Abstract
Primary myelofibrosis (MF) is a severe chronic myeloproliferative neoplasm, progressing towards a terminal stage with insufficient haematopoiesis and osteosclerotic manifestations. Whilst densitometry studies have showed MF patients to have elevated bone mineral density, data on bone geometry and micro-structure assessed with non-invasive methods are lacking. We measured areal bone mineral density (aBMD) using dual-energy X-ray absorptiometry (DXA). Bone geometry, volumetric BMD, and micro-architecture were measured using high-resolution peripheral quantitative computed tomography (HR-pQCT). We compared the structural parameters of bones by comparing 18 patients with MF and healthy controls matched for age, sex, and height. Blood was analysed for biochemical markers of bone turnover in patients with MF. There were no significant differences in measurements of bone geometry, volumetric bone mineral density, and micro-structure between MF patients and matched controls. Estimated bone stiffness and bone strength were similar between MF patients and controls. The level of pro-collagen type 1 N-terminal pro-peptide (P1NP) was significantly increased in MF, which may indicate extensive collagen synthesis, one of the major diagnostic criteria in MF. We conclude that bone mineral density, geometry, and micro-architecture in this cohort of MF patients are comparable with those in healthy individuals.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Primary myelofibrosis (MF) is one of the classical Philadelphia chromosome-negative chronic myeloproliferative neoplasms (CMPN) also including essential thrombocythemia (ET), polycythaemia vera (PV), and others [1]. With time a progression across the biological CMPN continuum from ET and PV to secondary myelofibrosis may evolve. Some patients undergo the full spectrum with the initial proliferative stage that gradually diminishes due to the increasing fibrosis eventually resulting in a burn-out terminal stage with bone marrow failure [2, 3]. Accordingly, manifestations of extra-medullar hematopoiesis arise, spleen size increases, and constitutional symptoms may compromise quality of life [4, 5]. As the name myelofibrosis refers, increasing reticulin or collagen fibres are discriminating features of the bone marrow [2]. Along with this, osteosclerotic changes appear in the bone tissue as a result of the growing increase and thickening of bone trabeculae thickness and focal osteosclerotic changes with osteoid seams and appositional new bone formation in budding plaques [6]. Bone density assessed using dual-energy X-ray absorptiometry (DXA) in MF patients has been found to be higher than the background population [7, 8]. Histomorphometric measurements in a cohort of 75 CMPN patients have showed that MF patients have significantly more bone tissue compared with other CMPN type patients, and a positive correlation between the amount of bone and the degree of fibrosis [9]. Radiological abnormalities visualized by conventional X-ray such as endosteal osteosclerosis have been reported, most common in the axial skeleton and the methaphyseal ends of the long bones [10].
Recently, we were the first to show that patients with the classical CMPNs have a higher incidence rate and risk of fractures than age- and sex-matched controls from the general population [11]. The CMPN patients in our previous study were defined by their WHO International Classification of Diseases ICD-8 or ICD-10 diagnosis code. Previously, a specific diagnosis code for MF was not comprised in the ICD; therefore, this CMPN subtype was not included. Also, due to the registry-based nature of the data on disease or treatment specific, details were not available for the included patients [11].
Osteoporosis is the characterized by low bone mass and micro-architectural deterioration [12]. It is well documented that areal bone mineral density (aBMD) measured by dual-energy X-ray absorptiometry (DXA) is an important factor in predicting osteoporotic fractures [13]. However, aBMD is limited by assessing only the bone mass and does not capture all fractures [14, 15]. Today it is possible to assess micro-architecture by high-resolution peripheral quantitative computed tomography (HR-pQCT) with distal measurements of volumetric BMD and compartmental architecture at the radius and tibia with a voxel size of 82 µm [16].
Further, with measurements of biochemical bone turnover markers (BTM), complementary information of bone metabolism can be achieved, with information of bone formation as well as resorption, reflecting the global bone metabolism, in contrast to imaging modalities measuring defined focal areas [17].
The absolute fracture risk in MF is unknown, but since MPNs are perceived as different phenotypic presentations of the same disease, the aim of this clinical study is to assess bone geometry, bone mineral density, and micro-architecture in patients with myelofibrosis and indirectly assess fracture risk [18]. We report results from bone strength and structure investigations amongst 18 patients with MF including dual-energy X-ray absorptiometry (DXA), and high-resolution peripheral quantitative computed tomography (HR-pQCT). The latter method has not been adopted amongst patients with MF previously. We also assess bone turnover by measurements of biochemical markers in peripheral blood.
Methods
We conducted a cross-sectional study including all patients with either a diagnosis of primary myelofibrosis or secondary myelofibrosis following PV or ET according to WHO 2008-criteria from the department of Haematology, Odense University Hospital, Denmark covering the period 2012–2014.
