Abstract
To develop effective alternatives for detecting genetically modified organisms, we constructed one loop-mediated isothermal amplification (LAMP) event-specific detection method for wheat B73-6-1 in this study. This event-specific LAMP assay can be performed within 38 min without any PCR equipment and the LAMP products can be directly observed by the naked eye instead of conventional gel electrophoresis analysis. The limits of detection of these established visual LAMP assays were about six copies of haploid wheat-genomic DNA. Furthermore, the high specificity of LAMP assays was determined. High specificity and sensitivity, cost-effectiveness, and low requirement of equipment of the developed event-specific LAMP assay of B73-6-1 allow this method to be useful in transgenic wheat samples analysis, especially in highly processed products.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
Biotech crop hectares increased by an unprecedented 100-fold from 1.7 million hectares in 1996, to over 170 million hectares in 2012 (James 2012). In recent years, special concerns have been raised about the safety assessment of foods and food ingredients derived from genetically modified organisms (GMOs). To strengthen the regulation of GMOs, more than 50 countries and areas have published a series of laws and rules for GMO regulation and labeling. Several countries have implemented mandatory labeling for foods derived from transgenic plants, for example, threshold levels for unintended mixing of GMOs in non-GMOs has been defined as 0.9 % in European Union, 3 % in Korea, 5 % in Japan, and 0 % in China. Since labeling of foods containing GMO ingredients is mandatory in many countries, the demand to develop easy and reliable detection methods for GMOs is very high. Consequently, the development of reliable methods of GMO detection, identification, tracing, and quantification has become increasingly important.
Now, lots of conventional and quantitative real-time PCR methods for GMOs have been developed and validated. Loop-mediated isothermal amplification (LAMP) is a novel nucleic acid amplification assay capable of providing results faster than PCR-based assays (Wang et al. 2012; Xu et al. 2010; Njiru 2012). LAMP generates a large amount of DNA with high specificity, allowing amplification to be detected by the naked eye as fluorescence or turbidity (Mori et al. 2013). LAMP has been used to detect several GMOs, including MS8 and RF3 oilseed rape (Lee et al. 2009), Roundup Ready soybeans (Fukuta et al. 2004; Liu et al. 2009), GTS 40-3-2 (Lan et al. 2008), and MON89788 (Fukuta et al. 2004), and has been proposed as a useful tool for the rapid and accurate diagnosis of pathogen (bacteria, virus, and parasite, etc.) in resource-limited laboratories (Fu et al. 2011).
Wheat is the most important crop in the world in terms of the area under cultivation, yield, and geographical distribution. Also, wheat is unique among cereals in that it can be used to make leavened bread. This results from the unusual biomechanical properties of the gluten proteins, which form a network in dough and confer elasticity and extensibility (Barro et al. 1997). So, wheat is widely used in food processing (e.g., for bread, pasta, noodles) and as feed for livestock (Alvarez et al. 2000). Wheat flour is different from other cereal flours, including maize, because it contains gluten that gives it the elasticity and extensity required for bread making (Barro et al. 1997). The elasticity of wheat dough depends mainly on the high molecular weight (HMW) glutenin, so they are important determinants of bread-making quality (Shewry et al. 2003). The transgenic wheat, B73-6-1, overexpresses an increased amount of a HMW glutenin subunit, which endues wheat B73-6-1 more elasticity than the non transgenic wheat. Nowadays, the transgenic wheat line B73-6-1 has been confirmed by large-scale testing of grain grown in field trials (Darlington et al. 2003), and has passed the intermediate testing phase followed by the environmental release phase. So, it is representative of the type of transgenic wheat which may grow commercially in the future (Zhang et al. 2012).
Currently, only two event-specific PCR (Zhang et al. 2012; Xu et al. 2013), one screening qualitative PCR (Luan et al. 2007), and one screening quantitative PCR (Bai et al. 2009) were established, and no detection method was developed for GM wheat B73-6-1. In this study, we developed a LAMP assay based on the 3′ integration flanking sequence of pHMW1Dx5 vector of B73-6-1 revealed by genome walking to rapidly identify B73-6-1 wheat (Zhang et al. 2012). This method is of high specificity, high sensitivity, and rapid identification for detection of GM wheat B73-6-1.
Materials and Methods
Plant Materials
GMO wheat B73-6-1, B72-8-11, B102-1-2, GTS 40-3-2, maize 3272, canola RT73, rice Bt63, and non-GMO wheat 523b, Nib8, 3-74-38, Jinghua 1, Jingdong 1, tomato, potato, cotton, and canola were supplied by Heilongjiang Entry–Exit Inspection.
