Abstract
A new method which utilizes a polypropylene amine dendrimers (POPAM)-grafted multi-walled carbon nanotubes (MWCNTs) hybrid materials as an effective sorbent in solid-phase extraction has been developed for separation and preconcentration of Au(III) and Pd(II) trace levels in food, water and soil samples. The optimum experimental conditions such as pH, flow rates, type, concentration, and volume of the eluent for elution of gold and palladium ions, breakthrough volume, and effect of potentially interfering ions on separation and determination of these noble metals were investigated. The extraction recoveries for the mentioned noble metals were greater than 98 % and the limits of detection were 0.08 and 0.12 ng mL−1 for gold and palladium, respectively. The relative standard deviations of the method were less than 4 % for eight separate column experiments for determination of 5.0 μg of gold and palladium ions. The adsorption capacity of the modified MWCNT was 92 mg g−1 for gold and 74 mg g−1 for palladium on POPAM-grafted MWCNTs. Validation of the suggested method was performed by analyzing certified reference materials. Finally, the proposed method was applied for determination of gold(III) and palladium(II) in real samples, including fish, shrimp, water, and soil.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
There is great interest in the removal and recovery of precious metals such as gold, palladium, platinum, and other noble metals in wastewater. The two most important reasons and motivations for the removal of precious metals are their economical impact of losing these metals and their environmental concerns (Elci et al. 2007). Trace amounts of these precious metals can be found in some wastewaters as a result of mining (Salih et al. 2007), electroplating industries (Ishikawa et al. 2002), and electronic and jewelry manufacturing. Removal and recovery of these noble metals from these manufacturing wastewaters for reuse can be financially beneficial and definitely decrease the production costs. Concentration of precious metals in environment and wastewaters is in trace levels, thus, an effective separation and preconcentration procedure prior to determination is considered an essential step. Palladium and gold are valuable mineral elements that exist together in nature; therefore, determination of their trace amounts is of great importance. Measurement of very low concentrations of Au and Pd usually requires separation and preconcentration steps (Saçmacı et al. 2013; Ebrahimzadeh et al. 2013). Many techniques are available for the separation and preconcentration of precious metals from wastewater, such as solvent extraction (Domı́nguez et al. 2002), membrane disk (Farhadi and Teimouri 2005; Bagheri et al. 2003), ion-exchange (Matsubara et al. 2000), selective precipitation (Dakshinamoorthy et al. 2008; Mauchauffée and Meux 2007; Sampaio et al. 2009), cloud point extraction (Tavakoli et al. 2008), electrodeposition (Komarek and Houserova 2003), leaching (Viñals et al. 2006), chlorination (Barefoot and Van Loon 1999), cyanidation (Kondos et al. 1995), and adsorption processes (Chang and Chen 2006). Each method has its own advantages and disadvantages. For instance, solid-phase extraction (SPE) based on an adsorption mechanism has been widely used for preconcentration of precious metals. This method is simple, rapid, and efficient with high preconcentration factor compared to the other separation techniques (Behbahani et al. 2012; Bagheri et al. 2012a, b, c; Behbahani et al. 2013).
More recently, great attention has been paid to the application of carbon nanotubes (CNTs) in several fields of chemical analysis (Barbosa et al. 2007) due to their special electronic, metallic, and structural properties. While recently, several works presented how to functionalize nanotubes with polymers, only a few reports have been presented for modification of carbon nanotubes with dendrimers (Sun et al. 2001; Holzinger et al. 2003; Campidelli et al. 2006; Antonia Herrero et al. 2009). The terminal groups on the dendrimer periphery can be tailored to control solubility of the hybrid nanocomposite and used as handles to facilitate linking to surfaces and other polymers (Zhao et al. 1997; Wells et al. 1996). These nanotubes are classified into multi-walled carbon nanotubes (MWCNTs) and single-walled carbon nanotubes according to the number of layers in the wall of nanotubes. Many applications of MWCNTs for preconcentration of heavy metal ions, organometallic compounds, and trace amounts of organic materials have been reported (Afzali and Mostafavi 2008; Bagheri et al. 2012a, b, c).
