Abstract
Climate change issues are calling for the design of renewable sources of energy. In particular, biomass energy from algae is encouraging because production of algae at the commercial scale can be done successfully with various techniques. Here, we review the conversion of algal biomass into energy by fast, slow, microwave and catalytic pyrolysis. The article details algae classification; cultivation of macroalgae and microalgae; pyrolysis parameters; production of biochar, bio-oil and biogas; and types of pyrolysis.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The twenty-first century has seen a rise in energy consumption worldwide. Most of the energy production in the current century is driven by the usage of conventional energy sources. The need for energy is particularly high in the developing nations, led by India and China (Hubacek et al. 2007). As of 2017, energy demands increased, with the world consumption of coal rising up by 25 million tonnes of oil equivalent (mtoe) (British Petroleum Co. 2018). The global oil price also increased from $43.73 to $54.19 per barrel, the first annual increase seen from 2012. Natural gas consumption rose by 96 billion cubic metres (bcm), the fastest since 2010. With growing economies, the worldwide requirement for energy is bound to increase. There has been an estimated amount of 1.1 trillion tonnes of coal resources found, enough to last for 150 years at current consumption rates (World Coal Association 2019). The preference of coal as a source of energy production is widely attributed to the cheapness of coal and to the ease in production of energy. However, the usage of coal generally brings about a lot of disadvantages, namely the increased release of sulphur and nitrogen oxides, particulate matter and carbon dioxide (Finkelman and Tian 2018). The reserves of coal in the world are mostly made up of high-ash-content coals, which release particulate matter. These particulate matters are adverse to human health, with a lot of instances being reported in the developing countries such as China (Finkelman et al. 2002) and India (Swer and Singh 2004).
Alternative sources of energy are wind, solar, hydroelectricity and biofuels. Theoretically, solar energy by itself has the potential to fulfil the energy needs of the entire world, with a maximum capacity of nearly 4 million exajoule (1 exajoule = 10^18 J) (Kabir et al. 2018). Solar energy appears to be promising, but suffers from some flaws such as high installation costs (Pillai 2015), which will be difficult for developing countries to afford. The usage of rare metals, such as tellurium, silver and indium, raises the cost of manufacturing a panel. Constant maintenance of solar panels is also required. The distribution of solar energy also is not uniform with some areas of the earth not getting consistently high amounts of solar radiation required for electricity production. A promising alternative appears to be harnessing the energy obtained by wind. Wind energy is being harnessed with wind power capacity reaching 539 GW (World Wind Energy Association 2018). Chief producers of wind energy are China, USA, Germany, Brazil, India and Canada. However, wind energy cannot be taken as a reliable source of energy, because of the uneven and seasonal distribution of winds. For an area to be chosen, it must be experiencing sufficient wind speeds required for electricity production. Also, wind farms face a similar problem faced by solar plants: cost and maintenance upkeep (Davy et al. 2018). Hydroelectricity involves harnessing the energy obtainable by running water. Currently, it is a popular source of renewable energy. Most of the countries in the world are blessed with rivers, which can be effectively harnessed. According to the International Energy Agency (IEA), in 2018, hydropower capacity was estimated to be at 630 GW, with a potential increase in production and usage (IEA 2018). Yet, hydroelectricity by itself suffers a lot of drawbacks. It generally disturbs the local ecological balance when dams are being created. It has adverse effects such as erosion of soil, flooding of valleys and mass displacement of local people in the area leading to societal problems. Also, hydroelectricity production does release greenhouse gases in some ways or the other from construction to operation (Sovacool and Walter 2019). Emissions are present in the construction stage by large-scale manufacturing of steel, cement and fuels. The operation part, which involves large-scale flooding, inundated trees. These rotting trees release CO2, which also causes more greenhouse gas emissions. Figure 1 shows the categories of energy distribution. The energy classification used here is on the basis of renewable and non-renewable energy. Further classification is based on the availability in organic and inorganic sources. Renewable inorganic energy is comprised of solar, wind, hydrothermal and oceanic energy, while non-renewable inorganic energy is comprised of nuclear energy. Renewable organic energy is present as bioenergy, while non-renewable energy from organic sources is coal and petroleum.
Bioenergy
Although people have used biomaterial, specifically wood and dead plants to give heat by combustion in the past, the use of bioenergy as an alternative fuel has begun recently. In simple terms, bioenergy refers to the energy obtainable from living organisms. Biomass, an organic feedstock, is derived from plant material, directly or indirectly, as a result of photosynthesis. Commonly used biomass for energy production includes agricultural waste, organic wastes, energy crops, sewage sludges and municipal green wastes. Proper combustion techniques can ensure that these raw materials can give heat, power and fuel. If produced on a large-level basis, these fuels can also be a notable agent in carbon dioxide emission reduction (Schuck 2006). Biomass is conceptualized as a renewable energy resource due to two major reasons, being the renewable nature of biomass, which is present in the earth and the capability of plants to capture solar energy and carbon from available CO2, which can be utilized into other forms of energy by combustion (Demirbas 2001).
There is a general consensus that utilization of bioenergy is very less than its actual potential. If the traditional use of biomass is included, then bioenergy contributes to an estimated 12.8% to the total finite energy consumption (REN21 2018). Bioenergy can be useful in many sectors, such as transport (short and long haul) and heating (domestic and industrial). While there may be debates on usage of certain feedstocks and also concerns regarding sustainability and profitability, the overall consensus is that biofuels can provide to the reduction in greenhouse gas emissions and can provide a wide range of socioeconomic benefits.
Bioenergy industry can be divided into solid biomass industry, liquid biofuel industry and gaseous biomass industry. The use of solid biomass to produce heat and electricity has been commonly used, since ancient times, but the specialized use of other feedstock, such as bagasse, corn, maize and sorghum, is gaining traction. Brazil uses agricultural residue to produce heat and electricity. Different areas of the world have begun experimenting with the production of energy by biofuels: examples include Sierra Leone, where Sunbird Energy Africa has successfully commissioned the country’s first bioenergy plant (32 MW), Mexico, where a 50 MW bagasse plant was completed in February 2018, to provide energy to sugarcane mills, Myanmar, which experimented generating bioenergy, by using rice husks as fuels (Renewable Global Statistics Report 2018a, b). Liquid biofuels are used widely in transportation, with biodiesel, ethanol and biogas, commonly called as ‘first-generation fuels’. Bio-ethanol is a petrol substitute and can be used in some ‘flex-fuel vehicles’. Biodiesel is a diesel substitute produced from transesterification of vegetable oils and residues. With some modifications, biodiesel can be used as a viable alternative to diesel. Biogas (biomethane) with some modifications can be used as a supplement in petrol vehicles (Fotiadis and Polemis 2018). ‘Second-generation fuels’ are those, which are produced with efficient techniques, and contribute to carbon neutral or even carbon negative emissions to the overall CO2 emission.