Primer Design
The B73-6-1 wheat event-specific sequences were selected as the target. All LAMP primers were designed by the online software http://primerexplorer.jp/e/ (Eiken Chemical Co., Ltd., Tokyo, Japan). Three primer sets were designed to select one set for the LAMP assay, including two outer primers (F3 and B3), a forward inner primer (FIP; F1c + F2) and a backward inner primer (BIP; B1c + B2) in one primer set. As “c” represents a complementary sequence, the F1c or B1c sequence is complementary to the F1 or B1 sequence, respectively. The location and detailed sequences of primer set 2 is shown in Table 1, and primer set 1 and primer set 3 are not shown.
DNA Extraction and Purification
DNAs from all plant materials were extracted from B73-6-1 seeds using Plant Genomic DNA Kit (Tiangen Biotech (Beijing) Co., Ltd). And the concentration of the sample has been accurately determined as 100 ng/μL by dilutions of template with ddH2O.
LAMP Assay
The LAMP reaction was performed in a total volume of 25-μL mixture, containing 1× ThermoPol buffer, 0.2-μM HMW-GS F3 and B3, 1.6-μM HMW-GS FIP and BIP, 1.6-mM dNTPs, 1.0-M betaine, 2.4-mM MgSO4, 16 U/μL Bst DNA polymerase, and 1 μL (100 ng) template DNA. The mixture was incubated at 63 °C for 70 min and then heated at 80 °C for 5 min to terminate the reaction. Template DNA was replaced by ddH2O as negative control. A portion of each product was analyzed by 2 % agarose gel electrophoresis stained with ethidium bromide. In addition, the LAMP-amplified products were directly observed by the naked eye through adding 2 μL 1,000 × SYBR Green I (Sigma) into the reaction mixture. The amplification product can also be visually inspected for the color change from orange to green, and the no template controls (NTC) reaction will remain orange. The tubes were observed by naked eyes and photographed under the natural light by obtaining fluorescence spectra (Realtime Turbidimeter LA-320c).
Results and Discussion
Design of the LAMP Primers
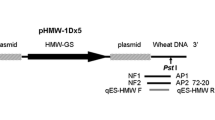
Notomi et al. developed the novel method that can amplify a few copies of DNA to 109 in less than an hour under isothermal conditions and with greater specificity. They described the mechanism, sensitivity, and specificity of this amplification method, termed LAMP (Notomi et al. 2000). One ideal LAMP assay relies on the concurrence of the Bst DNA polymerase with strand displacement activity and a set of four specially designed primers capable of recognizing a total of six distinct regions on the target DNA (Bi et al. 2012; Parida et al. 2008; Mori and Notomi 2009). The LAMP reaction requires a set of four primers, which are two inner primers (FIP and BIP) and two outer primers (F3 and B3) (Fig. 1a). FIP and BIP recognize two regions on the template DNA (Nagamine et al. 2002). LAMP primers of B73-6-1 event were designed based on the event-specific sequence of 3′ flanking sequence of foreign plasmid pHMW1Dx5 in B73-6-1 revealed by genome walking (Zhang et al. 2012). The primers HMW-F3, HMW-B3, HMW-FIP, and HMW-BIP recognizing a 217-bp target sequence (Fig. 1b). The nucleotide sequences of these primers are shown in Fig. 1b.
Evaluation of the Reaction Efficiency of LAMP Assays
The LAMP products are a mixture of stem-loop DNAs of various sizes and cauliflower-like structures with multiple loops induced by annealing between alternately inverted repeats of the target sequence in the same strand (Njiru et al. 2008). After amplification, LAMP products were electrophoresed in an agarose gel, and positive reactions yielded typical ladder-like bands (Fig. 2b). All positive samples detected by gel electrophoresis or in real time using fluorescence spectra monitor could also be detected visually by addition of SYBR Green I to the product. This ability highlights another advantage of LAMP technique: the results of amplification can visually be observed through the addition of a DNA intercalating dye (Fig. 2c), eliminating the need for gel electrophoresis and greatly reducing the time taken for result analysis.
This phenomenon allows easy and rapid visual identification that the target DNA was amplified by LAMP. Therefore, LAMP is a highly sensitive and specific DNA amplification technique suitable for detection of GMO (Guan et al. 2010; Fukuta et al. 2004; Lan et al. 2008; Liu et al. 2009) or diagnosis of an infectious disease (Iwamoto et al. 2003; Kuboki et al. 2003; Mori et al. 2001) both in well-equipped laboratories and in field situations.