In Denmark, the entire population has a unique and permanent 10-digit registry number (the CPR number), and each contact with the government-based healthcare system is registered by this number in both patient electronic files as well as the Danish National Registry of patients (DNRP) together with their diagnostic code [19]. This enables a linkage between specific diagnostic codes and individuals, and thereby identification of well-defined cohort of patients. The earlier diagnostic coding for MF was unspecific in the nomenclature “morbus myeloproliferativa chronicus”, and leads to mis-classifications, but with the latest classification named “primary or secondary myelofibrosis” the diagnostic coding improved. At our the clinic, a revision and update to the latest classification of all living MF patients have been performed, which allowed us to identify a complete group of patients diagnosed with myelofibrosis at the department of Haematology, and by their CPR number link to their medical files. The files of all eligible patients were reviewed to ensure all patients met the diagnostic WHO 2008 MF criteria. After review of files MF patients were disregarded if they met any of the following exclusion criteria: Dementia, terminal illness, age above 80 years (since matching controls were all under 80 years), pregnancy, Paget’s disease, multiple myeloma, hyperparathyroidism, osteomalacia, liver or kidney disease, medical treatment with anti-oestrogen drugs, anti-osteoporotic drugs or use of glucocorticoids more than 5 mg/day for 3 months or more. In all 27 MF eligible patients were identified. Of these, 18 were included in the study. Seven patients were excluded by pre-defined exclusion criteria (one dementia, five aged 80 years or more, and one terminally ill) and two declined participation.
Age-matched controls were identified in a database with normative and descriptive data of men and woman from a Caucasian population (N = 499) recruited from the general population of the municipality of Odense [20]. The population in this database has participated in previous studies to generate descriptions of bone structure and strength measures amongst the Danish population [20]. Each MF patient was matched with respect to age (±2 years), sex, and height (±5 cm).
Ethics
All participants were informed both in person by SF and written, and informed consent was obtained according to the National GCP Guidelines. The study was approved by Ethics Committee of Southern Denmark S-20110110 as well as the Danish data protecting agency nr. 2008-58-0035.
Diagnosis validation
All patients had previously at least one bone marrow examination done to meet the diagnostic criteria, and new bone marrow biopsies were not requested for this study. For the purpose of this study, all previous bone marrow biopsies were re-examined by one of the authors (CS) who is an experienced haematopathologist. This was done to confirm the histopathological MF diagnosis, to validate the classification of fibrosis grade, as well as the presence vs. the absence of osteosclerotic bone marrow changes. Grades of bone marrow fibrosis were after pathology review subsequently categorized in two groups: MF grade 1–2 and MF grade 3. Janus kinase 2 mutational status (JAK2) was known in all patients [21].
Dynamic International Prognostic Scoring System (DIPPS Plus score) that incorporates age >65, haemoglobin >10 g/L, leucocytes >25 × 109 g/L, circulating blasts >1 % and constitutional symptoms besides karyotype, platelet count, and transfusion status was assessed at all patients at the time of the inclusion in the study as a prognostic tool, to assess the severity of disease of the patients [22, 23]. Patients were stratified into four groups; low risk, intermediate 1, intermediate 2 and high risk.
Dual-energy X-ray absorptiometry (DXA)
Areal BMD (aBMD) were measured of the lumbar spine (L1–L4) and the non-dominant total hip region using DXA (Hologic Discovery, Waltham, MA, USA). Coefficient of variation (CV) for measurements of total hip and spine in our clinic was 1.5 % at each site.
High-resolution peripheral quantitative computed tomography (HR-pQCT)
Bone geometry, vBMD, micro-architecture, and bone strength were assessed using an HR-pQCT system (Xtreme CT, Scanco Medical, AG, Brüttisellen, Switzerland) at the non-dominant distal radius and distal tibia (the opposite limb in the presence of a previous fracture). The manufacturer default protocol for in vivo imaging that has been described previously [16] was applied, providing a 9.02-mm axial 3D representation at each anatomical site. Each measurement was initiated at 9.5 and 22.5 mm from the mid-endplate at the radius and tibia respectively and included 110 parallel slices in the axial direction providing images with an isotopic image voxel size of 82 µm. The following outcome variables were included in our analysis using the manufacturer’s standard software: bone geometrical measures including total, cortical and trabecular areas (mm2), volumetric BMD (vBMD) (mg hydroxyapatite/cm3) for the entire (Total vBMD), trabecular (Tb vBMD) and cortical regions (Ct vBMD), and trabecular micro-architectural parameters including trabecular bone volume to tissue volume fraction (Tb BV/TV), trabecular number (Tb.N, mm−1), thickness (Tb.Th, mm), separation (Tb.Sp, mm) and trabecular network inhomogeneity (standard deviation of 1/Tb.N, mm). In addition, periosteal and endosteal surfaces of the cortex were extracted using a specific cortical evaluation software allowing 3D measurement of cortical thickness (periosteal–endosteal distance, Ct.Th, mm) and cortical porosity (Ct.Po, %) as previously described [24, 25]. Finally, total bone stiffness (N/mm) and estimated failure load (kN) were assessed as indices of bone strength using a micro-FEA solver provided by the manufacturer (Finite Element analysis Software v1.15, Scanco Medical, Switzerland) [26, 27].
