Abstract
We have designed and synthesized a thermosensitive tri-block copolymer for selective trace extraction of Pb(II) ions from biological and food samples. The polymer was characterized by Fourier transform IR and NMR spectroscopy, and by gel permeation chromatography. The critical aggregation concentration and lower critical solution temperature were determined via fluorescence and UV spectrophotometry, respectively. The effects of solution pH value, amount of copolymer, of the temperature on extraction and on phase separation, and of the matrix on the extraction of Pb(II) were optimized. Pb(II) ions were then quantified by FAAS. The use of this copolymer resulted in excellent figures of merit including a calibration plot extending from 0.5 to 160 μg L−1 (with an R2 of >0.99), a limit of detection (LOD) as low as 90 pg L−1, an extraction efficiency of >98 %, and relative standard deviations of <4 % for eight separate extraction experiments.

In this paper, for the first time an intelligent system using a thermosensitive tri-block copolymer for selective trace removal of Pb(II) in biological and food samples was designed and its determination was carried out by flame atomic absorption spectrometry.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Heavy metals contamination is an important topic due to its various effects on human health and the environment, and furthermore, exposure to certain heavy metals at trace amounts can entail irrecoverable and severe effects [1–4]. In addition, the presence of heavy metals in water has been a major concern for many years due to their toxicity which endangers the aquatic-life, and there is a worldwide tendency to decrease the permitted level of contamination in drinking water and this has been a big challenge for the environmental researchers [5]. The sources of heavy metals pollution are mainly industries such as metal cleaning, mining activities, metal finishing, and etc. [6, 7].
The toxicity of lead which widely exists in the environment is worth mentioning. This metal can accumulate in the vital organs of human and animals and its cumulative poisoning effects can cause serious hematological damages such as brain damage, anemia and kidney malfunctioning. Lead concentration in natural waters is typically between 2 and 10 ng mL−1 for which the upper limit recommended by World Health Organization (WHO) is less than 10 ng mL−1 [8]. As it was previously stated, lead is one of the most ubiquitous elements in the environment, and has become recognized as a major health risk to the environment. Lead can release into the biosphere particularly as a fuel additive in large quantities as a result of its widespread industrial applications [9]. The primary sources of lead in humans and other animals include food, beverages, water, soil, and paint [10]. Therefore, the development of analytical methods that are rapid with low detection limits is necessary.
Analysis of trace heavy metals is difficult because of their very low concentrations in samples and high complexity of sample matrices. Therefore, preliminary preconcentration and matrix removal steps are highly needed to ensure the accuracy and precision of the analytical results [11]. Various preconcentration techniques such solvent extraction; coprecipitation, cloud point extraction, ion-exchange and solid phase extraction [12–16] have been developed for various environmental samples. Recently, the use of polymeric materials such as micelles and hydrogels for separation of heavy metals from natural water has been developed extensively. Some monomers which can act as ligands and form complexes with metals are more favorable for this purpose [17–20].
In this study, we focused on the design and synthesis of a system which, in addition to acting as ligand, it can respond to change in the environmental temperatures by altering its physiochemical structure from sol-to-crudy-to-syneresis in an aqueous medium and hence, extraction and separation of heavy metals can be tuned by regulation of temperature. For this reason, we used an atom transfer radical polymerization (ATRP) for synthesis of tri-block copolymer which consists of polyethyleneglycol (PEG) as a hydrophilic central block and poly(N-isopropylacrylamide) (PNIPAAm) as thermosensitive terminal blocks. The ATRP reaction was used to synthesize the block copolymers. By using this reaction, some polymerization limitations such as high poly dispersity and unwanted random or branched polymerization can be removed and hence, it allows adjusting the composition and temperature-induced phase transition behavior of the synthesized tri-block aqueous solution precisely [21–23]. PNIPAAm, the most popular thermosensitive and water-soluble polymer, shows reversible hydration–dehydration changes in response to small solution temperature changes [24–26]. PNIPAAm chains hydrate to form an expanded structure at temperatures lower than 32 °C but undergo a sharp phase transition at higher temperatures to form inter- and intra-chain associations which results in precipitation. A temperature showing hydration–dehydration changes is called the lower critical solution temperature (LCST) [27, 28]. This phase transition can be used for extraction and separation of impurities from aqueous media. Various factors affecting the adsorption and desorption steps for the lead were investigated and optimized. Finally, the mentioned copolymer was validated using Fourier Transform Infrared spectrometry (FT-IR), Gel permission chromatography (GPC), Nuclear Magnetic Resonance (NMR), and UV–vis spectrophotometry.