The physical and chemical composition of various biomass residues varies a lot. Thus, the suitable selection of biomass is often required in order to create a desired biofuel (Hoogwijk et al. 2003). Biomass, produced from residue, can be classified into three categories, primary, secondary and tertiary (Demirbas 2001). Primary residues are those which can be directly collected from nature. Secondary residues often are accounted for by agricultural waste. Tertiary residues are those which are derived from used biomass-derived commodities (Capodaglio and Callegari 2018). Crops which are used for both food and energy purposes include corn, maize, sorghum, rapeseed and wheat. Sometimes, perennial crops are planted and harvested regularly such as willows, poplars and eucalyptus. Figure 2 is a tabular explanation of the above-mentioned classification.
There is a growing interest in using algal biomass as a suitable biofuel agent. Algae are simple aquatic microscopic organisms, which convert sunlight, water and carbon dioxide into starch, and eventually algal biomass by photosynthesis (Demirbas 2010). While the concept of using algae for energy production has been around for quite some time (Vandna et al. 2015) using lipids in the algae as a source to produce liquid fuels is begun to be seriously considered. Given that algal growth has been seen everywhere, right from open ponds, to oceans, the possibility of harnessing algae as a biofuel feedstock appears to be viable.
Algae: classification and morphology
Algae can be classified very broadly into two categories: filamentous and phytoplankton. The smallest unit which cannot be seen by the naked eye are known as microalgae and those which can be seen by the eye are known as macroalgae (Sudhakar et al. 2018). On the basis of the pigment colour, macro- and microalgae are separated into three general groups, Rhodophyceae, Chlorophyceae and Phaeophyceae, with their pigment colours being red, green and brown, respectively. By their habitats, algae are classified as marine water and freshwater algae. The habitats of algae range from freshwater ponds and rivers to saline environments. Some algal species can also be found deep under the oceans of the earth. Chlorophytes are a wide clade, exhibiting great morphological differences (Domozych et al. 2012). The colonization of land by the green algae eventually led to the plant life seen today; as a result, it is very important for studies related to evolution.
Figure 3 shows the photographs associated with green algae and products. The photographs show macroalgal strands like Ulva and microalgal strands like Spirulina. The open ponds and methods used to harvest algae are shown, along with the major products of biochar and bio-oil. Algal blooms are also seen.
Chlorophytes can be further subdivided into three informal classes, Ulva, Trebouxio and Chlorophyceae (Kornprobst 2014). Each of them varies significantly, with pronounced morphological changes. While the Chloro and Trebouxio grew in freshwater and terrestrial habitats, Ulvaceae dominated in marine water. Chlorophyceae is of a wide variety, 100 families consisting of 700 genera in total. Some examples of Chlorophyceae include Chlamydomonas. Some land-based examples of Chlorophyceae also include with their growth conditions being reported in acidic soil (Darienko et al. 2015). Streptophyta is a clade comprising of Embryophyta and Charophyta. Chlorophyta and sister clade Streptophyta were the ancestors of modern-day plants. Chlorophyceae, eventually split up, and each of them developed differently, with the first split occurring in 470–450 MYA (Wichard et al. 2015). This leads to the overall split of deciding whether there is the algal habitat of freshwater and saltwater. Trebouxiophyceae usually are found as biofilms on soil and are also associated with mosses. Some of the species are also found in association with basidiomycetes. Representatives are characterized with small sizes with (3–6 µm) (Becker and Marin 2009). Ulvophyceae is predominantly marine algae, with multicellular structures, examples being Ulva.
Macroalgal and microalgal classification is of great importance. Macroalgae species may be unicellular or multicellular, with cell sizes sometimes being more than a millimetre. They can exist coenocytically, meaning that multiple nuclei can exist within single protoplasmic media. Taxonomically, they can be found in Ulvophyceae, Chlorophyceae and Charophyceae along with Rhodophyceae and Xanthophyceae. Some examples for Macroalgae would be Caulerpa, Vaucheria, Acetabularia, Emodesmis, Griffithsia and Chara (Darienko et al. 2015). Externally, they are very diverse, with the only common feature being macroscopic in size. Habitats vary from freshwater to marine saltwater (Starks et al. 1981). Microalgae, in contrast, are small, usually appearing in clusters, with habitats and morphology varying. They are similarly to macroalgae and are classified under the following phylums Chlorophyta, Rhodophyta, Haptophyta, Stramenopiles and Dinophyta (Mine et al. 2008). Some examples would be Chlamydomonas and Chlorella. Both macroalgae and microalgae play a crucial role in regulating the ecosystem of aquatic habitats, help in carbon fixation, produce oxygen to sustain life, are also used as food in many indigenous communities, and are also seen as a potential biofuel feedstock.
Conversion of algae into energy
The idea of using natural materials obtained from living organisms as feedstock existed well before antiquity, wood being the primary feedstock. The idea of using algae as a source of food, feed and energy is not a new one, going back to the late 1950s. The energy crisis of 1970s forced the governments to look for alternative fuel sources, such as methane and hydrogen. Methane from algae was seen to be promising, and algae as a bio-feedstock were begun to be seriously considered. From 1980 to 1996, the US Department of Energy supported the aquatic species programme (ASP), a relatively small effort (about $25 million over almost 20 years) with the specific goal of producing oil from microalgae. Research has been done on using microalgae and macroalgae to check whether they can produce biofuel at commercial levels. Various thermochemical conversion techniques have been used, such as pyrolysis, gasification, torrefaction, liquefaction and combustion (Heimann and Huerlimann 2015). These techniques have been researched upon exclusively for different species of microalgae and macroalgae, each giving their own sets of products depending on feedstock characteristics. In this review, we see the production of macroalgae and microalgae to be used as feedstock and the usage of pyrolysis as an effective thermochemical method for producing biofuel.
