Abstract
An effective sorbent of 1-(2-Pyridylazo)-2-naphthol-functionalized mesoporous silica has been prepared to simultaneous separation and preconcentration of lead and cadmium ions in aqueous solution. Structural characterization of 1-(2-Pyridylazo)-2-naphthol-functionalized organic–inorganic hybrid mesoporous materials was conducted by Fourier transform infrared spectroscopy, transmission electron microscopy, N2 adsorption–desorption measurement, X-ray diffraction, and elemental and thermal analysis, which confirmed the successful grafting of organic moiety on mesoporous silica. The affecting parameters on adsorption and desorption steps were optimized by Box-Behnken design through response surface methodology. Three variables (pH value, sorption time, and amount of the sorbent) were selected as the main factors affecting sorption step, while four variables (type of eluent, eluent volume, eluent concentration, and elution time) were selected for desorption step in the optimization study. The optimized values by this optimization method were 10 mg, 8 min, 6.3, HCl, 1.6 mL, 1.2 mol L−l HCl, and 10 min, for amount of sorbent, sorption time, pH of solution, type, volume, and concentration of the eluent, and elution time, respectively. Under the optimized conditions, the detection limits of the proposed method for lead and cadmium ions were found to be 0.9 and 0.04 μg L−1, respectively, while the relative standard deviation (RSD) for five replicate measurements was calculated to be <3 % for both ions. For proving that the proposed method is reliable, a wide range of food, soil, and water samples with different and complex matrixes was used.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Heavy metals like Cd(II) and Pb(II) are considered as dangerous toxic element to the environment and human health. The main sources of metal contamination are industrial development and human activities (Carbonell-Barrachina et al. 2002; Behbahani et al. 2014a; Bagheri et al. 2012a; Bagheri et al. 2012b). However, these heavy metals are entered into the human body by ingestion of contaminated food, air, and water, to create the vulnerability of vital organs of the human body such as liver, kidney tissues and brain (Chen and Teo 2001; Kazi et al. 2008; Duran et al. 2013; Behbahani et al. 2014b).
Sample preparation is one of the most important and critical step in analytical chemistry. A number of preconcentration methods, including cold-induced aggregation microextraction (Baghdadi and Shemirani 2008), head space liquid phase microextraction (Zhang and Lee 2010), in situ solvent formation microextraction (Galan-Cano et al. 2012), dispersive liquid-liquid microextraction (DLLME) (Asadollahzadeh et al. 2014; Tsenga et al. 2014; Soylak and Tuzen 2006; Duran et al. 2008; Soylak et al. 2003), solid phase extraction (SPE) (Sahmetlioglu et al. 2014; Behbahani et al. 2013a), and magnetic solid phase extraction (MSPE) (Bagheri et al. 2012c) have been widely used for preconcentration and detection of organic and inorganic analytes.
Among these techniques, SPE offers an excellent alternative to the conventional sample preparation methods because of its simplicity, consumption of small volumes of organic solvent, and ability to achieve a higher enrichment factor and sensitivity.
Mesoporous solids have been widely used in different fields such as catalysis and sorption materials because of their large surface area, high porosity, controlled morphology, and easy functionalization (Feng et al. 1997; Fryxell 2006). Specific syntheses and applications for the mesoporous materials have been increasing in recent years. They are widely applied in environmental treatment techniques especially because of their high sorption capacity and good dispersibility in aqueous solutions (Liu et al. 2000; Mureseanu et al. 2008; Wang et al. 2005; Gao et al. 2007; Zhang et al. 2007). Functionalized mesoporous materials have been used for the sorption of metals such as Cu(II) (Jeong et al. 2011), Hg(II) (Mureseanu et al. 2010), Pb(II) (Hajiaghababaei et al. 2011), Zn(II) (Dong et al. 2010), and Ag(I) (Ebrahimzadeh et al. 2010).
One major challenge in the utilization of extraction techniques is the selection of experimental conditions which can provide acceptable response at low analyte concentration. Chemometrics, a term originally coined by Wold in 1971 (Brereton 1990), is “a chemical discipline that uses mathematics, statistics, and formal logic; (I) to design or select optimal experimental procedures; (II) to provide maximum relevant chemical information by analyzing chemical data; and (III) to obtain knowledge about chemical systems”. Moreover, chemometrics tools through the development of mathematical models can assess the statistical significance of the independent variable effects being investigated as well as evaluate their interaction effects. If significant interaction effects between the examined variables exist, the optimal conditions indicated by the univariate studies will be much different from the correct results of the multivariate optimization (Ferreira et al. 2007). Box-Behnken design (BBD) is an efficient option (three-level factor quadratic design) at which the experimental points are located on the midpoints of the edges of a cube and at the center (central points). The special arrangement of the BBD levels allows the number of design points to increase at the same rate as the number of polynomial coefficients. The spherical nature of the BBD combined with the fact that the design is rotatable or nearly rotatable suggests that ample center runs should be used (Stalikas et al. 2009). An advantage of BBD is that it does not contain combinations for which all factors are simultaneously at their highest or lowest levels. Therefore, these designs are useful in avoiding experiments that would be performed under extreme conditions, for which unsatisfactory results might occur (Teixeira Tarley et al. 2009).