Image quality was assessed by one of the authors (VS) using the 5-step scale with 1 being the best and 5 being the worst [28] and images more than grade 3 were excluded from the analysis. A maximum of three scans per anatomical site was allowed to obtain images of optimal quality. In our unit, CVs for geometry, density, and micro-architecture parameters ranged from 0.4 to 7.2 % [29].
Biochemical analysis
Blood samples were obtained in a fasting state from the MF patients. A biochemical screen for conditions or endocrinal abnormalities that could influence normal bone homoeostasis was performed including haemoglobin, leucocytes, thrombocytes, lactate dehydrogenase (LDH), thyroid-stimulating hormone (TSH), parathyroid hormone (PTH), ionized calcium (Ca2+), phosphate (P), 25-hydroxyvitamin D (25-OH-vitamin D), and alkaline phosphatase (BASP). These analyses were done at the department of Clinical Biochemistry, Odense University Hospital.
We collected blood samples for the purpose of measuring the biochemical bone markers: pro-collagen type 1 N-terminal pro-peptide (P1NP), osteocalcin (OC), C-terminal cross-linking telopeptide of type 1 collagen (CTX), and bone-specific alkaline phosphatase (bALP). Blood samples were pipetted into small tubes and frozen to −80 °C and stored until time for analyses.
P1NP was analysed by manual enzyme-linked immunosorbent assay (ELISA) at Institute of Cancer and Inflammation, University of Southern Denmark [30]. OC was analysed by automated electro-chemiluminescence immunoassay (ECLIA) at Unilabs laboratories, Copenhagen; bALP was analysed by manual ELISA (MicroVue™ BAP) and CTX by automated Roche modular, β-cross Laps/serum immunoassay, both at department of Clinical Immunology and Biochemistry, Vejle Hospital). Variation of analysis and intra-individual biologic variations are specified from the individual labs, respectively, for P1NP: 5 and 5 %, for CTX 5 and 18 %, for bALP 7 and 6 % and not given for OC.
P1NP levels were compared with samples from department of Clinical Immunology, Odense University Hospital from 18 healthy blood donors (aged 50–70) who served as reference subjects; remaining BTM were compared with reference values directed from the respective laboratories.
Statistical analysis
Data are presented as medians with 25–75 % inter-quartile range (IQR). Wilcoxon non-parametric rank-sum test was used to compare results of imaging modalities between MF patients and healthy controls, as well as P1NP levels in MF patient and healthy blood donors. Due to the size of the data set, data were generally not normally distributed, and non-parametric methods were therefore used. STATA statistical software release 12.0 was used for the analyses [31]. Level of statistical significance was set at a two-sided P value <0.05.
Results
Patients
The MF patients included in the study had a median age of 68.2 years (IQR 63.8–71.3 years) and with an even distribution of males and females (Supplementary Table 1). Anthropometrics for MF patients and their respective control subjects are shown in Supplementary Table 1. MF patients and controls were comparable with regard to age, height, weight, and BMI. Patients were scanned 1341 (IQR 868–1877) days after diagnosis, ranging from 123 to 2608 days. Assessment with DIPPS plus categorized no patients as low risk, 4 patients as intermediate-1 (22.2 %), 11 patients as intermediate-2 (61.1 %), and 3 patients as high risk (16.7 %) Table 1.
Bone marrow evaluation
The diagnostic criteria as well as grading of myelofibrosis were confirmed in all patients after pathology review. JAK2 mutation was present in 12 patients. Nine patients were classified as grade MF 1–2 and nine patients as grade MF-3 fibrosis in their bone marrow biopsy. Osteosclerosis including focal osteosclerosis was observed in three of the patients with MF grade 1–2, and eight of the patients with MF grade 3.
Comparison of areal bone mineral density (aBMD) by DXA
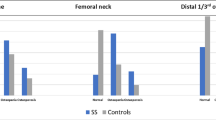
Results of the bone densitometry are shown in Fig. 1. As it can be seen aBMD in the hip was highly comparable between MF patients and controls, whilst measurements in the spine showed a possible increased aBMD of MF patients relative to controls, but did not reach statistical significance (Fig. 1).
