerts an antiapoptotic effect via augmenting HIF-1α and VEGF expressions in ischemic brain tissues. Supporting our justification, a recent study by Otsuka et al. (2019) showed an association between enhanced expression of HIF-1α and decreased neuronal apoptosis after brain ischemia. This consolidates the study Baranova et al. (2007) who showed that HIF-1α could upregulate apoptosis-related genes, like antiapoptotic protein (Bcl2) besides angiogenic factors including VEGF after brain ischemia.
In conclusion, this study showed that empagliflozin could attenuate neuronal apoptosis in experimental strokes in rats. Elevated HIF-1α/VEGF expression by empagliflozin treatment may play a significant role in the mitigation of cerebral I/R injury.
References
Andreadou I, Efentakis P, Balafas E, Togliatto G, Davos CH, Varela A, Dimitriou CA, Nikolaou P-E, Maratou E, Lambadiari V (2017) Empagliflozin limits myocardial infarction in vivo and cell death in vitro: role of STAT3, mitochondria, and redox aspects. Front Physiol 8:1077. https://doi.org/10.3389/fphys.2017.01077
Arafa NM, Ali EH, Hassan MK (2017) Canagliflozin prevents scopolamine-induced memory impairment in rats: comparison with galantamine hydrobromide action. Chem Biol Interact 277:195–203. https://doi.org/10.1016/j.cbi.2017.08.013
Baartscheer A, Schumacher CA, Wüst RC, Fiolet JW, Stienen GJ, Coronel R, Zuurbier CJ (2017) Empagliflozin decreases myocardial cytoplasmic Na+ through inhibition of the cardiac Na+/H+ exchanger in rats and rabbits. Diabetologia 60:568–573. https://doi.org/10.1007/s00125-016-4134-x
Baranova O, Miranda LF, Pichiule P, Dragatsis I, Johnson RS, Chavez JC (2007) Neuron-specific inactivation of the hypoxia inducible factor 1α increases brain injury in a mouse model of transient focal cerebral ischemia. J Neurosci 27:6320–6332. https://doi.org/10.1523/JNEUROSCI.0449-07.2007
Barnett AH, Mithal A, Manassie J, Jones R, Rattunde H, Woerle HJ, Broedl UC, investigators E-RRt (2014) Efficacy and safety of empagliflozin added to existing antidiabetes treatment in patients with type 2 diabetes and chronic kidney disease: a randomised, double-blind, placebo-controlled trial. Lancet Diabetes Endocrinol 2:369–384. https://doi.org/10.1016/S2213-8587(13)70208-0
Bederson JB, Pitts LH, Germano SM, Nishimura MC, Davis RL, Bartkowski HM (1986) Evaluation of 2,3,5-triphenyltetrazolium chloride as a stain for detection and quantification of experimental cerebral infarction in rats. Stroke 17:1304–1308. https://doi.org/10.1161/01.str.17.6.1304
Bergeron M, Yu AY, Solway KE, Semenza GL, Sharp FR (1999) Induction of hypoxia-inducible factor‐1 (HIF‐1) and its target genes following focal ischaemia in rat brain. Eur J Neurosci 11:4159–4170. https://doi.org/10.1046/j.1460-9568.1999.00845.x
Bessho R, Takiyama Y, Takiyama T, Kitsunai H, Takeda Y, Sakagami H, Ota T (2019) Hypoxia-inducible factor-1α is the therapeutic target of the SGLT2 inhibitor for diabetic nephropathy. Sci Rep 9:1–12. https://doi.org/10.1038/s41598-019-51343-1
Chang Y, Hsiao G, Chen S-h, Chen Y-c, Lin J-h, Lin K-h, Chou D-s, Sheu J-r (2007) Tetramethylpyrazine suppresses HIF-1α, TNF-α, and activated caspase-3 expression in middle cerebral artery occlusion-induced brain ischemia in rats. Acta Pharmacol Sin 28:327. https://doi.org/10.1111/j.1745-7254.2007.00514.x