In order to test the reaction efficiency of the developed LAMP assays, the different primer sets (sets 1, 2, and 3) were designed in B73-6-1 LAMP assay. The LAMP was conducted based on the change of turbidity (Parida et al. 2007). As shown in Fig. 2a, the event-specific LAMP products could be observed at 38-min amplification with primer set 2, whereas the LAMP products were observed only after 60 min amplification with primer sets 1 and 3.
Also, the results were obtained in agarose gel electrophoresis analysis (Fig. 2b) and visual observation using SYBR Green I (Fig. 2c). The primer sets’ screening results indicated that the established LAMP assay with primer set 2 has higher reaction efficiency than the assays with primer sets 1 and 3, suggesting that the primer set 2 (hereinafter referred to as “primer set”) is sufficient for rapid amplification of target DNAs.
Optimization of the LAMP Assays
The most significant advantage of LAMP is the ability to amplify specific sequences of DNA under isothermal conditions between 60 and 65 °C. This ability allows the method to be performed with only simple and cost-effective reaction equipment amenable to use in common laboratories (Han et al. 2007; Yoshikawa et al. 2004; Njiru et al. 2008). In the present study, we found that the amplified efficiency of LAMP assays using primer sets 1 and 3 were obviously lower than that using primer set 2 (Fig. 2a). The optimal reaction temperature of 63 °C was determined by comparing the LAMP-amplified efficiencies under various temperatures ranging from 60 to 65 °C (data not shown). In addition, different primer concentrations and the ratios between inner primers (HMW-FIP and HMW-BIP) and outer primers (HMW–F3 and HMW–B3) were also optimized. The LAMP assays for B73-6-1 events were established based on the optimized reaction conditions described in “Materials and Methods” section. In our experiment, SYBR Green I was added, which made it easy to judge the result by monitoring the amplification curve.
The LAMP reaction enabled detection within 1 h, and it needed only a conventional water bath or heating block, which was faster and less expensive than conventional PCR assays. Nagamine et al. (2002) accelerated the LAMP reaction through application of two loop primers to the reaction mixtures. This LAMP could be performed at 38 min from the beginning, and the derived LAMP products could be directly observed by naked eye employing SYBR Green I dye without any equipment instead of conventional gel electrophoresis analysis.
The accumulation of reaction product was completed in 5 to 10 min, and the amplicon was stable in the reaction system with additional incubation time. So, the analysis methods of LAMP results were flexible and more amendable to use in the field or in an underequipped laboratory.
Specificity of LAMP Assay
To evaluate the specificity of the developed LAMP assays, several GMO (wheat 73-6-1, wheat B72-11-8, wheat B102-1-2, GTS 40-3-2, GM 3272 maize, rice Bt63, and canola GT73) and non-GMO (wheat 523b, wheat Nib8, wheat 3-74-38, wheat Jinghua 1, wheat Jingdong 1, tomato, potato, cotton, canola) were used. In the specificity test, 100-ng total corresponding plant genomic DNA was used as the template in each LAMP assay. As expected, in the LAMP assay, the typical ladder-like pattern products and green color of the reactions were only obtained in the tests using wheat B73-6-1 genomic DNA samples as templates, no amplified products and orange color were observed in other GM and non-GM crops as well as the NTC (Fig. 3a). In addition, we found that all the results obtained from gel electrophoresis and visual observation by addition of SYBR Green I was consistent in the three LAMP assays (Fig. 3b). These data confirmed that the developed LAMP assays had high specificity for amplifying the target DNAs.