The primary aim of the current work is to synthesize an absorbent by modifying MCM-48 with 1-(2-Pyridylazo)-2-naphthol. The newly modified mesoporous material was employed in solid phase extraction for the extraction and preconcentration of lead and cadmium ions. Structural characterization of 1-(2-Pyridylazo)-2-naphthol-functionalized organic–inorganic hybrid mesoporous materials was conducted by Fourier transform infrared spectroscopy, transmission electron microscopy, N2 adsorption–desorption measurement, X-ray diffraction, and elemental and thermal analysis, which confirmed the successful grafting of organic moiety on mesoporous silica. BBD was used to optimize the influence of factors in adsorption and desorption steps, separately. Finally, the introduced solid phase extraction technique was successfully applied for the extraction and preconcentration of lead and cadmium ions in soil and water samples.
Experimental
Apparatus
Lead and cadmium concentration was determined by an AA-680 Shimadzu (Kyoto, Japan) flame atomic absorption spectrometer (FAAS) in an air-acetylene flame, according to the user’s manual, provided by the manufacturer. Lead and cadmium hollow cathode lamps were used as the radiation source with wavelength of 283.3 and 228.8 nm, respectively. A digital pH meter, WTW Metrohm 827 Ion analyzer (Herisau, Switzerland), equipped with a combined glass calomel electrode was used for the pH adjustments at 25 ± 1 °C temperature. Heidolph heater stirrer model MR 3001 (Germany) was employed for heating and stirring of the solutions. Fourier transform infrared (FT-IR) spectra (4,000–200 cm−1) in KBr were recorded using Bruker IFS66/S FT-IR spectrometer. The CHN analysis was performed on a Thermo Finnigan Flash EA112 elemental analyzer (Okehampton, UK). The specific surface areas were measured by nitrogen adsorption technique using a Micromeritics ASPS 2010 analyzer. The transmission electron microscopy (TEM) images were taken on a JEOL JEM-2100F field emission transmission electron microscope. Low-angle X-ray diffraction patterns were obtained on a Philips-PW 17C diffractometer with Cu Kα radiation. Thermogravimetry and differential scanning calorimetry (TGA/DSC) were carried out on a Bahr STA-503 instrument under air atmosphere.
Material and Reagents
Sodium nitrite, 4-nitrobenzaldehyde, 1-(2-pyridylazo)-2-naphthol (PAN), and sodium hydrosulfite were purchased from Aldrich Company. All other reagents used in this study (solvents, acids, 3-amino-propyltriethoxysilane, and stock solutions of metal ions) were of analytical grade and purchased from Merck Chemical Company (Darmstadt, Germany, www.merck.de/). Colloidal silica with particle size below 0.5 mm and surface area of 200 m2/g was purchased from Merck and used immediately. Double-distilled water from a Milli-Q purification system (Millipore, Bedford, MA, USA) was used for the preparation of solutions. Stock standard solutions (100 mg/L) of corresponding analytes were prepared with the addition of an appropriate amount of nitrate salts in ultra-pure water while working standard of metals were prepared from the dilution of stock standard solution with distilled water. Soil (NCS DC 73323) and ore polymetallic gold Zidarovo-PMZrZ (206 BG 326) from Bulgaria were prepared as the reference materials.
Preparation of 1-(2-Pyridylazo)-2-Naphthol-Functionalized Organic–Inorganic Hybrid Mesoporous Materials
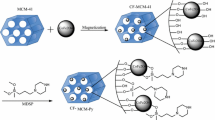
MCM-48 mesoporous silica was prepared according to the earlier report (Xu et al. 1998), and modified with amine group according to the published procedure (Johnson and Stein 2001). Briefly, 1.0 g of MCM-48 was suspended in 50 mL of toluene and then 3-amino-propyltriethoxysilane (2.0 mL) was added to the mixture and was refluxed for 24 h under N2 atmosphere. The resulted solid was suspended in 100 mL of toluene and an excess amount of 4-nitrobenzaldehyde (5.0 g) was added to the mixture. After refluxing the mixture for 24 h, the solid was removed from the solvent by filtration and suspended in 50 mL of water containing 5.0 g of sodium hydrosulfite. The resulted solid was filtrated, washed with water, and suspended in 50 mL of aqueous solution of acetic acid (1 %) in ice bath. Then, a total amount of 2.0 g of sodium nitrite was added to the mixture and stirred overnight. The solid was removed by filtration and again suspended in 50 mL of ethanol and then 0.50 g of PAN was added to the mixture in ice bath. After stirring the mixture for 24 h, light red solid was separated by filtration and washed several times with ethanol and water. The synthesis of 1-(2-pyridylazo)-2-naphthol-functionalized mesoporous silica (PAN-MCM-48) was confirmed by IR spectroscopy, low-angle X-ray diffraction, elemental and thermal analysis, and N2 adsorption surface area measurement.