Chang Y-K, Choi H, Jeong JY, Na K-R, Lee KW, Lim BJ, Choi DE (2016) Dapagliflozin, SGLT2 inhibitor, attenuates renal ischemia-reperfusion injury. PLoS ONE 11:e0158810. https://doi.org/10.1371/journal.pone.0158810
Déry M-AC, Michaud MD, Richard DE (2005) Hypoxia-inducible factor 1: regulation by hypoxic and non-hypoxic activators. Int J Biochem Cell Biol 37:535–540. https://doi.org/10.1016/j.biocel.2004.08.012
Dong Y, Li Y, Feng D, Wang J, Wen H, Liu D, Zhao D, Liu H, Gao G, Yin Z (2013) Protective effect of HIF-1α against hippocampal apoptosis and cognitive dysfunction in an experimental rat model of subarachnoid hemorrhage. Brain Res 1517:114–121. https://doi.org/10.1016/j.brainres.2013.04.024
Dong Y-s, Wang J-l, Feng D-y, Qin H-z, Wen H, Yin Z-m, Gao G-d, Li C (2014) Protective effect of quercetin against oxidative stress and brain edema in an experimental rat model of subarachnoid hemorrhage. Int J Med Sci 11:282. https://doi.org/10.7150/ijms.7634
Erdogan MA, Yusuf D, Christy J, Solmaz V, Erdogan A, Taskiran E, Erbas O (2018) Highly selective SGLT2 inhibitor dapagliflozin reduces seizure activity in pentylenetetrazol-induced murine model of epilepsy. BMC Neurol 18:81. https://doi.org/10.1186/s12883-018-1086-4
Ferrara N (2004) Vascular endothelial growth factor as a target for anticancer therapy. Oncologist 9:2–10. https://doi.org/10.1634/theoncologist.9-suppl_1-2
Garcia JH, Wagner S, Liu K-F, Hu X-j (1995) Neurological deficit and extent of neuronal necrosis attributable to middle cerebral artery occlusion in rats: statistical validation. Stroke 26:627–635. https://doi.org/10.1161/01.str.26.4.627
Harms KM, Li L, Cunningham LA (2010) Murine neural stem/progenitor cells protect neurons against ischemia by HIF-1α–regulated VEGF signaling. PLoS ONE 5:e9767. https://doi.org/10.1371/journal.pone.0009767
Heeba GH, El-Hanafy AA (2012) Nebivolol regulates eNOS and iNOS expressions and alleviates oxidative stress in cerebral ischemia/reperfusion injury in rats. Life Sci 90:388–395. https://doi.org/10.1016/j.lfs.2011.12.001
Heise T, Seman L, Macha S, Jones P, Marquart A, Pinnetti S, Woerle HJ, Dugi K (2013) Safety, tolerability, pharmacokinetics, and pharmacodynamics of multiple rising doses of empagliflozin in patients with type 2 diabetes mellitus. Diabetes Ther 4:331–345. https://doi.org/10.1007/s13300-013-0030-2
Ho VC, Fong G-H (2015) Vasculogenesis and angiogenesis in VEGF receptor-1 deficient mice. Methods Mol Biol 1332:161–176. https://doi.org/10.1007/978-1-4939-2917-7_12
Jin K, Mao X, Nagayama T, Goldsmith P, Greenberg D (2000a) Induction of vascular endothelial growth factor and hypoxia-inducible factor-1α by global ischemia in rat brain. Neuroscience 99:577–585. https://doi.org/10.1016/s0306-4522(00)00207-4
Jin K, Mao X, Nagayama T, Goldsmith P, Greenberg D (2000b) Induction of vascular endothelial growth factor receptors and phosphatidylinositol 3′-kinase/Akt signaling by global cerebral ischemia in the rat. Neuroscience 100:713–717. https://doi.org/10.1016/s0306-4522(00)00331-6
Jin K, Mao X, Batteur S, McEachron E, Leahy A, Greenberg D (2001) Caspase-3 and the regulation of hypoxic neuronal death by vascular endothelial growth factor. Neuroscience 108:351–358. https://doi.org/10.1016/s0306-4522(01)00154-3