The consideration of choosing algal strains for biofuel is to look for strains which are fast growing, capable of surviving in hardy climes, rich in lipid contents. Genetic engineering may be considered for enhancing capacity of the wild strains for greater lipid production (Peng et al. 2019).
Macroalgae cultivation
Macroalgae are seen as an upcoming bio-feedstock, when compared with the well-established microalgal field of study (Min et al. 2011). Classification of macroalgae cultivation can be divided into two subtopics, wild seaweed and aqua-cultured seaweed method. Primary natural sources of wild seaweed cultivation are from drift seaweeds. Aqua-cultured seaweed involves the use of ponds or tanks (land-based cultivation) or seas (ocean-based cultivation). Similar to microalgae, pond-based cultivation is seen to be an effective method of growth. Species which grow are Ulva rotundata (Petrusevski et al. 1995), Monostroma and Laminaria digitata (Taher et al. 2011). Seaweed cultivation in sea is done for Eucheuma, Undaria and Kappaphycus (Min et al. 2011). The cultivation of macroalgae is performed as follows. It involves setting up a pilot plant (Chen et al. 2015), consisting of a hatchery and an on growing site, either on land or on water. The hatchery has requirements such as filtering of sea water and air supply, proper lighting, chiller units, tanks and storage. The on-growth site has similar characteristics, having ropes, anchors and buoys. The cultivation is strongly dependent on the budget and the location of the site. The type of algae cultivated must be native to the land. Other factors influencing cultivation are water depth, nutrients, turbidity of water and temperature of water. The seeding is done with a view not to disturb the environmental considerations. The sampling of the growing biomass is crucial, to gauge the growth. A stable environment is necessary for the growth, with culture cabinet temperature of 10 °C with a thermostat alarm, and maintaining a steady air supply, with cleaning filters and water, which are some of the prerequisites. It is good practice to draw up a list of daily, weekly and monthly activities to ensure good growth.
Macroalgae harvesting
Macroalgae, due to their large size, are separated mainly by physical methods. Manual harvesting is preferred and has been used before the industrial age. At low tides, terrestrial vehicles are used across shore and used to collect the algae. Trawlers, boats and dredges are used as the vehicles for macroalgae harvesting (Brennan and Owende 2010a, b). Harvesting is usually performed when there is enough sunlight to ensure a sustained growth of epiphytic communities, and generally a very good spring and summer is crucial for the growth (Mooney-McAuley et al. 2016). The harvesting technique usually involves taking the thallus by hand, though this may vary for different species. Pre-treatment techniques of macroalgae are easier due to the relative size of the algae. Some algae are attached to rocks by holdfasts. In general, species cultivation differs for macroalgae due to morphology. Harvesting instruments used are drag rake, winchers, cutter blades and suction harvesters. In general, one can conclude that the harvesting of macroalgae is easier and economical than that of microalgae. However, it is labour intensive. Also, harvesting cannot be done on a regular basis. Care should be taken that regeneration of harvested population occurs regularly. A big advantage for harvesting macroalgae is that it can be easily grown in wastewater.
Microalgae cultivation
Microalgae cultivation techniques can be classified into two methods, open and closed cultivation systems (Burton et al. 2009). Open cultivation systems involve the algae being exposed to nature. Open pond cultivation is an example (Duran et al. 2018). Here, a pond is constructed with sufficient depth and is exposed to solar radiation. Nutrients are provided by channelling the water containing nitrogen and phosphorous. The pond is designed to meet local conditions of temperature, land and algal strains. Closed cultivation systems, on the other hand, deal with controlled conditions which optimize algal growth. Photobioreactors are very efficient, producing high biomass content with respect to smaller areas and lesser operating costs (Borowitzka 1999).
Microalgae harvesting
Harvesting techniques of microalgae can be broadly divided into two types of techniques, physical and chemical. Physical techniques involve solid–liquid separation of algae from water. Some techniques are sedimentation, filtration, centrifugation and floatation, while chemical techniques involve flocculation and electrophoresis.
In sedimentation, the solids and liquids are separated by using gravitational force and allowing them to settle down. Sedimentation of algal biomass depends on the density difference, particle size, cell age, temperature and light (Haarhoff and Maritz Rykaart 1995; Shelef et al. 1965; Danquah et al. 2009). Lamella separator and sedimentation tank are some of the common examples of sedimentation tanks. Filtration uses a medium that is permeable to the growth medium to separate algal biomass and media. Membrane plays an important role in separation and is classified on pore size. Microfiltration (0.1 to 10 µm), ultra-filtration (0.02–0.2 µm) and nano-filtration (less than 0.001 µm) are some of filtration techniques used which depend on pore size of membrane (Knuckey et al. 2006). Different types of filtration involve apparatus designed on pressure difference, types of filtrate and filter media. Vacuum filtration is a popular choice for separating microalgae due to their small size, via microfiltration. Energy consumption is usually about 0.1–5.9 kW/hm3 depending on filter type used (Al Hattab 2015). Other types of filtration include pressure filtration (use of plate and frame filter), cross-flow filtration. The third technique to be talked about is centrifugation. This process involves the use of a centrifugal force to separate the particles in media by causing separation in the disc stack, forcing the heavier particles (heavy phase and sludge) towards the periphery of the bowl, and is discharged automatically through ports, while the light phase flows towards the centre of the bowl. The efficiency of the process depends mainly on the centrifugal force involved. The two types of centrifuges used for microalgae harvesting are disc stack centrifuge and decanter centrifuge. The energy consumption for disc stack centrifuge varied from 0.53 to 5.5 kWh/m3, with (Mohn 1988) noting 12% suspended solid concentration of microalgae species Scenedesmus using a disc stack centrifuge with energy consumption of 1 kWh/m3. The decanter centrifuge energy consumption varied from 1.3 to 8 kWh/m3 (REN21 2018). The last physical harvesting method is flotation where low density of microalgae property is used. Gas bubbles are passed through a solid–liquid suspension, causing microalgae to float to the surface by adhering to the gas bubbles. Aeration is also useful to remove any residual compounds (Sim et al. 1988). Types of flotation techniques are dispersed air floatation, dissolved air floatation and fluidic oscillation. Factors influencing air floatation are pH, air flow rate, medium time and loading rate (Singh et al. 2011; Haarhoff and Maritz Rykaart 1995).