Experimental
Reagents
The information about reagents was described in the electronic supplementary material (ESM).
Instrumentation
The information about instrumentation was described in the electronic supplementary material (ESM).
Synthesis of Bromo-terminated PEG
Bromo-terminated Poly (ethylene glycol) (Br-PEG-Br) was synthesized by the esterification of the terminal hydroxyl groups of the PEG with 2-bromoisobutyryl bromide in the presence of triethylamine (TEA) in toluene [29]. Typically, 2.0 g of PEG (Mn = 2,000 g mol−1) was dissolved in 50 mL toluene in a 250 mL three-neck flask under azeotropic distillation. After trace removal of water, the TEA (0.8 g, 8 mmol, 1.11 mL) was added via a syringe and the mixed solution was cooled to 4 °C. Then, 2-Bromoisobutyryl bromide (1.84 g, 8 mmol, 1 mL) was added drop wise to the vigorously stirred solution at 4 °C over 1 h. The reaction mixture was stirred at room temperature for 24 h. The precipitate was removed by filtration, and most of the toluene was evaporated by rotary evaporator. The crude product was dissolved in 50 mL methylene chloride, and then washed consecutively with 1.0 mol L−1 NaHCO3 aqueous solution and distilled water, and finally it was dried over anhydrous MgSO4, filtered and concentrated before pouring into large amounts of ice-cooled diethyl ether to precipitate the product. The resulting white solid was dried under vacuum at room temperature for 24 h.
Synthesis of (PNIPAAm-b-PEG-b-PNIPAAm) tri-block copolymer
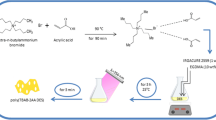
The (PNIPAAm-b-PEG-b-PNIPAAm) tri-block copolymer was prepared by ATRP of NIPAAm using dibromo-terminated PEG (Br-PEG-Br), as macroinitiator [29]. Briefly, 2 g Br-PEG2000-Br (Mn = 2,299.8 g mol−1, 1.74 mmol Br), 0.172 g of CuCl (1.74 mmol), 0.6 g PMDETA (3.44 mmol, 0.72 mL), 15.74 g NIPAAm (139.2 mmol) and 30 mL dry dioxane were added to a 50 mL Schlenk flask with a magnetic stirring bar, and oxygen was removed by bubbling the contents of the flask by nitrogen for 30 min. Then, the flask was placed in an oil bath thermostat under nitrogen flow at 70 °C to start the polymerization. Polymerization was performed at 70 °C for 24 h. The resultant solution was diluted with THF and passed through a silica gel column to remove the copper catalyst. The polymer was recovered by two times precipitation into cold diethyl ether and then dried under vacuum for 24 h. Figure 1 provides a schematic illustration of the synthesized (PNIPAAm-b-PEG-b-PNIPAAm) tri-block copolymer.