Katan M, Luft A (2018) Global burden of stroke. Seminars in neurology. Thieme Medical Publishers, New York, pp 208–211
Kepe V, Scafoglio C, Liu J, Yong WH, Bergsneider M, Huang S-C, Barrio JR, Wright EM (2018) Positron emission tomography of sodium glucose cotransport activity in high grade astrocytomas. J Neurooncol 138:557–569. https://doi.org/10.1007/s11060-018-2823-7
Khan M, Khan H, Singh I, Singh AK (2017) Hypoxia inducible factor-1 alpha stabilization for regenerative therapy in traumatic brain injury. Neural Regen Res 12:696. https://doi.org/10.4103/1673-5374.206632
Kim Y-W, Byzova TV (2014) Oxidative stress in angiogenesis and vascular disease. Blood 123:625–631. https://doi.org/10.1182/blood-2013-09-51274
Kramer CK, Zinman B (2019) Sodium–glucose cotransporter–2 (SGLT-2) inhibitors and the treatment of type 2 diabetes. Annu Rev Med 70:323–334. https://doi.org/10.1146/annurev-med-042017-094221
Lee HJ, Kim KS, Park IH, Kim SU (2007) Human neural stem cells over-expressing VEGF provide neuroprotection, angiogenesis and functional recovery in mouse stroke model. PLloS ONE 2:e156. https://doi.org/10.1371/journal.pone.0000156
Li J, Han B, Ma X, Qi S (2010) The effects of propofol on hippocampal caspase-3 and Bcl-2 expression following forebrain ischemia–reperfusion in rats. Brain Res 1356:11–23. https://doi.org/10.1016/j.brainres.2010.08.012
Li C, Zhang J, Xue M, Li X, Han F, Liu X, Xu L, Lu Y, Cheng Y, Li T (2019) SGLT2 inhibition with empagliflozin attenuates myocardial oxidative stress and fibrosis in diabetic mice heart. Cardiovasc Diabetol 18:15. https://doi.org/10.1186/s12933-019-0816-2
Liu G, Wang T, Wang T, Song J, Zhou Z (2013) Effects of apoptosis–related proteins caspase-3, Bax and Bcl-2 on cerebral ischemia rats. Biomed Rep 1:861–867. https://doi.org/10.3892/br.2013.153
Maae E, Nielsen M, Steffensen KD, Jakobsen EH, Jakobsen A, Sørensen FB (2011) Estimation of immunohistochemical expression of VEGF in ductal carcinomas of the breast. J Histochem Cytochem 59:750–760. https://doi.org/10.1369/0022155411412599
Mu D, Jiang X, Sheldon RA, Fox CK, Hamrick SE, Vexler ZS, Ferriero DM (2003) Regulation of hypoxia-inducible factor 1α and induction of vascular endothelial growth factor in a rat neonatal stroke model. Neurobiol Dis 14:524–534. https://doi.org/10.1016/j.nbd.2003.08.020
Navaratna D, Guo S, Arai K, Lo EH (2009) Mechanisms and targets for angiogenic therapy after stroke. Cell Adh Migr 3:216–223. https://doi.org/10.4161/cam.3.2.8396
Oerter S, Förster C, Bohnert M (2019) Validation of sodium/glucose cotransporter proteins in human brain as a potential marker for temporal narrowing of the trauma formation. Int J Legal Med 133:1107–1114. https://doi.org/10.1007/s00414-018-1893-
Ogle ME, Gu X, Espinera AR, Wei L (2012) Inhibition of prolyl hydroxylases by dimethyloxaloylglycine after stroke reduces ischemic brain injury and requires hypoxia inducible factor-1α. Neurobiol Dis 45:733–742. https://doi.org/10.1016/j.nbd.2011.10.020
Ojima A, Matsui T, Nishino Y, Nakamura N, Yamagishi S (2015) Empagliflozin, an inhibitor of sodium-glucose cotransporter 2 exerts anti-inflammatory and antifibrotic effects on experimental diabetic nephropathy partly by suppressing AGEs-receptor axis. Horm Metab Res 47:686–692. https://doi.org/10.1055/s-0034-1395609
