Abstract
With the ever-increasing environmental concerns and the rush to meet the United Nations’ sustainable development goals, it is an uphill task to find a single source of energy that may completely replace fossil fuels. Energy derived from biomass is an attractive alternative to transportation fuel along with electricity and heat generation. The bioenergy from agricultural biomass, food crops, forest residue, algae, and municipal waste can also allow sustainable waste management. However, most bioenergy conversion facilities are still in the research or pilot stage and have many technological and economical limitations. This critical review provides an insight into different recourses of biomass, bioenergy conversion routes, and other challenges to biofuel production. An attempt has been made to elucidate the novel technological advancements made in these processes like bio-chemical looping combustion, torrefaction, and photo- and dark fermentation. The integration of these systems with artificial intelligence and machine learning-based modeling and optimization is also discussed to bring insight to alternate advancement routes. A comparison of the conversion methods is attempted to bring insight into the feasibility, sustainability, and advancement of bioenergy production and its commercialization.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
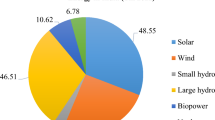
Coal, natural gas, petroleum products, and petrochemicals have been an ingrained part of human existence for over two centuries, driving the industrial revolution and all other subsequent technological innovations. However, concerns over the environment and climate changes have forced humankind to look for alternate energy resources. The Paris Energy Agreement in 2015, and the Sustainable Development Goals implemented by the United Nations General Assembly in 2015, established a framework for global cooperation in identifying and implementing a reliable, economical, and sustainable renewable energy source by 2030 [1]. With this framework, the uphill task is to identify energy sources to meet the household, industry, and transportation energy requirements of the world at an affordable price. Table 1 provides a glimpse of the total primary energy supply in 2017 globally and by the top five major energy-supplying countries as reported by the International Energy Agency (IEA) [2, 3].
It is quite interesting to note that in the current race to identify the most economical and efficient renewable energy resources, biofuels and biomass waste are one of the front-runners, with a ~ 11% contribution, along with solar, wind, and hydropower as can be seen from Table 1. Also, interestingly, India contributes around 21% of the total energy supply from biomass and waste. It is not a surprising figure as India generates 960 million tonnes of solid waste every year and 680 million people in India rely on biomass waste for traditional cooking [3, 4]. However, currently, a negligible amount of the biomass waste produced in India contributes to electricity generation or as a transportation fuel [3]. In China, currently, 3.7% of energy from biomass and waste comes from 650 million tonnes of coal equivalent of biomass waste being produced every year which contributes to 13% of electricity generation [5]. It is quite apparent that with the new energy policies and mandates in place, renewable energy is slowly picking up the pace; however, the major chunk of the total world’s energy supply (~ 81%) is still being provided by fossil fuel.
Biomass as a potential energy source has its major advantages in its global availability and ease of storage. It can also contribute toward all the commercial energy requirements like heat, electricity, and transportation fuel [6,7,8,9,10,11,12]. Nevertheless, traditional biomass has some major challenges which require immediate attention for it to become commercially viable. There is a lack of global standards, monitoring, and regulation for biofuel production with a certification of biomass origin and sources [8, 13, 14]. Due to multiple sources, there is always variability in biomass quality, composition, and properties, leading to variable product quality. The biomass (both agricultural and waste) collection, segregation, and transportation are a huge problem leading to uneven supply [8, 15, 16]. Furthermore, most of the bioenergy conversion technologies being developed are still in their nascent stage. Major technological interventions toward pre-treatment of lignocellulosic biomass, improving energy efficiency, and reducing the cost of production are required [17, 18].
Biomass to bioenergy conversion is a well-reviewed topic. Numerous reviews and journal articles have been published to elucidate the technologies and their limitations; however, most of these papers are specific to a type of conversion technologies. Also, very few review articles include more novel technologies like dark and photo-fermentation, bio-CLC, and torrefaction along with the traditional methods and analyze their feasibilities and challenges. Furthermore, an attempt has been made to understand the applicability of AI/ML-based modeling, simulation, and optimization to these processes to improve process design, productivity predictions, and biomass supply chain. The paper also strives to understand the feasibility of these processes based on economy, ease of operation, and scale-up and provide possible solutions to some of the existing challenges. This review is an attempt to bring the entire biomass to bioenergy conversion technologies, their prospects, challenges, and feasibility from feed to product in one framework.
Biomass as Feedstock and Classification of Biofuels
Biomass as a feedstock is as varied as its source. Biomass derived from agriculture or plant residues is rich in cellulose, hemicellulose, and lignin with varying percentages, whereas animal residues are mostly comprised of proteins, and cereals are composed of starch [19]. The biomass source from plants contains primary metabolites and secondary metabolites. The primary metabolites are lignin and carbohydrates (cellulose, hemicellulose, starch, etc.), which form the base of biofuels and the secondary metabolites are gums, resins, rubber, terpenoids, steroids, triglycerides, etc. that can be used to produce value-added chemicals like food flavors and pharmaceuticals [8, 20,21,22,23]. The physicochemical properties of the biomass like cellulose/lignin ratio, ash content, moisture content, calorific value, fixed carbon to volatile matter ratio, alkali metal content, and bulk density play key roles in identifying the biomass feedstock to be used for a certain form of bioenergy [17, 19, 24,25,26]. Each of these properties provides information about the quality of the fuel produced. If ash content in biomass is high, it means that the proportionate fuel produced will be low. A high calorific value indicates high heat release from fuel burning [17, 24]. High cellulose to lignin content indicates reduced pretreatment requirement for lignin removal and ease of conversion processes [24, 27]. Low carbon to volatile matter ratio means more ease of burning and high alkali metal content leads to processing problems [17, 24]. These properties also decide which technology will be employed for the conversion of biomass to fuel. For example, for high-moisture content, biomass like sugarcane is a better fit for aqueous conversion like fermentation into bioethanol, whereas dry biomass like wood is better suited for gasification or thermal conversion into bio-methanol. Similarly, if the cellulose to lignin ratio in biomass is low, then they are less suited for biochemical processes as the biodegradability of lignin is low compared to cellulose [17, 24]. Table 2 describes the chemical compositions of distinct groups of biomass feedstocks.
To better understand the source, composition, and application of the biomass, it can be classified into distinct groups based on (a) source of the feedstock, (b) vegetation type and (c) use and application. However, Tursi in his paper accepted that “there is no definite way of categorizing the biomass so they can be classified differently depending on the purpose and scope” [19]. The details of each of the below classifications are shown in Fig. 1a, b, and c.
Woody biomass is currently the most used source of energy (~ 30 EJ) as traditional wood burning for cooking and space heating is prevalent [19, 28, 30]. This mode of energy extraction is also leading to major environmental challenges globally [3, 31]. The agricultural residues are a reliable source of energy; however, their availability is varied across regions and is not well monitored and controlled. Aquatic biomass like algae on the other hand is an ideal source of biomass for biodiesel production as their productivity is higher compared to terrestrial crops and they do not compete with food crops [19, 28, 32,33,34,35,36,37].
Biofuels from biomass can be categorized into primary and secondary biofuels. The primary biofuels are used unprocessed for cooking and heating like firewood, wood chips, and pellets [20, 38, 39]. The secondary biofuels are further classified into (i) first-generation biofuels (1G), (ii) second-generation biofuels (2G), (iii) third-generation biofuels (3G), and (iv) fourth-generation biofuels (4G), based on the type of raw materials used and the techniques employed for their production. The classification of biofuels and details can be seen in Fig. 2. The first-generation biofuels are established processes, produced from starch, and sugar-based food crops by the process of fermentation or transesterification of vegetable oils, residue oils, and fats. However, they compete for land and water with food and have high production and processing cost [20, 40]. Around 2% of the agricultural land is used for biofuel edible feedstock which can feed half the current population of the world. This competition with the source as food and biofuel is predicted to increase the market price of these feedstocks and thus the need for second-generation biomass [41,42,43]. A comparison of all four generations of secondary biofuels is given in Table 3.
The second-generation biofuels are lignocellulosic, which are derived from dry products of agricultural wastes, and industrial and forest residues [20, 29, 40, 47]. Annually approximately, 5 to 8 million tons/year of lignocellulosic biomass get generated as forest and agricultural residue. Thus, their abundant availability makes them an attractive feedstock for bioenergy production [40, 59,60,61]. The USA and European Union have proposed many projects for lignocellulosic-based biofuels as an initiative to move from a fossil fuel-based economy to a more sustainable one [62]. The lignocellulosic biomass consists of cellulose (~ 40–50%), hemicellulose (~ 25–30%), and lignin (~ 15–25%) [63]. Because of the presence of lignin, the lignocellulosic biomass requires extensive pre-treatment before it can be processed into biofuels [64,65,66,67]. Biochemical, thermochemical, and hybrid processes like pyrolysis, thermochemical liquefaction, and torrefaction are a few conversion technologies employed to convert the lignin-cellulose-based biomass to biofuels [68, 69]. These methodologies are also known to have a higher yield compared to simple fermentation but at a higher cost due to the pre-treatment required for the feedstocks [49, 66]. Moreover, second-generation biofuels also require extremely high consumption of energy for the entire conversion process [66, 70, 71].
The limitations of the 1G and 2G biofuels led to the exploration of the third-generation biofuel feedstocks like microalgae, macroalgae, and phytoplankton [20, 40, 72,73,74]. Algae as a fuel source have proven to be lucrative due to its high cultivation rate, productivity, and ability to sequester carbon dioxide faster [32]. Also, they can be cultivated in moist land or wastewater [75]. Few microalgae species like Botryococcus braunii, Dunaliella salina, and Chlorella spp. contain 70–80% lipid (dry weight basis) which can be easily converted into useable biofuels using biochemical or thermo-chemical processes [32, 73, 74]. However, the lipid produced is sometimes highly volatile which affects the stability of the oil [76,77,78]. Also, the processing of the algal biomass requires a large input of energy, for drying of the algae as well as for the oil extraction and processing which leads to negative energy gain [44, 77, 79,80,81].
The fourth-generation biofuels concentrate on genetically modifying the microalgae to better sequester carbon dioxide and produce more lipid and oil [82, 83]. These biofuels also enable the integration of the algal generation process with wastewater treatment or flue gas utilization [84]. Both the 3G and 4G biomass feedstocks provide a sustainable source for biochemicals that can be converted into high-value food products, biochemicals, and biofuels [85, 86]. However, scientists are also skeptical regarding the environmental repercussions of genetically modified algal productions [58, 84, 87]. Table 5 provides a comparison of biofuel productivity/yield from different generations of biomass. It is evident from Tables 3 and 4 that second, third-, and fourth-generation biofuels provide better possibilities of being developed into a sustainable source for bioenergy and other value-added bioproducts compared to first-generation biofuels.
Energy from Biomass — Conversion Technologies
The process of conversion of biomass to biofuel, heat, chemicals, and electricity depends vastly on the origin of the biomass feedstock. A biorefinery is a facility that integrates all these processes to produce value-added products from biomass feedstock and wastes [19, 20, 65, 86, 97,98,99,100,101]. The biorefineries can be three types, based on the type of feedstock as well as the flexibility or ease of operation [86, 102, 103].
-
1.
The first kind utilizes dry grain as feedstock to produce bioethanol, dried distiller grain, and carbon dioxide in a fixed processing capacity [86, 102, 103].
-
2.
The second type of biorefinery produces starch, high fructose syrup, ethanol, carbon dioxide, etc., using dry grain feedstock but with a much more flexible processing capacity [86, 102, 103].
-
3.
The third type of biorefinery is advanced and uses mixed feedstock [97, 102]. They are based on high-value low volume and low-value high volume output principles and produce various fuels and value-added products by using a combination of technologies [97, 98, 102]. Lignocellulosic biomass refineries, algal-based biorefineries, waste biomass-based refineries, green biorefineries, intergraded biorefineries, etc. are a few examples of this type. The pre-treatment required for the processing of these biomasses and their conversion methodologies are complex and expensive but hold immense potential for sustainable bioenergy generation and bioeconomy [86, 99, 102,103,104,105,106].
Green biorefineries use natural wet feedstocks like grass, green plants, or green crops [97, 102]. These refineries are primarily treating the first-generation biomass for bioethanol production via fermentation, digestion, or esterification processes [97, 98, 102].
The lignocellulosic biorefineries can be developed into a sustainable production route for bioproducts as well as biofuels by process integration of various technologies [86, 101, 107,108,109,110,111]. Processes like extractive distillation with ionic liquids, adsorption with molecular sieve and biobased adsorbents, nanofiltration, extractive fermentation, and vacuum membrane distillation are a few advanced technologies that hold huge potential for the future of lignocellulosic biorefineries [102, 107, 108, 110, 112]. Critical analysis of lignocellulosic refineries shows that though they provide clean energy with sustainable agricultural development, they require high capital investments with an equally high operating cost [110].
Algal biorefineries which are based on third and advanced fourth-generation biomass require lower land and have higher productivity compared to lignocellulosic biorefineries [86, 89, 113,114,115,116]. However, very few biorefineries have been established with just algal biomass as the primary feedstock and are limited to extracting primary bioproducts [80, 86, 115, 116]. Nonetheless, research shows that more suitable technological developments and process integrations (like with wastewater treatment) will allow sustainable development in algal biorefineries [34, 80, 116,117,118,119,120].
The waste biorefineries use non-edible biomass and biogenic waste as the feedstock to sustainably convert them into biochemical, biopolymers, and biofuels [86, 104, 121]. This allows recycling and reusing of the waste as well as better waste management which is slowly becoming a global problem [86, 104, 122, 123]. To allow better conversion of waste to value-added bioproducts, proper characterization of the waste is required in synergy with the process of conversion [86, 124, 125]. Several types of waste like food waste, municipal solid waste, lignocellulosic waste, paper waste, and manure are being researched as a possible feedstock for waste refineries [64, 86, 104, 123, 126,127,128,129,130,131].
The biomass to biofuel conversion technologies employed for different generations of biomass and their products for several types of biorefineries are given in Fig. 3. The conversion technologies require an in-depth understanding of chemistry, pre-processing technologies, production technologies, conversion processes, economics, scale-up, and environmental effects, and policies to be developed into a large-scale commercialized biorefinery process [97, 98, 108, 112].
Biochemical Conversion Methods
The biochemical conversion methods are used to convert sugar, starch-based, and sometimes lignocellulosic-based biomass into grain-ethanol or bioethanol [19, 20]. Fermentation, anaerobic digestion, and enzymatic hydrolysis are a few conventional, well-established, and cost-effective processes that produce bioethanol, grain-ethanol, biogas, bio-oil, and electricity as fuel products with other value-added biochemicals [19, 20, 133, 135]. These processes allow chemical decomposition of the biomass into carbohydrates which then convert into liquid fuel or biogas [19, 136].
Fermentation
The fermentation is a chemical conversion process where simple sugars like hexoses (glucose/fructose) and pentoses (ribose) are converted into ethanol and CO2 under anaerobic conditions using microorganisms like yeast (Saccharomyces cerevisiae), bacteria (Zymomonas mobilis), and fungi (Fusarium avenaceum) [19, 102, 137,138,139,140]. The feedstock used for the process can be sugar or starch like corn and wheat producing grain-ethanol and lignocellulosic substrates producing 2G bioethanol [19, 41, 139]. Sugar feedstocks are simple to ferment and convert into ethanol; however, starch is a complex branched glucose polymer comprising amylose and amylopectin [140, 141]. These macromolecules need to be hydrolyzed into simple fermentable sugar like hexoses (glucose/fructose) and pentoses (ribose) by a process called mashing which typically contains 15–20% starch [44, 140, 141]. The simple sugars are then converted into ethanol using microorganisms under anaerobic conditions [137, 140, 141]. The reactions 1–3 below show the conversion stoichiometry for sugar to ethanol [19]. Theoretically, the conversion of sugar to ethanol is 51%; however, as the microorganisms utilize a part of the sugar for their metabolic activities, the fermentation efficiency is between 40 and 48% [19, 102]. The quality and productivity of the process depend upon feedstock, pH, agitation time, temperature, microorganism used, inoculum, and fermentation time [140].
The conversion of simple sugar to ethanol can take place via two different pathways depending upon the initial substrate. The pentose sugar follows the pentose-phosphate-pathway (PPP), whereas the hexose converts into ethanol via glycolysis or the Embden-Meyerhof pathway (EMP) [19, 142]. Microorganisms like Saccharomyces cerevisiae follow the EMP pathway and produce an ethanol concentration of 18% of the fermentation broth [137]. Few bacteria like the Zymomonas follow the Entner-Doudoroff pathway (EDP) as an additional metabolic pathway that adds more carbon to the fermentation process and yields half as much ATP per mole of glucose as the EMP [137]. The ethanol yield widely varies with the type of feedstock used and the fermenter parameters. Also, genetically modified microorganisms produce better yields compared to un-engineered species [41].
The processing of lignocellulosic biomass via fermentation is more complex compared to sugar- or starch-based feedstock because of the presence of carbohydrates like cellulose and hemicellulose, and lignin. Through the biological conversion process, the biomass is first delignified where the cellulose and hemicellulose bonds with lignin are broken. Then, the carbohydrates (cellulose and hemicellulose) are broken down into simple sugars (glucose, xylose, etc.) by hydrolysis. In this entire pre-treatment process, the delignification of biomass is the most complex, expensive, and rate-limiting in nature [137, 142,143,144]. A list of different pretreatment techniques for lignocellulosic biomass is given in Table 5. Recently, micro- and macroalgae are also being researched as feedstock for the fermentation process [34]. Different algal biomass consists of several types of polysaccharides (glucans) like green algae containing cellulose and starch and red algae containing cellulose and cellulose and Floridean starch. Along with glucans, some non-glucans are also present like agar, carrageenan, and alginate. For improved ethanol productivity, hydrolysis of both glucans and non-glucans is essential [34].
The fermentation process for biomass to bioethanol conversion follows the schematic given in Fig. 4. The size reduction and milling are the first unit operations where the biomass is ground and milled. The milling can be (i) dry milling and (ii) wet milling [142, 144]. Dry milling is when the biomass is milled into flour without separating the nutritional components and sent for processing as a whole crop. Wet milling is when the biomass is treated with water to separate starch and fiber, and only starch is further processed. The advantage of wet milling is the separation of different value-added products from the biomass before processing and has a higher production capacity [44, 142, 144]. Dry milling produces distillers’ dried grains which is an excellent animal fodder rich in proteins, fats, and carbohydrates [44]. Once, milling is done, then the processed biomass is sent to the pre-treatment chamber, where, based on the biomass type (sugar-based, starch-based, or lignocellulose-based), it is processed into simple sugar by hydrolysis. The simple sugar or saccharine is then sent for fermentation to be converted into grain/bioethanol [19, 137, 142, 144]. Typically, fermentation of sugar or starch-based feedstocks is done at 30–40 °C with 3.7–5.5 pH and under continuous stirring of 150–300 rpm [41].