Real samples pretreatment for Pb(II) analysis
Honey
The organic matrix of honey samples was mineralized in order to determine the total content of Pb(II). The digestion was carried by a microwave digestion unit Milestone MLS 1200, (Milestone, Italy) equipped with airtight closure (vessel) Mod. SV140. The operative conditions were referred to a 0.5 g of honey sample. The conditions were: sample acidification, by 4 mL of HNO3, 24 h, room temperature; first microwave treatment, 3 min at 25 % power and 1 min at 50 % power; after cooling, addition of 2 mL of H2O2 30 %; final microwave treatment, 3 min at 25 % power and 1 min at 50 % power. The mineralised honey solution was transferred into the vessel and then the solution was diluted to 20 mL with double distilled water, and finally, the pH value was adjusted to 7.0 with NaOH solution for further analysis [30].
Butter
Butter sample was purchased from a local supermarket in Tehran, Iran, and stored at 4 °C until the analysis. Fats were digested as follows: sample was accurately weighed on an analytical balance and then it was transferred to a flask. After that 150 mL of concentrated HNO3 was added, and the mixture was left at room temperature overnight. The flask was gradually heated to 150–160 °C on a hotplate, while hydrogen peroxide (1–5 mL) was periodically added to the solution until the digestion was completed. After cooling, the sample was transferred into a volumetric flask and diluted to 20 mL with double distilled water before adjusting the pH to 7.0 [31].
Tangerine
0.5 g of dried and grounded tangerine sample was put into burning cup with 15 mL of pure NHO3. The sample was incinerated in a MARS 5 microwave oven at 200 °C. After digestion treatment, the sample was filtrated through Whatman No. 42. After filtration, the obtained clear solution was diluted to 20 mL (pH of 7.0) for metal analysis [32].
Nail
Finger nails were clipped with a stainless steel cutting instrument providing a sample in amount of 1.0 g. For microwave assisted digestion of nail sample, approximately 1.0 g of the sample was weighed and transferred to a vessel followed by addition of 0.5 mL of HNO3 and 0.5 mL of H2O2. The vessel was then closed, mounted in sleeves (outer vessels) and heated in the microwave oven at 325 W for 30 min. After cooling to room temperature, the vessel was carefully vented in a fume hood and 9 mL of ultrapure water was added, resulting in a solution of 10 mL. The solution was then transferred to acid-cleaned (hot mixture of HNO3/HCl followed by soaking in 10 % HNO3 overnight) flask before adjusting the pH to 7.0 [33].
Urine
The urine sample was collected from a smoker person and the volunteer was asked to refrain from eating fish and seafood for 3 days prior to sample collection. Early morning, urine sample was collected in 50 mL polypropylene tubes and centrifuged at 1,500 rpm for 10 min to clear them of particulate matter. The sample (20 mL) was stored in acid-washed polypropylene tubes at −20 °C [34]. Before analysis, the pH of urine sample was adjusted to 7.0.
Extraction procedure
In the extraction procedure, 20 mL of an aqueous solution containing Pb(II) ions was prepared in room temperature and its pH was adjusted to 7.0 (optimum pH for extraction), then, 5 mg of PNIPAAm-PEG-PNIPAAm tri-block copolymer was dissolved in the solution. After dissolving the copolymer, the solution temperature was increased to 40 °C and the solution was stirred at this temperature for 30 s and the solution became cloudy, and for complete extraction, the solution was allowed to stir for another 30 s. After this time, the temperature of the solution was again increased up to 65 °C. At this temperature, the cloudy solution was become a solution containing second phase. After that, the stirring was stopped so that the second phase can be reached the surface of the solution, and consequently, it can be separated from the aqueous phase. Finally, the nanomicelles containing the adsorbed Pb(II) ions was separated from the aqueous solution and dissolved in 0.5 mL of distilled water (room temperature) and then the content of the solution was determined by flame atomic absorption spectrometry.