In this work, polypropylene amine dendrimers (POPAM)-grafted MWCNTs hybrid materials were synthesized and used as a sorbent for separation and preconcentration of trace amounts of Au(III) and Pd(II) ions in real samples prior to its determination by flame atomic absorption spectroscopy. All of the factors affecting the preconcentration of noble metals such as pH, flow rates, type, concentration, and volume of the eluent for elution of gold and palladium ions, breakthrough volume, and effect of coexisting ions on the separation and determination of these noble metals were studied and optimized. The developed method was validated by the analysis of Au(III) and Pd(II) in certified reference materials and real samples. The results demonstrated that POPAM-grafted MWCNTs hybrid materials are useful, convenient, and non-expensive adsorbents for separation of Au(III) and Pd(II) ions, and they can be used several times because of their recoverability.
Experimental
Materials
The used MWCNTs were prepared by chemical vapor deposition procedure in the presence of Co/Mo/MgO as catalyst at 900 °C. The outer diameter of MWCNTs was between 10–20 nm. MWCNTs were obtained from Research Institute of Petroleum Industry (Iran). All solvents and reagents were purchased from Aldrich or Merck and used without further purification unless otherwise stated.
Other reagents were used with analytical grades. Stock solutions of Cu(II), Cd(II), Mn(II), Ni(II), Cr(III), Al(III), Fe(II), Pb(II), Mg(II), Ca(II), Cs(I), Na(I), and K(I) were prepared from Titrisol solutions (Merck, Darmstadt, Germany). Working solutions were prepared by dilution of stock solutions (1,000 mg L−1) with deionized water. HCl, HNO3, and CH3COOH as the elution solvents were purchased from Merck Company (Whitehouse Station, NJ, USA), and 2-amino thiophenol was purified by distillation in the presence of zinc powder. Soil (NCS DC 73323) and mine stone (was also certified by Geological Survey of Iran) was used as the certified reference materials.
Apparatus
AA-680 Shimadzu (Kyoto, Japan) atomic absorption spectrometer equipped with deuterium background correction was used for determination of Au(III) and Pd(II). Gold and palladium hollow cathode lamps were used as the radiation source with wavelength of 242.8 and 244.8 nm, respectively. An air/acetylene flame was utilized in all measurements. All instrumental parameters were adjusted according to the recommendations of manufacturer. A digital pH meter, WTW Metrohm 827 Ion analyzer (Herisau, Switzerland), equipped with a combined glass calomel electrode was used for the pH adjustments at 25 ± 1 °C temperature. An ultrasonic bath (EUROSONIC® 4D ultrasound cleaner with a frequency of 50 kHz and an output power of 350 W) was used to disperse materials in solvents. The Fourier transform infrared (FT-IR) measurements were carried out using a BOMEM MB-Series FT-IR spectrometer in the form of KBr pellets. 1H NMR spectra were recorded with a BRUKER DRX-300 AVANCE spectrometer, and D2O or CDCl3 were used as solvents. Transmission electron microscopy (TEM) analyzes were performed by LEO 912AB electron microscope. The thermal stability of the nanocomposites was determined using a thermogravimetric analyzer (TGA/DTA BAHR: STA 503) under air and a heating rate of 10 °C min−1. XPS analysis was performed using a VG multilab 2000 spectrometer (ThermoVG scientific) in an ultra high vacuum.
Preparation of POPAM-grafted MWCNTs Hybrid Materials
Preparation of MWCNT-COCl
Typically, 0.6–1 g of purified MWCNTs-COOH species was stirred in a mixture of 160–200 mL of SOCl2 and 20 mL of DMF at 80 °C for 50 h. The MWCNTs material was isolated by vacuum filtration through a PTFE membrane (pore size of 0.2 mm), and washed with dry methylene chloride. MWCNTs-COCl was used almost immediately in the next reaction step after isolation in order to avoid the hydrolysis of the formed chloroanhydride.
Preparation of MWCNT-NH2
For the synthesis of MWCNTs-NH2, Curtius rearrangement was carried out. 1 g of MWCNTs-COCl and 2 g of NaN3 (30 mmol) were stirred in 800 mL of DMF at room temperature for 30 h. Then the temperature was raised up to 100 °C and the stirring was continued for an additional 20 h. The reaction product was isolated by filtration and after 10 h of sonication in concentrated hydrochloric acid, MWCNTs-NH2 was yielded.