Extraction Procedure
The optimized values by this optimization method were 10 mg, 8 min, 6.3, HCl, 1.6 mL, 1.2 mol L−l HCl, and 10 min, for amount of sorbent, retention time, pH of solution, type, volume, concentration of the eluent, and elution time, respectively. Batch experiments were used for investigation of factors in sorption and desorption steps. Simultaneous extraction of cadmium and lead ions from the solution is followed by two steps: sorption and desorption. In the sorption step, the pH of the sample solution was adjusted to 6.3 by drop wise addition of 2 mol L−1 sodium hydroxide or nitric acid solutions. Then, 10 mg of dried sorbent was suspended in aqueous solution containing 1 mg L−1 of cadmium and lead ions and stirred for 8 min with a magnetic stirrer. In the desorption step, simultaneous elution of lead and cadmium ions from the sorbent was performed by 1.6 mL of HCl (1.2 mol L−1). After 10 min, the concentration of cadmium and lead ions in this solution was determined by flame atomic absorption spectrometry (FAAS).
Real Sample Pretreatment
The tested water samples were distilled, tap (Tehran, Iran), and sea water (Persian Gulf and Caspian Sea). The water samples were collected in polyethylene bottles. They were cleaned with acid bath, and then filtered through nylon filters (Millipore, 0.22 μm) before the analysis. Soil samples were collected from Mouteh gold mine (Iran). A 0.2-g of homogenized soil sample and certified reference materials (CRM) was accurately weighed in a 100-mL beaker. The sample was digested by using a mixture of HNO3–HCl (aqua regia) on a hot plate at 100 °C for 1 h, till the clear transparent solutions were obtained. After cooling, the resulting solutions were filtered through a 0.45-mμ pore size membrane filter into a 100-mL conical bottom flask and were diluted with distilled water (diluted to 75 mL). Afterwards, pH of soil samples was adjusted to desired pH and then was subjected to developed procedure as described in Section 2.4 and subsequent determination of cadmium and lead ions by FAAS. Blanks were also treated in the same manner as samples were treated but without analyte.
The shrimps and fishes were bought from four local supermarkets in four different sites randomly near the Persian Gulf, Iran. After collection, four samples of seafood (fish and shrimp) from each supermarket were randomly selected, mixed, and transferred into an ice bag and transported to the laboratory and stored at −20 °C prior to analysis. The sampling was carried out on August 2012. The muscle tissues of the shrimps and fishes were separated and freeze-dried in order to obtain a constant weight, and were finely grounded. For metal analysis, approximately 1.0 g of the grounded samples was digested in 5 mL of concentrated nitric acid at 135 °C for 4 h, and then, 1 mL of hydrogen peroxide (30 %) and 1 ml of concentrated perchloric acid were added, and the temperature maintained at 150 °C until the liquid was clear (Zhou et al. 1998; Wua and Yang 2011). Then, the resulted solution was filtered and after the filtration, the clear solution was diluted to 75 mL and the pH was adjusted to 6.3 using NaOH or HNO3 for further analysis.