LAMP specificity for different crops. a Agarose gel electrophoresis detection. Lane 1 wheat B72-8-11; lane 2 wheat B102-1-2; lane 3 wheat 523b; lane 4 wheat Nib8; lane 5 wheat 3-74-38; lane 6 wheat Jinghua 1; lane 7 wheat Jingdong 1; lane 8 GTS 40-3-2; lane 9 maize 3272; lane 10 canola RT73; lane 11, rice Bt63; lane 12 tomato; lane 13 potato; lane 14 cotton; lane 15 canola seed; lane 16 50-bp DNA ladder marker; lane 17 wheat B73-6-1; lane 18 NTC. b Visual observation of LAMP reaction. 1 wheat B73-6-1; 2 wheat B72-8-11; 3 wheat B102-1-2; 4 wheat 523b; 5 wheat Nib8; 6 wheat 3-74-38; 7 wheat Jinghua 1; 8 wheat Jingdong 1; 9 GTS 40-3-2; 10 maize 3272; 11 canola RT73; 12 rice Bt63; 13 tomato; 14 potato; 15 cotton; 16 canola seed; and 17 NTC
Limit of Detection of LAMP Assay
For GMOs detection, high sensitivity is important and necessary because the degradation of low-quantity DNA derived from GMOs often occurs in practical detection. Lee et al. assessed the LAMP protocol for the detection of transgenic MS8 and RF3 oilseed. And the results show that detection of 0.01 % GMO in equivalent background DNA was possible and dilutions of template suggested that detection from single copies of the template could be possible using LAMP (Lee et al. 2009). The limit of detection (LOD) of visual LAMP assays established for transgenic soybeans (GTS 40-3-2 and MON89788) were about four copies of haploid soybean genomic DNA and much higher than those of reported conventional PCR assays (Guan et al. 2010).
To test the LOD of B73-6-1 LAMP assay, non-GM wheat-genomic DNA was serially diluted with ddH2O to final concentrations of 5.0, 1.0, 0.5, 0.1, and 0.05 % (w/w). One microliter (100 ng) diluted DNA sample was used as template in each reaction, and the corresponding copy number of the transformation event B73-6-1 were 300, 60, 30, 6, and 3 copies in each reaction, respectively. As shown in Fig. 4, the amplified LAMP products and green color were observed in each dilution by three replicates except for the levels of 0.05 %. Thus, the LOD of LAMP assay was as low as 0.1 % (0.1 ng), corresponding to about six copies.
LOD of LAMP assay by agarose gel electrophoresis analysis and visual observation using SYBR Green I. a. Agarose gel electrophoresis detection. Lane 1 NTC; lanes 2–4 three replicates of GM of 5.0 % (300 copies) content; lanes 5–7, three replicates of GM of 1.0 % (60 copies) content; lanes 8–10, three replicates of GM of 0.5 % (30 copies) content; lanes 11–13 three replicates of GM of 0.1 % (6 copies) content; lanes 14–16 three replicates of GM of 0.05 % (3 copies) content; lane 17 50-bp DNA ladder marker. b Visual observation of LAMP reaction. 1 NTC; 2–4 three replicates of GM of 5.0 % (300 copies) content; lanes 5–7 three replicates of GM of 1.0 % (60 copies) content; lanes 8–10 three replicates of GM of 0.5 % (30 copies) content; lanes 11–13 three replicates of GM of 0.1 % (6 copies) content; lanes 14–16 three replicates of GM of 0.05 % (3 copies) content
Repeatability of LAMP Assay
Repeatability and reproducibility of B73-6-1 event-specific LAMP assay were determined using the B73-6-1 DNA with different contents of 5.0 % (300 copies), 1.0 % (60 copies), 0.5 % (30 copies), 0.1 % (6 copies), and 0.05 % (3 copies) by three parallels replications (Fig. 4), and the results of the repeatability and reproducibility tests indicated that the B73-6-1 event-specific LAMP assay was reliable in B73-6-1 wheat detection.
Testing of Blind Samples by LAMP Assay
Mixed samples were prepared to evaluate the accuracy and precision of the established real-time PCR methods in this study. GM B73-6-1 wheat DNA samples BS1, BS2, and BS3 with 75.0, 50.0, and 5.0 % (w/w) purity, were artificially prepared by mixing the pure B73-6-1 DNA with non-GM wheat genomic DNA on a genome/genome basis and were used for detection in developed B73-6-1 event-specific LAMP assay. All the blind samples could be detected as positive by B73-6-1 event-specific LAMP assay, which indicates that the developed B73-6-1 LAMP assay is creditable and suitable for the detection of wheat B73-6-1 and its derivates (Fig. 5).
Conclusion
In this study, we developed the visual and rapid detection methods for wheat B73-6-1 using the optimized LAMP method. LAMP is one isothermal nucleic acids amplification technique. This LAMP assay can be performed at 63 °C without PCR thermal cyclers within shorter time (38 min in this study). In combination with SYBR Green I dye, the amplified products can be directly detected by the naked eye instead of the conventional gel electrophoresis analysis or fluorescent detection. In addition, the LOD of developed wheat B73-6-1 LAMP assay with the value of six copies are remarkably sensitive. All the results demonstrated that the developed visual LAMP assay is convenient and rapid for GMOs detection. Furthermore, high specificity and sensitivity, cost-effectiveness, and low requirement of equipment of the developed visual LAMP assay allow this method to be useful in transgenic wheat samples analysis, especially in highly processed products.