Total spine aBMD was 1.01 g/cm2 (IQR 0.87–1.15 g/cm2) in MF patients and 0.92 g/cm2 (IQR 0.83–0.99 g/cm2) in healthy controls, P = 0.08. Total hip aBMD was 0.95 g/cm2 (IQR 0.86–1.05 g/cm2) in patients compared to 0.89 g/cm2 (IQR 0.79–1.03 g/cm2) in the controls, P = 0.35.
Geometry, vBMD, and micro-architecture by HR-pQCT
Bone geometry, volumetric BMD, micro-architecture, and bone strength were obtained by the HR-pQCT, and data are shown in Table 2 for distal radius and in Table 3 for distal tibia. There were no differences between MF patients and healthy controls in any of the measured parameters in both radius and tibia. At the tibia, there was a trend towards an increase in trabeculae number (P = 0.09) and decrease in trabeculae thickness (P = 0.14) and trabeculae spacing (P = 0.10) in MF patients in comparison to controls (Table 3).
Similarly, total bone stiffness and estimated failure load were not significantly different between MF patients and the healthy controls in either of the skeletal sites examined (Tables 2, 3). It was not possible to obtain sufficient image quality in two patients at radial site and in one patient at tibial site due to motion artefact.
Biochemical analysis
Biochemical measurements amongst MF patients revealed decreased haemoglobin and elevated lactate dehydrogenase, whilst leucocytes and platelets were within normal range (Table 1).
Thyroid-stimulating hormone, ionized calcium, phosphate, creatinine, and alkaline phosphatase were within reference values. PTH was marginally above reference values amongst 6 patients and the level of 25-OH-vitamin D was below reference values amongst 10 patients.
Levels of biochemical bone turnover markers are listed in Table 1. MF patients had significantly higher levels of P1NP compared to the reference level (P < 0.001), whilst the remaining markers bALP, OC, and CTX were within reference values.
Discussion
This study is the first assessing the bone structure with HR-pQCT in a small cohort of patients diagnosed with myelofibrosis by a direct comparison to a matched group of healthy volunteers. Our data revealed no differences between patients and healthy volunteers with regard to bone geometry, volumetric bone mineral density, and micro-structure measured at distal tibia and distal radius. Estimated bone stiffness and bone strength were similar between MF patients and healthy controls.
DXA BMD
Previous studies of BMD amongst patients with MF have been mainly small cross-sectional studies including MF patients compared with a matched group from the background population, with up to nine participants or case reports [7, 8]. Diamond et al. [8] reported increased bone mass in a series of four MF patients compared to normative data from the Australian population. Mellibovsky et al. [7] showed in a study that hip BMD was above average in eight of nine patients with MF when compared with individuals matched for height and age. The same study also observed that MF patients in disease stages one or two had lower BMD than those with stage three. Secondary osteoporosis and osteolytic lesions are extremely rare findings in myelofibrosis, but have been described in case reports [10, 32, 33].
In our study the areal bone mineral density, estimated by DXA, as well as the volumetric bone mineral density assessed using HR-pQCT in patients with myelofibrosis were comparable with the background population, although osteosclerotic manifestations were observed in the bone marrow biopsies. Elevated BMD in the spine may reflect an artefact from degenerative conditions, as seen in the elderly populations [34]. Artefact may also partly explain the splenomegaly reported associated with elevated BMD at lumbar site [35, 36]. This was found in a patient with Gaucher’s disease who also had coexistent low hip BMD, possibly reflecting the high glycolipid load or secondary calcification of the spleen [35]. Of note Gaucher’s disease often results in bone manifestations where increased risk of osteoporosis and fractures is a common feature [37]. Splenomegaly is an almost universal finding in MF and therefore spine BMD may not be as accurate as hip BMD in our patient group. In situations with discrepancy between BMD measurements of spine and hip in MF patients, an artefact from splenomegaly must be considered.
CMPNs and bone pathophysiology
Patients with systemic mastocytosis (SM), another rare chronic myeloproliferative neoplasm has decreased bone mass and increased risk of osteoporosis [38]. Conversely, osteosclerotic changes amongst patients with SM have also been described. In spite of this, these CMPN type patients were reported to have increased bone mass with increased trabecular volume, increased cortical thickness and narrowing of marrow spaces, and osteosclerosis and this was associated with a poorer disease outcome [38–40]. The pathogenesis of the affected bone metabolism in SM is unknown. The neoplastic mast cell has been suggested to be involved as well as inflammatory mediators such as IL-6 may contribute to the bone pathophysiology [41, 42]. The mast cell and its mediator histamine do not play an important role in the classical CMPNs. A common feature between SM and the classical CMPNs may however be the chronic inflammation of the bone marrow which again may affect bone metabolism. Elevation of IL-6 is also found amongst the classic CMPN patients [43, 44]. Possibly, chronic inflammation may cause a bone loss in CMPN leading to an increased risk of fractures but the loss may be masked by an elevation of BMD which is caused by osteosclerosis. Of note, glucocorticoids may be used as a symptom-directed therapy in SM, whereas treatment of the classical CMPN patients does not include corticosteroids or other agents known to affect bone metabolism [45].