Results and Discussion
Characterization of 1-(2-Pyridylazo)-2-Naphthol-Functionalized Organic–Inorganic Hybrid Mesoporous Materials
The MCM-48 mesoporous silica was synthesized according to the earlier report by the hydrothermal method. The formation of MCM-48 was confirmed by low-angle X-ray powder diffraction and nitrogen adsorption analysis. The N2 adsorption analysis showed a surface area of 1,026 m2 g−1 for the mesoporous silica which indicates that the material has a mesopore structure. After functionalization of the MCM-48 with 3-amino-propyltriethoxysilane agent [34], the amine functionalized material was reacted with the aldehyde group of 4-nitrobenzaldehyde. This reaction led to the formation of the nitro functionalized MCM-48 (Fig. 1). The subsequent reactions which resulted in the formation of PAN-modified MCM-48 were carried out according to previous report (Liu et al. 1995). Formation of PAN-MCM-48 mesoporous silica was confirmed by IR spectroscopy, low-angle X-ray diffraction, elemental and thermal analysis, and N2 adsorption surface area measurement. The FT-IR spectra of PAN-MCM-48 showed stretching vibration bands of PAN at 1,608 cm−1 (N = N), 1,598 cm−1 (C-C aromatic ring), 1,281 cm−1 (C-N), 2,983 cm−1 (aliphatic C-H), and 3,027 cm−1 (aromatic C-H) and confirmed the presence of PAN groups in the sorbent. Elemental analysis of the PAN-functionalized MCM-48 showed 0.86 mmol g−1 of PAN in the sorbent. According to the N2 adsorption results and decrease of the surface area from 1,026 to 574 m2 g−1 after functionalization, PAN is mainly grafted within the channels of mesoporous MCM-48. The low-angle X-ray diffraction pattern of PAN-MCM-48 showed slight shifts in peaks and decrease in intensity in comparison to un-functionalized MCM-48 (Fig. 2). According to this pattern, the mesopore structure of MCM-48 remains intact after functionalization. For further investigation of the mesoporous structure of the sorbent which is a key point in its efficiency, the TEM study was carried out and the result also confirmed that the mesoporous structure of the MCM-48 (Fig. 3a) has been retained after functionalization (Fig. 3b). The pores (bright spots) and walls (dark spots) are clearly visible from TEM micrographs in Fig. 3. The thermal stability of PAN-MCM-48 has been investigated by TGA/DSC analysis. According to the TGA/DSC analysis, the sorbent is stable up to 160 °C. Moreover, about 37 % of loss weigh is in good agreement with the elemental analysis results about PAN concentration grafted on mesoporous silica (Fig. 4).
Optimization Experiments
The affecting parameters on adsorption and desorption steps were optimized by a Box-Behnken design through response surface methodology. Three variables (pH value, extraction time, and amount of the synthesized sorbent) were selected as the main factors affecting the sorption step, while four variables (type of eluent, volume of the eluent, concentration of the eluent, and elution time) were selected for the desorption step in the optimization study.
Optimization of Retention Parameters
The Box-Behnken design (BBD) is an efficient option (three-level factor quadratic design) in which the experimental points are located on the midpoints of the edges of a cube and at the center (central points). The levels of factors selected based on primary experiments based on one variable at the time are shown in Table 1. In a system involving three independent variables ×1, ×2, and ×3 the responses of the Box-Behnken design can be expressed by the following quadratic polynomial equation:
where y is the response, β0 is a constant and β1–β33 are the regression coefficients. The number of experimental points (N) can be defined by the following expression:
where K is the number of parameters and C0 is the number of center points. In this step, K and C0 were set at 3 and therefore 15 experiments had to be done. The effects of variables on the retention step by using the Pareto chart are shown in Fig. 5a. The bars, extending beyond the line, correspond to effects that are statistically significant at the 95 % confidence level. Enhancement or reduction of the response are shown with the positive or negative sign (corresponding to a colorless or colored), respectively. The results expressed that the pH of solution has the main effect on the retention step. The best pH for simultaneous sorption of analyte was found to be 6.3. This observation could be attributed to the fact that in high pH, the analyte ions precipitate as their hydroxide salts which cause reducing of the retention. Moreover, in low pH, the active groups of 1-(2-Pyridylazo)-2-naphthol will be protonated and could not coordinate to lead and cadmium ions. A sorption time of about 8 min was required for quantitative retention of the analytes from solution into solid phase. Furthermore, the amount of PAN-MCM-48 has a negative insignificant effect on the extraction process. Due to the high viscosity of the solution, the retention of cadmium and lead ions decreased in higher amounts of the sorbent. The response surface methodology (RSM) in Fig. 5b represents simultaneous effects of two variables (amount of sorbent and sorption time) on the retention of cadmium and lead ions. According to the overall results of the optimization study, pH = 6.3, sorption time = 8 min, and amount of sorbent = 10 mg were chosen for the sorption step.
a Pareto chart of the main effects in the BBD. AA, BB, and CC are the quadratic effects of the pH, amount of the sorbent and sorption time, respectively. AB, AC, and BC are the interaction effects between pH and amount of the sorbent, pH and sorption time, and amount of the sorbent and sorption time, respectively. b The estimated response surface of amount of the sorbent and sorption time
Selection of Elution Solvent Type
Complete removal of lead and cadmium ions from synthesized sorbent is a very crucial step to guarantee the absence of memory effect. For investigation of eluent’s type, elution of metal ions from the sorbent was studied using 2 mL of three solvents such as HCl, HNO3, and CH3COOH. Based on the results in Table 2, HCl showed a better recovery compared to other solvents. Therefore, HCl was selected as the eluent for further studies.