References
Alvarez M, Guelman S, Halford N, Lustig S, Reggiardo M, Ryabushkina N, Shewry P, Stein J, Vallejos R (2000) Silencing of HMW glutenins in transgenic wheat expressing extra HMW subunits. Theor Appl Genet 100(2):319–327
Bai Y, Luan F, Wang X (2009) Detection limits of PCR and real time PCR for genetically modified components in transgenic wheat. J Triticeae Crop 29(2):199–205, Article in Chinese
Barro F, Rooke L, Bekes F, Gras P, Tatham A, Fido R (1997) Transformation of wheat with high-molecular-weight subunit genes results in improved functional properties. Nat Biotechnol 15:1295–1299
Bi A, Nakajima C, Fukushima Y, Tamaru A, Sugawara I, Kimura A, Kawahara R, Hu Z, Suzuki Y (2012) A rapid loop-mediated isothermal amplification assay targeting hspX for the detection of Mycobacterium tuberculosis complex. Jpn J Infect Dis 65:247–251
Darlington H, Fido R, Tatham A, Jones H, Salmon S, Shewry P (2003) Milling and baking properties of field grown wheat expressing HMW subunit transgenes. J Cereal Sci 38(3):301–306
Fu S, Qu G, Guo S, Ma L, Zhang N, Zhang S, Gao S, Shen Z (2011) Applications of loop-mediated isothermal DNA amplification. Appl Biochem Biotechnol 163:845–850
Fukuta S, Mizukami Y, Ishida A, Ueda J, Hasegawa M, Hayashi I, Hashimoto M, Kanbe M (2004) Real-time loop-mediated isothermal amplification for the CaMV-35S promoter as a screening method for genetically modified organisms. Eur Food Res Technol 218(5):496–500
Guan X, Guo J, Shen P, Yang L, Zhang D (2010) Visual and rapid detection of two genetically modified soybean events using loop-mediated isothermal amplification method. Food Anal Methods 3:313–320
Han E, Watanabe R, Sattabongkot J, Khuntirat B, Sirichaisinthop J, Iriko H, Jin L, Takeo S, Tsuboi T (2007) Detection of four plasmodium species by genus- and species-specific loop-mediated isothermal amplification for clinical diagnosis. J Clin Microbiol 45(8):2521–2528
Iwamoto T, Sonobe T, Iwamoto T, Sonobe T, Hayashi K (2003) Loop-mediated isothermal amplification for direct detection of Mycobacterium tuberculosis complex, M. avium, and M. intracellul are in sputum samples loop-mediated isothermal amplification for direct Detection of Mycobacterium tuberculosis complex. M J Clin Microbiol 41(6):2616–2622
James C (2012) Global status of commercialized biotech/GM crops: 2012. ISAAA brief no. 44
Kuboki N, Inoue N, Sakurai T, Cello F, Grab D, Suzuki H, Sugimoto C, Igarashi I (2003) Loop-mediated isothermal amplification for detection of African Trypanosomes. J Clin Microbiol 41(12):5517–5524
Lan Q, Wang Y, Zhao X, Zhu Z, Cheng Y (2008) Application of LAMP in cp4-epsps gene detection of roundup ready soybean. J Anhui Agri Sci 36(24):10377–10378, 10390 (Article in Chinese)
Lee D, Mura M, Allnutt T, Powell W (2009) Detection of genetically modified organisms (GMOs) using isothermal amplification of target DNA sequences. BMC Biotechnol 9(7):1–7
Liu C, Liang C, Xu B, Gao H, Lin C, Sun M (2009) Detection of genetically modified soybean and products by LAMP reaction. Soybean Sci 28(2):305–309, Article in Chinese
Luan F, Zhang H, Bai Y (2007) Screening and detecting of genetically modified components in transgenic wheat by PCR. J Triticeae Crops 27:378–381, Article in Chinese
Mori Y, Notomi T (2009) Loop-mediated isothermal amplification (LAMP): a rapid, accurate, and cost-effective diagnostic method for infectious diseases. J Infect Chemother 15:62–69
Mori Y, Nagamine K, Tomita N, Notomi T (2001) Detection of Loop-mediated isothermal amplification reaction by turbidity derived from magnesium pyrophosphate formation. Biochem Bioph Res Co 289(1):150–154
Mori Y, Kanda H, Notomi T (2013) Loop-mediated isothermal amplification (LAMP): recent progress in research and development. J Infect Chemother. doi:10.1007/s10156-013-0590-0