In the case of lignocellulose-based biomass, sometimes, saccharification and fermentation are combined into the simultaneous saccharification and fermentation (SSF) process. This is done because, when cellulose is hydrolyzed using cellulase enzyme, glucose inhibits its activity. SSF process keeps the concentration of glucose low allowing low inhibition and better ethanol conversion [137, 143, 147]. Compared to the two-staged process, SSF yields higher ethanol concentration (~ 40%) with a shorter fermentation time and less contamination [137, 147]. Also, to effectively increase the production of 2G ethanol using lignocellulosic feedstock, it is advisable to maximize the conversion of xylose sugar present in hemicellulose using engineered Saccharomyces cerevisiae. The process where saccharification and co-fermentation of xylose to 2G ethanol occurs is called the saccharification and co-fermentation process (SSCF) by co-culture of two recombinant yeasts [76, 148,149,150]. Furthermore, research shows that efficient removal of lignin, increase in the cellulose porosity, and reduction of cellulose crystallinity during pre-treatment improve the efficiency of hydrolysis by many folds [76, 102, 151]. The fermented product thus obtained is sent for distillation where 90–95% hydrated bioethanol is obtained, which is then dehydrated to obtain 99.99% pure bioethanol [41, 138, 146].
Recently, the solid-state or solid substrate fermentation process is also employed on agricultural and industrial waste which occurs in the absence or near absence of water. The process is known to enhance the production of various value-added products and biofuels at a lower cost of operation [152]. Photo-fermentation and dark fermentation are the other two novel fermentation techniques being researched to convert biomass into bio-hydrogen. Dark fermentation is an anaerobic fermentation process occurring in the absence of light at temperatures between 25 and 80 °C. Photo-fermentation is a catalytic conversion of biomass into hydrogen by nitrogenase bacteria using solar energy under a nitrogen-deficient medium. These conversion processes have several constraints like time-consuming, expensive, and high-energy demand, which limits their applicability [153].
The fermentation as a process is commercially well established and can yield high productivity with high purity when first-generation biomass is used (~ 450 l of grain-ethanol can be produced per ton of dry corn) [17]. However, pre-treatment of lignocellulosic biomass and hydrolysis becomes a problem when fermentation is employed for second-generation biomass [41, 146]. The lignocellulosic biomass processing techniques are not well developed and still at the laboratory or pilot plant scale [131]. Also, fermentation being a biochemical process requires numerous chemical and biological parameters to be controlled and optimized to be sustainable.
Anaerobic Digestion
Anaerobic digestion is a multi-staged biochemical process that is commercially established for high-moisture content waste (~ 80–90% moisture) treatment as well as for bioenergy generation [17, 102, 154]. Agricultural residue, municipal solid waste, sewage sludge, etc. are a few feedstocks commonly used for the anaerobic digestion process. The process can directly convert biomass to biogas (60–70% methane and ~ 30% CO2 with small quantities of other gases like H2S) and digestate [155, 156]. The conversion occurs through a series of biochemical reactions occurring via metabolic pathways of bacteria under anaerobic conditions which breaks down the macromolecules into simpler molecules that converts into biogas [17, 19, 102, 155].
The schematic for the anaerobic digestion process is given in Fig. 5. The biomass feedstock is first made into a slurry, before feeding into a digester. In the digester, the biomass converts into biogas and is digested in the following four steps [157, 158]
-
i.
Hydrolysis: Biomass is consisting of macromolecules like fats, carbohydrates, and proteins. In the first step of conversion, these large organic polymers are hydrolyzed into smaller compounds like fatty acids, monosaccharides, amino acids, and peptides using fermentative bacteria. Hydrogen and acetate are some by-products resulting from this rate-limiting stage. The hydrolysis occurs at a temperature between 30 and 50 °C and an optimum pH of 5–7.
-
ii.
Acidogenesis: In the second step of anaerobic digestion, the products of hydrolysis are picked up by acidogenic microorganisms and converted into lighter volatile fatty acids, H2, NH3, CO2, H2S, carbonic acids, and alcohols. The more is the lighter volatile fatty acids formed in this stage, the more will be the formation of acetic acid in the next stage.
-
iii.
Acetogenesis: Acetogenesis is the third phase of digestion, where acetogenic microorganisms catabolize the products from the acidogenesis stage into acetic acid, carbon dioxide, and H2. This step facilitates the methanogenesis process to produce the final product as methane.
-
iv.
Methanogenesis: In the last step of the digestion process, methane is produced by hydrogenotrophic methanogens and acetotrophic methanogens from acetic acid, carbon dioxide, and H2 via two reaction mechanisms, (a) acetoclastic methanogenesis and (b) hydrogenotrophic methanogenesis, as shown in reactions 4 and 5 [154]. The methanogens need to be maintained under anaerobic conditions with a pH between 6.5 and 7.5 to enable proper conversion.
$$Acetoclastic\:methanogenesis: {\mathrm{CH}}_{3}\mathrm{COOH}\to {\mathrm{CH}}_{4}+{\mathrm{CO}}_{2}$$(4)$$Hydrogenotrophic\:methanogenesis: {\mathrm{CO}}_{2}+{4\mathrm{H}}_{2}\to {\mathrm{CH}}_{4}+2{\mathrm{H}}_{2}\mathrm{O}$$(5)
After digestion, two products are formed: (a) biogas and (b) digestate. The biogas is sent to a collection tank, where it may be further distributed for electricity production or other household usage. The digestate is sent to a separator, where the wastewater is sent for treatment and the solid residue may be used as compost or biofertilizers [17, 19, 102, 159]. Feedstock composition and size, inoculum to substrate ratio, liquid recirculation, rate, bed compaction, and use of bulking agents are some of the parameters that affect the performance of the digester [158, 160].
Different technological advancements are being made to improve the anaerobic digestion process to enhance methane formation. Electrical treatments, biological pre-treatment of the substrate, thermal hydrolysis, etc. are a few methodologies employed to enhance the rate-limiting hydrolysis process. Improved hydrolysis allows the better formation of micro-molecules which further enhances the acidogenesis and acetogenesis steps and the products [161]. For lignocellulosic biomass, pre-treatment and removal of lignin again become important as lignin adversely affects the hydrolysis stage of the digestion process [162, 163]. The choice of enhancement and pre-treatment methodologies is influenced by economic and energy efficiency analysis [145]. Many new reactor designs have also been proposed which improve the efficiency of the process. Zhang et al. have developed a three-staged digester with each step of digestion, hydrolysis, acidification, and methanogenic, occurring in three independent chambers. This design improves the yield by 24–54% over a single-phase or two-phase single-chamber process [164]. Digesters with high-pressure biological membrane systems also show a significant effect on methane yield during the methanogenesis step [157]. Integration of the fermentation process and anaerobic digestion of fermentation residue to produce ethanol and methane is also found to increase the decomposition rate of food waste by 27% and reduce the energy requirement by 52% [157]. Nowadays, a lot of research is being done to study anaerobic digestion with microalgal biomass as a feedstock or as a co-substrate [143, 165].
One of the major advantages of this process is that biogas produced can be directly used for electricity generation with overall biomass to electricity conversion efficiency which is about 10–16%. It can also be upgraded to higher quality natural gas by removing carbon dioxide from the mixture [17]. The application of anaerobic digestion in landfills to process municipal solid wastes generates an equal amount of methane (CH4) and carbon dioxide (CO2). These gases along with trace amounts of nitrogen, oxygen, and other volatile organic contaminants like hydrogen sulfide (H2S) and vinyl chloride (C2H3Cl) are known as landfill gas (LFG) [157]. The usage of LFG for electricity generation and other applications requires efficient treatment of the LFG to remove the containments and carbon dioxide [166]. Lately, a lot of effort is being made to produce liquid fuel in the form of methanol instead of gaseous fuel using anaerobic digestion as a treatment of biogas/methane and its storage is expensive [157, 166]. The liquid fuel is easy to manage, store, and distribute. Also, it has low ash and sulfur content compared to biogas [166].
Enzymatic Hydrolysis
The enzymatic hydrolysis process is normally always combined with fermentation for the conversion of biomass into bioenergy. As the name suggests, the hydrolysis stage of converting carbohydrates into simple sugar is facilitated by enzymatic activities. Enzymatic hydrolysis is very often preferred for starch-based and lignocellulosic biomass feedstock and has recently been researched on algal biomass hydrolysis [34, 167]. For starch enzymatic hydrolysis, amylase is the first enzyme that decomposes the starch macromolecules into short chains of glucose. The amylase enzyme liberates “maltodextrin” oligosaccharides which are then further hydrolyzed by enzymes like pullulanase and glucoamylase in a process called saccharification. During saccharification, all the dextrin is converted into glucose and maltose which are then fermented to produce ethanol using microorganisms [102]. In lignocellulosic biomass, the cellulose consists of glucose, and hemicellulose is made up of pentoses (D-xylose in abundance and D-arabinose) and hexoses (D-mannose, D-glucose, and D-galactose) [63]. Lignin is composed of three aromatic alcohols, p-coumaryl alcohol, coniferyl alcohol, and sinapyl alcohol [63]. Pre-treatment allows the cellulose and the hemicellulose to be available for easy hydrolysis. During the enzymatic hydrolysis process, cellulolytic (cellulase) enzyme hydrolyzes the cellulose into glucose and xylanases break down hemicellulose into xylose which can be then co-fermented to produce 2G ethanol [63, 168].
A novel approach to improve the economic viability of the process is to increase the solid loading of the process called “high-solids” enzymatic hydrolysis. The process can be considered high solids when the solid content is more than 15% (w/w) dry matter and there is no free water present at the onset of the hydrolysis process. This methodology improves the energy conversion at lower capital and operating cost and reduces the energy input requirement [51]. As, with other biochemical processes, the abundance of lignocellulosic biomass and its usability has made it a preferred feedstock choice for enzymatic hydrolysis. However, the scale-up of the lignocellulose pre-treatment processes and the cost of enzymes are the major limitations to its commercial success [51]. Many new techniques for lignin pre-treatment like benzenesulfonic acid-induced hydrotropic fractionation [169] and supercritical carbon dioxide pre-treatment [170] are also employed in integration with enzymatic hydrolysis to improve energy yield.
Physicochemical Conversion Processes
The physicochemical conversion process like transesterification leads to high-density biofuels like biodiesel [19]. Biodiesel is an attractive substitute for diesel as it is non-toxic, has high oxygen content, and has better lubrication properties which allow efficient combustion in diesel engines [19, 29, 102].
Transesterification/Esterification Process
The oil-containing first-generation crops like Jatropha, palm, rapeseed, and sunflower oil, waste vegetable oil, and microalgae, can be used as the feedstock for the conversion of oil to biodiesel (fatty acid alkyl esters) by the transesterification process [19, 29, 171]. Currently, 80–85% and 10–15% of the total biodiesel production in the world are produced from rapeseeds and sunflower seeds respectively [19]. The oil extracted from crops, algae, and the waste vegetable oil is composed of triglycerides, which, when burned as fuel, lead to incomplete combustion and deposition inside the combustion engine [29]. Thus, conversion of triglycerides into biodiesel is a required step. The reversible chemical reaction of triglycerides with alkyl alcohol to form fatty acids alkyl esters and glycerol in the presence of a catalyst is called transesterification [171]. The process occurs at atmospheric pressure and 50–70 °C temperature, in the presence of excess methyl or ethyl alcohol to increase the forward reaction rate as shown in reactions 6–8 [171].
-
Step 1: Conversion of triglycerides into diglycerides
$$\begin{array}{c}\begin{array}{cc}\begin{array}{cc}{\mathrm{C}}_{6}{\mathrm{H}}_{5}{\mathrm{O}}_{6}{\mathrm{R}}_{3}& +\end{array}& \begin{array}{ccc}\begin{array}{cc}\mathrm{R}-\mathrm{OH}& \stackrel{catalyst}{\leftrightarrow }\end{array}& \begin{array}{cc}{\mathrm{C}}_{5}{\mathrm{H}}_{6}{\mathrm{O}}_{5}{\mathrm{R}}_{2}& +\end{array}& \mathrm{R}1-\mathrm{COO}-\mathrm{R}\end{array}\end{array}\\ \begin{array}{cc}\begin{array}{cc}\mathrm{Triglycerides}& \end{array}& \begin{array}{ccc}\begin{array}{cc}\mathrm{Alcohol}& \end{array}& \begin{array}{cc}\mathrm{Diglycerides}& \end{array}& \mathrm{Fatty\:acid\:ester}\end{array}\end{array}\end{array}$$(6) -
Step 2: Conversion of diglycerides into monoglycerides
$$\begin{array}{c}\begin{array}{cc}\begin{array}{cc}{\mathrm{C}}_{5}{\mathrm{H}}_{6}{\mathrm{O}}_{5}{\mathrm{R}}_{2}& +\end{array}& \begin{array}{ccc}\begin{array}{cc}\mathrm{R}-\mathrm{OH}& \stackrel{catalyst}{\leftrightarrow }\end{array}& \begin{array}{cc}{\mathrm{C}}_{4}{\mathrm{H}}_{7}{\mathrm{O}}_{4}{\mathrm{R}}_{1}& +\end{array}& {\mathrm{R}}_{1}-\mathrm{COO}-\mathrm{R}\end{array}\end{array}\\ \begin{array}{cc}\begin{array}{cc}\mathrm{Diglycerides}& \end{array}& \begin{array}{ccc}\begin{array}{cc}\mathrm{Alcohol}& \end{array}& \begin{array}{cc}\mathrm{Monoglycerides}& \end{array}& \mathrm{Fatty\:acid\:ester}\end{array}\end{array}\end{array}$$(7) -
Step 3: Conversion of monoglycerides into glycerol and fatty acids alkyl esters
$$\begin{array}{c}\begin{array}{cc}\begin{array}{cc}{\mathrm{C}}_{4}{\mathrm{H}}_{7}{\mathrm{O}}_{4}{\mathrm{R}}_{1}& +\end{array}& \begin{array}{ccc}\begin{array}{cc}\mathrm{R}-\mathrm{OH}& \stackrel{catalyst}{\leftrightarrow }\end{array}& \begin{array}{cc}{\mathrm{C}}_{3}{\mathrm{H}}_{8}{\mathrm{O}}_{3}& +\end{array}& {\mathrm{R}}_{1}-\mathrm{COO}-\mathrm{R}\end{array}\end{array}\\ \begin{array}{cc}\begin{array}{cc}\mathrm{Monoglycerides}& \end{array}& \begin{array}{ccc}\begin{array}{cc}\mathrm{Alcohol}& \end{array}& \begin{array}{cc}\mathrm{Glycerol}& \end{array}& \mathrm{Fatty\:acid\:ester}\end{array}\end{array}\end{array}$$(8)
Homogeneous catalysts like liquid acids (HCl, H2SO4, etc.) or liquid bases (NaOH, KOH, etc.) can be used for the conversion; however, their activity reduces in the presence of excessive free fatty acids. Moreover, transesterification reaction with homogeneous catalysts produces a huge amount of wastewater and the catalysts are corrosive and non-eco-friendly [172]. Heterogeneous catalysts on the other hand are preferred as they can simultaneously esterify fatty acids and transesterify triglycerides [173]. Heterogeneous catalysts can be solid acids or solid bases and have immense potential as they are easy to separate and have fewer environmental repercussions [173, 174]. Also, the reusability of the catalyst and less consumption make biodiesel production more economical compared to the homogeneous catalyzed process. In the case of the vegetable oil transesterification process, solid acid catalysts are preferred because the base catalysts are known to cause saponification of the free fatty acids, which reduces biodiesel formation and increases the cost of production [171, 173].
The process of transesterification starts with the extraction of oil from the biomass feedstock as shown in the schematic in Fig. 6. The extraction of the oil can be done using various methods like solvent extraction, supercritical fluid extraction, ultrasonic extraction, microwave extraction, osmotic shock, and enzymatic extraction [78, 171]. The oil extracted is then sent for transesterification. The glycerol formed as the by-product of transesterification is a much denser compound compared to fatty acid esters and can be easily separated. The fatty acid esters once separated are sent for distillation, where the excess alcohol is removed. The distilled biodiesel is sent for a final water washing where the residual catalyst and soap are removed [19, 174].
Integration of different processes together to enhance the production and treat mixed biomass feedstock has also been researched. Karpagam et al. observe that integration of transesterification with biochemical processes enhances bioethanol and biodiesel production for algal biomass [175]. Similar observations have also been made by Jung et al. and Sundaramahalingam et al. when they combined transesterification with thermal enhancement and ultrasound effects and reported a biodiesel yield of 59.3% and 94.7% respectively [172, 176]. A considerable amount of research is needed to identify reusable catalysts and economical downstream purification processes. It is also evident that single process conversion of vegetable/crop oil to biodiesel is not economical and proper integration of processes is advisable [174].
Chemical Conversion Methods
Hydrolysis
The chemical hydrolysis process is the pre-fermentation step of converting long-chain carbohydrates into simple sugars. Acid hydrolysis or acidolysis can be employed for starch-based, lignocellulosic, and microalgal biomass [177,178,179]. Inorganic acids like HCl, H2SO4, nitric acid, and phosphoric acid and organic acids like citric acid, oxalic acid, and acetic acid can be used for the process [178]. Acid concentration, temperature, time, and surface-to-volume ratios are important parameters that affect the hydrolysis process [177, 178, 180]. Hong and Wu reviewed that when microalgae G. verrucose is treated with 0.1 M HCl at 121 °C, it yields 34.9% of hydrolysate, whereas when it is treated with 0.1 M citric acid using 10% biomass at 150 °C for 60 min, it yields 57.8% hydrolysate [178]. Thus, the feasibility of acidolysis using organic acids is economical and environmentally friendly [178]. Integration of acid hydrolysis with ultrasound and microwave is currently being researched to improve the yield of the process [178, 181]. Ultrasound causes shearing of the cell wall of biomass due to cavitation which enhances the release of low-molecular-weight sugars from polysaccharides [181]. Microwave-assisted hydrolysis on the other hand improves hydrolytic efficiencies by enabling better temperature and heating control [178]. Though the processes are well tested, their commercial applications are still at their initial stages [178].