Optimization of Desorption Step
In this step, the Box-Behnken design was employed for desorption step while other factors were optimized in the previous section. Eluent concentration (mol L−1), eluent volume (mL), and elution time (min) were investigated in the elution step using the Box-Behnken design. According to BBD, eluent concentration and volume showed positive and significant effect on the recovery of target ions but elution time showed a negative and non-significant effect [Pareto chart (Fig. 6a)]. Simultaneous effects of eluent concentration and eluent volume on the response were investigated with the aid of RSM (Fig. 6b). According to the overall results, eluent concentration = 1.2 mol L−1, elution volume = 1.6 mL, and elution time = 10 min were chosen for desorption of target ions from the synthesized sorbent.
a Pareto chart of the main effects in the BBD. AA, BB, and CC are the quadratic effects of the eluent concentration, eluent volume, and elution time, respectively. AB, AC, and BC are the interaction effects between eluent concentration and eluent volume, eluent concentration and elution time, and eluent volume and elution time, respectively. b The estimated response surface of eluent concentration and eluent volume
Effect of Sample Volume
In the analysis of real samples, the sample volume is one of the important parameters influencing the preconcentration factor. Therefore, the effect of sample volume on simultaneous quantitative retention of cadmium and lead ions by the synthesized sorbent was investigated. For this purpose, 10 mg of the sorbent was suspended in different sample volumes (25, 50, 100, 200, 300, 350, 400, 425, 450, 475, and 500 mL) containing 16 μg of cadmium and lead ions. All solutions were extracted under optimum condition by the method and then eluted under optimized desorption parameters. It was found that the simultaneous quantitative recovery (Fig. 7) of cadmium (300 mL) and lead (450 mL) ions on PAN-MCM-48 could be obtained for sample volume up to 300 mL.
Sorption Capacity of the 1-(2-Pyridylazo)-2-Naphthol-Functionalized Organic–Inorganic Hybrid Mesoporous Materials
The sorption capacity defined as the maximum amount of metal ions sorbed per gram of the sorbent is an important factor for evaluation of the synthesized mesoporous material. To evaluate this factor, 100 ml of a solution containing 30 mg cadmium and lead ions was applied to the extraction method and the sorption capacity was calculated at the optimized extraction conditions. In order to evaluate the maximum adsorption capacity, the difference between concentration of the solution before extraction and the concentration of the solution after extraction was calculated. The sorption capacities of the sorbent were calculated to be 210 and 254 mg g−1 for cadmium and lead ions, respectively. Obviously, the adsorption capacity of the synthesized mesoporous sorbent was larger than previous synthesized sorbent.
The Effect of the Potentially Interfering Ions
To investigate the effect of various cations found in natural samples, elements that are known as alkaline, alkaline earth, and transition metals were added to 50 mL of solution containing 0.5 μg of cadmium and 0.5 μg of lead ions. The degree of tolerance for some alkaline, alkaline earth, and transition metal ions are presented in Table 3. From the tolerance data, it can be seen that the potentially interfering ions have no significant effects on preconcentration of cadmium and lead ions at pH of 6.3.
Reusability of the Solid Material
The long-term stability of the sorbent as solid phase was investigated by successive sorption and elution cycles of solutions of 100 μg L−1 Pb and Cd ions at the optimum condition. Afterwards, the adsorbed metal ions were eluted with 1.2 mL of 1.6 mol L−1 HCl solution. By monitoring the change in the recoveries of the extraction of Pb and Cd, the stability of the sorbent was estimated. The results show that the sorbent is stable after eight and ten adsorption–desorption cycles for cadmium and lead ions, respectively.
Statistical and Calibration Parameters
The performance of PAN-functionalized organic–inorganic hybrid mesoporous materials was investigated at the optimum conditions. The limit of detection (LOD), regression equations, coefficient of determination (R 2), linear dynamic ranges (LDRs), preconcentration factors (PFs), and relative recoveries (RR %) were obtained. The limits of detection, defined as C LOD = 3S b/m, where S b is the standard deviation of seven replicate blank signals and m is the slope of the linear section of calibration curve after preconcentration for a sample volume of 75 mL. Precision was expressed as a mean percentage of the relative standard deviation (RSD %). Also, the preconcentration factor calculated as the ratio between the slopes of the calibration curves acquired by the proposed method (Y = 1.819 X + 0.0005 for lead ions and Y = 29.403 X + 0.0015 for cadmium ions) and through the direct analysis of cadmium and lead ions by FAAS (Y = 0.0143 X + 0.0063 for lead ions and Y = 0.08 X + 0.001) was found to be 127.2 (1.819/0.0143 = 127.2) and 367.5 (29.403/0.08 = 367.5) for lead and cadmium ions, respectively. The analytical performances of the proposed method were summarized in Table 4.