Otsuka S, Sakakima H, Terashi T, Takada S, Nakanishi K, Kikuchi K (2019) Preconditioning exercise reduces brain damage and neuronal apoptosis through enhanced endogenous 14-3-3γ after focal brain ischemia in rats. Brain Struct Funct 224:727–738. https://doi.org/10.1007/s00429-018-1800-4
Plate KH, Beck H, Danner S, Allegrini PR, Wiessner C (1999) Cell type specific upregulation of vascular endothelial growth factor in an MCA-occlusion model of cerebral infarct. J Neuropathol Exp Neurol 58:654–666. https://doi.org/10.1097/00005072-199906000-00010
Poppe R, Karbach U, Gambaryan S, Wiesinger H, Lutzenburg M, Kraemer M, Witte OW, Koepsell H (1997) Expression of the Na+-D‐glucose cotransporter SGLT1 in neurons. J Neurochem 69:84–94. https://doi.org/10.1046/j.1471-4159.1997.69010084.x
Radak D, Katsiki N, Resanovic I, Jovanovic A, Sudar-Milovanovic E, Zafirovic S, Mousad A, Isenovic SR (2017) Apoptosis and acute brain ischemia in ischemic stroke. Curr Vasc Pharmacol 15:115–122. https://doi.org/10.2174/1570161115666161104095522
Ramakrishnan S, Anand V, Roy S (2014) Vascular endothelial growth factor signaling in hypoxia and inflammation. J Neuroimmune Pharmacol 9:142–160. https://doi.org/10.1007/s11481-014-9531-7
Robinson PJ, Hack C, Merrill EA, Mattie DR (2017) Mathematical model of HIF-1 alpha pathway, oxygen transport and hypoxia. Henry M. Jackson Foundation For the Advancement of Military Medicine Wright-Patterson AFB
Rosen LS (2002) Clinical experience with angiogenesis signaling inhibitors: focus on vascular endothelial growth factor (VEGF) blockers. Cancer Control 9:36–44. https://doi.org/10.1177/107327480200902S05
Ryou M-G, Liu R, Ren M, Sun J, Mallet RT, Yang S-H (2012) Pyruvate protects the brain against ischemia–reperfusion injury by activating the erythropoietin signaling pathway. Stroke 43:1101–1107. https://doi.org/10.1161/STROKEAHA.111.620088
Sanguanmoo P, Tanajak P, Kerdphoo S, Jaiwongkam T, Pratchayasakul W, Chattipakorn N, Chattipakorn SC (2017) SGLT2-inhibitor and DPP-4 inhibitor improve brain function via attenuating mitochondrial dysfunction, insulin resistance, inflammation, and apoptosis in HFD-induced obese rats. Toxicol Appl Pharmacol 333:43–50. https://doi.org/10.1016/j.taap.2017.08.005
Sanganalmath SK, Gopal P, Parker JR, Downs RK, Parker JC, Dawn B (2017) Global cerebral ischemia due to circulatory arrest: insights into cellular pathophysiology and diagnostic modalities. Mol Cell Biochem 426:111–127. https://doi.org/10.1007/s11010-016-2885-9
Seman L, Macha S, Nehmiz G, Simons G, Ren B, Pinnetti S, Woerle HJ, Dugi K (2013) Empagliflozin (BI 10773), a potent and selective SGLT2 inhibitor, induces dose-dependent glucosuria in healthy subjects. Clin Pharmacol Drug Dev 2:152–161. https://doi.org/10.1002/cpdd.16
Simard JM, Geng Z, Woo SK, Ivanova S, Tosun C, Melnichenko L, Gerzanich V (2009) Glibenclamide reduces inflammation, vasogenic edema, and caspase-3 activation after subarachnoid hemorrhage. J Cereb Blood Flow Metab 29:317–330. https://doi.org/10.1038/jcbfm.2008.120
Sun Y, Jin K, Xie L, Childs J, Mao XO, Logvinova A, Greenberg DA (2003) VEGF-induced neuroprotection, neurogenesis, and angiogenesis after focal cerebral ischemia. J Clin Invest 111:1843–1851. https://doi.org/10.1172/JCI17977