Solvent Extraction
Solvent extraction of oil or lipid from oil-containing biomass like rapeseeds, palm seeds, corn, soybean, Jatropha, micro-, and macroalgae is a conventional method being commercially used over the last 50 years [182]. Solvent extraction is also extensively employed to extract secondary metabolites from biomass like terpenoids, waxes, resins, sterols, and alkaloids [182]. Extraction is a chemical process in which the solute (oil or lipid) is separated from the carrier (biomass) by allowing the solute to selectively dissolve in the solvent. For efficient extraction, the solvent needs to penetrate the biomass and match the polarity of the solute (lipid/oil). The choice of solvent is essential in this process as extraction and further separation of solvent from the extracted oil are both required [102]. Also, for the process to be cost-effective, the solvent must be inexpensive and easily available. Organic solvents like benzene, hexane, cyclohexane, acetone, and chloroform are effective in extracting oil from the plant and algal biomass by degrading the cell wall [183]. Microalgal species like Botryococcus braunii actively secrete oil, which can be then recovered without damaging the cell wall using solvents like decane [183].
The process of solvent extraction follows the schematic given in Fig. 7. The biomass is initially dried and then sent for extraction using a choice of solvent. The extracted oil and solvent mixture is then sent for separation and the de-oil biomass meal is sent for toasting [102]. Integration of solvent extraction with processes like hydrolysis, fermentation, and transesterification is also being applied in biorefineries. The solvent extraction removes the secondary metabolites from the biomass, and hydrolysis and fermentation of the remaining biomass meal lead to bio-oil production. Frequently, mechanical extraction is combined with solvent extraction to enhance the oil extraction process. The mechanical extraction processes like bead milling or wet milling allow the plant cell wall to be disrupted and ease the solvent extraction process. Mercer and Armenta report an increase in oil extraction from 5.6 to 18.8% when bead milling is combined with extraction from Chlorella protothecoides using hexane solvent [183].
Schematic for the solvent extraction process [19]
Microwave-assisted, ultrasound-assisted, and high-shear-assisted extraction processes are a few advanced processes that enhance the overall yield of bio-oil [184,185,186]. Supercritical extraction using supercritical CO2 is another alternative to improve the yield of oil [183]. This method of extraction is completely free of solvent and thus yields pure products. Solvent extraction efficiencies can also be improved by a process called “Accelerated solvent extraction,” where the organic solvent is used at temperature and pressure above its boiling point [182, 186]. The increase in extraction temperature and improved contact between the solvent and biomass shortens the time required for extraction. However, thermal degradability and oxidation of lipids are a few critical shortcomings of the process. Also, the conventional solvent extraction method may not be efficient in removing lipid from plant cells. Furthermore, parameters like solvent-to-sample ratios, sample sizes, extraction temperatures, and extraction cycles need to be optimized to make the process commercially viable [182].
Supercritical Conversion of Biomass
Supercritical conversion of biomass is an efficient alternative to the chemical or enzymatic hydrolysis process. Chemical hydrolysis is expensive and enzymatic hydrolysis requires pre-treatment of the lignocellulosic biomass [187]. Contrary to these, supercritical water or CO2 can easily convert biomass into a mixture of oils, alcohol, organic acids, and methane and cellulose into sugar [187]. A supercritical fluid is a state of matter that is at a temperature and pressure condition above the critical point. At the supercritical state (water, 644 K and 22 MPa; and CO2, 304 K and 7.4 MPa), the fluid is neither liquid nor gas [102, 115, 188]. Water under supercritical conditions is present in its ionic H+ and OH− form and dissolves separately in the lignocellulosic biomass enabling faster rupture of the bonds and formation of simple sugars. The simple sugars (glucose and xylose) get converted into bioethanol and the lignin into bio-oil [102, 115, 188].
Supercritical water gasification technology can convert cellulose into glucose in 10–20 s and produces bio-hydrogen [189]. When the temperature of the supercritical water is increased up to 873 K, the water acts as a strong oxidant leading to the complete decomposition of biomass. The oxygen atom from water reacts with the biomass carbon atom, allowing the free hydrogen atom to form bio-hydrogen. This method is effective for biomass with moisture content and does not require any drying pre-treatment [189]. Also, the reaction medium being water allows better mass transfer and reduced coke formation. This method produces high energy-dense renewable hydrogen gas at low purification and downstream separation cost. However, due to the requirement of elevated temperature and pressure requirement, the process is not yet industrially established [189].
Thermochemical Conversion Methods
The thermochemical processes of converting biomass to energy apply thermal and chemical decomposition methodologies under varied oxygen supply and temperature conditions. Some of the methods like liquefaction, pyrolysis, and torrefaction are modern technologies with numerous benefits like small carbon footprint, short reaction time, and capability of handling several types of biomass feedstocks [105]. However, most of these methods are still in the pilot or research stages of development.
Liquefaction
The liquefaction or hydrothermal liquefaction process converts biomass into stable liquid hydrocarbons with a high H/C ratio under moderate-temperature (~ 280–370 °C) and high-pressure (10–25 MPa) conditions [19, 190,191,192]. The fuel obtained has a high heating value and low oxygen content making it a stable energy source. Lignocellulosic biomass (dry biomass) and algal biomass (wet biomass) are the preferable feedstocks for the process with an adequate pre-treatment [190, 193, 194]. Prestigiacomo et al. have recently studied hydrothermal liquefaction of municipal sludge as a feedstock in a stirred reactor [195]. The process of liquefaction is either direct or indirect in the presence of alkalis, glycerine, and propanol or butanol [196]. In direct liquefaction, fast pyrolysis of biomass occurs producing liquid tar/oil and condensable gases, whereas indirect pyrolysis requires catalysts to convert the non-condensable gaseous products into liquid fuel [196]. The biofuel produced is highly viscous and water-insoluble and requires solvents, reducing gases like CO and H2 and the presence of a catalyst to upgrade its properties. Alkali salts like sodium carbonate and potassium carbonate can act as a catalyst for the lignocellulose liquefaction process, which converts cellulose and hemicellulose into simple compounds by depolymerization and deoxygenation [196]. Nagappan et al. have reported that usage of heterogenous catalysts like Ni/Al2O3 or Mo/Al2O3 improves selectivity and thus improves yield [197]. Ni/Al2O3 is known to be more selective toward lipid for deoxygenation and Mo/Al2O3 facilitates the deoxygenation of carbohydrates. Also, heterogeneous catalysts are easy to recover and are less corrosive [197].
The presence of water, alkalis, glycerol, and propanol/butanol during indirect liquefaction facilitates different degradation processes [198]. In the presence of glycerol and alkali salts, glycerol enables a reduction in surface tension of the solvent at a higher temperature, thus allowing the alkali salts to penetrate the lignocellulosic biomass and break the lignin bonds [102, 196]. In the aqueous liquefaction of lignocellulosic biomass, the water molecules cause desegregation of the wood structure followed by partial depolymerization of the compounds. It is observed that the bio-crude yield from aqueous liquefaction is higher in the presence of a catalyst (~ 63%) compared to its absence (~ 31%) [102].
The process of liquefaction, in general, follows three major steps: (i) depolymerization, (ii) decomposition, and (iii) recombination. Initially, the biomass depolymerizes and decomposes into smaller compounds. However, the presence of free radicals causes these simple compounds to repolymerize and recombine into bio-crude and solid residues. In depolymerization, long chains of hydrocarbons break down into smaller chains under high pressure and temperature conditions, mimicking the natural process of fossil fuel production. The decomposition step involves the removal of water molecules (dehydration), removal of amino acids (deamination), and loss of CO2 molecules (carboxylation). The dehydration and decarboxylation processes facilitate the removal of oxygen from the biomass. The recombination or repolymerization of molecules occurs post decomposition due to the presence of excessive free radicals and the absence of hydrogen molecules. If hydrogen molecules are freely present during the liquefaction process, then it reduces the free radical activities enabling more stable molecular weight species yield [190]. The complete absence of free hydrogen leads to more coke formation. Parameters like temperature, pressure, residence time, and biomass type significantly affect the process kinetics and product composition [190, 199, 200]. It is observed that elevated temperature (> 350 °C) yields gaseous products whereas low temperature (150–200 °C) favors solid formation, with bio-oil production maximized at moderate temperature (250–300 °C) [199].
The lignocellulosic biomass is liquefied at 350 °C and 150 bar pressure for 15 min in a liquefaction unit in either a batch or continuous manner [190]. Elliot et al. have studied hydrothermal liquefaction of algal biomass in batch and continuous systems at 523–653 K and 1.0 MPa with a residence time of 3–5 min [201]. A spontaneous phase change of biomass occurs under these process conditions producing CO2, bio-crude, water, and solid residues [202]. It is observed that the more the lignin content in the biomass, the more will be solid residue production. The solid residues formed can be used as biofertilizers or biofuels. The bio-crude produced is sent for further processing and upgrading as can be seen in Fig. 8 [190, 202]. The upgrading of the bio-oil obtained can be done by esterification, catalytic cracking, hydrogenation, molecular distillation, and catalytic pyrolysis [199]. Lignocellulosic biomass can also be directly converted to liquid hydrocarbons or bio-crude by reacting it with syngas in the presence of a catalyst [196]. The use of subcritical and supercritical solvents and water for direct liquefaction of lignocellulosic biomass is also known to yield fuel with 80% energy efficiency [203, 204].
Microwave-assisted liquefaction for algal biomass, simultaneous hydrothermal liquefaction, esterification for sugarcane bagasse, and liquefaction by plasma electrolysis are a few technical integrations currently being researched [205,206,207]. Araujo et al. show that the integration of simultaneous liquefaction with esterification of sugarcane bagasse biomass yields 91% bio-oil and 9% biochar [207]. One of the major benefits of the liquefaction process is the generation of bio-crude which can be upgraded to replace fossil fuel. Also, the process has an energy efficiency of 85–90% and can recover 70% of the carbon content of the feedstock [190]. Furthermore, the bio-crude generated does not require extensive treatment or upgrading for commercial utilization [190]. However, the economics and scale-up of the process become an issue due to the high-pressure and high energy input requirements [190, 203, 204, 208]. The process is still at the lab-scale research stage and requires further understanding of chemistry, kinetics, catalysts, hydrodynamics, and economics before it can be made commercially viable [190].
Pyrolysis
Pyrolysis is the process of thermal degradation of biomass in the absence of oxygen to produce bio-oil, biochar, and gaseous fuel [209]. The fuel obtained has a medium–low calorific value [210]. The high temperature of ~ 500 °C facilitates breakage of the bonds and the release of volatile substances which are condensed into liquid fuel [19, 209]. The pyrolysis oil obtained can be utilized as transportation fuel, electricity generation, and heating [211]. Algal biomass, forest residue, municipal sludge, agricultural residue, waste cooking oil, and lignocellulosic biomass are the possible feedstock for the pyrolysis process [212,213,214].
The process of pyrolysis occurs in stages, where primary phase decomposition at ~ 450–550 °C releases all the volatile matters present in the woody biomass and forms non-condensable gases like CO, CO2, and CH4. The secondary decomposition occurs at temperatures ~ 400–500 °C, causing cracking of the bonds and releasing vapors that can be condensed to form bio-oil. Finally, some extent of repolymerization of the small chain hydrocarbon occurs to form char, bio-oil, and gaseous products [215]. For lignocellulosic biomass, lignin is known to decompose over a larger range of temperature (~ 550–770 K) compared to cellulose (~ 510–620 K) and hemicellulose (~ 470–530 K) which decomposes over shorter temperature ranges [210]. The kinetics, temperature for decomposition, the extent of decomposition, and product composition vastly vary with biomass feedstock, reactor type, temperature, heating rates, and pressure [210]. Extensive research is being done to understand the correlations between biomass type, pyrolysis pathways, and kinetics, and the suitable reactor designs to improve pyrolysis conversion [216]. Based on the operating conditions, the pyrolysis process can be categorized into five types [209].
-
i.
Slow or conventional pyrolysis: The pyrolysis occurs at a low heating rate (~ 0.1–1 °C/s) with a vapor residence time of 10–60 min [209]. In slow pyrolysis, the first stage of decomposition, called pre-pyrolysis, leads to the internal arrangement and breakage of bonds, the release of water molecules, and the formation of free radicals, carbonyl, and carboxyl groups. In the second stage, fast decomposition of the solid state occurs forming the pyrolysis products. In the third stage, the char decomposes at a slow rate forming carbon-rich solid residue [210]. This process is ideal for producing biochar. Studies show that yield of bio-oil is maximum (~ 24–43 wt%) at an optimum temperature of 500 °C with 34 to 63 wt% of biochar formation [209]. The major disadvantage of this process is excessive cracking of the primary feedstock leading to low-quality bio-oil production [215].
-
ii.
Fast pyrolysis: Fast pyrolysis occurs under rapid heating rate (1000 °C/s), higher temperature (500–650 °C), low residence time (0.5–10 s), and using fine particle (< 1 mm) [19, 102, 209, 215]. This process is recommended for producing bio-oil [17]. In fast pyrolysis, the biomass rapidly decomposes to form vapors, aerosols, and a small amount of char. The vapor and aerosols can then be condensed to form bio-oil with a heating value of about half of the conventional fuels [102, 215]. Fast pyrolysis yields a product with 60–75% bio-oil, 15–25% biochar, and 10–20% non-condensed gases depending upon feedstocks [211].
-
iii.
Flash pyrolysis: Flash pyrolysis occurs at an extremely high temperature (450–1000 °C), short residence time (< 0.5 s), and very high heating rate with very fine biomass particle size (< 0.2 mm) [102, 215]. Flash pyrolysis can produce bio-oil fractions up to 75% with 80% efficiency [17, 211]. Fluidized bed reactors are preferred for both fast and flash pyrolysis processes [209].
-
iv.
Catalytic pyrolysis: Catalytic pyrolysis is done to enhance the bio-oil quality and reduce the oxygen content of the bio-oil. The catalyst also alters the pyrolysis pathway and allows the process to occur at a lower temperature (300–600 °C). Acid and base catalysts are used for the process, with acid catalysts facilitating the production of more biochar, and base catalysts producing more bio-oil [209, 217]. Among all the catalysts, nickel catalysts are found to be better as they activate decarboxylation and decarbonylation reactions during the hydrodeoxygenation process [209].
-
v.
Hydro-pyrolysis: This is a novel pyrolysis process that occurs in high-pressure hydrogen conditions with nitrogen used as the carrier gas. Compared to other pyrolysis processes, this method produces hydrocarbon with better structural stability and less oxygen content. The optimum condition for maximized bio-oil and biogas production is 310 °C, 3 MPa, and 60 min. The addition of catalysts and conversion of the process to fast hydro-pyrolysis have been shown to improve the overall bio-oil productivity [209].
Figure 9 shows the schematic for the pyrolysis process. Microwave-assisted pyrolysis is an advancement made in the conversion methodology [209, 218, 219]. This process is slowly gaining importance because of its ability for mass conversion, uniform heating, and easy controllability. However, the process is expensive and is still in the lab scale of operation [209, 219]. Solar pyrolysis is another advancement that has gained importance in recent times due to its usage of solar as the source of energy [220, 221]. The bio-oil obtained from pyrolysis can be used as a feedstock for biorefineries and can also be used in engines and turbines. However, poor thermal stability, corrosive nature, and extensive upgradation requirements function as major roadblocks in its commercial application [222]. Oxygen content reduction and alkali removal by dehydrogenation and catalytic cracking are a few treatments needed for the fuel produced to be commercially applicable [223]. Hydrotreating of pyrolysis oil or catalytic cracking can produce naphtha, high octane gasoline, and fuel oil [223, 224].
Schematic for the pyrolysis conversion process [19]
Torrefaction
Solid biomass has low energy density, high moisture content, low bulk density, low compositional homogeneity, and low shelf life (easily biodegradable) which are hurdles in its applicability as an efficient fuel in the industry [105]. Torrefaction is a thermochemical conversion technology that is applied to upgrade biomass and improve biochar quality as an alternative to coal [225]. Like liquefaction and pyrolysis, torrefaction also occurs in the absence of oxygen-producing solid biomass like biochar or coke as the primary product. The torrefaction of biomass (lignocellulosic, algal, municipal waste, etc.) may occur via (i) wet torrefaction, (ii) dry torrefaction, and (iii) steam torrefaction [226]. The upgraded biomass from the torrefaction process may be commercially used for cofiring or combustion, as a feed for pyrolysis or gasification, as adsorbents for pollution, etc. [105, 227, 228].
Dry torrefaction occurs at 200–300 °C under either oxidative or non-oxidative conditions. In non-oxidative states, nitrogen and CO2 act as the carrier gas to sweep the biomass during the thermal degradation process [105, 229]. In oxidative torrefaction, air or flue gas may be used as a carrier. The presence of oxygen enables oxidative torrefaction to have a higher reaction rate compared to non-oxidative torrefaction, thus reducing the time for degradation [230]. However, oxidative torrefaction yields lower biochar compared to non-oxidative torrefaction. It is also found that biomass torrefied with higher oxygen concentration and temperature below 300 °C displays a lower heating value. Non-oxidative torrefaction requires high energy input and nitrogen separation from air compared to oxidative state processing [105, 230, 231].
Torrefaction of biomass occurring in the presence of water or dilute acid at 180–260 °C and reaction time of 5–240 min is called wet torrefaction. The solid generated as the product of wet torrefaction is called “hydro-char” [105, 229, 232]. The properties of water like density, viscosity, diffusivity, and dielectric constant change drastically with an increase in temperature which affects the biochar quality during the torrefaction process. Therefore, wet torrefaction is preferably operated under conditions near the subcritical state [233]. It is observed that when biomass is treated with hot compressed water at 180 °C, volatile acids like aldehydes and furfural derivatives get generated which enhances the torrefaction process [105, 234]. The addition of acids like sulfuric acid and acetic acid, to water, is also known to improve the process [235]. A major advantage of wet torrefaction is the non-requirement of any drying pre-processing. Thus, wet biomass feed like sludge, manure, and sewage can be considered for this process. Furthermore, with wet torrefaction, one can obtain a product with higher energy density and mass yield compared to dry torrefaction [236]. Another difference between wet and dry torrefaction is the ash content of the biochar. Ash is inert and its composition in the upgraded biochar increases proportionally after dry torrefaction. However, with wet torrefaction due to the dissolution of minerals in ash into the aqueous phase, the final ash content in the biochar is hugely reduced. The reduction in ash content prevents agglomeration, corrosion, fouling, and slagging during the hydro-char conversion processes [105, 225, 237].