Bone remodelling and bone turnover markers
Bone turnover in our study was assessed with biochemical markers, reflecting the global bone status, as recommended by International Osteoporosis Foundation and International Federation of Clinical Chemistry and Laboratory Medicine in clinical studies [46]. BTM in MF patients have been investigated in several studies [7, 8, 47, 48]. In the study by Mellibovsky et al. [7] osteocalcin was within normal range in the MF patients, consistent with our results. Diamond et al. [8] found elevated markers of bone formation; alkaline phosphatase and bone gla-protein as well as elevated markers of bone resorption; carboxy telepeptidase (ICTP); and deoxypyridinoline in their case report of 4 MF patients. P1NP is a direct marker for collagen synthesis, and therefore used as a proxy marker of bone formation in clinical studies. However, this marker is likely to also reflect the neoplastic driven bone marrow fibrosis in MF. Patients with myelofibrosis have excess collagen type I and type III fibres in the bone marrow [2, 49] which may explain the significant elevation of P1NP in our study. Studies have shown that pro-collagen III peptide (P3NP)—a marker of collagen 3 syntheses—is elevated in CMPN patients compared with controls, and that the level is highest in MF patients. Also P3NP increases with the degree of reticulin fibrosis, and thus correlates with disease activity in patients with CMPN [47, 48]. Furthermore, a marker of collagen degradation, carboxy-terminal telopeptide of type I collagen (S-ICTP), is significantly higher in MF patients compared with controls [48].
Limitations
An overlap exists within the different CMPN entities in ICD-10 and diagnostic misclassification can occur [50]. In this study, all MF diagnoses were re-evaluated by review of both the medical files and the bone marrow biopsies. Since the bone marrow biopsies were not performed at the time of the scanning, some patients may have progressed in fibrosis grade or developed osteosclerosis since the last bone marrow biopsy. A possible explanation for the lack of differences may be that the peripheral scanning measurements are underestimating the actual new bone formation and the biomechanical properties in the central skeleton, including the location of haematopoietic tissue, even though it has been shown in healthy individuals that distal measurements by HR-pQCT of radius and tibia are associated with bone density, micro-structural, and mechanical properties of the central skeleton [51]. However, at the present time the high resolution of the HR-pQCT cannot be obtained when measuring central anatomical locations.
Previous, associations between fracture risk and estimated failure load have been demonstrated [52]. In the perspective of increased fracture risk found amongst other types of CMPN patients, none of the structural parameters in this study are indicating an increased risk of fractures in MF patients [11]. The reason for this potential discrepancy is unknown, but since MF is a rare disease with an incidence ranging from 0.1 to 1.0 per 100,000 person years and a median survival of 6.6 years [53–55], we may have had inadequate statistical power to detect differences between groups. Figure 1 shows a possible increased aBMD in the spine of MF patients relative to control. Given the modest number of patients included, it is possible that aBMD are higher in MF patients, and that this could have been demonstrated in a larger patient population. The distribution of the patients in DIPSS plus categories did not allow comparison of aBMD or other measures across categories.
Perspectives and conclusion
A collaborative study is to be considered in the future to achieve a larger patient cohort, but is restricted by the availability of HR-pQCT scans at present. In a cross-sectional study design, the structural changes expected along with progression of the MF are not evaluated. Mellibovsky et al. [7] suggest that the coupling between progression of MF and BMD could constitute a non-invasive method for follow-up of MF. Ultimately, non-invasive bone assessment in addition with biochemical markers of fibrosis may prove to be a way of monitoring disease and treatment response amongst patients with MF in the future to minimize the number of invasive bone marrow biopsies.
In summary, data from HR-pQCT in a cohort of 18 patients with myelofibrosis regarding bone geometry, bone mineral density, and micro-architecture were comparable with the background population, despite manifestations of osteosclerosis observed in bone marrow biopsies of the patients. P1NP were significant elevated and may be a marker of formation of fibrosis to include in further studies.
References
Swerdlow SHCE, Harris NL et al. WHO classification of tumours of the haematopoietic and lymphoid tissues. France: IARC; 2008.