Certified Reference Material Analysis
Soil (NCS DC 73323) and ore polymetallic gold Zidarovo-PMZrZ (206 BG 326) with a certified cadmium and lead content were applied as certified reference material. As Table 5 shows, a good correlation was obtained between the certified amounts and the amounts found by the method. Therefore, PAN-functionalized organic–inorganic hybrid mesoporous materials can be used as a reliable solid phase technique for separation and trace determination of cadmium and lead ions in environmental samples.
Real Sample Analysis
To evaluate the capability of this solid phase extraction technique for trace analysis of lead and cadmium ions in real samples with different matrices containing various amounts of diverse ions, the method was used for the extraction of cadmium and lead ions from different samples (Table 6). The relative recoveries percentage of lead and cadmium ions from the real and spiked samples varied in the range of 96.0–102 %. The results clearly indicate the suitability of the PAN-functionalized organic–inorganic hybrid mesoporous materials for the preconcentration and determination of cadmium and lead ions at trace levels in environmental samples.
Comparison of the Synthesized PAN-Functionalized Organic–Inorganic Hybrid Mesoporous Materials with Literature
The proposed method was compared with other reported methods in literatures. According to Table 7, the introduced solid phase extraction method is clearly better than other reports in LOD, sorption capacity, PF, RSD, and extraction time (Parham et al. 2009; Salarian et al. 2014; Nabid et al. 2012; Bagheri et al. 2012a; Behbahani et al. 2013a; Behbahani et al. 2013b).
Conclusion
In the present study, an application of 1-(2-Pyridylazo)-2-naphthol-functionalized organic–inorganic hybrid mesoporous material was introduced in the area of solid phase extraction. The mesoporous structure of PAN-MCM-48 can cause the fast adsorption of lead and cadmium ions. The affecting parameters on adsorption and desorption steps were optimized by the Box-Behnken design through response surface methodology. Determination of the lead and cadmium ions resulted in good quantitative recoveries in food, water, and soil samples. In addition, PAN-MCM-48 showed high tolerance to interferences from the matrix ions, and finally, the important features of the proposed method were its high adsorption capacity, good preconcentration factor, and low detection limit which is better than other solid phase extraction methods.
References
Asadollahzadeh M, Tavakoli H, Torab-Mostaedi M, Hosseini G, Hemmati A (2014) Response surface methodology based on central composite design as a chemometric tool for optimization of dispersive-solidification liquid–liquid microextraction for speciation of inorganic arsenic in environmental water. Talanta 123:25–31
Baghdadi M, Shemirani F (2008) Cold-induced aggregation microextraction: a novel sample preparation technique based on ionic liquids. Anal Chim Acta 613:56–63
Bagheri A, Behbahani M, Amini MM, Sadeghi O, Taghizade M, Baghayi L, Salarian M (2012a) Simultaneous separation and determination of trace amounts of Cd (II) and Cu (II) in environmental samples using novel diphenylcarbazide modified nanoporous silica. Talanta 89:455–461
Bagheri A, Behbahani M, Amini MM, Sadeghi O, Tootoonchi A, Dahaghin Z (2012b) Preconcentration and separation of ultra-trace palladium ion using pyridine-functionalized magnetic nanoparticles. Microchim Acta 178:261–268
Bagheri A, Taghizadeh M, Behbahani M, Asgharinezhad AA, Salarian M, Dehghani A, Ebrahimzadeh H, Amini MM (2012c) Synthesis and characterization of magnetic metal-organic framework (MOF) as a novel sorbent, and its optimization by experimental design methodology for determination of palladium in environmental samples. Talanta 99:132–139
Behbahani M, Najafi M, Amini MM, Sadeghi O, Bagheri A, Salarian M (2013a) Dithizone-modified nanoporous fructose as a novel sorbent for solid-phase extraction of ultra-trace levels of heavy metals. Microchim Acta 180:911–920
Behbahani M, Salarian M, Amini MM, Sadeghi O, Bagheri A, Bagheri S (2013b) Application of a new functionalized nanoporous silica for simultaneous trace separation and determination of Cd(II), Cu(II), Ni(II), and Pb(II) in food and agricultural products. Food Anal Methods 6:1320–1329