Steam torrefaction is a process where high-temperature and high-pressure steam explosion is used to torrefy the biomass. In this process, the lignocellulosic biomass is treated in a chamber at 200–260 °C using high-pressure and high-temperature steam [105, 238]. The pressure inside the chamber is slowly increased which caused the biomass to swell and disintegrate into separate components. The volatile matter present in the biomass also gets removed by a steam explosion which increases the carbon content and calorific value of the biochar and decreases its mean particle size and bulk density [227, 231, 232, 239]. Furthermore, the biochar derived from steam torrefaction has higher elasticity and mechanical strength compared to wet and dry torrefaction. However, steam torrefaction requires a high energy supply and is expensive [105, 232, 239].
The commercial development of the torrefaction process is still at its initial stages. Various technical aspects like high ash content in the biochar, emission of dibenzofurans and polychlorinated dibenzo-p-dioxins during torrefaction, formation of tar as a by-product (~ 2000–8000 tons/year of tar gets generated during torrefaction), and scale-up are a few roadblocks in the commercial feasibility of the process [105, 240].
Hydrothermal carbonization (HTC) is a thermochemical conversion process similar to wet torrefaction technology. Many studies have discussed “wet torrefaction” under the terminology of “hydrothermal carbonization” [192, 229, 241, 242]. However, the products formed at the end of both these processes are different in characteristics and usability. The wet torrefaction produces upgraded solid fuels, whereas the hydrothermal carbonization produces charcoal with high carbon content, which can be used as activated charcoal, fertilizers, catalysts, biosensing, supercapacitors, and fuel [192, 229, 243]. The HTC is a process of removing oxygen from biomass via dehydration and decarboxylation reactions (reducing the molar ratios of O/C and H/C), producing a more coal-like product [192, 242, 244,245,246]. Analysis of the various process parameters shows that increasing the residence time and operating the reactor under optimum temperature (180–260 °C) and pressure (< 300 bar) conditions can improve the “hydro char” characteristics [192]. A lot of the current research is focused on integrating HTC with wastewater treatment and municipal solid waste treatment to improve energy production and recovery. Industries and researchers are working on developing portable and flexible HTC processes for these integrated systems [242]. However, the treatment and processing of the contaminated water received from HTC as a by-product remains a major challenge for this process [192, 242].
Combustion
Combustion is an exothermic reaction process where the biomass reacts with oxygen (air) at high temperatures to form carbon dioxide, water vapor, and chemical heat. This process accounts for 90% of the total renewable energy generated from biomass [19, 102]. The heat produced from combustion can be converted into useful mechanical and electrical energy [228]. Dry wood, dry leaves, hard vegetable shells, agricultural residues (rice/wheat straws), etc. are some of the feedstocks that are used for the process to produce around 20 MJ/kg biomass of thermal energy [247]. The combustion is carried out inside a combustion chamber at 800–1000 °C for biomass with moisture content less than 50% as shown in Fig. 10 [19]. High moisture content biomass is better suited for biochemical conversions. The generated heat from combustion is used to produce steam which is fed to a turbine to generate electricity.
Schematic for the combustion process [19]
The current biomass combustion plants generate 20–50 MW of electricity with electrical conversion efficiencies of 25–30%. With the incorporation of processing techniques like fluidized bed systems and improved gas processing, the production can be upgraded to 100–3000 MW with conversion efficiencies up to 30–40% [17, 19, 248, 249]. Integration of biomass combustion with coal-fired power generation is also attractive as it improves the conversion efficiencies [105, 250, 251]. The process of converting biomass to energy by combustion is a conventional route that is widely implemented both at the commercial and household level. However, the emissions of particulate matter and CO2 are the major concerns with the process [247, 252]. Chemical looping combustion (CLC) integrated with biomass feedstock (bio-CLC) which combines bioenergy with CO2 sequestration is a novel technique to reduce CO2 emissions [253]. The CLC consists of air and a fuel reactor. Oxygen carriers in the form of metal oxides carry oxygen from the air reactor to that of the fuel reactor, where it reacts with the fuel to produce H2O and CO2. This process enables pure CO2 generation which can be easily sequestered without any further processing [253, 254].
Gasification
Gasification is the conventional process of converting biomass into the combustible gas mixture (CO, H2, CO2, CH4, and N2) called syngas or synthesis gas and biochar by partial oxidation at a high temperature of around 800–1100 °C [255]. Syngas, which normally have the lowest heating value (LHV) of 4–13 MJ/N m3, can be used to generate electricity, petrochemical products, methanol, and hydrogen [255]. The char produced contains carbon, unconverted organic residue, and ash and has an average LHV of 25–30 MJ/N m3 [255]. The composition of the char majorly depends upon the gasification methodology and biomass type and quality. Lignocellulosic biomass, forest residues, agricultural residue, etc. can be as feedstock for gasification [256, 257]. Pre-treatment and drying of the biomass are required before it can be converted. It is observed that the initial moisture content of the biomass adversely affects the LHV of the biofuel produced [255].
Gasification is an endothermic process and is conducted in an air-tight chamber under air suction or low air pressure condition as shown in Fig. 11. The heat energy required for the process is derived from partial oxidation of the biomass feed. The gasification process follows five major steps in series [255]
-
i.
Oxidation to generate heat: The partial oxidation of the biomass is an essential step to generate the heat required for gasification and maintaining the temperature. The oxidation occurring in absence of oxygen (given in reactions 9–11) produces CO, CO2, H2O, and heat [255]. Though all the carbonaceous components present participate in the reactions, it can be simplified to consider the involvement of only char and hydrogen contained in the syngas [255].
Schematic for biomass gasification process [19]
Char combustion:
Partial oxidation:
Hydrogen combustion:
-
ii.
Drying: Drying is an essential step for removing moisture from the biomass. The amount of heat required for drying is proportionately dependent on the amount of moisture content. The process of drying can be considered complete when the biomass temperature reaches the temperature of 150 °C [255].
-
iii.
Pyrolysis: This is the stage where thermochemical decomposition of the biomass takes place at 250–700 °C. The long chains of hydrocarbon break down into shorter chains of lower molecular weight compounds producing solid, liquid, and gaseous fractions. The solid (biochar) yield is around 5–10 wt% for fluidized bed gasifiers and 20–25 wt% for fixed bed gasifiers. The liquid (tar) yield in downdraft gasifiers is around 1 wt%, 1–5 wt% in bubbling bed gasifiers, and 10–20 wt% for updraft gasifiers. The gaseous fraction (pyrolysis gas) is typically 70–90 wt% and comprises incondensable gases like hydrogen, CO, CO2, and light hydrocarbons. The process of pyrolysis is complex and is governed by numerous factors like heat transfer, diffusion, and kinetics. At low temperatures, the process is kinetic controlled, but it becomes heat transfer controlled at higher temperatures [255]. The overall reaction for pyrolysis is as given below in reaction 12.
$$\mathrm{Biomass}\leftrightarrow {\mathrm{H}}_{2}+\mathrm{CO}+{\mathrm{CO}}_{2}+{\mathrm{CH}}_{4}+{\mathrm{H}}_{2}\mathrm{O}(\mathrm{g})+\mathrm{Char}+\mathrm{Tar}$$(12) -
iv.
Reduction: In the reduction step, the char and gaseous products from the above two stages react together to form the syngas as given in reactions 13–16. As the reactions are reversible, the temperature of the reduction stage defines the composition of the syngas. Higher temperature reduces char formation and increases tar and syngas fraction. However, it may also lead to increased ash sintering and a reduction in the energy content of the syngas [255].
Boudouard reaction:
Reforming of the char:
Water gas shift reaction:
Methanation reaction:
-
v.
Tar decomposition: The tar formed during pyrolysis also decomposes and contributes to the reduction step. CH4 and short-chain hydrocarbons are formed as products from this stage as given in reaction 17.
$${\mathrm C}_{\mathrm n}{\mathrm H}_{\mathrm m}\leftrightarrow{\mathrm C}_{\mathrm n-\mathrm x}{\mathrm H}_{\mathrm m-\mathrm y}+{\mathrm H}_2+\mathrm C+{\mathrm{CH}}_4$$(17)
The syngas via gasification can be produced by either the catalytic or non-catalytic route. The non-catalytic process occurs at an extremely high temperature of ~ 1300 °C, whereas the catalytic gasification can be done at a lower temperature of ~ 800 °C [102, 189, 258]. Hu et al. are researching chemical loop biomass gasification (CLG) using F2O3/CaO catalyst to produce hydrogen-rich syngas. They observe that the hydrogen production using CLG is 1.88 times more compared to normal steam gasification [258]. For transportation liquid fuel production, Fischer–Tropsch (FT) synthesis or methanol synthesis route of syngas conversion is employed [257]. Recently, efforts are being made to produce renewable aviation fuel via gasification and FT synthesis [257]. Macri et al., in their work, proposed supercritical water gasification (SCWG) as the route to improve bio-hydrogen production [259]. They observe that the presence of excess water during gasification promotes water gas shift reaction as well as steam reforming during the reduction stage increasing hydrogen production. Furthermore, SCWG can be employed on biomass with high moisture content like algal biomass [259]. Biomass integrated gasification with combined cycle (BIG/CC) can convert syngas into electricity at a high conversion efficiency of ~ 40–60% for a plant capacity of 30–60 MW. This is a process that utilizes purified syngas thus reducing further processing costs. However, this process is still in the pilot stage [260, 261]. Similarly, microwave-assisted gasification processes are also being researched at a lab scale [262]. Suárez-Almeida et al. are currently studying solar gasification of biomass in a dual fluidized bed gasifier where the solid particle acts as the thermal energy carrier [263].
Gasification for syngas production or electricity production is at pilot or small-scale developmental stages. One of the major disadvantages is that the producer gas contains contaminants such as particulates, tar, alkali metals, H2S, and NH3, which causes blockage and corrosion problems and requires extensive processing before the application [264, 265]. Also, technological advancements are needed to develop compatible engines for syngas applications [255, 259].
Physical Conversion Methods
Mechanical Extraction
The crude oil from crops and microalgae can be extracted by applying mechanical pressure using a screw press [102, 171]. The mechanical pressing can be done either by (i) full pressing or (ii) pre-pressing method. The full pressing employs 95,000 kPa of pressure on the oilseeds to extract up to 3–5% of residual oil. Pre-pressing is normally employed in integration with the solvent extraction process, where 18–20% of oil is removed from the crop using pressing and the rest of it by solvent extraction [102, 266]. This process is commonly employed for biomass with high oil content (~ 30–40%) [102]. Wu et al. have combined enzymatic hydrolysis with intermittent ball milling to increase the lignocellulosic biomass conversion to 84.7% [267]. Mechanical extraction is normally combined with the transesterification process to convert the extracted oil into biodiesel and other value-added bioproducts. This method of oil extraction is well established and orthodox but is often time-consuming and energy inefficient, and demonstrates low yield [268, 269].
Briquetting/Pelleting
The biomass received in bulk is often pre-processed into briquettes and pellets for transportation, storage, and application in biorefineries as feedstock [270]. The most common pre-processing required is the densification of the biomass either by (i) pressing or (ii) maceration (chopping, grinding, etc.). By pressing, the density of the biomass increases proportionately to the amount of pressure inflected [271]. The briquetted biomass is also recommended for usage as primary biofuel for traditional cooking and space heating as it increases burning efficiency and reduces emissions and pollution [272, 273]. The process of briquetting is conventional and widely used but is expensive and needs economic analysis and technical improvements to have commercial success [271].
Distillation
Steam distillation and hydro-distillation are two of the most extensively used techniques to extract oils, essential oils, and many other value-added products from biomass [274]. The volatile matters present in the biomass are allowed to vaporize using steam and then collected and processed [275]. A more recent development is the use of molecular distillation for the extraction of temperature-sensitive components where conventional methods cannot be applied [276, 277]. In molecular distillation, the distance between the evaporation and condensation surfaces is less than the mean free path of the molecules [277].
Feasibility Analysis — Prospects and Challenges
The biomass to energy conversion technologies is at various stages of development. Table 6 provides a detailed comparison between different biomass to bioenergy conversion technologies, their economics, scale-up possibilities, commercial feasibility, and the current state of development.
Conversion processes like fermentation and anaerobic digestion are well established and economic, and have high commercial feasibility for 1G biomass conversion. However, as first-generation biomass feedstocks, like sugarcane and corn, compete for both food and fuel, their viability for fuel production is low. This is one of the major reasons why, despite being the second-largest producer of sugarcane, countries like India, China, and Brazil are yet to establish commercial large-scale plants for 1G bioethanol production. Lignocellulosic or algal biomass conversion to energy is more lucrative because of their abundant availability; however, their pre-treatment processes and scale-up are a major bottleneck. Furthermore, algal biomass technology is still at its preliminary stages of development and its conversion methodologies are extremely energy-intensive making its commercial feasibility low. Thus, fermentation and anaerobic digestion for 2G bioethanol production have a high possibility but moderate feasibility until the lignin pre-treatments and its scale-up are improved. Viabilities of chemical hydrolysis and enzymatic hydrolysis are also largely decided by the pre-treatment technologies and the cost of production.
Supercritical conversion and liquefaction technologies have enormous potential in terms of fuel production efficiency and fuel quality. However, the high-pressure requirement is a huge barrier to their large-scale productivity. The development of catalysts to improve the reaction pathway and reduction in pressure and temperature requirements for these processes may enhance the possibility. Similarly, processes like combustion and gasification are well understood and are currently used but have problems with GHG emissions. Integration of these processes with CO2 sequestration and their economic analysis is needed to make the process environment-friendly. Processes like torrefaction, HTC, and pyrolysis are still in the lab and pilot stages of development and lack cost information, optimized process parameters, and post-treatment methodologies leading to uncertainties about their commercial applicability. However, appropriate technological interventions like reactor design and catalyst development for bio-oil upgradation will allow the processes to be commercially developed. Thermochemical and biochemical conversion processes integrated with microwave technology or ultrasound technologies are novel and they improve the conventional methods; however, their scale-up is a challenge. The energy analysis of each of these processes is required to understand the energy efficiencies. Many of these processes like gasification, microwave or ultrasound-assisted processes, or processing that require drying of biomass will have huge energy input. Thus, if this energy is derived from fossil fuels, then they do not serve the purpose. Alternatively, the integration of renewable energy resources is needed to make the system self-sustainable.
Modeling and simulation-based analysis of the bioenergy conversion processes is an alternate route to understand the behavior of the systems, perform parametric analysis and optimization, and improve the design, technology, and overall productivity [32, 33, 278]. These modeling techniques allow holistic development of the processes, reduce time and labor-intensive experiments, and lead to rapid technological advancements. In recent times, a lot of emphases are given to integrating artificial intelligence (AI)/artificial neural networks (ANN) and machine learning (ML) frameworks into these modeling techniques to enhance their performance [278]. Application of various optimization techniques like ant colony algorithm (ACA), genetic algorithm (GA), fuzzy logic, and particle swarm optimization (PSO) to the biological and chemical processes allows improved optimization of parameters like temperature, pH, hydraulic retention time, and substrate concentration [278, 279]. Machine learning frameworks with various optimization algorithms have allowed researchers to optimize different bioenergy conversion technologies like pyrolysis, anaerobic digestion, and the supply chain for biofuel processing involving biomass cultivation, feedstock quality control, processing, and emissions [280,281,282,283,284]. Khan et al. and Ullah et al. applied ANN integrated with various optimization algorithms like GA, PSO, and grey wolf optimization, to predict bio-fuel formation in the pyrolysis process using biomass characteristic and pyrolysis condition data [282,283,284]. Similarly, Aniza et al. integrated supervised ANN with the Taguchi method to maximize the bio-oil and bio-char yield for pyrolysis and torrefaction processes [285]. Pereira et al. applied AI with a PSO algorithm to optimize industrial bioethanol production [286]. The results show that optimization of the input parameters like biomass purity and pH, fermentation time, and temperature leads to a 10% increase in bioethanol productivity [286].
Thus, these techniques can be successfully implemented to develop a better understanding of the processes and improve their engineering at a reduced cost, time, risk, and labor [278]. These models can be used to optimize the problems of biomass collection, transportation, and segregation [280, 287]. However, validation of these models will require lab-scale and pilot-scale experimentations. Moreover, all the AI/ML-based models require a huge amount of data for model development. The authenticity and availability of the data will limit the usability and validity of these models. Thus, authentic data collection, data validation, data cleaning, and sorting will be crucial for these techniques to be successful. Suitable identification of the parameters, network architecture, AI/ML framework, and model validations are also critical when implementing these technology advancements [280, 281, 287].
The commercial feasibility of biomass to energy conversion technology depends upon the type of biomass feed and its cost, ease of biomass collection, segregation, storage and transportation, ease of operation, cost of production, ease of scale-up, product quality, and environmental and government policies.
-
a)
The entire biomass supply chain starting with biomass collection and segregation followed by storage and transportation to the point of conversion is currently an extremely cost-intensive process [288]. The agricultural biomass collection and segregation are normally done at the point of farming. However, collection and segregation of forest residue and municipal waste collection are a major logistics problem due to lack of access and cost [289, 290]. Many technological interventions like chipper, tractor and guillotine blades, multi-tree handling devices, harvesters, and forwarders are employed for forest residue collection; however, the process is cost-intensive and lacks global standardization [289]. The storing of the biomass collected is the next logistic challenge. Biomass containing 15–20% moisture may be stored without drying at a reduced cost of storage, but material loss during handling and loss of heating value needs to be analyzed [288]. However, biomass feedstock with high moisture content requires drying before storage and expensive storage facilities to avoid biomass degradation [288, 290]. Multi-agricultural biomass approach is suggested by Rentizelas et al., to reduce the storage requirement and the cost by combining different agricultural biomass to be stored and transported together [288]. Finally, the transportation of the biomass to the conversion facility is governed by the distance and location of the conversion facility, biomass density, load capacity of the vehicle, and traveling speed [290,291,292,293,294]. The preferable logistics and methodology for collection, storage, and transportation are decided by the cost of the process. AI/ML-based supply chain modeling and analysis can help decide the most optimized options [280, 290].