In chemical harvesting methods, flocculation and electrophoresis are the two major methods for microalgae harvest. Flocculation means the aggregation of fine particles in a colloidal solution by the use of a chemical agent. Generally, microalgae are seen as particles dispersed in water, the dispersion phase, so flocculation is a viable method. In this, the algal culture is allowed to flocculate, and then, the clumps of algae are removed by dewatering.
There are three types of flocculation, chemical, autoflocculation and bioflocculation (Bruhn et al. 2011). Chemical flocculation is commonly seen and is achieved with the use of a flocculating agent (Brennan and Owende 2010a, b). The nature of flocculent is important. It can be briefly divided into organic and inorganic flocculants, with some examples being chitosan and praestol for organic and aluminium and iron sulphate salts for inorganic. Generally, algal harvesting is preferred due to its non-toxic nature (Smith and Miettinen 2006). Autoflocculation is seen in some microalgal species which can spontaneously flocculate due to environmental stress (Vandamme et al. 2011). Environmental stresses include dissolved oxygen content, ionic content of calcium and magnesium, pH and nitrogen concentration. Bio-flocculation refers to the use of microorganisms to induce flocculation in microalgal culture (Horiuchi et al. 2003). This works by making sure that the microorganisms adhere to the cell wall of the microalgae, causing weight to increase and sedimentation to occur. In general, flocculation depends on size of particle, ion charge, pH and concentration of dissolved oxygen. Electrophoresis is used to eliminate toxic and costly chemicals from microalgal solution, by the use of an applied electric field (Shelef et al. 1965). Coagulation, flocculation and flotation are three methods used in electrophoresis. The process involves the use of iron or aluminium electrode, and current is passed through the solution. The algal solution behaves like a negatively charged species and is attracted to the cathode. A combination of methods is performed usually where sedimentation or flotation with filtration is performed with flocculation (Molina Grima et al. 2003). The overall advantages and disadvantages of each method are given in Table 1 (Mooney-McAuley et al. 2016). A brief overview of various thermochemical and biochemical conversions of biomass is required to know the different methods of extracting bioenergy from algal sources. The thermal methods which are usually employed to convert biomass into biofuel are gasification, combustion, pyrolysis, liquefaction and torrefaction. The biochemical methods used to convert are anaerobic digestion and transesterification (Pourkarimi et al. 2019a, b; Kirubakaran et al. 2009).
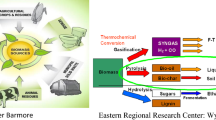
Figure 4 shows an overall view of conversion of energy techniques. Cultivation techniques are covered in open and closed. Harvest is seen by physical and chemical techniques. The algae are to be pre-treated with techniques mentioned above. The various thermochemical methods are further explained.
Thermochemical conversion techniques
Gasification refers to the conversion of biomass feedstock into hydrogen (H2), carbon dioxide (CO2) and methane (CH4) with a wide range of hydrocarbon products formed. Catalysts are used to enhance hydrocarbon production. The process involves heating the biomass to high temperature (> 700 °C) without combustion or with a controlled amount of oxygen and steam. The resulting gas mixture is known as syngas (Kirubakaran et al. 2009; Ebadi and Ebadi 2017). This process has seen increased use in algal biomass conversion, but potential disadvantages that occur are due to the gaseous nature of the product formed, which prevents easier storage and transport. The next method discussed is combustion. Combustion refers to the oxidation occurring when a substance is reacted with sufficient amounts of oxygen, producing amounts of heat and light rapidly. The products of combustion are similar to that of gasification, with a notable difference being the production of solid residues. Algal biomass is considered to be a potential candidate for combustion due to the fact that it has a higher heating value and more efficiency, however, due to its large amounts of nitrogen (Lane et al. 2013), which results in the formation of nitrous oxides. The higher moisture content of algae also prevents it from being used successfully with regards to large-scale industrial usage (Vassilev and Vassileva 2016). Pyrolysis refers to the thermochemical degradation of a substance in the absence of oxygen at elevated temperatures. The by-products of pyrolysis include a viscous liquid known as bio-oil, a dark black charry substance known as biochar and a gaseous mixture known as pyrogas (Chiaramonti et al. 2017). The bio-oil formed can be used as an intermediate for downstream processing in biofuel production. There has been a lot of research regarding algal pyrolysis. However, one disadvantage for algal pyrolysis is that it requires the feedstock to be dry, and this results in spending energy for pre-processing the feedstock. Torrefaction is a special case of mild pyrolysis which is used for augmenting the biomass characteristic. It is performed at temperatures lesser than pyrolysis range (Luque et al. 2012). Hydrothermal liquefaction is one of the procedures where wet biomass can be used. It provides a direct pathway for liquid biocrude production. This liquid product is a complex mixture of oxygenated hydrocarbons, and in the case of algae biomass, it contains substantial nitrogen as well (Mwangi et al. 2015). This method gains popularity from the fact that drying of biomass is not a prerequisite and it is energy efficient. The oil gained from the liquefaction process is used as a biofuel intermediate. However, the economics of the process needs to be looked into in detail (Elliott et al. 2013).
Biochemical techniques
Some of the biochemical processes that will be discussed about in brief are anaerobic digestion and transesterification.
Anaerobic digestion refers to the process where biomass is broken down into simpler molecules that eventually yields bio-products in the absence of oxygen. The potential of algae to be used as a biomass is vast with many algal species releasing methane upon digestion. Some advantages of the process include reduction in electrical and thermal losses usually associated with other methods of conversions, the solid residue being used as a fertilizer for farms and a viable methane source, along with liquid bio-oil. Disadvantages with this process with respect to algal feedstock arise from technical restraints including low concentration of digestible biodegradable substrate, recalcitrant substrate constituents and low-carbon-to-nitrogen ratio (Ward et al. 2014). Transesterification refers to the process where a glyceride combines with an alkyl alcohol of low molecular mass to produce fatty acid methyl esters (FAME) and glycerol. Algal transesterification has been an upcoming field to produce biodiesel from algal bio-oil produced from pyrolysis or liquefaction (Amin 2009). Disadvantages of the transesterification process by itself are that the production costs of biodiesel make the process economically unfeasible for large scale. Table 2 shows how microalgae and macroalgae have their element composition distributed.