Behbahani M, Gorji T, Mahyari M, Salarian M, Bagheri A, Shaabani A (2014a) Application of polypropylene amine dendrimers (POPAM)-grafted MWCNTs hybrid materials as a new sorbent for solid-phase extraction and trace determination of gold (III) and palladium (II) in food and environmental samples. Food Anal Method 7:957–966
Behbahani M, Sadeghi Abandansari H, Salarian M, Babapour M, Bagheri A, Nabid MR (2014b) Synthesis and application of a thermosensitive tri-block copolymer as an efficient sample treatment technique for preconcentration and ultra-trace detection of lead ions. Microchim Acta 181:129–137
Brereton RG (1990) Chemometrics. Ellis Horwood, Chichester
Carbonell-Barrachina AA, Garcia E, SanchezSoriano J, Aracil P, Burlo F (2002) Effects of raw materials, ingredients, and production lines on arsenic and copper concentrations in confectionery products. J Agric Food Chem 50:3738–3742
Chen J, Teo KC (2001) Determination of cadmium, copper, lead and zinc in water samples by flame atomic absorption spectrometry after cloud point extraction. Anal Chim Acta 450:215–222
Dong ZP, Dong ZH, Wang P, Tian X, Geng HM, Li R, Ma JT (2010) A fluorescent probe for zinc detection based on organically functionalized SBA-15. Appl Surf Sci 257:802–806
Duran A, Soylak M, Tuncel SA (2008) Poly(vinyl pyridine-poly ethylene glycol methacrylate-ethylene glycol dimethacrylate) beads for heavy metal removal. J Hazard Mater 155(1–2):114–120
Duran A, Tuzen M, Soylak M (2013) Evaluation of metal concentrations in food packaging materials: relation to human health. At Spectrosc 34:99–101
Ebrahimzadeh H, Shekari N, Tavassoli N, Amini MM, Adineh M, Sadeghi O (2010) Extraction of trace amounts of silver on various amino-functionalized nanoporous silicas in real samples. Microchim Acta 170:171–178
Feng XD, Fryxell GE, Wang LQ, Kim AY, Liu J, Kemner KM (1997) Functionalized monolayers on ordered mesoporous supports. Science 276:923–926
Ferreira SLC, Bruns RE, Ferreira HS, Matos GD, David JM, Brandão GC, da Silva EGP, Portugal LA, dos Reis PS, Souza AS, dos Santos WNL (2007) Box-Behnken design: an alternative for the optimization of analytical methods. Anal Chim Acta 597:179–186
Fryxell GE (2006) The synthesis of functional mesoporous materials. Inorg Chem Commun 9:1141–1150
Galan-Cano F, Lucena R, Cardenas S, Valcarcel M (2012) Ionic liquid based in situ solvent formation microextraction coupled to thermal desorption for chlorophenols determination in waters by gas chromatography/mass spectrometry. J Chromatogr A 1229:48–54
Gao ZF, Wang LN, Qi T, Chu JL, Zhang Y (2007) Synthesis, characterization, and cadmium(II) uptake of iminodiacetic acid-modified mesoporous SBA-15. Colloids Surf A Physicochem Eng Asp 304:77–81
Hajiaghababaei L, Badiei A, Ganjali MR, Heydari S, Khaniani Y, Ziarani GM (2011) Highly efficient removal and preconcentration of lead and cadmium cations from water and wastewater samples using ethylenediamine functionalized SBA-15. Desalination 266:182–187
Jeong EY, Ansari MB, Mo YH, Park SE (2011) Removal of Cu(II) from water by tetrakis(4-carboxyphenyl) porphyrin-functionalized mesoporous silica. J Hazard Mater 185:1311–1317
Johnson JS, Stein A (2001) Surface modification of mesoporous, macroporous, and amorphous silica with catalytically active polyoxometalate clusters. Inorg Chem 40:801–808
Kazi TG, Jalbani N, Kazi N, Arain MB, Jamali MK, Afridi HI, Kandehro GA (2008) Evaluation of toxic metals in blood and urine samples of chronic renal failure patients, before and after dialysis. Ren Fail 30:737–745
Liu F, Li KA, Wu YS, Wang X, Tong SY (1995) Study on preconcentration and separation of trace Pd(II) and Pt(IV) with silica gel bonded by aminopropyl-benzoylazo-1-(2 pyridylazo)-2-naphthol. Microchem J 52:274–281
Liu AM, Hidajat K, Kawi S, Zhao DY (2000) A new class of hybrid mesoporous materials with functionalized organic monolayers for selective adsorption of heavy metal ions. Chem Commun 13:1145–1146
Mureseanu M, Reiss A, Stefanescu I, David E, Parvulescu V, Renard G, Hulea V (2008) Modified SBA-15 mesoporous silica for heavy metal ions remediation. Chemosphere 73:1499–1504