Preparation of POPAM-grafted MWCNTs Hybrid Materials
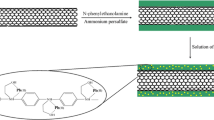
First-, second-, and third-generation POPAM dendrimers were synthesized on the amino-functionalized MWCNTs. The amino-functionalized MWCNTs (0.8 g) were added in portions at ambient temperature in a solution of acrylonitrile (3.44 mL, 80 mmol) and methanol (10 mL) in a 100-mL round-bottomed flask under stirring. The reaction mixture was stirred at ambient temperature under nitrogen atmosphere for 5 days. After the reaction, excess reactants and solvents were removed under vacuum. The product was washed with methanol, dichloromethane, and acetone (3 mL × 20 mL), and it was dried under vacuum for 24 h. Then, the product (0.8 g) and CoCl2·6H2O (23.8 g, 100 mmol) in MeOH (300 ml) were added to NaBH4 (19 g, 500 mmol) in portions with stirring at 20 °C. After 24 h, the stirred mixture was acidified with HCl (3 mol L−1, 100 mL). Then, the mixture was stirred at ambient temperature for 4 days to ensure reaction completion. The resulted mixture was filtered under vacuum, washed with methanol, acetone, and diethyl ether (3 mL × 20 mL) and dried under vacuum for 24 h. Figure 1 provides the images of synthesized POPAM-grafted MWCNTs hybrid materials after each step.
Real Samples Pre-treatment
The shrimps and fishes were bought from four local supermarkets in four different sites randomly near Persian Gulf, Iran. After collection, four samples of shrimp from each supermarket were randomly selected, mixed, and transferred into an ice bag and transported to the laboratory and stored at −20 °C prior to analysis. The sampling was carried out in August 2012. The muscle tissues of the shrimps and fishes were separated and freeze-dried in order to obtain a constant weight, and were finely ground. For metal analysis, approximately 0.5 g of the grounded samples was digested in 5 mL of concentrated nitric acid at 135 °C for 4 h, and then, 1 mL of hydrogen peroxide (30 %) and 1 ml of concentrated perchloric acid were added, and the temperature maintained at 150 °C until the liquor was clear and all particles turned to white or gray. After the filtration, the clear solution was diluted to 250 ml and the pH was adjusted to 3.0 for further analysis.
The soil sample was taken from the fields in the vicinity of a gold making workshop in Tehran, Iran, and then approximately 50 mg of the soil sample was weighted and added to 20 ml of concentrated hydrochloric acid solution and stirred at 50 °C. After standing for 4 h, the solution was centrifuged at 2,000 rpm, and the clear solution was transferred into a 500-ml volumetric flask and diluted to 250 mL and the pH was adjusted to 3.0 for further analysis.
Water samples were collected from four different sites in Iran. One of them was taken from a river near Sarcheshmeh copper mine (Kerman, Iran). Another one was taken from the waste of a gold-making workshop. The third one was collected from Caspian Sea in Northern Iran, and the last one was taken from the tap water of the laboratory. First, these four water samples were filtered and then 250 mL of each sample was used for analysis before adjusting the pH to 3.0.
Results and Discussion
Characterization of the Adsorbent
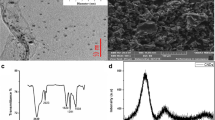
The resulting POPAM-grafted MWCNTs hybrid materials were characterized by FT-IR spectroscopy, TEM, NMR, thermal gravimetric analysis (TGA), and X-ray photoelectron spectroscopy (XPS).
FT-IR spectra provide valuable information on the functional groups (–NH2) of the first-, second-, and third-generation of POPAM. The frequencies related to –NH2 bending vibrations (1,380 cm−1) were intensified from MWCNT-NH2 to G3. This amount of sharpness and increase in intensity are related to the increase of NH2 functional groups in the surface of MWCNT. Furthermore, the increase in intensity of frequencies related to –NH2 stretching vibrations (3,440 cm−1) from MWCNT-NH2 to G3 can confirm the successful formation of G3 (Fig. 2).
Figure 3 shows 1H NMR spectra of third-generation POPAM-grafted MWCNTs in CDCl3. In this figure, peaks correspond to NH2, CH2N, and CH2CH2CH2 can be observed and they demonstrate that third-generation POPAM-grafted MWCNTs were synthesized successfully. XPS data show N (1 s) and C (1 s) signals which justify the presence of carbon and nitrogen and the successful modification of MWCNT with third-generation POPAM dendrimers (Fig. 4).