Green algae pyrolysis
The reason why algae are considered as a potential biomass agent for pyrolysis is due to greater bio-oil production seen, especially in microalgae. Macroalgae have been looked as potential fuel, and research has been done on that field. There exists a significant amount of literature on algal pyrolysis, of varying algae, of those belonging to red, brown and green algae. While studies have been performed on red algae with prominent example species such as Gracilaria and Porphyria (Francavilla et al. 2015; Bae et al. 2011) and brown algae species such as Sargassum and Saccharina (Kim et al. 2012, 2013), a thorough literature study and a review of green algal pyrolysis techniques have not been compiled.
Green algae, especially microalgae, have been seen to produce high amounts of bio-oil, making them a field of potential interest. Studies on Spirulina and Chlorella have been done extensively with about 5000 research papers on pyrolysis, but a systematic review of green algal pyrolysis for various micro- and macroalgae has not been done yet. Green microalgae have already been tested upon for bio-oil yield, and the results have been encouraging. Reasons for using green macroalgae and microalgae as a potential fuel source are more. Although it is widely present, it is underused, with less than 1% being used, with macroalgae being found to be having chemical rich bio-oils. These bio-oils are rich in aromatics, sugars and other high-value chemicals. These can be used to produce biodiesel, by various chemical conversion techniques (Budarin et al. 2011). Algae have been seen as third-generation biofuels, meaning that they are used to enhance the performance of biofuels processed from existing feedstock. Integration of macroalgae into bio-refineries has been prioritized, with studies being performed on it (Golberg et al. 2018). Green microalgae have consistently been seen as sources to produce bio-oil.
The need to exclusively focus on green algae lies on two major counts. Green algae are seen as the direct ancestors to modern-day green plants, due to the presence of chlorophyll A and chlorophyll B. The yield of bio-oil from terrestrial plant-based life forms has been generally inadequate, and green algae, being the precursors, are checked to see whether they have any potential in bio-oil formation. Also, green algae are ubiquitous, being part of many ocean niches. The growth and the role of green algae that plays in the marine ecosystem are beneficial. This is the fact that their rapid regeneration in climes and also their relative usefulness for other produces has made green algae a tempting research field.
Maximum bio-oil yields have been reported from Spirulina and Chlorella (Lin et al. 2014; Chaiwong et al. 2013; Jena et al. 2011). Pyrolysis on green algae species has been performed extensively, and a review on the various pyrolysis techniques and the methods used in pyrolysis of green algae thus becomes necessary. This review covers the topics of different pyrolysis techniques, viz. slow, fast, microwave and catalytic pyrolysis, and various parameters that affect pyrolysis, viz. temperature, particle size, reaction time and nature of algae. Table 3 shows how the biochemical fractions are placed in green algal species.
Chemistry of pyrolysis
Pyrolysis mechanism
As mentioned above, pyrolysis refers to the process where a solid or a liquid undergoes thermal degradation into smaller molecules at elevated temperatures in the presence of inert atmosphere of nitrogen or very less stoichiometric quantities of oxygen. Pyrolysis can produce many different thermal degradation products. A point to be noted is that pyrolysis is a chemical process, but is not a phase change. The thermal decomposition of algae can be divided into three main steps:
- (1)
Dehydration of algae at temperatures below than about 200 °C,
- (2)
Devolatilization that is the main pyrolysis process and occurs at 200–550 °C
- (3)
Solid decomposition at temperatures above 550 °C.
During algae pyrolysis, decomposition of proteins and carbohydrates takes place at the temperature below than 400 °C, while the lipids mainly decompose by raising the temperature to about 550 °C. However, further heating the biomass to higher temperatures (600 °C) increases secondary cracking reactions wherein larger molecular weight hydrocarbons are broken down to smaller ones and therefore the bio-oil products decrease.
- (1)
Removal of bound water moieties from algae at 150 °C
- (2)
Removal of volatiles from the organic matter starting at 200, going up to 550 °C
- (3)
Temperatures greater than 600 °C see solid decomposition
Pyrolysis of biomolecules in algae present begins with the decomposition of carbohydrates and proteins which is seen at the temperature below than 400 °C. Lipid decomposition is seen at 550 °C, significantly higher than proteins or carbohydrates. Higher temperatures above 600 °C show less bio-oil production and greater gas release, as the hydrocarbon chain gets fragmented into simpler linear chains (Pourkarimi et al. 2019a, b).
The mechanism of pyrolysis involves the breakdown of matter by three methods, random scission and side-group scission and monomer reversion. Generally for biomass pyrolysis, we see monomer reversion and random scission. In random scission, the carbon chain is broken randomly by the action of heat, and this occurs in biomass where we have uniform composition like cellulose. Side-group scission is seen where the carbon-side element bond is broken, rather than the main chain. Some examples are seen in plastic waste pyrolysis, such as polyethene. Monomer reversion sees the complete breakdown of polymer into constituent monomers (Stauffer et al. 2008).
The general reaction of biomass pyrolysis can be said as follows: (Angin 2013).
- (1)
Biomass → Vapours + Residue, which is unreacted
- (2)
Unreacted residue → Char + Volatiles + Gases
- (3)
(Char) 1 → (Volatile + Gases) 2 + (Char) 2.