-
b)
Once biomass feedstock reaches the location, the conversion process is decided based on the type of biomass, product requirement, and cost of production. As discussed previously, lignocellulosic biomass, municipal waste, agricultural and forest residues, etc. have immense potential to become the preferred source of bioenergy provided the pre-treatment processes are made more economical and their technological barriers to scale-up are removed. Biochemical and thermochemical processes like fermentation (normal, dark, and photo), anaerobic digestion, and torrefaction are the most scalable processes that can be commercialized to produce bio-methanol, bio-ethanol, and bio-hydrogen. Large-scale algal biomass generation is also a lucrative option if the challenges of huge water and nutrient requirements, and large energy need for drying are addressed. Moreover, integrated processes and integrated biorefineries demonstrate improved productivities compared to standalone conversion methods but require more technological interventions.
-
c)
Most of the conversion processes being researched are energy-intensive. Drying the biomass for energy conversion requires a huge energy input, which is currently derived from fossil fuels. Also, though we consider biomass as CO2 neutral process, many of the conversion technologies like pyrolysis, combustion, and gasification produces CO2 during the process. Furthermore, burning of the bio-char or hydro-char will also release CO2 and other GHG. Mat Aron et al. has provided a detailed analysis of GHG emissions from different conversion process and concluded that there is no conversion process with net-zero GHG emissions; however, the emissions are much lesser compared to fossil fuel burning [40]. Thus, analysis of energy requirements for the process and the GHF emissions during the process and post-processing is imperative to choose the most energy-efficient and environment-friendly process. Furthermore, fourth-generation (genetically modified) algal biomass may be developed to sequester the GHG and increase lipid production [40].
-
d)
Finally, the policies and government interventions for standardization of biomass price and quality control are necessary. Government subsidies, support plans, and price incentives are also required to encourage industries to invest in the commercialization processes [295]. Policy interventions are also essential to encourage the 1G ethanol producers to move toward 2G ethanol production, thus increasing food security [295, 296]. Another approach to expanding the biofuel sector is to establish small-scale biofuel plants in rural settings to meet their local energy needs via clean and sustainable routes [296]. Finally, to improve the global market for biofuels and create a global need, international collaborations and global market-oriented policies for the biofuel sector are crucial [296, 297].
Conclusions
Biomass as an alternate energy source is lucrative and has immense potential to be developed into a commercially viable solution to energy challenges and waste management. Biomass is abundantly available throughout the world which allows biorefineries to be established at any geographical location. Biomass as a source of energy can fulfill the electricity, heat, and transportation fuel needs. However, policy interventions are required to standardize biomass feedstock collection, distribution, transportation, and cost. Efforts are also needed to make conversion technologies more economic, easy to scale up, user-friendly, and with improved productivity. More emphasis is needed to improve the lignocellulosic biomass, forest and agricultural residues, and municipal waste pre-treatment and conversion processes. The choice of the best conversion technology will be decided by the ease of operation and cost of production along with its energy efficiency and environmental impact. Based on the current analysis, 2G biomass conversion via biochemical and thermochemical pathways holds the most prospects. However, this study is limited to bringing an overview of the entire biomass to energy process and the technological interventions. Some limited discussions on the collection, segregation, transportation of biomass, AI/ML-based modeling, and optimization routes and policies are presented. However, the biomass pricing and economics of the processes are not reviewed here. Furthermore, a more detailed discussion is entailed on challenges in storage and transportation of biomass, policies, and other modeling and simulation-based approaches to improve bioenergy generation.
Abbreviations
- 1G:
-
First generation
- 2G:
-
Second generation
- 3G:
-
Third generation
- 4G:
-
Fourth generation
- AI:
-
Artificial intelligence
- ANN:
-
Artificial neural network
- ATP:
-
Adenosine triphosphate
- BIG/CC:
-
Biomass integrated gasification with combined cycle
- CLC:
-
Chemical looping combustion
- CLG:
-
Chemical loop biomass gasification
- CSIR:
-
Council of Scientific & Industrial Research
- EDP:
-
Entner-Doudoroff pathway
- EMP :
-
Embden-Meyerhof pathway
- FT:
-
Fischer-Tropsch
- GHG:
-
Greenhouse gas
- IEA:
-
International Energy Agency
- LFG:
-
Landfill gas
- LHV:
-
Lowest heating value
- MMT:
-
Million metric ton
- ML:
-
Machine learning
- PPP:
-
Pentose-phosphate pathway
- SCWG:
-
Supercritical water gasification
- SSCF:
-
Simultaneous saccharification and co-fermentation
- SSF:
-
Simultaneous saccharification and fermentation
References
Gielen D, Boshell F, Saygin D et al (2019) The role of renewable energy in the global energy transformation. Energy Strat Rev 24:38–50. https://doi.org/10.1016/j.esr.2019.01.006
International Energy Agency (2019) World energy balances: An Overview. Paris 2019. https://www.iea.org/reports/world-energy-balances-overview
International Energy Agency (2020) India 2020 - Energy Review Policy. https://www.iea.org/reports/india-2020
Pappu A, Saxena M, Asolekar SR (2007) Solid wastes generation in India and their recycling potential in building materials. Build Environ 42:2311–2320. https://doi.org/10.1016/j.buildenv.2006.04.015
Xingang Z, Wang J, Liu X, Liu P (2012) China’s wind, biomass, and solar power generation: what the situation tells us? Renew Sustain Energy Rev 16:6173–6182. https://doi.org/10.1016/j.rser.2012.07.020
van Dael M, van Passel S, Pelkmans L et al (2013) A techno-economic evaluation of a biomass energy conversion park. Appl Energy 104:611–622. https://doi.org/10.1016/j.apenergy.2012.11.071
Sriram N, Shahidehpour M (2005) Renewable biomass energy. In: IEEE Power Engineering Society General Meeting, 2005. IEEE, pp 612–617. https://www.semanticscholar.org/paper/Renewable-biomass-energy-Sriram-Shahidehpour/8d5c1eea82cfdb059c76f1d6b506a474ac8ad758
Long SP, Karp A, Buckeridge MS et al (2015) Feedstocks for biofuels and bioenergy. In: van Sluys M-A (ed) Bioenergy & sustainability: bridging the gaps. Scientific Committee on Problems of the Environment, pp 302–346. http://bioenfapesp.org/scopebioenergy/images/chapters/bioen-scope_chapter10.pdf
Vassilev SV, Vassileva CG, Vassilev VS (2015) Advantages and disadvantages of composition and properties of biomass in comparison with coal: an overview. Fuel 158:330–350. https://doi.org/10.1016/j.fuel.2015.05.050
Hoang AT, Sirohi R, Pandey A et al (2022) Biofuel production from microalgae: challenges and chances. Phytochem Rev. https://doi.org/10.1007/s11101-022-09819-y
Chen H, Xia A, Zhu X et al (2022) Hydrothermal hydrolysis of algal biomass for biofuels production: a review. Biores Technol 344:126213. https://doi.org/10.1016/j.biortech.2021.126213
Yana S, Nizar M, Irhamni MD (2022) Biomass waste as a renewable energy in developing bio-based economies in Indonesia: a review. Renew Sustain Energy Rev 160:112268. https://doi.org/10.1016/j.rser.2022.112268
Saravanan A, Senthil Kumar P, Jeevanantham S et al (2022) Recent advances and sustainable development of biofuels production from lignocellulosic biomass. Biores Technol 344:126203. https://doi.org/10.1016/j.biortech.2021.126203
Ashokkumar V, Venkatkarthick R, Jayashree S et al (2022) Recent advances in lignocellulosic biomass for biofuels and value-added bioproducts - a critical review. Biores Technol 344:126195. https://doi.org/10.1016/j.biortech.2021.126195
Amjith LR, Bavanish B (2022) A review on biomass and wind as renewable energy for sustainable environment. Chemosphere 293:133579. https://doi.org/10.1016/j.chemosphere.2022.133579
Lu H, Yadav V, Zhong M et al (2022) Bioengineered microbial platforms for biomass-derived biofuel production – a review. Chemosphere 288:132528. https://doi.org/10.1016/j.chemosphere.2021.132528
McKendry P (2002) Energy production from biomass (part 2): conversion technologies. Biores Technol 83:47–54. https://doi.org/10.1016/S0960-8524(01)00119-5
Ebhodaghe SO, Imanah OE, Ndibe H (2022) Biofuels from microalgae biomass: a review of conversion processes and procedures. Arab J Chem 15:103591. https://doi.org/10.1016/j.arabjc.2021.103591
Tursi A (2019) A review on biomass: importance, chemistry, classification, and conversion. Biofuel Res J 6:962–979. https://doi.org/10.18331/BRJ2019.6.2.3
Chakraborty S, Aggarwal V, Mukherjee D, Andras K (2012) Biomass to biofuel: a review on production technology. Asia-Pac J Chem Eng 7:S254–S262. https://doi.org/10.1002/apj.1642
Clark JH, Deswarte FEI (2008) The biorefinery concept-an integrated approach. In: Introduction to Chemicals from Biomass. Wiley, Chichester, pp 1–20. https://doi.org/10.1002/9780470697474.ch1
Haldar D, Purkait MK (2020) Lignocellulosic conversion into value-added products: a review. Process Biochem 89:110–133. https://doi.org/10.1016/j.procbio.2019.10.001
Antar M, Lyu D, Nazari M et al (2021) Biomass for a sustainable bioeconomy: an overview of world biomass production and utilization. Renew Sustain Energy Rev 139:110691. https://doi.org/10.1016/j.rser.2020.110691
McKendry P (2002) Energy production from biomass (part 1): overview of biomass. Biores Technol 83:37–46. https://doi.org/10.1016/S0960-8524(01)00118-3
Hartley DS, Thompson DN, Griffel LM et al (2020) Effect of biomass properties and system configuration on the operating effectiveness of biomass to biofuel systems. ACS Sustain Chem Eng 8:7267–7277. https://doi.org/10.1021/acssuschemeng.9b06551
Yakoyama S, Yukihiko M (2008) The Asian biomass handbook - a guide for biomass production and utilization. The Japan Institute of Energy. https://www.jie.or.jp/relays/download/?file=/files/libs/732/201708300901271178.pdf
Gani A, Naruse I (2007) Effect of cellulose and lignin content on pyrolysis and combustion characteristics for several types of biomass. Renew Energy 32:649–661. https://doi.org/10.1016/j.renene.2006.02.017
Vassilev SV, Baxter D, Andersen LK, Vassileva CG (2010) An overview of the chemical composition of biomass. Fuel 89:913–933. https://doi.org/10.1016/j.fuel.2009.10.022
Joshi G, Pandey JK, Rana S, Rawat DS (2017) Challenges and opportunities for the application of biofuel. Renew Sustain Energy Rev 79:850–866. https://doi.org/10.1016/j.rser.2017.05.185
Lauri P, Havlík P, Kindermann G et al (2014) Woody biomass energy potential in 2050. Energy Policy 66:19–31. https://doi.org/10.1016/j.enpol.2013.11.033
Kumar M, Kumar S, Tyagi SK (2013) Design, development and technological advancement in the biomass cookstoves: a review. Renew Sustain Energy Rev 26:265–285. https://doi.org/10.1016/j.rser.2013.05.010
Banerjee N (2021) Predictive model development and simulation of photobioreactors for algal biomass growth estimation. Int J Chem Reactor Eng 19:139–153. https://doi.org/10.1515/ijcre-2020-0218
Banerjee N (2020) Design of photobioreactors for algal biomass growth. SSRN Electron J:1–17.https://doi.org/10.2139/ssrn.3728496
Ramachandra TV, Hebbale D (2020) Bioethanol from macroalgae: prospects and challenges. Renew Sustain Energy Rev 117:109479. https://doi.org/10.1016/j.rser.2019.109479
Vu HP, Nguyen LN, Vu MT et al (2020) A comprehensive review on the framework to valorise lignocellulosic biomass as biorefinery feedstocks. Sci Total Environ 743:140630. https://doi.org/10.1016/j.scitotenv.2020.140630
Peng L, Fu D, Chu H et al (2020) Biofuel production from microalgae: a review. Environ Chem Lett 18:285–297. https://doi.org/10.1007/s10311-019-00939-0
Aravind S, Kumar PS, Kumar NS, Siddarth N (2020) Conversion of green algal biomass into bioenergy by pyrolysis. A review. Environ Chem Lett 18:829–849. https://doi.org/10.1007/s10311-020-00990-2
Huang HJ, Ramaswamy S, Tschirner UW, Ramarao BV (2008) A review of separation technologies in current and future biorefineries. Sep Purif Technol 62:1–21. https://doi.org/10.1016/j.seppur.2007.12.011
Yadav VG, Yadav GD, Patankar SC (2020) The production of fuels and chemicals in the new world: critical analysis of the choice between crude oil and biomass vis-à-vis sustainability and the environment. Clean Technol Environ Policy 22:1757–1774. https://doi.org/10.1007/s10098-020-01945-5
Mat Aron NS, Khoo KS, Chew KW et al (2020) Sustainability of the four generations of biofuels – a review. Int J Energy Res 44:9266–9282. https://doi.org/10.1002/er.5557
Ayodele BV, Alsaffar MA, Mustapa SI (2020) An overview of integration opportunities for sustainable bioethanol production from first-and second-generation sugar-based feedstocks. J Clean Prod 245:118857
Chew KW, Chia SR, Show PL et al (2018) Effects of water culture medium, cultivation systems and growth modes for microalgae cultivation: a review. J Taiwan Inst Chem Eng 91:332–344. https://doi.org/10.1016/j.jtice.2018.05.039
Haji Esmaeili SA, Szmerekovsky J, Sobhani A et al (2020) Sustainable biomass supply chain network design with biomass switching incentives for first-generation bioethanol producers. Energy Policy 138:111222. https://doi.org/10.1016/j.enpol.2019.111222
Alalwan HA, Alminshid AH, Aljaafari HASS (2019) Promising evolution of biofuel generations. Subject review. Renew Energy Focus 28:127–139. https://doi.org/10.1016/j.ref.2018.12.006
Cai H, Dunn JB, Wang Z et al (2013) Life-cycle energy use and greenhouse gas emissions of production of bioethanol from sorghum in the United States. Biotechnol Biofuels 6:141. https://doi.org/10.1186/1754-6834-6-141
Borzęcka-Walker M, Faber A, Pudełko R et al (2011) Life cycle assessment (LCA) of crops for energy production. J Food Agric Environ 9:698–700
Rahim AHA, Khoo KS, Yunus NM, Hamzah WSW (2019) Ether-functionalized ionic liquids as solvent for Gigantochloa scortechini dissolution. In: AIP Conference Proceedings. AIP Publishing LLC, p 020025. https://doi.org/10.1063/1.5126560
Gnansounou E (2010) Production and use of lignocellulosic bioethanol in Europe: current situation and perspectives. Biores Technol 101:4842–4850. https://doi.org/10.1016/j.biortech.2010.02.002
Sánchez ÓJ, Cardona CA (2008) Trends in biotechnological production of fuel ethanol from different feedstocks. Biores Technol 99:5270–5295. https://doi.org/10.1016/j.biortech.2007.11.013
Bhatia SK, Jagtap SS, Bedekar AA et al (2020) Recent developments in pretreatment technologies on lignocellulosic biomass: effect of key parameters, technological improvements, and challenges. Biores Technol 300:122724. https://doi.org/10.1016/j.biortech.2019.122724
da Silva ASA, Espinheira RP, Teixeira RSS et al (2020) Constraints and advances in high-solids enzymatic hydrolysis of lignocellulosic biomass: a critical review. Biotechnol Biofuels 13:1–28. https://doi.org/10.1186/s13068-020-01697-w
Balan V (2014) Current challenges in commercially producing biofuels from lignocellulosic biomass. ISRN Biotechnol 2014:1–31. https://doi.org/10.1155/2014/463074
Tang DYY, Khoo KS, Chew KW et al (2020) Potential utilization of bioproducts from microalgae for the quality enhancement of natural products. Biores Technol 304:122997. https://doi.org/10.1016/j.biortech.2020.122997
Yi-Feng C, Wu Q (2011) Production of biodiesel from algal biomass. In: Biofuels. Elsevier, pp 399–413
Bennion EP, Ginosar DM, Moses J et al (2015) Lifecycle assessment of microalgae to biofuel: comparison of thermochemical processing pathways. Appl Energy 154:1062–1071. https://doi.org/10.1016/j.apenergy.2014.12.009
Dasan YK, Lam MK, Yusup S et al (2019) Life cycle evaluation of microalgae biofuels production: effect of cultivation system on energy, carbon emission and cost balance analysis. Sci Total Environ 688:112–128. https://doi.org/10.1016/j.scitotenv.2019.06.181
Abdullah B, Syed Muhammad SAF, Shokravi Z et al (2019) Fourth generation biofuel: a review on risks and mitigation strategies. Renew Sustain Energy Rev 107:37–50. https://doi.org/10.1016/j.rser.2019.02.018
Hannon M, Gimpel J, Tran M et al (2010) Biofuels from algae: challenges and potential. Biofuels 1:763–784. https://doi.org/10.4155/bfs.10.44
Kumar S, Sani RK (2018) Biorefining of biomass to biofuels. Springer International Publishing, Cham