Results and discussion
Characterization of the(PNIPAAm-b-PEG-b-PNIPAAm) tri-block copolymer
FTIR, NMR and GPC analysis
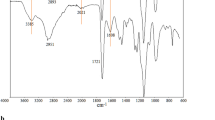
The dibromo-terminated PEG macroinitiator and thermo-responsive PNIPAAm-PEG-PNIPAAm tri-block copolymerswere prepared according to the reaction sequence shown in Fig. 1. The chemical structures of the synthesized copolymers were characterized by 1H NMR and FT-IR spectroscopy. The molecular weight and polydispersity of the synthesized copolymer were alsodetermined by gel permission chromatography (GPC). First, the starting dibromo-terminated PEG as ATRP macroinitiator was prepared by the esterification reaction of the terminal hydroxyl groups of PEG with 2-bromoisobutyryl bromide. The PNIPAAm-PEG-PNIPAAm tri-block copolymer was synthesized via ATRP of NIPAAm in dioxane at 60 °C from the Br-PEG-Br macroinitiator units. The FT-IR spectrum of PNIPAAm-PEG-PNIPAAm tri-block copolymer shows one band at 1,111 cm−1 related to C–O–C stretching vibrations of the repeated-OCH2CH2 units in PEG. PNIPAAm block shows characteristic peaks at 1,652, 1,541, and 1,245 cm−1, which can be attributed to C = O stretching and N-H bending of amide group and a methyl group in -CH(CH3)2, respectively. A broad band in the range of 3,200–3,600 cm−1 belongs to N–H stretching vibration. Figure 1S (ESM) shows the 1H NMR spectrum of the Br-PEG-Br macroinitiator and PNIPAAm-PEG-PNIPAAm tri-block copolymer. For Br-PEG-Br, there are two signals which correspond to methylene protons of the PEG (Ha at 3.59 ppm) and methyl protons of the 2-bromoisobutyrate group (Hb at 1.88 ppm) indicating that the terminal hydroxyl groups were substituted. As shown in the 1H NMR spectrum of the PNIPAAm-PEG-PNIPAAm tri-block copolymer, a broad peak at around 6.50 ppm was obtained which can be attributed to the characteristic signal of hydrogen of NH in the PNIPAAm. Moreover, the signals at 1.09 and 3.95 ppm are assigned to methyl protons (NH-CH-(CH 3 )2) and methylidyne proton (NH-CH–(CH3)2) of isopropyl unit, respectively. The chemical shifts at 1.60 and 2.09 ppm are attributed to the methylene (CH 2 -CH–C = O) and methylidyne (CH2-CH–C = O) protons adjacent to the carbonyl group. The composition of the block polymers was also calculated from the area ratio of peaks (a) and (g) in Figure 1S (ESM) and is summarized in Table 1S (ESM). The results in table 1S (ESM) illustrate that the results from NMR are in good agreement with those obtained from GPC (Figure 2S (ESM)).
CAC determination
The information about CAC determination was described in the electronic supplementary material.
LCST determination of PNIPAAm-b-PEG-b-PNIPAAmtri-block copolymer
The transition phenomena can be easily characterized by optical property change (turbidimetry) of polymeric solution [35]. The effect of the temperature on the absorbance of visible light (λ = 500 nm) through the synthesized tri-block copolymer solution (0.025 wt%) is shown in Fig. 2. Based on the Fig. 2, the LCSTs were determined at the temperature with a half of the optical transmittance change between below and above the transitions. It is evident that the polymer solution phase transition slowly increases with temperature, and at 33 °C, a sharp increase indicates the formation and low aggregation of micelles. Therefore, the LCST of tri-block copolymer is about 33.5 °C and is more than LCST of the PNIPAAm chain alone which is about 32 °C. The addition of hydrophilic PEG block to the PNIPAAm chain increased the LCST of PNIPAAm since the hydrophilic block in this structure prevents the dehydration of the polymer chain and expands the collapsed structure [24, 25].