Composition of any biomass including algal biomass consists of following major compounds, cellulose, hemicellulose and lignin. The concentrations of these compounds vary with the type of the biomass used, such as wood, rice husk or algae. For cellulose, which is the major constituent of green algal biomass, the mechanisms of pyrolysis are based on hypothetical assumptions rather than unambiguous proof. Nevertheless, it can be agreed that there are two competing mechanisms for cellulose breakdown, fragmentation reaction to produce char and light volatile products such as gases, aldehydes and ketones. The second mechanism involves the formation of levoglucosan and other anhydromonosaccharides. The first mechanism is usually seen in slow pyrolysis, while the second one is seen at high temperatures and heating rates. Volatiles are generally thought to form by free radical mechanisms, primarily by scission. It is theorized that the formation of various products happens as parallel and not competing reactions, and the reactions are of first order in nature (Jakab 2015; White et al. 2011). The overall mechanism of algal pyrolysis is based on the relative protein, lipid and hydrocarbons. The general method to derive the mechanism of reaction is thermogravimetric analysis. Generally, mass loss for macroalgae represents the decomposition of carbohydrates. Subsequent heating involves the pyrolysis of proteins, with depolymerization being the main factor. The release of nitrogen in algal pyrolysis is mainly seen as a result of protein depolymerization; however, significant amounts of ammonia and nitrous oxides are released. Lipid breakdown is usually seen by hemicellulose mechanism formation (Debiagi et al. 2017). The reaction conditions of producing exclusively biochar, bio-oil and pyrogas vary. To make sure that more yield is obtained, sometimes materials are subjected to a slow form of mild pyrolysis called torrefaction, where the biomass is subjected to 200–250 °C at inert atmosphere to remove volatiles. This refined biomass is then taken for pyrolysis.
Temperature dependence of pyrolysis
Temperature plays a major role in pyrolysis. The nature of the product depends mainly on the heating rate. Slow heating rates tend to avoid cracking rates and increase biochar yield. Rapid heating rate increases the possibility of the biomass decomposition into gaseous and avoids the repolymerization of the algae lipids vapours even at low temperatures which increase the bio-oil yield (Pourkarimi et al. 2019a, b). When compared to red or brown algae, green algae tend to have more water content (Fathy 2007); however, pre-treatment studies have shown that green algae lose a lot of water while drying. Green algae tend to pyrolyse at the optimum temperature of 500–550 °C. Temperatures above 550 °C significantly promote secondary cracking reactions wherein larger molecular weight hydrocarbons are broken down to smaller ones resulting in decreasing bio-oil (Norouzi et al. 2016). Further increasing the temperature leads to an increase in the carbon content of the yields and formation poly aromatic hydrocarbons. Norouzi et al. (2016) shows that the heating value of Cladophora glomerata, a macroalgae commonly seen, is 14.97 MJ/kg, comparable to agricultural wastes. For scaling up of pyrolysis for macroalgae, the heating rate, particle size and temperature are factors to be noted. Microalgal pyrolysis has shown the fact of greater ash production when compared to terrestrial biomass pyrolysis. Kebelmann et al. (2013) show that the heating value of Chlamydomonas reinhardtii and Chlorella vulgaris is 23 and 18 MJ/kg, higher than some macroalgae species. This is seen due to higher ash content seen in microalgae when compared to macroalgae.
Effect of particle size
It is generally seen that pyrolysis is seen to be most effective when particle size is seen to be small, for producing bio-oil. As seen from general biomasses, reported by Kaur et al. (2015), various biomasses such as hazelnut, rapeseed and sunflower had best pyrolysis conditions at particle sizes ranging from 0.1 to 0.5 mm. The particle size also varies with different reaction conditions such as type of pyrolysis and the reactor. The reason for small particle size being used is that larger particles require more heat, and the activation energy too subsequently increases. For smaller particles, the energy required is less, but requires various commutation techniques which increases costs and processing time. Optimum particle size is required to increase bio-oil yield. For slow pyrolysis, which is the main pathway to char, large particle size (> 2 mm) and slow heating rates (< 10 °C) are common parameters (Kocer et al. 2018).
Hu et al. (2013) found that the maximum bio-oil yield of blue green algae blooms can be achieved by smaller particle size without considering the cost of grinding. When the algal particle size was increased, the bio-oil yield was significantly reduced from 54.97% to 42.86%, and the gas yield was decreased slightly from 20.47% to 18.98%, while the biochar yield was increased considerably from 24.56% to 38.16% which were mainly associated with the heating rate of the particles. With increasing the particle size, the heat resistance distance from the algae particle surface to its centre is increased which hinders the rapid heat transfer from the hot material to cold biomass and therefore the incomplete pyrolysis reaction occurred.
Bio-oil yield of blue green algal blooms was shown to be achieved by Hu et al. (2012). This was tested with the parameter of particle size. The smaller the particle size, the greater the yield. Hu et al. (2013) observed in their experiments with algal pyrolysis that a greater particle size involved lesser oil yield from 54.97 to 42.86%. The gas yield too decreases slightly, from 20.47 to 18.98%. The char production was seen to increase dramatically from 24.6 to 38.2%. An explanation could be suggested that particle size increase results in an increase in heat resistance, as the distance from surface to centre of algal particle increases. Larger distance travelled would decrease the heat transfer capability. This would thus result in an incomplete heat transfer, and subsequently, complete pyrolysis does not occur. A complete pyrolysis process is characterized by the production of gas and liquid seen by solid decomposition, but this might not be the case here. With respect to algal pyrolysis, research is needed to conclusively prove that particle size does matter.
Effect of feedstock on pyrolysis
Green macroalgae and microalgae vary considerably in their feedstock composition. As seen from microalgae (Scenedesmus sp.) taken, they have considerable amounts of carbohydrate and protein amounts of 29.3% and 36.4%, indicating the amounts of high nitrogen content at 7.25%, which may not be beneficial for fuel-based purpose. The percentage of ash content in microalgae is generally less, though higher than macroalgae, usually found in the range of 7 to 10% (Chaiwong et al. 2013). The prospect of higher oil production is seen from microalgal pyrolysis. General trends of bio-oil produced from microalgae and macroalgae are put as a table here.
When we analyse the tables of microalgae and macroalgae oil production, the higher oil content is found in microalgae because of greater lipid content. This makes microalgae to be a potential candidate for oil production, but as mentioned, the cultivation and harvesting process takes a longer time than macroalgae. The feedstock is ultimately selected based on various considerations such as the oil productivity, ease of availability and processing techniques, financial constraints and the viability to scale up the reactions occurring during the testing phase.