Stark A (2011) Ionic liquids in the biorefinery: a critical assessment of their potential. Energy Environ Sci 4:19–32. https://doi.org/10.1039/C0EE00246A
Baruah J, Nath BK, Sharma R et al (2018) Recent trends in the pretreatment of lignocellulosic biomass for value-added products. Front Energy Res 6:141. https://doi.org/10.3389/fenrg.2018.00141
Hassan SS, Williams GA, Jaiswal AK (2019) Lignocellulosic biorefineries in Europe: current state and prospects. Trends Biotechnol 37:231–234
Houfani AA, Anders N, Spiess AC et al (2020) Insights from enzymatic degradation of cellulose and hemicellulose to fermentable sugars– a review. Biomass Bioenergy 134:105481. https://doi.org/10.1016/j.biombioe.2020.105481
Singh A, Rodríguez Jasso RM, Gonzalez-Gloria KD et al (2019) The enzyme biorefinery platform for advanced biofuels production. Bioresour Technol Rep 7:100257. https://doi.org/10.1016/j.biteb.2019.100257
Hassan SS, Williams GA, Jaiswal AK (2019) Moving towards the second generation of lignocellulosic biorefineries in the EU: drivers, challenges, and opportunities. Renew Sustain Energy Rev 101:590–599. https://doi.org/10.1016/j.rser.2018.11.041
Cheah WY, Sankaran R, Show PL et al (2020) Pretreatment methods for lignocellulosic biofuels production: current advances, challenges and future prospects. Biofuel Res J 7:1115–1127. https://doi.org/10.18331/BRJ2020.7.1.4
Ahorsu R, Medina F, Constantí M (2018) Significance and challenges of biomass as a suitable feedstock for bioenergy and biochemical production: a review. Energies 11:3366. https://doi.org/10.3390/en11123366
Fisher T, Hajaligol M, Waymack B, Kellogg D (2002) Pyrolysis behavior and kinetics of biomass derived materials. J Anal Appl Pyrol 62:331–349. https://doi.org/10.1016/S0165-2370(01)00129-2
Yang H, Yan R, Chen H et al (2007) Characteristics of hemicellulose, cellulose and lignin pyrolysis. Fuel 86:1781–1788. https://doi.org/10.1016/j.fuel.2006.12.013
Klein-Marcuschamer D, Oleskowicz-Popiel P, Simmons BA, Blanch HW (2012) The challenge of enzyme cost in the production of lignocellulosic biofuels. Biotechnol Bioeng 109:1083–1087. https://doi.org/10.1002/bit.24370
Carriquiry MA, Du X, Timilsina GR (2011) Second generation biofuels: economics and policies. Energy Policy 39:4222–4234. https://doi.org/10.1016/j.enpol.2011.04.036
Shah SH, Raja IA, Mahmood Q, Pervez A (2016) Improvement in lipids extraction processes for biodiesel production from wet microalgal pellets grown on diammonium phosphate and sodium bicarbonate combinations. Biores Technol 214:199–209. https://doi.org/10.1016/j.biortech.2016.04.036
Hoh D, Watson S, Kan E (2016) Algal biofilm reactors for integrated wastewater treatment and biofuel production: a review. Chem Eng J 287:466–473. https://doi.org/10.1016/j.cej.2015.11.062
Faried M, Samer M, Abdelsalam E et al (2017) Biodiesel production from microalgae: processes, technologies and recent advancements. Renew Sustain Energy Rev 79:893–913. https://doi.org/10.1016/j.rser.2017.05.199
Wang Y, Ho S-H, Cheng C-L et al (2016) Perspectives on the feasibility of using microalgae for industrial wastewater treatment. Biores Technol 222:485–497. https://doi.org/10.1016/j.biortech.2016.09.106
Halder P, Azad K, Shah S, Sarker E (2019) Prospects and technological advancement of cellulosic bioethanol ecofuel production. In: Advances in Eco-Fuels for a Sustainable Environment. Elsevier, pp 211–236. https://doi.org/10.1016/B978-0-08-102728-8.00008-5
Bhargavi G, Rao PN, Renganathan S (2018) Review on the extraction methods of crude oil from all generation biofuels in last few decades. In: IOP Conference Series: Materials Science and Engineering. IOP Publishing, p 12024. https://iopscience.iop.org/article/10.1088/1757-899X/330/1/012024
Rodionova MV, Poudyal RS, Tiwari I et al (2017) Biofuel production: challenges and opportunities. Int J Hydrogen Energy 42:8450–8461. https://doi.org/10.1016/j.ijhydene.2016.11.125
Khoo KS, Lee SY, Ooi CW et al (2019) Recent advances in biorefinery of astaxanthin from Haematococcus pluvialis. Biores Technol 288:121606. https://doi.org/10.1016/j.biortech.2019.121606
Liu Z, Wang K, Chen Y et al (2020) Third-generation biorefineries as the means to produce fuels and chemicals from CO2. Nat Catal 3:274–288. https://doi.org/10.1038/s41929-019-0421-5
Banerjee N, Sukichandran P, Chaudhari P et al (2022) Energy analysis and feasibility studies for algal biomass and biofuels. Mater Today Proc 57:1448–1454. https://doi.org/10.1016/j.matpr.2021.11.223
Zhu LD, Li ZH, Hiltunen E (2016) Strategies for lipid production improvement in microalgae as a biodiesel feedstock. Biomed Res Int 2016:7–9. https://doi.org/10.1155/2016/8792548
Zhu B, Chen G, Cao X, Wei D (2017) Molecular characterization of CO2 sequestration and assimilation in microalgae and its biotechnological applications. Biores Technol 244:1207–1215. https://doi.org/10.1016/j.biortech.2017.05.199
Adeniyi OM, Azimov U, Burluka A (2018) Algae biofuel: current status and future applications. Renew Sustain Energy Rev 90:316–335. https://doi.org/10.1016/j.rser.2018.03.067
Jiang R, Ingle KN, Golberg A (2016) Macroalgae (seaweed) for liquid transportation biofuel production: what is next? Algal Res 14:48–57. https://doi.org/10.1016/j.algal.2016.01.001
Ubando AT, Felix CB, Chen WH (2020) Biorefineries in circular bioeconomy: a comprehensive review. Bioresour Technol 299.https://doi.org/10.1016/j.biortech.2019.122585
Searchinger T, Heimlich R, Houghton RA et al (2008) Use of U.S. croplands for biofuels increases greenhouse gases through emissions from land-use change. Science 319:1238–1240. https://doi.org/10.1126/science.1151861
Cheroennet N, Suwanmanee U (2017) Net energy gain and water footprint of corn ethanol production in Thailand. Energy Procedia 118:15–20. https://doi.org/10.1016/j.egypro.2017.07.003
da Silva ARG, Torres Ortega CE, Rong B-G (2016) Techno-economic analysis of different pretreatment processes for lignocellulosic-based bioethanol production. Biores Technol 218:561–570. https://doi.org/10.1016/j.biortech.2016.07.007
Rooni V, Raud M, Kikas T (2017) The freezing pre-treatment of lignocellulosic material: a cheap alternative for Nordic countries. Energy 139:1–7. https://doi.org/10.1016/j.energy.2017.07.146
Wu X-F, Yin S-S, Zhou Q et al (2019) Subcritical liquefaction of lignocellulose for the production of bio-oils in ethanol/water system. Renew Energy 136:865–872. https://doi.org/10.1016/j.renene.2019.01.041
Zhang H, Zhang P, Ye J et al (2018) Comparison of various pretreatments for ethanol production enhancement from solid residue after rumen fluid digestion of rice straw. Biores Technol 247:147–156. https://doi.org/10.1016/j.biortech.2017.09.065
Cardona E, Llano B, Peñuela M et al (2018) Liquid-hot-water pretreatment of palm-oil residues for ethanol production: an economic approach to the selection of the processing conditions. Energy 160:441–451. https://doi.org/10.1016/j.energy.2018.07.045
Shah SH, Raja IA, Rizwan M et al (2018) Potential of microalgal biodiesel production and its sustainability perspectives in Pakistan. Renew Sustain Energy Rev 81:76–92. https://doi.org/10.1016/j.rser.2017.07.044
Abu-Ghosh S, Fixler D, Dubinsky Z, Iluz D (2015) Energy-input analysis of the life-cycle of microalgal cultivation systems and best scenario for oil-rich biomass production. Appl Energy 154:1082–1088. https://doi.org/10.1016/j.apenergy.2015.02.086
Stephenson AL, Kazamia E, Dennis JS et al (2010) Life-cycle assessment of potential algal biodiesel production in the United Kingdom: a comparison of raceways and air-lift tubular bioreactors. Energy Fuels 24:4062–4077. https://doi.org/10.1021/ef1003123
Kamm B, Gruber PR, Kamm M (2016) Biorefineries-industrial processes and products. Ullmann’s Encyclopedia of Industrial Chemistry. Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim, pp 1–38
Kamm B, Kamm M (2004) Principles of biorefineries. Appl Microbiol Biotechnol 64:137–145. https://doi.org/10.1007/s00253-003-1537-7
Hassan SS, Williams GA, Jaiswal AK (2019) Lignocellulosic biorefineries in Europe: current state and prospects. Trends Biotechnol 37:231–234. https://doi.org/10.1016/j.tibtech.2018.07.002
Mariana OS, Camilo STJ, Ariel CAC (2021) A comprehensive approach for biorefineries design based on experimental data, conceptual and optimization methodologies: the orange peel waste case. Biores Technol 325:124682. https://doi.org/10.1016/j.biortech.2021.124682
Stafford W, de Lange W, Nahman A et al (2020) Forestry biorefineries. Renew Energy 154:461–475. https://doi.org/10.1016/j.renene.2020.02.002
Naik SN, Goud VV, Rout PK, Dalai AK (2010) Production of first and second generation biofuels: a comprehensive review. Renew Sustain Energy Rev 14:578–597. https://doi.org/10.1016/j.rser.2009.10.003
Fernando S, Adhikari S, Chandrapal C, Murali N (2006) Biorefineries: current status, challenges, and future direction. Energy Fuels 20:1727–1737
Alibardi L, Astrup TF, Asunis F et al (2020) Organic waste biorefineries: looking towards implementation. Waste Manage 114:274–286. https://doi.org/10.1016/j.wasman.2020.07.010
Chen WH, Lin BJ, Lin YY et al (2021) Progress in biomass torrefaction: principles, applications and challenges. Prog Energy Combust Sci 82:100887. https://doi.org/10.1016/j.pecs.2020.100887
Dragone G, Kerssemakers AAJ, Driessen JLSP et al (2020) Innovation and strategic orientations for the development of advanced biorefineries. Biores Technol 302:122847. https://doi.org/10.1016/j.biortech.2020.122847
de Bhowmick G, Sarmah AK, Sen R (2018) Lignocellulosic biorefinery as a model for sustainable development of biofuels and value added products. Biores Technol 247:1144–1154. https://doi.org/10.1016/j.biortech.2017.09.163
Clauser NM, Felissia FE, Area MC, Vallejos ME (2021) A framework for the design and analysis of integrated multi-product biorefineries from agricultural and forestry wastes. Renew Sustain Energy Rev 139:110687. https://doi.org/10.1016/j.rser.2020.110687
Galbe M, Wallberg O (2019) Pretreatment for biorefineries: a review of common methods for efficient utilisation of lignocellulosic materials. Biotechnol Biofuels 12:1–26. https://doi.org/10.1186/s13068-019-1634-1
Chandel AK, Garlapati VK, Singh AK et al (2018) The path forward for lignocellulose biorefineries: bottlenecks, solutions, and perspective on commercialization. Biores Technol 264:370–381. https://doi.org/10.1016/j.biortech.2018.06.004
Yamakawa CK, Qin F, Mussatto SI (2018) Advances and opportunities in biomass conversion technologies and biorefineries for the development of a bio-based economy. Biomass Bioenergy 119:54–60. https://doi.org/10.1016/j.biombioe.2018.09.007
Severo IA, Siqueira SF, Deprá MC et al (2019) Biodiesel facilities: what can we address to make biorefineries commercially competitive? Renew Sustain Energy Rev 112:686–705. https://doi.org/10.1016/j.rser.2019.06.020
Chew KW, Yap JY, Show PL et al (2017) Microalgae biorefinery: high value products perspectives. Biores Technol 229:53–62. https://doi.org/10.1016/j.biortech.2017.01.006
de Bhowmick G, Sarmah AK, Sen R (2019) Zero-waste algal biorefinery for bioenergy and biochar: a green leap towards achieving energy and environmental sustainability. Sci Total Environ 650:2467–2482. https://doi.org/10.1016/j.scitotenv.2018.10.002
Liu J, Wang D, Yu C et al (2021) A two-step process for energy-efficient conversion of food waste via supercritical water gasification: process design, products analysis, and electricity evaluation. Sci Total Environ 752:142331. https://doi.org/10.1016/j.scitotenv.2020.142331
Torres MD, Kraan S, Domínguez H (2019) Seaweed biorefinery. Rev Environ Sci Biotechnol 18:335–388. https://doi.org/10.1007/s11157-019-09496-y
Sosa-Hernández J, Romero-Castillo K, Parra-Arroyo L et al (2019) Mexican microalgae biodiversity and state-of-the-art extraction strategies to meet sustainable circular economy challenges: high-value compounds and their applied perspectives. Mar Drugs 17:174. https://doi.org/10.3390/md17030174
Walmsley TG, Varbanov PS, Su R et al (2018) Frontiers in process development, integration and intensification for circular life cycles and reduced emissions. J Clean Prod 201:178–191. https://doi.org/10.1016/j.jclepro.2018.08.041
Uggetti E, García J, Álvarez JA, García-Galán MJ (2018) Start-up of a microalgae-based treatment system within the biorefinery concept: from wastewater to bioproducts. Water Sci Technol 78:114–124. https://doi.org/10.2166/wst.2018.195
Japar AS, Takriff MS, Yasin NHM (2017) Harvesting microalgal biomass and lipid extraction for potential biofuel production: a review. J Environ Chem Eng 5:555–563. https://doi.org/10.1016/j.jece.2016.12.016
Venkata Mohan S, Dahiya S, Amulya K et al (2019) Can circular bioeconomy be fueled by waste biorefineries — a closer look. Bioresour Technol Rep 7:100277. https://doi.org/10.1016/j.biteb.2019.100277
Minelgaitė A, Liobikienė G (2019) Waste problem in European Union and its influence on waste management behaviours. Sci Total Environ 667:86–93. https://doi.org/10.1016/j.scitotenv.2019.02.313
Cristóbal J, Caldeira C, Corrado S, Sala S (2018) Techno-economic and profitability analysis of food waste biorefineries at European level. Biores Technol 259:244–252. https://doi.org/10.1016/j.biortech.2018.03.016
Chandak SP, Chari KR, Memon MA (2015) Converting waste agricultural biomass into energy: experiences and lessons learnt from a capacity building and technology demonstration project in India. J Jpn Inst Energy 94:1129–1147. https://doi.org/10.3775/jie.94.1129
Skaggs RL, Coleman AM, Seiple TE, Milbrandt AR (2018) Waste-to-energy biofuel production potential for selected feedstocks in the conterminous United States. Renew Sustain Energy Rev 82:2640–2651. https://doi.org/10.1016/j.rser.2017.09.107
Bastidas-Oyanedel J-R, Schmidt J (2018) Increasing profits in food waste biorefinery—a techno-economic analysis. Energies (Basel) 11:1551. https://doi.org/10.3390/en11061551
Schebek L, Mrani O (2014) Environmental and sustainability assessment of biorefineries. In: Advances in Biorefineries. Elsevier, pp 67–88. https://doi.org/10.1533/9780857097385.1.67
Serrano A, Fermoso FG, Alonso-Fariñas B et al (2017) Olive mill solid waste biorefinery: high-temperature thermal pre-treatment for phenol recovery and biomethanization. J Clean Prod 148:314–323. https://doi.org/10.1016/j.jclepro.2017.01.152
Adu C, Jolly M, Thakur VK (2018) Exploring new horizons for paper recycling: a review of biomaterials and biorefinery feedstocks derived from wastepaper. Curr Opin Green Sustain Chem 13:21–26. https://doi.org/10.1016/j.cogsc.2018.03.003
Barampouti EM, Mai S, Malamis D et al (2019) Liquid biofuels from the organic fraction of municipal solid waste: a review. Renew Sustain Energy Rev 110:298–314. https://doi.org/10.1016/j.rser.2019.04.005
Nizami AS, Rehan M, Waqas M et al (2017) Waste biorefineries: enabling circular economies in developing countries. Biores Technol 241:1101–1117. https://doi.org/10.1016/j.biortech.2017.05.097
Mesa L, González E, Ruiz E et al (2010) Preliminary evaluation of organosolv pre-treatment of sugar cane bagasse for glucose production: application of 23 experimental design. Appl Energy 87:109–114. https://doi.org/10.1016/j.apenergy.2009.07.016
Lebaka VR (2013) Potential bioresources as future sources of biofuels production: an overview. Biofuel Technologies. Springer Berlin Heidelberg, Berlin, pp 223–258
Portha J-F, Parkhomenko K, Kobl K et al (2017) Kinetics of methanol synthesis from carbon dioxide hydrogenation over copper–zinc oxide catalysts. Ind Eng Chem Res 56:13133–13145. https://doi.org/10.1021/acs.iecr.7b01323
Gaurav N, Sivasankari S, Kiran GS et al (2017) Utilization of bioresources for sustainable biofuels: a review. Renew Sustain Energy Rev 73:205–214. https://doi.org/10.1016/j.rser.2017.01.070
Mahalaxmi S, Williford C (2012) Handbook of climate change mitigation. Springer US, New York
Lin Y, Tanaka S (2006) Ethanol fermentation from biomass resources: current state and prospects. Appl Microbiol Biotechnol 69:627–642. https://doi.org/10.1007/s00253-005-0229-x
Callegari A, Bolognesi S, Cecconet D, Capodaglio AG (2020) Production technologies, current role, and future prospects of biofuels feedstocks: a state-of-the-art review. Crit Rev Environ Sci Technol 50:384–436. https://doi.org/10.1080/10643389.2019.1629801
Khan Z, Dwivedi AK (2013) Fermentation of biomass for production of ethanol: a review abstract: 2. Potential of biomass. Univers J Environ Res Technol 3:1–13
Strezov V (2014) Properties of biomass fuels. In: Strezov V, Evans TJ (eds) Biomass processing technologies., Illustrate. CRC Press, Boca Raton, pp 1–32
Yu W, Quek WP, Li C et al (2018) Effects of the starch molecular structures in barley malts and rice adjuncts on brewing performance. Fermentation 4. https://doi.org/10.3390/fermentation4040103
Taherzadeh M, Karimi K (2008) Pretreatment of lignocellulosic wastes to improve ethanol and biogas production: a review. Int J Mol Sci 9:1621–1651. https://doi.org/10.3390/ijms9091621