Also, the TG analysis of the sorbent can justify the modification of MWCNTs with dendrimer after each step (Fig. 5).
Column Preparation
One hundred milligrams of POPAM-grafted MWCNTs as sorbent was slurred in water and then poured into a glass column (120 mm × 20 mm) with a porous disk. The columns were used repeatedly after washing with distilled water. It was determined that the columns are stable up to seven adsorption–elution cycles without any noticeable decrease in the recovery of palladium and gold. The column was dried in vacuum oven at 60 °C for 3 h, and it was used in the following optimization conditions.
Optimization of the Retention Conditions
Among the tested variables, pH was found to be the most critical parameter for adsorption of metals on the POPAM-grafted MWCNTs. To evaluate the effect of pH on the retention efficiency, the pH of 50 mL sample solution containing 2 mg L−1 of gold and palladium ions was adjusted to fit in the range of 2–8. The obtained results in Fig. 6 indicate that the Au(III) and Pd(II) ions could be retained quantitatively on POPAM-grafted MWCNTs in the pH range of 2–5. The results suggest that adsorption has two mechanisms: ion pair and coordination ability. In pH ranges from 2 to 5, amine groups on the adsorbent are protonated and two ion pair complexes with PdCl4 2− and AuCl4 − form. Formation of these ion pair complexes increases the sorption efficiency. In high pH conditions, amine groups are deprotonated and cannot produce an ion pair complex with PdCl4 2− and AuCl4 −. Therefore, pH 3 was chosen as the optimum pH for further studies (Bagheri et al. 2012a, b, c; Ebrahimzadeh et al. 2011).
To optimize the sample flow rate, 50 mL solutions of 2 mg L−1 gold and palladium ions were adjusted to pH of 3, and then passed through the column at flow rates in the range of 1–20 mL min−1 with a peristaltic pump. The results in Fig. 7a demonstrated that sample flow rate variation in the ranges of 1–14 mL min−1 had no effect on the retention of these metals on POPAM-grafted MWCNTs.
Optimization of the Elution Conditions
For desorption of gold and palladium ions from POPAM-grafted MWCNTs, a series of selected eluent solutions, such as HNO3, HCl, HCl/thiourea, HNO3/thiourea, and CH3COOH were used. As shown in Table 1, it was eventually found that 0.4 mol L−1 thiourea in 1 mol L−1 HCl provided an effective elution of gold and palladium ions from POPAM-grafted MWCNTs. The effect of eluent volume on the recovery of the noble metals was also studied. As Table 1 shows, quantitative recovery could be obtained with 2.5 mL of 0.4 mol L−1 thiourea in 1 mol L−1 HCl. Therefore, volumes of 2.5 mL of eluent for desorption of gold and palladium ions were used in the remaining experiments.
The influence of the eluent flow rate on metals recovery was also studied. The results in Fig. 7b demonstrated the quantitative recoveries for gold and palladium ions which obtained at a flow rate range of 0.5–2 mL min−1 with 0.4 mol L−1 thiourea in 1 mol L−1 HCl.
Effect of the Volume of Sample Solutions
A higher preconcentration factor can be obtained by increasing the sample to eluent volume ratio by either decreasing the eluent volume and/or increasing the sample volume. Therefore, the maximum volume of sample solution was investigated by increasing the volume of metal ion solution with a constant amount of ions (0.1 mg of gold and palladium ions). Samples solution volumes of 25, 100, 250, 500, 750, 800, 900, 1,000, 1,100, 1,200 mL containing gold and palladium ions were passed through the column. The results (Fig. 8) demonstrated that the dilution effect was not significant for sample volumes of 900 mL for gold and 1,100 mL for palladium ions on POPAM-grafted MWCNTs. It was found that the simultaneous quantitative recovery of gold and palladium ions on POPAM-grafted MWCNTs can be obtained for sample volumes up to 900 mL. As the elution volume was 2.5 mL for gold and palladium, an enrichment factor of 360 was obtained for simultaneous preconcentration of palladium and gold ions.