Thiele J, Kvasnicka HM. The 2008 WHO diagnostic criteria for polycythemia vera, essential thrombocythemia, and primary myelofibrosis. Current Hematol Malig Rep. 2009;4(1):33–40.
Dameshek W. Some speculations on the myeloproliferative syndromes. Blood. 1951;6(4):372–5.
Scherber R, Dueck AC, Johansson P, Barbui T, Barosi G, Vannucchi AM, et al. The myeloproliferative neoplasm symptom assessment form (MPN-SAF): international prospective validation and reliability trial in 402 patients. Blood. 2011;118(2):401–8.
Mesa RA, Niblack J, Wadleigh M, Verstovsek S, Camoriano J, Barnes S, et al. The burden of fatigue and quality of life in myeloproliferative disorders (MPDs): an international Internet-based survey of 1179 MPD patients. Cancer. 2007;109(1):68–76.
Tefferi A. Myelofibrosis with myeloid metaplasia. New Engl J Med. 2000;342(17):1255–65.
Mellibovsky L, Marinoso ML, Cervantes F, Besses C, Nacher M, Nogues X, et al. Relationship among densitometry, bone histomorphometry, and histologic stage in idiopathic myelofibrosis. Bone. 2004;34(2):330–5.
Diamond T, Smith A, Schnier R, Manoharan A. Syndrome of myelofibrosis and osteosclerosis: a series of case reports and review of the literature. Bone. 2002;30(3):498–501.
Poulsen LW, Melsen F, Bendix K. A histomorphometric study of haematological disorders with respect to marrow fibrosis and osteosclerosis. APMIS Acta Pathologica, Microbiologica, et Immunologica Scandinavica. 1998;106(4):495–9.
Guermazi A, de Kerviler E, Cazals-Hatem D, Zagdanski AM, Frija J. Imaging findings in patients with myelofibrosis. Eur Radiol. 1999;9(7):1366–75.
Farmer S, Horvath-Puho E, Vestergaard H, Hermann AP, Frederiksen H. Chronic myeloproliferative neoplasms and risk of osteoporotic fractures; a nationwide population-based cohort study. Br J Haematol. 2013;163(5):603–10.
Consensus development conference. diagnosis, prophylaxis, and treatment of osteoporosis. Am J Med. 1993;94(6):646–50.
Marshall D, Johnell O, Wedel H. Meta-analysis of how well measures of bone mineral density predict occurrence of osteoporotic fractures. BMJ. 1996;312(7041):1254–9.
Stone KL, Seeley DG, Lui LY, Cauley JA, Ensrud K, Browner WS, et al. BMD at multiple sites and risk of fracture of multiple types: long-term results from the study of osteoporotic fractures. J Bone Miner Res Off J Am Soc Bone Miner Res. 2003;18(11):1947–54.
Schuit SC, van der Klift M, Weel AE, de Laet CE, Burger H, Seeman E, et al. Fracture incidence and association with bone mineral density in elderly men and women: the Rotterdam Study. Bone. 2004;34(1):195–202.
Boutroy S, Bouxsein ML, Munoz F, Delmas PD. In vivo assessment of trabecular bone microarchitecture by high-resolution peripheral quantitative computed tomography. J Clin Endocrinol Metab. 2005;90(12):6508–15.
Vasikaran S, Eastell R, Bruyere O, Foldes AJ, Garnero P, Griesmacher A, et al. Markers of bone turnover for the prediction of fracture risk and monitoring of osteoporosis treatment: a need for international reference standards. Osteoporos Int. 2011;22(2):391–420 (A Journal established as result of cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA).
Campbell PJ, Green AR. The myeloproliferative disorders. New Engl J Med. 2006;355(23):2452–66.
Schmidt M, Pedersen L, Sorensen HT. The danish civil registration system as a tool in epidemiology. Eur J Epidemiol. 2014;29(8):541–9.
Hansen S, Shanbhogue V, Folkestad L, Nielsen MM, Brixen K. Bone microarchitecture and estimated strength in 499 adult danish women and men: a cross-sectional population-based high-resolution peripheral quantitative computed tomographic study on peak bone structure. Calcified tissue international. 2013;94:269.
Levine RL, Pardanani A, Tefferi A, Gilliland DG. Role of JAK2 in the pathogenesis and therapy of myeloproliferative disorders. Nature Rev Cancer. 2007;7(9):673–83.
Gangat N, Caramazza D, Vaidya R, George G, Begna K, Schwager S, et al. DIPSS plus: a refined dynamic international prognostic scoring system for primary myelofibrosis that incorporates prognostic information from karyotype, platelet count, and transfusion status. J Clin Oncol Off J Am Soc Clin Oncol. 2011;29(4):392–7.