Proposed mechanism for extraction of Pb(II)
The synthesized PNIPAAm-PEG-PNIPAAm tri-block copolymer consists of two types of thermosensitive blocks (PEG and PNIPAAm). In temperatures below 33.5 °C, PEG and PNIPAAm blocks are hydrophilic due to formation of hydrogen bonding between their heteroatoms and H2O, and therefore, they can be easily dissolved in aqueous solutions. In temperatures ranging from 33.5 °C to 65 °C, although the hydrogen bonding of PEG block remains stable, hydrogen bonding between the amide group of N-isopropylacrylamide in PNIPAAm and H2O breaks, therefore, PEG group remains hydrophilic but PNIPAAm block becomes hydrophobic. Thus, between 33.5 °C and 65 °C, the hydrophobic blocks aggregate together and formation of micelles and micelles aggregation was beginning and solution was being turbid. By formation of these micelles aggregate, lead ions due to interactions with N-H groups of PNIPAAm can be trapped in the core of aggregated micelles and extracted from the aqueous solution. In temperatures higher than 65 °C, due to decrease in hydrophilic characteristic of PEG group, the overall hydrophobic property of aggregated micelles increases, consequently, initially aggregated micelles come closer to each other and high micelle aggregation occurs. This high micelle aggregation causes the formation of a second phase at the surface of the solution which can be easily separated from the aqueous phase without the need for centrifugation. Figure 3 demonstrated a scheme of proposed mechanism for extraction of lead ions.
The effect of solution’s pH
Among the tested parameters, pH was found to be one of the most important variables affecting the selective adsorption of lead on PNIPAAm-PEG-PNIPAAm tri-block copolymer. To assess the mentioned effect, 5 mg of copolymer was dissolved in 20 mL of solution containing lead ions (2 mg L−1) and then the pH was tested in the ranges from 2 to 9. In the experiment, after dissolving the desired amount of copolymer in the solution, and adjusting the pH, the solution temperature was increased to 40 °C and the solution was stirred for 60 s, then, the temperature was again increased to 65 °C for better separation of the formed extraction phase from the aqueous phase. Finally, in order to measure the lead ions of the solution with flame atomic absorption spectrometry and for injection to the instrument, the separated phase was dissolved in 0.5 mL of distilled water (room temperature) and then, the content of solution was determined using FAAS. As can be seen in Figure 4, maximum quantitative recovery of lead ions on the copolymer was obtained in the pH of 7.0. Lowering the pH value of the solution decreased the quantitative recovery of the copolymer due to the electrostatic repulsion of the protonated active sites on the copolymer with the positively charged lead species. Due to hydrolysis, pH above 9.0 was not tested.
The effect of amount of copolymer
In order to achieve maximum quantitative recovery for extraction of Pb(II), the amount of PNIPAAm-PEG-PNIPAAm tri-block copolymer was optimized. Therefore, different amounts of copolymer ranging from 2–10 mg was dissolved in 20 mL of solution consisting of Pb(II) (2 mg L−1) after adjusting the pH to 7.0. The same extraction procedure mentioned in section extraction procedure was used. The results illustrated that; 5 mg of the copolymer can provide quantitative adsorption of lead ions and in concentrations higher that the recovery remained constant. Consequently, this amount of copolymer (5 mg) was selected for further experiments.
The effect of temperature on extraction
Temperatures between 32 and 45 °C were tested in order to achieve an optimum temperature for extraction of Pb(II) on PNIPAAm-PEG-PNIPAAm tri-block copolymer with best quantitative recovery. According to the results, best recovery for extraction of Pb(II) ions can be obtained in 40 °C and in temperatures higher than that the recovery remained constant. Therefore, the mentioned temperature was used in the following experiments.
The effect of temperature on phase separation
In order to separate the extraction phase from the aqueous phase easily, temperatures between 50 and 75 °C were examined. Easy, fast and complete separation of extraction phase from the aqueous phase can be obtained in 65 °C and in temperatures higher than that the recovery remained constant. Therefore, the optimum temperature of 65 °C was used in further experiments in order to have a complete separation PNIPAAm-PEG-PNIPAAm tri-block copolymer from the aqueous phase.