Nagamine K, Hase T, Notomi T (2002) Accelerated reaction by loop-mediated isothermal amplification using loop primers. Mol Cell Probes 16(3):223–229
Njiru Z (2012) Loop-mediated isothermal amplification technology: towards point of care diagnostics. PLoS Negl Trop Dis 6(6):e1572. doi:10.1371/journal.pntd.0001572
Njiru Z, Stanislaw A, Mikosza J, Armstrong T, Enyaru J, Mathu J, Thompson A (2008) Loop-mediated isothermal amplification (LAMP) method for rapid detection of Trypanosoma brucei rhodesiense. PLoS Negl Trop Dis 2(2):e147
Notomi T, Okayama H, Masubuchi H, Yonekawa T, Watanabe K, Amino N, Hase T (2000) Loop-mediated isothermal amplification of DNA. Nucleic Acids Res 28(12):e63
Parida M, Santhosh S, Dash P, Tripathi N, Lakshmi V, Mamidi N, Shrivastva A, Gupta N, Saxena P, Pradeep Babu J, Lakshmana Rao L, Morita K (2007) Rapid and real-time detection of Chikungunya virus by reverse transcription loop-mediated isothermal amplification assay rapid and real-time detection of Chikungunya virus by reverse transcription loop-mediated isothermal amplification assay. J Clin Microbiol 45(2):351–357
Parida M, Sannarangaiah S, Dash P, Rao P, Morita K (2008) Loop mediated isothermal amplification (LAMP): a new generation of innovative gene amplification technique; perspectives in clinical diagnosis of infectious diseases. Rev Med Virol 18(6):407–421
Shewry P, Halford N, Tatham A, Popineau Y, Lafiandra D, Belton P (2003) The high molecular weight subunits of wheat glutenin and their role in determining wheat processing properties. Adv Food Nutr Res 45:221–302
Wang X, Zhang Q, Zhang F, Ma F, Zheng W, Zhao Z, Bai Y, Zheng L (2012) Visual detection of the human metapneumovirus using reverse transcription loop-mediated isothermal amplification with hydroxynaphthol blue dye. Virol J 9:138–143
Xu J, Rong R, Zhang H, Shi C, Zhu X, Xia C (2010) Sensitive and rapid detection of Schistosoma japonicum DNA by loop-mediated isothermal amplification (LAMP). Int J Parasitol 40(3):327–331
Xu J, Cao J, Cao D, Zhao T, Huang X, Zhang P, Luan F (2013) Flanking sequence determination and event-specific detection of genetically modified wheat B73-6-1. Acta Bioch Bioph Sin 45(5):416–421
Yoshikawa T, Ihira M, Akimoto S, Miyake F, Suga S, Enomoto Y, Suzuki R, Nishiyama Y, Asano Y (2004) Detection of human Herpesvirus 7 DNA by loop-mediated isothermal amplification. J Clin Microbiol 42(3):1348–1352
Zhang M, Huo N, Liu Y, Qiu Y, Ao J, Luan F, Li Q, Gao J (2012) Event-specific detection of genetically modified wheat B73-6-1 based on the 3′ -flanking sequence. Eur Food Res Technol 235(6):1149–1159
Acknowledgments
This work was supported by General Administration of Quality Supervision, Inspection and Quarantine of P. R. C. (2011B286k), the National Natural Science Foundation of China (J1210069/J0106) and Heilongjiang Bureau Program of China (2013HK001).
Conflict of Interest
Yang Cheng declares that she has no conflict of interest. Minghui Zhang declares that she has no conflict of interest. Kun Hu declares that she has no conflict of interest. Fangda Sun declares that he has no conflict of interest. Ran Tao declares that she has no conflict of interest. Xuejun Gao declares that he has no conflict of interest. Fengxia Luan declares that she has no conflict of interest. This article does not contain any studies with human or animal subjects.
Author information
Authors and Affiliations
Corresponding author
Additional information
Yang Cheng and Minghui Zhang contributed equally to this work.
Rights and permissions
About this article
Cite this article
Cheng, Y., Zhang, M., Hu, K. et al. Loop-Mediated Isothermal Amplification for the Event-Specific Detection of Wheat B73-6-1. Food Anal. Methods 7, 500–505 (2014). https://doi.org/10.1007/s12161-013-9718-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12161-013-9718-1