Effect of time on the pyrolysis process
The nature of pyrolysis is decided by the time the feedstock spends in the reactor. The criterion for slow and fast pyrolysis depends on the residence time of the feedstock. The vapour residence time refers to the time which the primary vapours and char of pyrolysis interact. Higher vapour time leads to greater char production because greater time allows for the complete repolymerization of the biomass components, while shorter time leads to lesser yield (Pourkarimi et al. 2019a, b). The heating rate also plays a crucial role where low heating rates for long periods give greater char and higher heating rates and shorter periods give more oil (Tripathi et al. 2016). (Wang et al. 2013) show that the residence time of vapour has significant impact on seaweed. Longer vapour residence time also has the possibility of secondary reaction of volatiles occurring more frequently, and number of alcohols, ketones and hydrocarbons are enhanced.
Dependence on reactor type
Type of reactors
There are two types of reactors that are generally used for algal pyrolysis: fixed bed and fluidized bed reactors (Zhao et al. 2013).
A fixed bed reactor is usually long and cylindrical in shape and has a gas cooling and cleansing system. The materials used are firebricks, steel or concrete. The catalyst is usually arranged as a bed. The reaction is controlled and the heat supply is derived from an external furnace. A gas cylinder is attached externally so that the inert atmospheric conditions can be maintained. Nitrogen is the preferred inert carrier gas. A cooling system is attached to the reactor to condense the vapours to pyrolysis oil. They are suitable for a low-scale production. Fixed bed reactors are designed for a given pressure drop, and the catalyst mass filling the reactor should not exceed that specification (Worstell 2014). The various levels in which they are modelled are laboratory, pilot and commercial scale.
Fluidized bed reactors function on the principle of a stationary bed being made into a liquid-like continuum (called fluidization) when a fluid of high velocity is used to make the bed of particles into individual entities in the fluid. This allows for the better heat and mass transfer. The reasons to a fluidized bed being preferred are that it has ease of operation, good temperature control and scale-up capability. The fluidized bed reactor consists of a fluid–solid mixture wherein a stable bed of biomass particles is retained using an inert fluidizing gas such as nitrogen. The pyrolysis reactor sand particles warm up by gas before loading of the reactor with biomass. The reactor is equipped with a cyclone to separate the solid particles (char) from vapour. The condensable liquid–vapours leaving the cyclone are quickly cooled and collected in condenser, while the non-condensable gasses are vented out. The design of fluidized bed reactors enables decreasing catalytic cracking of pyrolysis vapour by char due to rapid separation of char by cyclone separator.
Fixed bed reactors
Industrial-grade fixed bed reactor systems are tubular in nature. They are equipped with gas cooling systems to regulate heat transfer rates, and a cleansing system present to remove impurities. The catalyst is usually arranged as a bed. Construction of these reactors is made with materials that can withstand high temperatures, viz. firebricks, steel or concrete. The gas cylinder, containing inert elements such as nitrogen or helium, is attached to system to maintain non-reactive atmosphere. Heat supply is provided externally. Gas cooling systems are used to get pyrolysis oil. These systems are designed in general for a given pressure drop. The catalyst mass drop should not exceed the specification (Worstell 2014). Bench-scale studies are also performed.
Fluidized bed reactors
Fluidized bed reactors work with the principle that a high-velocity fluid is able to make a stationary bed into a liquid-like continuum. The phenomena are called fluidization. The subsequent intermixing of the bed particles and fluid is desired as they allow good heat and mass transfer. Commercial preference of using fluidized bed reactors is given due to reasons such as ease of operation, good temperature control and scale-up mobility. The composition of the reactor is a stable bed of biomass particles and catalyst if needed, along with an inert fluidizing gas. The inert gas is mixed with sand and is then heated. This gas mix is let into the pyrolysis chamber, to react with the biomass. A cyclone separator is used to separate solid particles (char) from the gas. A condenser is used to gather the liquid vapours which are condensable, while the non-condensable ones are let out at exhaust. The design of the reactor enables a very important feature. It lessens catalytic cracking occurring in pyrolysis by rapid separation of char and vapour.
Pyrolysis products
Biochar
Biochar refers to the solid residue obtained from pyrolysis process, which is brown and charry in nature. It has a high amount of coke content in it. Algal biochar contains carbon, hydrogen and nitrogen, along with inorganic elements such as potassium, sodium, calcium and magnesium. As mentioned, due to the higher amounts of ash and fixed content, the production of biochar seen is higher than that of terrestrial plants. Studies of using algal biochar as an adsorbent have been performed on both macroalgae and microalgae. Bird et al. (2011), in his experiment with green macroalgal species, observe that biochar produced from species of Cladophora, Chaetomorpha, Ulva and Caulerpa shows that the char has comparably less carbon content than microalgal char and also has low surface area and cation exchange capability. However, they have high pH and higher nutrient contents, thus making them apt for soil nutrition purposes (Torri et al. 2011). Yu et al. (2018) show that the biochar production from microalgal species of Chlorella vulgaris shows an alkaline pH value of 8.1, making it suitable for agricultural soil as a supplement. The decrease in volatile matter fraction makes algal biochar useful for coal fuelled boilers without any conversion process (Gong et al. 2014a, b). When comparing the higher heating values of microalgae and macroalgae biochars, the microalgae biochar has values similar to that of coal (25 MJ/kg), thus suggesting its potential use as an alternative energy source. Higher surface area in microalgal biochar is seen as a reason for microalgal biochar to be considered as an adsorbent, with the scanning electron microscope (SEM) observation attesting to the fact that the surface is highly porous and fragmented, and may contain active binding sites. Fourier transform infrared spectroscopy (FTIR) of biochars, formed at high temperatures exceeding 600 °C, shows the presence of polycyclic aromatic rings, with the complete breakdown of hemicellulose and cellulose structures. Biochars impregnated with the catalyst also show the presence of hetero-atoms such as sulphur and bromine present. Since biochars are not comprised of only one single compound, various bonds such as C–H stretch, C = C alkene bonds and aromatic linkages are also observed (Balaji and Niju 2019).
Bio-oil
Bio-oil produced during pyrolysis refers to a viscous liquid, rich in organic compounds. The nature of bio-oil produced varies with respect to the feedstock with coal showing it as a darkish black liquid, wood showing as a dark brown liquid and algae showing a greenish tinge due to the presence of nitrogen. The oil contains two phases, aqueous and tar phase, with them containing oxygenated hydrocarbons and various compounds such as aldehydes, ketones, esters and phenols.