Effect of the Potentially Interfering Ions
To investigate the effect of various cations found in natural samples, elements that are known as alkaline, alkaline earth, and transition metals were added to 100 mL of solution containing 0.01 mg gold and palladium ions. The degree of tolerance for some alkaline, alkaline earth, and transition metal ions are presented in Table 2. From the tolerance data, it can be seen that the external ions have no significant effects on preconcentration of gold and palladium ions at pH 3.
Adsorption Capacity
The adsorption capacity of POPAM-grafted MWCNTs for gold and palladium ions was investigated by passing 250 mL portions of aqueous solutions containing 10 mg of gold and palladium ions at optimal pH through the column. In order to evaluate the maximum adsorption capacity, the difference between concentration of the solution before passing through the column and the concentration of the solution after passing through the column was calculated. The obtained capacities of POPAM-grafted MWCNTs were found to be 92 and 74 mg g−1 for gold and palladium ions, respectively.
Analytical Performance
Under the optimized conditions, calibration curves were sketched for determination of gold and palladium ions, according to the general procedure (conditions, 100.0 mg of modified MWCNTs; sample pH, 3; sample volume, 250 mL). Linearity was maintained in the range of 0.3–140 ng mL−1 for gold and 0.7–130 ng mL−1 for palladium in initial solution. The correlation of determination (r 2) was 0.998 for gold and 0.997 for palladium ions. The limit of detection which is defined as CLOD = 3S b / m, where S b is the standard deviation of seven replicate blank signals and m is the slope of the calibration curve after preconcentration, for a sample volume of 250 mL, was found to be 0.08 ng mL−1 for gold and 0.12 ng mL−1 for palladium ions, respectively. The relative standard deviations for eight separate column experiments for determination of 5.0 μg gold and palladium ions in 100 mL of water was 3.2 and 2.4 %, respectively.
Method Accuracy
The concentration of gold and palladium ions that obtained by POPAM-grafted MWCNTs was compared with the certified reference materials. For this reason, the concentration of these metals ions was determined at optimum conditions in certified reference materials. As it can be seen in Table 3, good correlation was obtained between estimated content by the present method and reference materials. In order to digest the certified reference materials, 50 mg from each of them was digested with 6 mL of HCl (37 %) and 2 mL of HNO3 (65 %) in a microwave digestion system. The digestion was carried out for 2 min at 250 W, 2 min at 0 W, 6 min at 250 W, 5 min at 400 W, 8 min at 550 W and then venting for 8 min. The resulted solution from digestion was then diluted to 250 mL with deionized water before adjusting the pH to 3.0.
Therefore, POPAM-grafted MWCNTs can be used as a reliable solid-phase for extraction and determination of gold and palladium ions in water and soil samples.
Determination of Gold and Palladium in Fish, Shrimp, Water, and Soil Samples
Since natural samples have complex matrices, non-specific background absorption was caused by interfering species from the sample matrix. To reduce this undesirable effect, POPAM-grafted MWCNTs were applied for selective extraction of gold and palladium ions in pH 3.0. Table 4 shows the gold and palladium ions recovery in fish, shrimp, water, and soil samples.
Conclusion
The proposed SPE method is simple, fast, and accurate for the flame atomic absorption spectrometric determination of traces of gold and palladium in water and soil samples. This SPE technique was successfully applied for separation, determination, and preconcentration of gold and palladium in environmental samples. Furthermore, external ions have no significant effects on preconcentration of gold and palladium ions at pH 3. In comparison with other solid phases, POPAM-grafted MWCNTs have the advantages of high adsorption capacity and low limit of detection (Table 5). In the optimum condition, the sorbent was stable for six times. Due to a relatively high enrichment factor (almost 360), trace amounts of heavy metals at nanogram-per-milliliter levels in high volume samples can be determined and separated by POPAM-grafted MWCNTs.