The effect of potentially interfering ions on extraction of Pb(II)
Because of the presence of other elements in real samples, pre-concentration of lead-containing samples is difficult. Therefore, the effects of common potentially interfering cations and anions on the selective extraction of lead ions on the thermosensitive tri-block copolymer were investigated. The degree of tolerance for some alkaline, alkaline earth and transition metal ions are presented in Table 1. From the tolerance data, it can be seen that the potentially interfering ions have no significant effects on preconcentration of lead ions at pH 7.0.
Validation of the method
Certified reference material (ore polymetallic gold zidarovo-PMZrZ (206 BG 326)) was used for the validation of the method. As Table 2S (ESM) shows, the measured mean value is not significantly different from the certified value [36]. Therefore, the system can be used as a reliable extraction method for determination of lead ions in food and biological samples.
Extraction of lead in different samples
Since natural samples have complex matrices, non-specific background absorption was caused by interfering species of the sample matrix. To reduce this undesirable effect, tri-block copolymer was applied for selective extraction of lead ions in pH of 7.0 (20 mL of sample). As Table 2 shows, the newly introduced microextraction system has high recovery for extraction of lead ions even in complex matrices.
Comparison with other extraction methods
The microextraction system was compared to a variety of extraction methods reported recently in the references. The distinct features are summarized in Table 3. As can be seen in the table, it is evident that extraction time, relative standard deviation and detection limit obtained by the system is far better than most of the other extraction techniques. Furthermore, the mentioned system does not need toxic solvents (acidic and organic) for elution of lead form the structure of copolymer and instead the only solvent used was distilled water; therefore, it can be referred as a green microextraction system.
Conclusion
We describe here a new sample preparation system based on formation of thermosensitive tri-block copolymer. The designed method has various and important advantages and benefits compared to other extraction methods. First, the mentioned system does not need toxic solvents (acidic and organic) for elution of lead from the structure of copolymer and instead the only solvent used was distilled water; therefore, it can be referred as a ligandless-green microextraction system. Second, in solid phase extraction (SPE) methods high volumes of real samples are needed, however, in this system low volume of the real sample (20 mL) was used. This low volume allows the system to be used in biological samples (urine) easily. Third, it is a very simple, fast, and accurate for Pb(II) extraction, and finally, the designed system is smart due to the thermosensitive characteristic of the copolymer since with temperature change fast extraction of lead can occur. Furthermore, determination of the metal ion yielded good quantitative recoveries in a wide range of real samples with complex matrices. The synthesized copolymer also showed good selectivity towards Pb(II) among other heavy metals in pH of 7.0. In addition, the copolymer showed high tolerance to interferences from the matrix ions. The important features of the method were its simplicity and low detection limit which is distinctive among other extraction methods.