Lin CY, Lu C (2021) Development perspectives of promising lignocellulose feedstocks for production of advanced generation biofuels: a review. Renew Sustain Energy Rev 136:110445. https://doi.org/10.1016/j.rser.2020.110445
Mohanty SK, Swain MR (2019) Bioethanol production from corn and wheat: food, fuel, and future. Elsevier Inc. https://doi.org/10.1016/C2017-0-00234-3
Paudel SR, Banjara SP, Choi OK et al (2017) Pretreatment of agricultural biomass for anaerobic digestion: current state and challenges. Biores Technol 245:1194–1205. https://doi.org/10.1016/j.biortech.2017.08.182
Faustine AS, Djamaan A (2021) Bioethanol production from various agricultural waste substrate using Saccharomyces cerevisiae. IOSR J Pharm Biol Sci 16:7–13. https://doi.org/10.9790/3008-1601030713
Szambelan K, Nowak J, Szwengiel A et al (2018) Separate hydrolysis and fermentation and simultaneous saccharification and fermentation methods in bioethanol production and formation of volatile by-products from selected corn cultivars. Ind Crops Prod 118:355–361. https://doi.org/10.1016/j.indcrop.2018.03.059
Li H, Mei X, Liu B et al (2019) Insights on acetate-ethanol fermentation by hydrogen-producing Ethanoligenens under acetic acid accumulation based on quantitative proteomics. Environ Int 129:1–9. https://doi.org/10.1016/j.envint.2019.05.013
Li W-C, Zhu J-Q, Zhao X et al (2019) Improving co-fermentation of glucose and xylose by adaptive evolution of engineering xylose-fermenting Saccharomyces cerevisiae and different fermentation strategies. Renew Energy 139:1176–1183. https://doi.org/10.1016/j.renene.2019.03.028
Zhang Y, Wang C, Wang L et al (2017) Direct bioethanol production from wheat straw using xylose/glucose co-fermentation by co-culture of two recombinant yeasts. J Ind Microbiol Biotechnol 44:453–464
Ye G, Zeng D, Zhang S et al (2018) Ethanol production from mixtures of sugarcane bagasse and Dioscorea composita extracted residue with high solid loading. Biores Technol 257:23–29. https://doi.org/10.1016/j.biortech.2018.02.008
Sadh PK, Duhan S, Duhan JS (2018) Agro-industrial wastes and their utilization using solid state fermentation: a review. Bioresour Bioprocess 5:1–15. https://doi.org/10.1186/s40643-017-0187-z
Lepage T, Kammoun M, Schmetz Q, Richel A (2021) Biomass-to-hydrogen: a review of main routes production, processes evaluation and techno-economical assessment. Biomass Bioenergy 144:105920. https://doi.org/10.1016/j.biombioe.2020.105920
Zamani A (2015) Introduction to lignocellulose-based products. In: Karimi K (ed) Lignocellulose-based bioproducts. Springer, Berlin, pp 1–36
Sharma VK (2015) Technology development and innovation for production of next-generation biofuel from lignocellulosic wastes. In: Sharma A, Kar S (eds) Energy sustainability through green energy. Springer, Berlin, pp 315–350
Zamri MFMA, Hasmady S, Akhiar A et al (2021) A comprehensive review on anaerobic digestion of organic fraction of municipal solid waste. Renew Sustain Energy Rev 137:110637. https://doi.org/10.1016/j.rser.2020.110637
Ren Y, Yu M, Wu C et al (2018) A comprehensive review on food waste anaerobic digestion: research updates and tendencies. Biores Technol 247:1069–1076. https://doi.org/10.1016/j.biortech.2017.09.109
Meegoda JN, Li B, Patel K, Wang LB (2018) A review of the processes, parameters, and optimization of anaerobic digestion. Int J Environ Res Public Health 15. https://doi.org/10.3390/ijerph15102224
Atelge MR, Atabani AE, Banu JR et al (2020) A critical review of pretreatment technologies to enhance anaerobic digestion and energy recovery. Fuel 270:117494. https://doi.org/10.1016/j.fuel.2020.117494
Rocamora I, Wagland ST, Villa R et al (2020) Dry anaerobic digestion of organic waste: a review of operational parameters and their impact on process performance. Bioresour Technol 299. https://doi.org/10.1016/j.biortech.2019.122681
Li Y, Chen Y, Wu J (2019) Enhancement of methane production in anaerobic digestion process: a review. Appl Energy 240:120–137. https://doi.org/10.1016/j.apenergy.2019.01.243
Wu Y, Wang S, Liang D, Li N (2020) Conductive materials in anaerobic digestion: from mechanism to application. Bioresour Technol 298. https://doi.org/10.1016/j.biortech.2019.122403
Kainthola J, Kalamdhad AS, Goud VV (2019) A review on enhanced biogas production from anaerobic digestion of lignocellulosic biomass by different enhancement techniques. Process Biochem 84:81–90. https://doi.org/10.1016/j.procbio.2019.05.023
Zhang J, Li W, Lee J et al (2017) Enhancement of biogas production in anaerobic co-digestion of food waste and waste activated sludge by biological co-pretreatment. Energy 137:479–486. https://doi.org/10.1016/j.energy.2017.02.163
Solé-Bundó M, Passos F, Romero-Güiza MS et al (2019) Co-digestion strategies to enhance microalgae anaerobic digestion: a review. Renew Sustain Energy Rev 112:471–482. https://doi.org/10.1016/j.rser.2019.05.036
Rasapoor M, Young B, Brar R et al (2020) Recognizing the challenges of anaerobic digestion: critical steps toward improving biogas generation. Fuel 261:116497. https://doi.org/10.1016/j.fuel.2019.116497
Alavijeh RS, Karimi K, Wijffels RH et al (2020) Combined bead milling and enzymatic hydrolysis for efficient fractionation of lipids, proteins, and carbohydrates of Chlorella vulgaris microalgae. Biores Technol 309:123321. https://doi.org/10.1016/j.biortech.2020.123321
Liu W, Wu R, Wang B et al (2020) Comparative study on different pretreatment on enzymatic hydrolysis of corncob residues. Biores Technol 295:122244. https://doi.org/10.1016/j.biortech.2019.122244
Zhou X, Liu J, Huang T et al (2020) Near-complete enzymatic hydrolysis efficiency of Miscanthus using hydrotropic fractionation at atmospheric pressure. Ind Crops Prod 149:112365. https://doi.org/10.1016/j.indcrop.2020.112365
Putrino FM, Tedesco M, Bodini RB, de Oliveira AL (2020) Study of supercritical carbon dioxide pretreatment processes on green coconut fiber to enhance enzymatic hydrolysis of cellulose. Biores Technol 309:123387. https://doi.org/10.1016/j.biortech.2020.123387
Sharma YC, Singh V (2017) Microalgal biodiesel: a possible solution for India’s energy security. Renew Sustain Energy Rev 67:72–88. https://doi.org/10.1016/j.rser.2016.08.031
Sundaramahalingam MA, Karthikumar S, Shyam Kumar R et al (2021) An intensified approach for transesterification of biodiesel from Annona squamosa seed oil using ultrasound-assisted homogeneous catalysis reaction and its process optimization. Fuel 291:120195. https://doi.org/10.1016/j.fuel.2021.120195
Murguía-Ortiz D, Cordova I, Manriquez ME et al (2021) Na-CaO/MgO dolomites used as heterogeneous catalysts in canola oil transesterification for biodiesel production. Mater Lett 291:129587. https://doi.org/10.1016/j.matlet.2021.129587
Bhatia SK, Bhatia RK, Jeon J-M et al (2021) An overview on advancements in biobased transesterification methods for biodiesel production: oil resources, extraction, biocatalysts, and process intensification technologies. Fuel 285:119117. https://doi.org/10.1016/j.fuel.2020.119117
Karpagam R, Jawaharraj K, Gnanam R (2021) Review on integrated biofuel production from microalgal biomass through the outset of transesterification route: a cascade approach for sustainable bioenergy. Sci Total Environ 766:144236. https://doi.org/10.1016/j.scitotenv.2020.144236
Jung S, Kim M, Lin K-YA et al (2021) Biodiesel synthesis from bio-heavy oil through thermally induced transesterification. J Clean Prod 294:126347. https://doi.org/10.1016/j.jclepro.2021.126347
Świątek K, Gaag S, Klier A et al (2020) Acid hydrolysis of lignocellulosic biomass: sugars and furfurals formation. Catalysts 10:1–18. https://doi.org/10.3390/catal10040437
Hong Y, Wu YR (2020) Acidolysis as a biorefinery approach to producing advanced bioenergy from macroalgal biomass: a state-of-the-art review. Biores Technol 318:124080. https://doi.org/10.1016/j.biortech.2020.124080
Mariano APB, Unpaprom Y, Ramaraj R (2020) Hydrothermal pretreatment and acid hydrolysis of coconut pulp residue for fermentable sugar production. Food Bioprod Process 122:31–40. https://doi.org/10.1016/j.fbp.2020.04.003
Mateo S, Mateo P, Barbanera M et al (2020) Acid hydrolysis of olive tree leaves: preliminary study towards biochemical conversion. Processes 8:1–13. https://doi.org/10.3390/PR8080886
Joshi SM, Gogate PR (2020) Intensification of dilute acid hydrolysis of spent tea powder using ultrasound for enhanced production of reducing sugars. Ultrason Sonochem 61:104843. https://doi.org/10.1016/j.ultsonch.2019.104843
Chen W, Liu Y, Song L et al (2020) Automated accelerated solvent extraction method for total lipid analysis of microalgae. Algal Res 51:102080. https://doi.org/10.1016/j.algal.2020.102080
Mercer P, Armenta RE (2011) Developments in oil extraction from microalgae. Eur J Lipid Sci Technol 113:539–547. https://doi.org/10.1002/ejlt.201000455
Gumaling RP, Agusan JR, Ellacer NVCR et al (2018) Increased bio-oil yield from Swietenia macrophylla seeds through microwave pretreatment and ultrasonic-assisted solvent extraction. Sustain Environ Res 28:430–437. https://doi.org/10.1016/j.serj.2018.06.003
Kwak M, Roh S, Yang A et al (2019) High shear-assisted solvent extraction of lipid from wet biomass of Aurantiochytrium sp. KRS101. Sep Purif Technol 227:115666. https://doi.org/10.1016/j.seppur.2019.06.004
Baskar G, Kalavathy G, Aiswarya R, Abarnaebenezer Selvakumari I (2019) Advances in bio-oil extraction from nonedible oil seeds and algal biomass. Elsevier Ltd. https://doi.org/10.1016/B978-0-08-102728-8.00007-3
Okolie JA, Nanda S, Dalai AK et al (2020) A review on subcritical and supercritical water gasification of biogenic, polymeric and petroleum wastes to hydrogen-rich synthesis gas. Renew Sustain Energy Rev 119:109546. https://doi.org/10.1016/j.rser.2019.109546
Wang C, Jin H, Feng H et al (2020) Study on gasification mechanism of biomass waste in supercritical water based on product distribution. Int J Hydrogen Energy 45:28051–28061. https://doi.org/10.1016/j.ijhydene.2020.02.146
Hu Y, Gong M, Xing X et al (2020) Supercritical water gasification of biomass model compounds: a review. Renew Sustain Energy Rev 118:109529. https://doi.org/10.1016/j.rser.2019.109529
Gollakota ARK, Kishore N, Gu S (2018) A review on hydrothermal liquefaction of biomass. Renew Sustain Energy Rev 81:1378–1392. https://doi.org/10.1016/j.rser.2017.05.178
Leng L, Zhang W, Peng H et al (2020) Nitrogen in bio-oil produced from hydrothermal liquefaction of biomass: a review. Chem Eng J 401:126030. https://doi.org/10.1016/j.cej.2020.126030
Lachos-Perez D, César Torres-Mayanga P, Abaide ER et al (2022) Hydrothermal carbonization and liquefaction: differences, progress, challenges, and opportunities. Bioresour Technol 343. https://doi.org/10.1016/j.biortech.2021.126084
Djandja OS, Wang Z, Chen L et al (2020) Progress in hydrothermal liquefaction of algal biomass and hydrothermal upgrading of the subsequent crude bio-oil: a mini review. Energy Fuels 34:11723–11751. https://doi.org/10.1021/acs.energyfuels.0c01973
Mahima J, Sundaresh RK, Gopinath KP et al (2021) Effect of algae (Scenedesmus obliquus) biomass pre-treatment on bio-oil production in hydrothermal liquefaction (HTL): biochar and aqueous phase utilization studies. Sci Total Environ 778:146262. https://doi.org/10.1016/j.scitotenv.2021.146262
Prestigiacomo C, Laudicina VA, Siragusa A et al (2020) Hydrothermal liquefaction of waste biomass in stirred reactors: one step forward to the integral valorization of municipal sludge. Energy 201:117606. https://doi.org/10.1016/j.energy.2020.117606
Demirbas A (2008) Liquefaction of biomass using glycerol. Energy Sources A Recov Util Environ Effects 30:1120–1126. https://doi.org/10.1080/15567030601100654
Nagappan S, Bhosale RR, Nguyen DD et al (2021) Catalytic hydrothermal liquefaction of biomass into bio-oils and other value-added products – a review. Fuel 285:119053. https://doi.org/10.1016/j.fuel.2020.119053
Yin S, Tan Z (2012) Hydrothermal liquefaction of cellulose to bio-oil under acidic, neutral and alkaline conditions. Appl Energy 92:234–239. https://doi.org/10.1016/j.apenergy.2011.10.041
Baloch HA, Nizamuddin S, Siddiqui MTH et al (2018) Recent advances in production and upgrading of bio-oil from biomass: a critical overview. J Environ Chem Eng 6:5101–5118. https://doi.org/10.1016/j.jece.2018.07.050
Basar IA, Liu H, Carrere H et al (2021) A review on key design and operational parameters to optimize and develop hydrothermal liquefaction of biomass for biorefinery applications. Green Chem 23:1404–1446. https://doi.org/10.1039/D0GC04092D
Elliott DC, Biller P, Ross AB et al (2015) Hydrothermal liquefaction of biomass: developments from batch to continuous process. Biores Technol 178:147–156. https://doi.org/10.1016/j.biortech.2014.09.132
Zhu Y, Biddy MJ, Jones SB et al (2014) Techno-economic analysis of liquid fuel production from woody biomass via hydrothermal liquefaction (HTL) and upgrading. Appl Energy 129:384–394. https://doi.org/10.1016/j.apenergy.2014.03.053
Toor SS, Rosendahl L, Rudolf A (2011) Hydrothermal liquefaction of biomass: a review of subcritical water technologies. Energy 36:2328–2342. https://doi.org/10.1016/j.energy.2011.03.013
Sharma K, Shah AA, Toor SS et al (2021) Co-hydrothermal liquefaction of lignocellulosic biomass in supercritical water. Energies (Basel) 14:1708. https://doi.org/10.3390/en14061708
Zhang X, Bo C, Xi D et al (2021) Liquefaction of biomass by plasma electrolysis in alkaline condition. Renew Energy 165:174–181. https://doi.org/10.1016/j.renene.2020.10.142
Yang J, He Q (Sophia), Niu H et al (2020) Microwave-assisted hydrothermal liquefaction of biomass model components and comparison with conventional heating. Fuel 277:118202. https://doi.org/10.1016/j.fuel.2020.118202
Araújo MFRS, Cardoso PL, Souza GLR et al (2021) Simultaneous thermal liquefaction of sugarcane bagasse and esterification with ethanol and fusel oil: one-step process for biofuel production. Chem Eng J 413. https://doi.org/10.1016/j.cej.2020.127432
Beims RF, Hu Y, Shui H, Xu C (Charles) (2020) Hydrothermal liquefaction of biomass to fuels and value-added chemicals: products applications and challenges to develop large-scale operations. Biomass Bioenergy 135:105510. https://doi.org/10.1016/j.biombioe.2020.105510
Sekar M, Mathimani T, Alagumalai A et al (2021) A review on the pyrolysis of algal biomass for biochar and bio-oil – bottlenecks and scope. Fuel 283:119190. https://doi.org/10.1016/j.fuel.2020.119190
Demirbas A, Arin G (2013) An overview of biomass pyrolysis. Energy Sources 24:471–482. https://doi.org/10.1080/00908310252889979
Patel A, Agrawal B, Rawal BR (2020) Pyrolysis of biomass for efficient extraction of biofuel. Energy Sources A Recov Util Environ Effects 42:1649–1661. https://doi.org/10.1080/15567036.2019.1604875
Djandja OS, Wang ZC, Wang F et al (2020) Pyrolysis of municipal sewage sludge for biofuel production: a review. Ind Eng Chem Res 59:16939–16956. https://doi.org/10.1021/acs.iecr.0c01546
Ganesan R, Manigandan S, Samuel MS et al (2020) A review on prospective production of biofuel from microalgae. Biotechnol Rep 27:e00509. https://doi.org/10.1016/j.btre.2020.e00509
Wu Z, Abramova A, Nikonov R, Cravotto G (2020) Sonozonation (sonication/ozonation) for the degradation of organic contaminants – a review. Ultrason Sonochem 68:105195. https://doi.org/10.1016/j.ultsonch.2020.105195
Jahirul MI, Rasul MG, Chowdhury AA, Ashwath N (2012) Biofuels production through biomass pyrolysis-a technological review. Energies (Basel) 5:4952–5001. https://doi.org/10.3390/en5124952
Murillo JD, Biernacki JJ, Northrup S, Mohammad AS (2017) Biomass pyrolysis kinetics: a review of molecular-scale modeling contributions. Braz J Chem Eng 34:1–18. https://doi.org/10.1590/0104-6632.20170341s20160086
Onarheim K, Hannula I, Solantausta Y (2020) Hydrogen enhanced biofuels for transport via fast pyrolysis of biomass: a conceptual assessment. Energy 199. https://doi.org/10.1016/j.energy.2020.117337
Zhang Y, Cui Y, Liu S et al (2020) Fast microwave-assisted pyrolysis of wastes for biofuels production – a review. Biores Technol 297:122480. https://doi.org/10.1016/j.biortech.2019.122480
Ge S, Yek PNY, Cheng YW et al (2021) Progress in microwave pyrolysis conversion of agricultural waste to value-added biofuels: a batch to continuous approach. Renew Sustain Energy Rev 135:110148. https://doi.org/10.1016/j.rser.2020.110148
Rony AH, Mosiman D, Sun Z et al (2018) A novel solar powered biomass pyrolysis reactor for producing fuels and chemicals. J Anal Appl Pyrol 132:19–32. https://doi.org/10.1016/j.jaap.2018.03.020
Zeng K, Gauthier D, Soria J et al (2017) Solar pyrolysis of carbonaceous feedstocks: A review. Sol Energy 156:73–92. https://doi.org/10.1016/j.solener.2017.05.033