Change history
20 April 2024
This article has been retracted. Please see the Retraction Notice for more detail: https://doi.org/10.1007/s12161-024-02627-4
References
Afzali D, Mostafavi A (2008) Potential of modified multiwalled carbon nanotubes with 1-(2-pyridylazo)-2-naphtol as a new solid sorbent for the preconcentration of trace amounts of cobalt(II) ion. Anal Sci 24:1135–1139
Antonia Herrero M, Toma FM, Al-Jamal KT, Kostarelos K, Bianco A, Da Ros T, Bano F, Casalis L, Scoles G, Prato M (2009) Synthesis and characterization of a carbon nanotube–dendron series for efficient siRNA delivery. J Am Chem Soc 131:9843–9848
Bagheri M, Mashhadizadeh MH, Razee S (2003) Solid phase extraction of gold by sorption on octadecyl silica membrane disks modified with pentathia-15-crown-5 and determination by AAS. Talanta 60:839–845
Bagheri A, Behbahani M, Amini MM, Sadeghi O, Taghizade M, Baghayi L, Salarian M (2012a) Simultaneous separation and determination of trace amounts of Cd(II) and Cu(II) in environmental samples using novel diphenylcarbazide modified nanoporous silica. Talanta 89:455–461
Bagheri A, Behbahani M, Amini MM, Sadeghi O, Tootoonchi A, Dahaghin Z (2012b) Preconcentration and separation of ultra-trace palladium ion using pyridine-functionalized magnetic nanoparticles. Microchim acta 178:261–268
Bagheri A, Taghizadeh M, Behbahani M, Asgharinezhad AA, Salarian M, Dehghani A, Ebrahimzadeh H, Amini MM (2012c) Synthesis and characterization of magnetic metal-organic framework (MOF) as a novel sorbent, and its optimization by experimental design methodology for determination of palladium in environmental samples. Talanta 99:132–139
Barbosa AF, Segatelli MG, Pereira AC (2007) Solid-phase extraction system for Pb (II) ions enrichment based on multiwall carbon nanotubes coupled on-line to flame atomic absorption spectrometry. Talanta 71:1512–1519
Barefoot RR, Van Loon JC (1999) Recent advances in the determination of the platinum group elements and gold. Talanta 49:1–14
Behbahani M, Taghizadeh M, Bagheri A, Hosseini H, Salarian M, Tootoonchi A (2012) A nanostructured ion-imprinted polymer for the selective extraction and preconcentration of ultra-trace quantities of nickel ions. Microchim Acta 178:429–437
Behbahani M, Bagheri A, Amini MM, Sadeghi O, Salarian M, Najafi F, Taghizadeh M (2013) Application of multiwalled carbon nanotubes modified by diphenylcarbazide for selective solid phase extraction of ultra traces Cd(II) in water samples and food products. Food Chem 141:48–53
Campidelli S, Sooambar C, Lozano-Diz E, Ehli C, Guldi DM, Prato M (2006) Dendrimer-functionalized single-wall carbon nanotubes: synthesis, characterization, and photo induced electron transfer. J Am Chem Soc 128:12544–12552
Chang YC, Chen DH (2006) Recovery of gold (III) ions by a chitosan-coated magnetic nano-adsorbent. Gold Bull 39:98–102
Dakshinamoorthy A, Dhami PS, Naik PW, Dudwadkar NL, Munshi SK, Dey PK, Venugopal V (2008) Separation of palladium from high level liquid waste of PUREX origin by solvent extraction and precipitation methods using oximes. Desalination 232:26–36
Domı́nguez M, Anticó E, Beyer L, Aguirre A, Garcı́a-Granda S, Salvadó V (2002) Liquid–liquid extraction of palladium(II) and gold(III) with N-benzoyl-N′,N′-diethylthiourea and the synthesis of a palladium benzoylthiourea complex. Polyhedron 21:1429–1435
Ebrahimzadeh H, Tavassoli N, Sadeghi O, Amini MM, Jamali M (2011) Comparison of novel pyridine-functionalized mesoporous silicas for Au(III) extraction from natural samples. Microchim Acta 172:479–487
Ebrahimzadeh H, Moazzen E, Amini MM, Sadeghi O (2013) Novel ion imprinted polymer coated multiwalled carbon nanotubes as a high selective sorbent for determination of gold ions in environmental samples. Chem Eng J 215:315–321
Elci L, Sahan D, Basaran A, Soylak M (2007) Solid phase extraction of gold(III) on Amberlite XAD-2000 prior to its flame atomic absorption spectrometric determination. Environ Monit Assess 132:331–338