Theis V, Theiss C (2018) VEGF-a stimulus for neuronal development and regeneration in the CNS and PNS. Curr Protein Pept Sci 19:589–597. https://doi.org/10.2174/1389203719666180104113937
Thomas L, Grempler R, Eckhardt M, Himmelsbach F, Sauer A, Klein T, Eickelmann P, Mark M (2012) Long-term treatment with empagliflozin, a novel, potent and selective SGLT-2 inhibitor, improves glycaemic control and features of metabolic syndrome in diabetic rats. Diabetes Obes Metab 14:94–96. https://doi.org/10.1111/j.1463-1326.2011.01518.x
Uthman L, Baartscheer A, Bleijlevens B, Schumacher CA, Fiolet JW, Koeman A, Jancev M, Hollmann MW, Weber NC, Coronel R (2018) Class effects of SGLT2 inhibitors in mouse cardiomyocytes and hearts: inhibition of Na+/H+ exchanger, lowering of cytosolic Na+ and vasodilation. Diabetologia 61:722–726. https://doi.org/10.1007/s00125-017-4509-7
Wang S, Fan F (2019) Oral antihyperglycemic therapy with a SGLT2 inhibitor reverses cognitive impairments in elderly diabetics. Hypertension 74:A051–A051. https://doi.org/10.1161/hyp.74.suppl_1.051
Wang L, Wang F, Liu S, Yang X, Yang J, Ming D (2018) VEGF attenuates 2-VO induced cognitive impairment and neuronal injury associated with the activation of PI3K/Akt and Notch1 pathway. Exp Gerontol 102:93–100. https://doi.org/10.1016/j.exger.2017.12.010
Wright EM, Loo DD, Hirayama BA (2011) Biology of human sodium glucose transporters. Physiol Rev 91:733–794. https://doi.org/10.1152/physrev.00055.2009
Ye Y, Jia X, Bajaj M, Birnbaum Y (2018) Dapagliflozin attenuates Na+/H+ exchanger-1 in cardiofibroblasts via AMPK activation. Cardiovasc Drugs Ther 32:553–558. https://doi.org/10.1007/s10557-018-6837-3
Yu AS, Hirayama BA, Timbol G, Liu J, Diez-Sampedro A, Kepe V, Satyamurthy N, Huang S-C, Wright EM, Barrio JR (2012) Regional distribution of SGLT activity in rat brain in vivo. Am J Physiol Cell Physiol 304:C240–C247. https://doi.org/10.1152/ajpcell.00317.2012
Zapata-Morales JR, Galicia-Cruz OG, Franco M, y Morales FM (2013) HIF-1α diminishes SGLT1 and SGLT2 expression in renal epithelial tubular cells (LLC-PK1) under hypoxia. J Biol Chem M113:526814. https://doi.org/10.1074/jbc.M113.526814
Zapata-Morales JR, Galicia-Cruz OG, Franco M, y Morales FM (2014) Hypoxia-inducible factor-1α (HIF-1α) protein diminishes sodium glucose transport 1 (SGLT1) and SGLT2 protein expression in renal epithelial tubular cells (LLC-PK1) under hypoxia. J Biol Chem 289:346–357. https://doi.org/10.1074/jbc.M113.526814
Zhou H, Ma Y, Zhou Y, Liu Z, Wang K, Chen G (2003) Effects of magnesium sulfate on neuron apoptosis and expression of caspase-3, bax and bcl-2 after cerebral ischemia-reperfusion injury. Chin Med J 116:1532–1534
Zhang Y, Nakano D, Guan Y, Hitomi H, Uemura A, Masaki T, Kobara H, Sugaya T, Nishiyama A (2018) A sodium-glucose cotransporter 2 inhibitor attenuates renal capillary injury and fibrosis by a vascular endothelial growth factor-dependent pathway after renal injury in mice. Kidney Int 94:524–535. https://doi.org/10.1016/j.kint.2018.05.002
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Abdel-latif, R.G., Rifaai, R.A. & Amin, E.F. Empagliflozin alleviates neuronal apoptosis induced by cerebral ischemia/reperfusion injury through HIF-1α/VEGF signaling pathway. Arch. Pharm. Res. 43, 514–525 (2020). https://doi.org/10.1007/s12272-020-01237-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12272-020-01237-y