Passamonti F, Cervantes F, Vannucchi AM, Morra E, Rumi E, Pereira A, et al. A dynamic prognostic model to predict survival in primary myelofibrosis: a study by the IWG-MRT (International Working Group for Myeloproliferative Neoplasms Research and Treatment). Blood. 2010;115(9):1703–8.
Burghardt AJ, Kazakia GJ, Ramachandran S, Link TM, Majumdar S. Age- and gender-related differences in the geometric properties and biomechanical significance of intracortical porosity in the distal radius and tibia. J Bone Miner Res Off J Am Soc Bone Miner Res. 2010;25(5):983–93.
Nishiyama KK, Macdonald HM, Buie HR, Hanley DA, Boyd SK. Postmenopausal women with osteopenia have higher cortical porosity and thinner cortices at the distal radius and tibia than women with normal aBMD: an in vivo HR-pQCT study. J Bone Miner Res Off J Am Soc Bone Miner Res. 2010;25(4):882–90.
Pistoia W, van Rietbergen B, Lochmuller EM, Lill CA, Eckstein F, Ruegsegger P. Image-based micro-finite-element modeling for improved distal radius strength diagnosis: moving from bench to bedside. J Clin Densitom Off J Int Soc Clin Densitom. 2004;7(2):153–60.
Macneil JA, Boyd SK. Bone strength at the distal radius can be estimated from high-resolution peripheral quantitative computed tomography and the finite element method. Bone. 2008;42(6):1203–13.
Pialat JB, Burghardt AJ, Sode M, Link TM, Majumdar S. Visual grading of motion induced image degradation in high resolution peripheral computed tomography: impact of image quality on measures of bone density and micro-architecture. Bone. 2012;50(1):111–8.
Hansen S, Hauge EM, Beck Jensen JE, Brixen K. Differing effects of PTH 1-34, PTH 1-84, and zoledronic acid on bone microarchitecture and estimated strength in postmenopausal women with osteoporosis: an 18-month open-labeled observational study using HR-pQCT. J Bone Miner Res Off J Am Soc Bone Miner Res. 2013;28(4):736–45.
Orum O, Hansen M, Jensen CH, Sorensen HA, Jensen LB, Horslev-Petersen K, et al. Procollagen type I N-terminal propeptide (PINP) as an indicator of type I collagen metabolism: ELISA development, reference interval, and hypovitaminosis D induced hyperparathyroidism. Bone. 1996;19(2):157–63.
Seung H, Medige J, Ziv I. Combined models of ultrasound velocity and attenuation for predicting trabecular bone strength and mineral density. Clin Biomech. 1996;11(6):348–53.
Merry GM, Aronowitz PB. Myelofibrosis with massive hepatosplenomegaly and osteolytic bone lesions. J Hosp Med Off Publ Soc Hosp Med. 2010;5(3):E27–8.
Assous N, Foltz V, Fautrel B, Rozenberg S, Etchepare F, Bourgeois P. Bone involvement in myelofibrosis: effectiveness of bisphosphonates. Joint Bone Spine Revue du Rhumatisme. 2005;72(6):591–2.
Blake GM, Fogelman I. An update on dual-energy X-ray absorptiometry. Semin Nucl Med. 2010;40(1):62–73.
Gregson CL, Steel SA, O’Rourke KP, Allan K, Ayuk J, Bhalla A, et al. Sink or swim: an evaluation of the clinical characteristics of individuals with high bone mass. Osteoporos Int. 2012;23(2):643–54 (A Journal established as result of cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA).
Gregson CL, Hardcastle SA, Cooper C, Tobias JH. Friend or foe: high bone mineral density on routine bone density scanning, a review of causes and management. Rheumatology. 2013;52(6):968–85.
Guggenbuhl P, Grosbois B, Chales G. Gaucher disease. Joint Bone Spine Revue du Rhumatisme. 2008;75(2):116–24.
Rossini M, Zanotti R, Bonadonna P, Artuso A, Caruso B, Schena D, et al. Bone mineral density, bone turnover markers and fractures in patients with indolent systemic mastocytosis. Bone. 2011;49(4):880–5.
Johansson C, Roupe G, Lindstedt G, Mellstrom D. Bone density, bone markers and bone radiological features in mastocytosis. Age Ageing. 1996;25(1):1–7.
Barete S, Assous N, de Gennes C, Grandpeix C, Feger F, Palmerini F, et al. Systemic mastocytosis and bone involvement in a cohort of 75 patients. Annals Rheum Dis. 2010;69(10):1838–41.
van der Veer E, van der Goot W, de Monchy JG, Kluin-Nelemans HC, van Doormaal JJ. High prevalence of fractures and osteoporosis in patients with indolent systemic mastocytosis. Allergy. 2012;67(3):431–8.