Lee XJ, Ong HC, Gan YY et al (2020) State of art review on conventional and advanced pyrolysis of macroalgae and microalgae for biochar, bio-oil and bio-syngas production. Energy Convers Manage 210:112707. https://doi.org/10.1016/j.enconman.2020.112707
Gupta S, Mondal P, Borugadda VB, Dalai AK (2021) Advances in upgradation of pyrolysis bio-oil and biochar towards improvement in bio-refinery economics: a comprehensive review. Environ Technol Innov 21:101276. https://doi.org/10.1016/j.eti.2020.101276
Sorunmu Y, Billen P, Spatari S (2020) A review of thermochemical upgrading of pyrolysis bio-oil: techno-economic analysis, life cycle assessment, and technology readiness. GCB Bioenergy 12:4–18. https://doi.org/10.1111/gcbb.12658
Ong HC, Chen WH, Singh Y et al (2020) A state-of-the-art review on thermochemical conversion of biomass for biofuel production: a TG-FTIR approach. Energy Convers Manage 209:112634. https://doi.org/10.1016/j.enconman.2020.112634
Kwoczynski Z, Čmelík J (2021) Characterization of biomass wastes and its possibility of agriculture utilization due to biochar production by torrefaction process. J Clean Prod 280:124302. https://doi.org/10.1016/j.jclepro.2020.124302
Zhang R, Zhang J, Guo W et al (2021) Effect of torrefaction pretreatment on biomass chemical looping gasification (BCLG) characteristics: gaseous products distribution and kinetic analysis. Energy Convers Manage 237:114100. https://doi.org/10.1016/j.enconman.2021.114100
Vamvuka D, Loukakou E, Sfakiotakis S, Petrakis E (2020) The impact of a combined pre-treatment on the combustion performance of various biomass wastes and their blends with lignite. Thermochim Acta 688:178599. https://doi.org/10.1016/j.tca.2020.178599
Bach QV, Tran KQ, Khalil RA et al (2013) Comparative assessment of wet torrefaction. Energy Fuels 27:6743–6753. https://doi.org/10.1021/ef401295w
Basu P, Dhungana A, Rao S, Acharya B (2013) Effect of oxygen presence in torrefier. J Energy Inst 86:171–176. https://doi.org/10.1179/1743967113Z.00000000060
Wang Q, Sun S, Zhang X et al (2021) Influence of air oxidative and non-oxidative torrefaction on the chemical properties of corn stalk. Biores Technol 332:125120. https://doi.org/10.1016/j.biortech.2021.125120
Zhang J, Cui Y, Zhang T et al (2021) Food waste treating by biochar-assisted high-solid anaerobic digestion coupled with steam gasification: enhanced bioenergy generation and porous biochar production. Biores Technol 331:125051. https://doi.org/10.1016/j.biortech.2021.125051
Adeleke AA, Odusote JK, Ikubanni PP et al (2021) Essential basics on biomass torrefaction, densification and utilization. Int J Energy Res 45:1375–1395. https://doi.org/10.1002/er.5884
Shi N, Liu Q, Cen H et al (2020) Formation of humins during degradation of carbohydrates and furfural derivatives in various solvents. Biomass Convers Biorefinery 10:277–287. https://doi.org/10.1007/s13399-019-00414-4
Lynam JG, Coronella CJ, Yan W et al (2011) Acetic acid and lithium chloride effects on hydrothermal carbonization of lignocellulosic biomass. Biores Technol 102:6192–6199. https://doi.org/10.1016/j.biortech.2011.02.035
Acharya B, Dutta A, Minaret J (2015) Review on comparative study of dry and wet torrefaction. Sustain Energy Technol Assess 12:26–37. https://doi.org/10.1016/j.seta.2015.08.003
Bach Q-V, Skreiberg Ø (2016) Upgrading biomass fuels via wet torrefaction: a review and comparison with dry torrefaction. Renew Sustain Energy Rev 54:665–677. https://doi.org/10.1016/j.rser.2015.10.014
Singh D, Yadav S (2021) Steam gasification with torrefaction as pretreatment to enhance syngas production from mixed food waste. J Environ Chem Eng 9:104722. https://doi.org/10.1016/j.jece.2020.104722
Zhang D, Chen X, Qi Z et al (2021) Superheated steam as carrier gas and the sole heat source to enhance biomass torrefaction. Biores Technol 331:124955. https://doi.org/10.1016/j.biortech.2021.124955
Trubetskaya A, Johnson R, Monaghan RFD et al (2021) Combined analytical strategies for chemical and physical characterization of tar from torrefaction of olive stone. Fuel 291:120086. https://doi.org/10.1016/j.fuel.2020.120086
Duman G, Balmuk G, Cay H et al (2020) Comparative evaluation of torrefaction and hydrothermal carbonization: effect on fuel properties and combustion behavior of agricultural wastes. Energy Fuels 34:11175–11185. https://doi.org/10.1021/acs.energyfuels.0c02255
Aragón-Briceño CI, Pozarlik AK, Bramer EA et al (2021) Hydrothermal carbonization of wet biomass from nitrogen and phosphorus approach: a review. Renew Energy 171:401–415. https://doi.org/10.1016/j.renene.2021.02.109
Ischia G, Fiori L (2021) Hydrothermal carbonization of organic waste and biomass: a review on process, reactor, and plant modeling. Waste Biomass Valoriz 12:2797–2824. https://doi.org/10.1007/s12649-020-01255-3
Cao Y, He M, Dutta S et al (2021) Hydrothermal carbonization and liquefaction for sustainable production of hydrochar and aromatics. Renew Sustain Energy Rev 152:111722. https://doi.org/10.1016/j.rser.2021.111722
Zhuang X, Liu J, Zhang Q et al (2022) A review on the utilization of industrial biowaste via hydrothermal carbonization. Renew Sustain Energy Rev 154:111877. https://doi.org/10.1016/j.rser.2021.111877
Axelsson L, Franzén M, Ostwald M et al (2012) Perspective: Jatropha cultivation in southern India: assessing farmers’ experiences. Biofuels Bioprod Biorefin 6:246–256. https://doi.org/10.1002/bbb
Oluwoye I, Altarawneh M, Gore J, Dlugogorski BZ (2020) Products of incomplete combustion from biomass reburning. Fuel 274:117805. https://doi.org/10.1016/j.fuel.2020.117805
Kanwal F, Ahmed A, Jamil F et al (2021) Co-combustion of blends of coal and underutilised biomass residues for environmental friendly electrical energy production. Sustainability 13:4881. https://doi.org/10.3390/su13094881
da Costa TP, Quinteiro P, Arroja L, Dias AC (2020) Environmental comparison of forest biomass residues application in Portugal: electricity, heat and biofuel. Renew Sustain Energy Rev 134:110302. https://doi.org/10.1016/j.rser.2020.110302
Falk J, Skoglund N, Grimm A, Marcus O (2020) Fate of phosphorus in fixed bed combustion of biomass and sewage sludge. Energy Fuels. https://doi.org/10.1021/acs.energyfuels.9b03976
Lisý M, Lisá H, Jecha D et al (2020) Characteristic properties of alternative biomass fuels. Energies (Basel) 13:1448. https://doi.org/10.3390/en13061448
Demirbas A (2004) Combustion characteristics of different biomass fuels. Prog Energy Combust Sci 30:219–230. https://doi.org/10.1016/j.pecs.2003.10.004
Gogolev I, Soleimanisalim AH, Linderholm C, Lyngfelt A (2021) Commissioning, performance benchmarking, and investigation of alkali emissions in a 10 kWth solid fuel chemical looping combustion pilot. Fuel 287:119530. https://doi.org/10.1016/j.fuel.2020.119530
Lyngfelt A (2020) Chemical looping combustion: status and development challenges. Energy Fuels 34:9077–9093. https://doi.org/10.1021/acs.energyfuels.0c01454
Molino A, Chianese S, Musmarra D (2016) Biomass gasification technology: the state of the art overview. J Energy Chem 25:10–25. https://doi.org/10.1016/j.jechem.2015.11.005
Lapuerta M, Hernández JJ, Pazo A, López J (2008) Gasification and co-gasification of biomass wastes: effect of the biomass origin and the gasifier operating conditions. Fuel Process Technol 89:828–837. https://doi.org/10.1016/j.fuproc.2008.02.001
Shahabuddin M, Alam MT, Krishna BB et al (2020) A review on the production of renewable aviation fuels from the gasification of biomass and residual wastes. Biores Technol 312:123596. https://doi.org/10.1016/j.biortech.2020.123596
Hu Q, Shen Y, Chew JW et al (2020) Chemical looping gasification of biomass with Fe2O3/CaO as the oxygen carrier for hydrogen-enriched syngas production. Chem Eng J 379:122346. https://doi.org/10.1016/j.cej.2019.122346
Macrì D, Catizzone E, Molino A, Migliori M (2020) Supercritical water gasification of biomass and agro-food residues: energy assessment from modelling approach. Renew Energy 150:624–636. https://doi.org/10.1016/j.renene.2019.12.147
Indrawan N, Kumar A, Moliere M et al (2020) Distributed power generation via gasification of biomass and municipal solid waste: a review. J Energy Inst 93:2293–2313. https://doi.org/10.1016/j.joei.2020.07.001
Farzad S, Mandegari MA, Görgens JF (2016) A critical review on biomass gasification, co-gasification, and their environmental assessments. Biofuel Res J 3:483–495. https://doi.org/10.18331/BRJ2016.3.4.3
Zhang Y, Ke C, Fu W et al (2020) Simulation of microwave-assisted gasification of biomass: a review. Renew Energy 154:488–496. https://doi.org/10.1016/j.renene.2020.03.056
Suárez-Almeida M, Gómez-Barea A, Ghoniem AF, Pfeifer C (2021) Solar gasification of biomass in a dual fluidized bed. Chem Eng J 406:126665. https://doi.org/10.1016/j.cej.2020.126665
Larsson A, Kuba M, Berdugo Vilches T et al (2021) Steam gasification of biomass – typical gas quality and operational strategies derived from industrial-scale plants. Fuel Process Technol 212:106609. https://doi.org/10.1016/j.fuproc.2020.106609
Jeong YS, Choi YK, Kang BS et al (2020) Lab-scale and pilot-scale two-stage gasification of biomass using active carbon for production of hydrogen-rich and low-tar producer gas. Fuel Process Technol 198:106240. https://doi.org/10.1016/j.fuproc.2019.106240
Bhuiya MMK, Rasul M, Khan M et al (2020) Comparison of oil extraction between screw press and solvent (n-hexane) extraction technique from beauty leaf (Calophyllum inophyllum L.) feedstock. Ind Crops Prod 144:112024. https://doi.org/10.1016/j.indcrop.2019.112024
Wu Y, Ge S, Xia C et al (2021) Application of intermittent ball milling to enzymatic hydrolysis for efficient conversion of lignocellulosic biomass into glucose. Renew Sustain Energy Rev 136:110442. https://doi.org/10.1016/j.rser.2020.110442
Mwaurah PW, Kumar S, Kumar N et al (2020) Novel oil extraction technologies: process conditions, quality parameters, and optimization. Compr Rev Food Sci Food Saf 19:3–20. https://doi.org/10.1111/1541-4337.12507
Lužaić T, Romanić R, Grahovac N et al (2021) Prediction of mechanical extraction oil yield of new sunflower hybrids: artificial neural network model. J Sci Food Agric n/a. https://doi.org/10.1002/jsfa.11234
Tumuluru JS, Fillerup E (2020) Briquetting characteristics of woody and herbaceous biomass blends: impact on physical properties, chemical composition, and calorific value. Biofuels, Bioprod Biorefin 14:1105–1124. https://doi.org/10.1002/bbb.2121
Kpalo SY, Zainuddin MF, Manaf LA, Roslan AM (2020) A review of technical and economic aspects of biomass briquetting. Sustainability 12:4609. https://doi.org/10.3390/su12114609
Suresh R, Singh VK, Malik JK et al (2016) Evaluation of the performance of improved biomass cooking stoves with different solid biomass fuel types. Biomass Bioenergy 95:27–34. https://doi.org/10.1016/j.biombioe.2016.08.002
Jeuland MA, Pattanayak SK, Samaddar S et al (2020) Adoption and impacts of improved biomass cookstoves in rural Rajasthan. Energy Sustain Dev 57:149–159. https://doi.org/10.1016/j.esd.2020.06.005
Chan YH, Loh SK, Chin BLF et al (2020) Fractionation and extraction of bio-oil for production of greener fuel and value-added chemicals: recent advances and future prospects. Chem Eng J 397:125406. https://doi.org/10.1016/j.cej.2020.125406
Kaya DA, Ghica M v, Dănilă E et al (2020) Selection of optimal operating conditions for extraction of Myrtus Communis L. Essential Oil by the Steam Distillation Method. Molecules 25:2399. https://doi.org/10.3390/molecules25102399
Deng W, Liu K, Cao S et al (2020) Chemical composition, antimicrobial, antioxidant, and antiproliferative properties of grapefruit essential oil prepared by molecular distillation. Molecules 25:217. https://doi.org/10.3390/molecules25010217
Dantas TNC, Cabral TJO, Dantas Neto AA, Moura MCPA (2020) Enrichmnent of patchoulol extracted from patchouli (Pogostemon cablin) oil by molecular distillation using response surface and artificial neural network models. J Ind Eng Chem 81:219–227. https://doi.org/10.1016/j.jiec.2019.09.011
Sewsynker-Sukai Y, Faloye F, Kana EBG (2017) Artificial neural networks: an efficient tool for modelling and optimization of biofuel production (a mini review). Biotechnol Biotechnol Equip 31:221–235. https://doi.org/10.1080/13102818.2016.1269616
Meena M, Shubham S, Paritosh K et al (2021) Production of biofuels from biomass: predicting the energy employing artificial intelligence modelling. Biores Technol 340:125642. https://doi.org/10.1016/j.biortech.2021.125642
Andrade Cruz I, Chuenchart W, Long F et al (2022) Application of machine learning in anaerobic digestion: perspectives and challenges. Biores Technol 345:126433. https://doi.org/10.1016/j.biortech.2021.126433
Ahmad I, Sana A, Kano M et al (2021) Machine learning applications in biofuels’ life cycle: soil, feedstock, production, consumption, and emissions. Energies (Basel) 14:5072. https://doi.org/10.3390/en14165072
Khan M, Ullah Z, Mašek O et al (2022) Artificial neural networks for the prediction of biochar yield: a comparative study of metaheuristic algorithms. Bioresour Technol 355. https://doi.org/10.1016/j.biortech.2022.127215
Ullah Z, Khan M, Raza Naqvi S et al (2021) A comparative study of machine learning methods for bio-oil yield prediction – a genetic algorithm-based features selection. Biores Technol 335:125292. https://doi.org/10.1016/j.biortech.2021.125292
Ullah Z, Khan M, Naqvi SR et al (2022) An integrated framework of data-driven, metaheuristic, and mechanistic modeling approach for biomass pyrolysis. Process Saf Environ Prot 162:337–345. https://doi.org/10.1016/j.psep.2022.04.013
Aniza R, Chen WH, Yang FC et al (2022) Integrating Taguchi method and artificial neural network for predicting and maximizing biofuel production via torrefaction and pyrolysis. Biores Technol 343:126140. https://doi.org/10.1016/j.biortech.2021.126140
Pereira RD, Badino AC, Cruz AJG (2020) Framework based on artificial intelligence to increase industrial bioethanol production. Energy Fuels 34:4670–4677. https://doi.org/10.1021/acs.energyfuels.0c00033
Nayak M, Dhanarajan G, Dineshkumar R, Sen R (2018) Artificial intelligence driven process optimization for cleaner production of biomass with co-valorization of wastewater and flue gas in an algal biorefinery. J Clean Prod 201:1092–1100. https://doi.org/10.1016/j.jclepro.2018.08.048
Rentizelas AA, Tolis AJ, Tatsiopoulos IP (2009) Logistics issues of biomass: the storage problem and the multi-biomass supply chain. Renew Sustain Energy Rev 13:887–894
Ghaffariyan MR, Brown M, Acuna M et al (2017) An international review of the most productive and cost effective forest biomass recovery technologies and supply chains. Renew Sustain Energy Rev 74:145–158. https://doi.org/10.1016/j.rser.2017.02.014
Miao Z, Shastri Y, Grift TE et al (2012) Lignocellulosic biomass feedstock transportation alternatives, logistics, equipment configurations, and modeling. Biofuels Bioprod Biorefin 6:351–362. https://doi.org/10.1002/bbb.1322
Searcy E, Flynn P, Ghafoori E, Kumar A (2007) The relative cost of biomass energy transport. Appl Biochem Biotechnol 137–140:639–652. https://doi.org/10.1007/s12010-007-9085-8
Gonzales D, Searcy EM, Ekşioĝlu SD (2013) Cost analysis for high-volume and long-haul transportation of densified biomass feedstock. Transp Res A Policy Pract 49:48–61. https://doi.org/10.1016/j.tra.2013.01.005
Martinez-Hernandez E, Amezcua-Allieri MA, Aburto J (2021) Assessing the cost of biomass and bioenergy production in agroindustrial processes. Energies (Basel) 14. https://doi.org/10.3390/en14144181
Carneiro P, Ferreira P (2012) The economic, environmental and strategic value of biomass. Renew Energy 44:17–22. https://doi.org/10.1016/j.renene.2011.12.020
Ranjbari M, Shams Esfandabadi Z, Ferraris A et al (2022) Biofuel supply chain management in the circular economy transition: an inclusive knowledge map of the field. Chemosphere 296:133968. https://doi.org/10.1016/j.chemosphere.2022.133968
Bardhan P, Deka A, Bhattacharya SS et al (2022) Economical aspect in biomass to biofuel production. Value-Chain of Biofuels, pp 395–427. https://doi.org/10.1016/B978-0-12-824388-6.00003-8
Demirbas A (2008) Biofuels sources, biofuel policy, biofuel economy and global biofuel projections. Energy Convers Manage 49:2106–2116. https://doi.org/10.1016/j.enconman.2008.02.020
Author information
Authors and Affiliations
Contributions
Dr. Nilanjana Banerjee: conceptualization, data collection, writing and reviewing of the paper. This is a single authorship paper.
Corresponding author
Ethics declarations
Conflict of Interest
The author declares no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Banerjee, N. Biomass to Energy — an Analysis of Current Technologies, Prospects, and Challenges. Bioenerg. Res. 16, 683–716 (2023). https://doi.org/10.1007/s12155-022-10500-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12155-022-10500-7