References
Jamil M, Zia MS, Qasim M (2010) Contamination of agro-ecosystem and human health hazards from waste water used for irrigation. J Chem Soc Pak 32:370
Khan S, Cao Q, Zheng YM, Huang YZ, Zhu YG (2008) Health risks of heavy metals in contaminated soils and food crops irrigated with wastewater in Beijing. China Environ Pollut 152:686
Lesmana SO, Febriana N, Soetaredjo FE, Sunarso J, Ismadji S (2009) Studies on potential applications of bio mass for the separation of heavy metals from water and wastewater. Biochem Eng J 44:19
Debelius B, Forja JM, Valls AD, Lubian LM (2009) Toxicity and bioaccumulation of copper and lead in five marine microalgae. Ecotoxicol Environ Saf 72:1503
Jia K, Pan B, Lva L, Zhang Q, Wang X, Pan B, Zhang W (2009) Impregnating titanium phosphate nanoparticles onto a porous cation exchanger for enhanced lead removal from waters. J Colloid Interface Sci 331:453
Wei BG, Yang LS (2010) A review of heavy metal contaminations in urban soils, urban road dusts and agricultural soils from China. Microchem J 94:99–107
Fu FL, Wang Q (2011) Removal of heavy metal ions from wastewaters: a review. J Environ Manage 92:407
Behbahani M, Bagheri A, Taghizadeh M, Salarian M, Sadeghi O, Adlnasab L, Jalali K (2013) Synthesis and characterisation of nano structure lead (II) ion-imprinted polymer as a new sorbent for selective extraction and preconcentration of ultra trace amounts of lead ions from vegetables, rice, and fish samples. Food Chem 138:2050
Ryan JA, Scheckel KG, Berti WR, Brown SL, Casteel SW, Chaney RL, Hallfrisch J, Doolan M, Grevatt P, Maddaloni M, Mosby D (2004) Reducing children’s risk from lead in soil. Environ Sci Technol 38:18
Mahaffey KR (1995) Environ. Nutrition and lead: strategies for public health. Health Perspect 103:191
Behbahani M, Najafi M, Amini MM, Sadeghi O, Bagheri A, Salarian M (2013) Dithizone-modified nanoporous fructose as a novel sorbent for solid-phase extraction of ultra-trace levels of heavy metals. Microchim Acta 180:911
Hemmatkhah P, Bidari A, Jafarvand S, Milani Hosseini MR, Assadi Y (2009) Speciation of chromium in water samples using dispersive liquid–liquid microextraction and flame atomic absorption spectrometry. Microchim Acta 166:69
Saçmacı Ş, Kartal Ş (2010) Determination of some trace metal ions in various samples by FAAS after separation/preconcentration by copper(II)-BPHA coprecipitation method. Microchim Acta 170:75
Liang P, Li J, Yang X (2005) Cloud point extraction preconcentration of trace cadmium as 1-Phenyl-3-methyl-4-benzoyl-5-pyrazolone complex and determination by flame atomic absorption spectrometry. Microchim Acta 152:47
Zhu X, Zhao L, Wang B (2006) Speciation analysis of inorganic tin (Sn(II)/Sn(IV)) by graphite furnace atomic absorption spectrometry following ion-exchange separation. Microchim Acta 155:459
Bagheri A, Behbahani M, Amini MM, Sadeghi O, Tootoonchi A, Dahaghin Z (2012) Preconcentration and separation of ultra-trace palladium ion using pyridine-functionalized magnetic nanoparticles. Microchim Acta 178:261
Wang JS, Chiu KH (2009) Metal extraction from solid matrices using a two-surfactant microemulsion in neat supercritical carbon dioxide. Microchim Acta 167:61
Esser-Kahn AP, Iavarone AT, Francis MB (2008) Metallothionein-cross-linked hydrogels for the selective removal of heavy metals from water. J Am Chem Soc 130:15820
Dave N, Chan MY, Huang PJJ, Smith BD, Liu J (2010) Regenerable DNA-functionalized hydrogels for ultrasensitive, instrument-free mercury(II) detection and removal in water. J Am Chem Soc 132:12668
Hazer O, Kartal S (2010) Use of amidoximated hydrogel for removal and recovery of U(VI) ion from water samples. Talanta 82:1974
Xu FJ, Li J, Yuan SJ, Zhang ZX, Kang ET, Neoh KG (2008) Thermo-responsive porous membranes of controllable porous morphology from triblock copolymers of polycaprolactone and Poly(N-isopropylacrylamide) prepared by atom transfer radical polymerization. Biomacromolecules 9:331