Theoharides TC, Boucher W, Spear K. Serum interleukin-6 reflects disease severity and osteoporosis in mastocytosis patients. Int Arch Allergy Immunol. 2002;128(4):344–50.
Hasselbalch HC. Perspectives on chronic inflammation in essential thrombocythemia, polycythemia vera, and myelofibrosis: is chronic inflammation a trigger and driver of clonal evolution and development of accelerated atherosclerosis and second cancer. Blood. 2012;119(14):3219–25.
Panteli KE, Hatzimichael EC, Bouranta PK, Katsaraki A, Seferiadis K, Stebbing J, et al. Serum interleukin (IL)-1, IL-2, sIL-2Ra, IL-6 and thrombopoietin levels in patients with chronic myeloproliferative diseases. Br J Haematol. 2005;130(5):709–15.
Pardanani A. Systemic mastocytosis in adults: 2011 update on diagnosis, risk stratification, and management. Am J Hematol. 2011;86(4):362–71.
Vasikaran S, Cooper C, Eastell R, Griesmacher A, Morris HA, Trenti T, et al. International osteoporosis foundation and international federation of clinical chemistry and laboratory medicine position on bone marker standards in osteoporosis. Clin Chem Lab Med CCLM/FESCC. 2011;49(8):1271–4.
Barosi G, Costa A, Liberato LN, Polino G, Spriano P, Magrini U. Serum procollagen-III-peptide level correlates with disease activity in myelofibrosis with myeloid metaplasia. Br J Haematol. 1989;72(1):16–20.
Jensen MK, Riisbro R, Holten-Andersen MN, Brown Pde N, Junker P, Brunner N, et al. Collagen metabolism and enzymes of the urokinase plasminogen activator system in chronic myeloproliferative disorders: correlation between plasma-soluble urokinase plasminogen activator receptor and serum markers for collagen metabolism. Eur J Haematol. 2003;71(4):276–82.
Kuter DJ, Bain B, Mufti G, Bagg A, Hasserjian RP. Bone marrow fibrosis: pathophysiology and clinical significance of increased bone marrow stromal fibres. Br J Haematol. 2007;139(3):351–62.
Barbui T, Thiele J, Vannucchi AM, Tefferi A. Problems and pitfalls regarding WHO-defined diagnosis of early/prefibrotic primary myelofibrosis versus essential thrombocythemia. Leukemia. 2013;27(10):1953–8.
Liu XS, Cohen A, Shane E, Yin PT, Stein EM, Rogers H, et al. Bone density, geometry, microstructure, and stiffness: relationships between peripheral and central skeletal sites assessed by DXA, HR-pQCT, and cQCT in premenopausal women. J Bone Miner Res Off J Am Soc Bone Miner Res. 2010;25(10):2229–38.
Boutroy S, Van Rietbergen B, Sornay-Rendu E, Munoz F, Bouxsein ML, Delmas PD. Finite element analysis based on in vivo HR-pQCT images of the distal radius is associated with wrist fracture in postmenopausal women. J Bone Miner Res Off J Am Soc Bone Miner Res. 2008;23(3):392–9.
Moulard O, Mehta J, Fryzek J, Olivares R, Iqbal U, Mesa RA. Epidemiology of myelfibrosis (Mf), essential thrombocythemia (Et), and polycythemia vera (Pv) in the european union (Eu). Eur J Haematol. 2013.
Mehta J, Wang H, Iqbal SU, Mesa R. Epidemiology of myeloproliferative neoplasms in the United States. Leukemia Lymphoma. 2013.
Cervantes F, Dupriez B, Passamonti F, Vannucchi AM, Morra E, Reilly JT, et al. Improving survival trends in primary myelofibrosis: an international study. J Clin Oncol Off J Am Soc Clin Oncol. 2012;30(24):2981–7.
Acknowledgments
We thank the staff at the Osteoporosis Clinic, Odense University Hospital, for performing the scans. This work has supported by grants from Danish Cancer Society/Kræftens bekæmpelse (R90-A6062-14S29) and the Region of Southern Denmark (j.nr. 11/28457).
Conflict of interest
The authors report no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
This study has been registered at www.clinicaltrials.gov NCT01816022.
Electronic supplementary material
Below is the link to the electronic supplementary material.
About this article
Cite this article
Farmer, S., Vestergaard, H., Hansen, S. et al. Bone geometry, bone mineral density, and micro-architecture in patients with myelofibrosis: a cross-sectional study using DXA, HR-pQCT, and bone turnover markers. Int J Hematol 102, 67–75 (2015). https://doi.org/10.1007/s12185-015-1803-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12185-015-1803-3