Farhadi K, Teimouri G (2005) Flame atomic absorption determination of palladium in solutions after preconcentration using octadecyl silica membrane disks modified by thioridazine HCl. Talanta 65:925–929
Holzinger M, Abraham J, Whelan P, Graupner R, Ley L, Hennrich F, Kappes M, Hirsch A (2003) Functionalization of single-walled carbon nanotubes with (R-)oxycarbonyl nitrenes. J Am Chem Soc 125:8566–8580
Ishikawa S, Suyama K, Arihara K, Itoh M (2002) Uptake and recovery of gold ions from electroplating wastes using eggshell membrane. Bioresour Technol 81:201–207
Komarek J, Houserova P (2003) Determination of gold by electrothermal atomic absorption spectrometry after electrodeposition on a graphite tube. Spectrochim Acta: Part B 58:1525–1530
Kondos PD, Deschenes G, Morrison RM (1995) Process optimization studies in gold cyanidation. Hydrometallurgy 39:235–250
Matsubara I, Takeda Y, Ishida K (2000) Improved recovery of trace amounts of gold (III), palladium (II) and platinum (IV) from large amounts of associated base metals using anion-exchange resins. Fresenius J Anal Chem 366:213–218
Mauchauffée S, Meux E (2007) Use of sodium decanoate for selective precipitation of metals contained in industrial wastewater. Chemosphere 69:763–768
Ruhela R, Singh KK, Tomar BS, Sharma JN, Kumar M, Hubli RC, Suri AK (2012) Amberlite XAD-16 functionalized with 2-acetyl pyridine group for the solid phase extraction and recovery of palladium from high level waste solution. Sep Purif Technol 99:36–43
Saçmacı Ş, Kartal Ş (2013) Determination of palladium by on-line flow-injection direct spectrophotometry in environmental samples using 2,2′-furyldioxime as a chelator. Talanta 109:26–30
Salih B, Çelikbıçak Ö, Döker S, Doğan M (2007) Matrix elimination method for the determination of precious metals in ores using electrothermal atomic absorption spectrometry. Anal Chim Acta 587:272–279
Sampaio RMM, Timmers RA, Xu Y, Keesman KJ, Lens PNL (2009) Selective precipitation of Cu from Zn in a pS controlled continuously stirred tank reactor. J Hazard Mater 165:256–265
Sun YP, Huang W, Lin Y, Fu K, Kitaygorodskiy A, Riddle LA, Joy Yu Y, Carroll DL (2001) Soluble dendron-functionalized carbon nanotubes: preparation, characterization, and properties. Chem Mater 13:2864–2869
Tavakoli L, Yamini Y, Ebrahimzadeh H, Nezhadali A, Shariati S, Nourmohammadian F (2008) Development of cloud point extraction for simultaneous extraction and determination of gold and palladium using ICP-OES. J Hazard Mater 152:737–743
Viñals J, Juan E, Ruiz M, Ferrando E, Cruells M, Roca A, Casado J (2006) Leaching of gold and palladium with aqueous ozone in dilute chloride media. Hydrometallurgy 81:142–151
Wells M, Crooks RM (1996) Interactions between organized, surface- confined monolayers and vapor-phase probe molecules. 10. Preparation and properties of chemically sensitive dendrimer surfaces. J Am Chem Soc 118:3988–3989
Zhao M, Tokuhisa H, Crooks RM (1997) Molecule-sized gates based on surface-confined dendrimers. Angew Chem 36:2596–2598
Compliance with ethics Requirements
Conflict of Interest
Mohammad Behbahani declares that he has no conflict of interest. Tayebeh Gorji declares that he has no conflict of interest. Mojtaba Mahyari declares that he has no conflict of interest. Mani Salarian declares that he has no conflict of interest. Akbar Bagheri declares that he has no conflict of interest. Ahmad Shaabani declares that he has no conflict of interest. This article does not contain any studies with human or animal subjects.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Behbahani, M., Gorji, T., Mahyari, M. et al. RETRACTED ARTICLE: Application of Polypropylene Amine Dendrimers (POPAM)-Grafted MWCNTs Hybrid Materials as a New Sorbent for Solid-Phase Extraction and Trace Determination of Gold(III) and Palladium(II) in Food and Environmental Samples. Food Anal. Methods 7, 957–966 (2014). https://doi.org/10.1007/s12161-013-9698-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12161-013-9698-1