Xia Y, Yin X, Burke NAD, Stover HDH (2005) Thermal response of narrow-disperse poly(N-isopropylacrylamide) prepared by atom transfer radical polymerization. Macromolecules 38:5937
Song J, Zhou J, Duan H (2012) Self-assembled plasmonic vesicles of SERS-encoded amphiphilic gold nanoparticles for cancer cell targeting and traceable intracellular drug delivery. J Am Chem Soc 134:13458
Garbern JC, Minami E, Stayton PS, Murry CE (2011) Delivery of basic fibroblast growth factor with a pH-responsive, injectable hydrogel to improve angiogenesis in infarcted myocardium. Biomaterials 32:2407
Garbern JC, Hoffman AS, Stayton PS (2010) Injectable pH- and temperature-responsive poly(N-isopropylacrylamide-co-propylacrylic acid) copolymers for delivery of angiogenic growth factors. Biomacromolecules 11:1833
Sun P, Zhang Y, Shi L, Gan Z (2010) Thermosensitive nanoparticles self-assembled from PCL-b-PEO-b-PNIPAAm triblock copolymers and their potential for controlled drug release. Macromol Biosci 10:621
Jeong B, Kim SW, Bae YH (2002) Thermosensitive sol–gel reversible hydrogels. Adv Drug Deliv Rev 54:37
He C, Kim SW, Lee DS (2008) In situ gelling stimuli-sensitive block copolymer hydrogels for drug delivery. J Control Release 127:189
Chen J, Liu M, Gong H, Huang Y, Chen C (2011) Synthesis and self-assembly of thermoresponsive PEG-b-PNIPAM-b-PCL ABC triblock copolymer through the combination of atom transfer radical polymerization, ring-opening polymerization, and click chemistry. J Phys Chem B 115:14947
Sanna G, Pilo MI, Piu PC, Tapparo A, Seeber R (2000) Determination of heavy metals in honey by anodic stripping voltammetry at microelectrodes. Anal Chim Acta 415:165
Szłyk E, Szydłowska-Czerniak A (2004) Determination of cadmium, lead, and copper in margarines and butters by galvanostatic stripping chronopotentiometry. J Agric Food Chem 52:4064
Hamurcu M, Özcan MM, Dursun N, Gezgin S (2010) Mineral and heavy metal levels of some fruits grown at the roadsides. Food Chem Toxicol 48:1767
Rodushkin I, Axelsson MD (2000) Application of double focusing sector field ICP-MS for multielemental characterization of human hair and nails. Part I. Analytical methodology. Sci Total Environ 250:83
Mortada WI, Sobh MA, El-Defrawy MM, Farahat SE (2002) Reference intervals of cadmium, lead, and mercury in blood, urine, hair, and nails among residents in Mansoura city, Nile Delta, Egypt. Environ Res 90:104
Li CM, Tang YQ, Armes SP, Morris CJ, Rose SF, Lloyd AW, Lewis AL (2005) Synthesis and characterization of biocompatible thermo-responsive gelators based on ABA triblock copolymers. Biomacromolecules 6:994
Surme Y, Narin I, Soylak M, Yuruk H, Dogan M (2007) Cloud point extraction procedure for flame atomic absorption spectrometric determination of lead(II) in sediment and water samples. Michrochim Acta 157:193
Ghanemi K, Nikpour Y, Omidvar O, Maryamabadi A (2011) Sulfur-nanoparticle-based method for separation and preconcentration of some heavy metals in marine samples prior to flame atomic absorption spectrometry determination. Talanta 85:763
Yousefi SR, Shemirani F (2010) Development of a robust ionic liquid-based dispersive liquid–liquid microextraction against high concentration of salt for preconcentration of trace metals in saline aqueous samples: application to the determination of Pb and Cd. Anal Chim Acta 669:25
Acknowledgments
We gratefully acknowledge financial support from the Research Council of Shahid Beheshti University.
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOC 619 kb)
Rights and permissions
About this article
Cite this article
Behbahani, M., Abandansari, H.S., Salarian, M. et al. Synthesis and application of a thermosensitive tri-block copolymer as an efficient sample treatment technique for preconcentration and ultra-trace detection of lead ions. Microchim Acta 181, 129–137 (2014). https://doi.org/10.1007/s00604-013-1079-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00604-013-1079-3