Mureseanu M, Reiss A, Cioatera N, Trandafir I, Hulea VJ (2010) Mesoporous silica functionalized with 1-furoyl thiourea urea for Hg(II) adsorption from aqueous media. J Hazard Mater 182:197–203
Nabid MR, Sedghi R, Bagheri A, Behbahani M, Taghizadeh M, Abdi Oskooie H, Heravi MM (2012) Preparation and application of poly(2-amino thiophenol)/MWCNTs nanocomposite for adsorption and separation of cadmium and lead ions via solid phase extraction. J Hazard Mater 203–204:93–100
Parham H, Pourreza N, Rahbar N (2009) Solid phase extraction of lead and cadmium using solid sulfur as a new metal extractor prior to determination by flame atomic absorption spectrometry. J Hazard Mater 163:588–592
Sahmetlioglu E, Yilmaz E, Aktas E, Soylak M (2014) Polypyrrole/multi-walled carbon nanotube composite for the solid phase extraction of lead(II) in water samples. Talanta 119:447–451
Salarian M, Ghanbarpour AR, Behbahani M, Bagheri S, Bagheri A (2014) A metal-organic framework sustained by a nanosized Ag12 cuboctahedral node for solid-phase extraction of ultra traces of lead(II) ions. Microchim Acta 181:999–1007
Soylak M, Tuzen M (2006) Diaion SP-850 resin as a new solid phase extractor for preconcentration-separation of trace metal ions in environmental samples. J Hazard Mater 137(3):1496–1501
Soylak M, Karatepe AU, Elçi L, Doǧan M (2003) Column preconcentration/separation and atomic absorption spectrometric determinations of some heavy metals in table salt samples using Amberlite XAD-1180. Turk J Chem 27(2):235–242
Stalikas C, Fiamegos Y, Sakkas V, Albanis T (2009) Developments on chemometric approaches to optimize and evaluate microextraction. J Chromatogr A 1216:175–189
Teixeira Tarley CR, Silveira G, Lopes dos Santos WN, Matos GD, Paranhos da Silva EG, Bezerra MA, Miro M, Costa Ferreira SL (2009) Chemometric tools in electroanalytical chemistry: methods for optimization based on factorial design and response surface methodology. Microchem J 92:58–67
Tsenga WC, Chenb PS, Huang SD (2014) Optimization of two different dispersive liquid–liquid microextraction methods followed by gas chromatography–mass spectrometry determination for polycyclic aromatic hydrocarbons (PAHs) analysis in water. Talanta 120:425–432
Wang XG, Lin KSK, Chan JCC, Cheng S (2005) Direct synthesis and catalytic applications of ordered large pore aminopropyl-functionalized SBA-15 mesoporous materials. J Phys Chem B 109:1763–1769
Wua X, Yang Y (2011) Heavy metal (Pb, Co, Cd, Cr, Cu, Fe, Mn and Zn) concentrations in harvest-size white shrimp Litopenaeus vannamei tissues from aquaculture and wild source. J Food Compos Anal 24:62–65
Xu J, Luan Z, He H, Zhou W, Kevan L (1998) A reliable synthesis of cubic mesoporous MCM-48 molecular sieve. Chem Mater 10:3690–3698
Zhang J, Lee HK (2010) Headspace ionic liquid-based microdrop liquid-phase microextraction followed by microdrop thermal desorption-gas chromatographic analysis. Talanta 81:537–542
Zhang LX, Yu CC, Zhao WR, Hua ZL, Chen HR, Li L, Shi JL (2007) Preparation of multi-amine-grafted mesoporous silica and their application to heavy metal ions adsorption. J Non-Cryst Solids 353:4055–4061
Zhou HY, Cheung RYH, Chan KM, Wong MH (1998) Metal concentrations in sediments and tilapia collected from inland waters of Hong Kong. Water Res 32:3331–3340
Acknowledgments
We gratefully acknowledge financial support from the Young Researchers and Elite Club, Damghan Branch, Islamic Azad University, Damghan, Iran.
Compliance with Ethics Requirements
ᅟ
Conflict of Interest
Hamid Reza Fouladian declares that he has no conflict of interest. Mohammad Behbahani declares that he has no conflict of interest. This article does not contain any studies with human or animal subjects.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Fouladian, H.R., Behbahani, M. Solid Phase Extraction of Pb(II) and Cd(II) in Food, Soil, and Water Samples Based on 1-(2-Pyridylazo)-2-Naphthol-Functionalized Organic–Inorganic Mesoporous Material with the aid of Experimental Design Methodology. Food Anal. Methods 8, 982–993 (2015). https://doi.org/10.1007/s12161-014-9981-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12161-014-9981-9