The characteristics of bio-oils vary among different species of macro- and microalgae, and the composition varies depending on the conditions during pyrolysis. For example, (Adamakis et al. 2018) report the bio-oil composition for the thermal pyrolysis of two sets of Chlorella vulgaris grown in nitrogen-rich and nitrogen-starved condition. He finds that while a nitrogen-rich medium promotes relatively high productivity (1.5 g of dry biomass per litre of media), the highest lipid condition (36% of dry biomass) was achieved for nitrogen-depleted medium conditions. The bio-oil contains a complex mixture of fatty acids, phenolics, pyrroles and amides, present at different concentrations. Studies by Babich et al. (2011), Francavilla et al. (2015) and Thangalazhy-Gopakumar et al. (2012) for different species of green microalgae show the presence of the compounds in different proportions. Oxygenated content present in bio-oil arises usually from catalytic pyrolysis, which will be explained. In general, pathways to bio-oil production are manipulated in such a way that we either get feedstock suitable to be converted to biodiesel or to be used as it is. Table 4 shows a proper distribution of how bio-oil, char and gas are formed at different conditions of pyrolysis. Macroalgal species too have oils varying in compositional percentages. However, the commonly seen compounds include toluene, ethylbenzene, styrene, phenols, indole, pentadecane, neophytadiene, pyridine and some other nitrogen-containing compounds. It should be noted that although gas chromatography–mass spectrometry (GC–MS) analysis is the most used method in the literature for analysing oil, only 25–40% of oil compounds can be identified by gas chromatography methods because some components of oils such as long-chain lignin and carbohydrates are not volatile enough to be detected by gas chromatography.
The commercial viability of bio-oil is being currently investigated. Although they have heating values comparable to terrestrial bio-feedstock, the higher amount of oxygen indicates a lesser stability and higher reactivity. More amounts of saturated and unsaturated fatty acids lead to corrosiveness which makes storage difficult. Greater nitrogen compounds seen during pyrolysis are also to be considered, as they release NOx emissions. Table 5 shows how biochar, oil and gas are formed from known samples of green macroalgae.
Biogas
Biogas refers to the volatile fraction recovered from pyrolysis and is composed primarily of low molecular weight compounds such as CH4, CO, CO2 and H2 which can be used as potential feed. The algal pyrolysis gives the same gaseous products such as other feedstock. Temperature, time and reaction conditions all play an important role in algal pyrolysis. Secondary volatiles are further released during pyrolysed conditions. Figure 4 is a culmination of how the yields are distributed in green algal species. We see that bio-oil is higher in quantity in microalgae than macroalgae.
Types of pyrolysis
Fast pyrolysis
Fast pyrolysis is the process of rapidly heating biomass to high temperatures in the absence of air. The temperature range of operation is around 500 °C and has very short reaction times of between 1 and 5 s. The process prior to fast pyrolysis requires that the biomass be dried and grinded to a particle size of less than 1 mm, to account for larger-surface-to-volume ratio which allows better heat transfer. The yield is considerably lower than that of slow and catalytic pyrolysis. The average yield of bio-oil by pyrolysis has been estimated to be 60–75 wt%, and it has a significant increase in energy density from the starting material. The rest of the yield consists of 15–25 wt% of solid and 10–20 wt% non-condensable gases. The heating rate generally used is 100 s in the absence of an oxidizing agent (Balat et al. 2009). The desired yield is bio-oil, and the oils obtained here are higher than that obtained in slow pyrolysis. Green microalgae have shown higher oil content production by fast pyrolysis, when compared to macroalgae.
Maximization of oil yield can be done by the following steps
- (1)
High heating and heat transfer rates,
- (2)
Optimal heating and vapour-phase temperature (500*C),
- (3)
Short residence and reaction time (< 2 h is preferable),
- (4)
Greater surface area of biomass,
- (5)
Rapid cooling of pyrolysis vapours,
- (6)
Usage of fluidized bed reactor (Pattiya 2017).
In the fast pyrolysis of C. protothecoides (Miao and Wu 2004a, b), they showed the dependence of pyrolysis on temperature and saw that the bio-oil yield changed with an increase in temperature from 400 to 500 and there was a decrease in the yield as it was increased to near 600 °C. The heterotrophic cells of the algae considered gave a yield 3.4 times better than that from autotrophic cells. The bio-oil from HC had lower oxygen content, lower viscosity and lower density than that from AC. This has also been confirmed by Elliott who has confirmed that there is a correlation between chemical composition and operating temperatures (Pattiya 2017; Elliott 1988). (Harman-Ware et al. 2013) confirmed that bio-oil from microalgae was stable. From the table above, it is clear that the average yield from pyrolysis is between 18 and 70% and the higher heating value (HHV) ranges.
Slow pyrolysis
The term slow pyrolysis refers to the conditions of a slow heating rate, longer holding time and lower temperatures. This leads to the formation of biochar over bio-oil. The decomposition temperature is seen to be around 400–500 °C, significantly lesser than that seen in fast and flash pyrolysis. Due to the lesser temperature involved and longer heating rates, char production is preferred. The stages seen in slow pyrolysis generally include water removal, organic fraction breaking down, to release primary volatiles, leaving behind carbon-rich residues. Gaseous components produced are CO2, CH4 and H2 (Luo et al. 2004; Belotti et al. 2014).
Microwave pyrolysis
Pyrolysis with microwave-assisted heating is known as microwave pyrolysis. Microwave radiation has been replacing conventional methods of heating due to carbon materials being very good absorbents of microwaves, which can be repurposed to indirectly heat other materials (Menéndez et al. 2010; Zhang et al. 2016).
One of the main advantages of microwave-assisted pyrolysis is the ease of control with the on/off system. The operating temperature is between the ranges of 500 and 800 °C at a power supply range of 500–2250 W. The yield of bio-oil from microwave-assisted pyrolysis is between the ranges of 18 and 57% as per the table. The yield value is lower than that obtained from fast pyrolysis. The heating values ranges from 27,900 to 32,000 kJ/kg. From the work done by Du et al. (2011) and Borges et al. (2014), we infer that microwave-assisted pyrolysis has the following advantages over conventional pyrolysis i) instantaneous response for rapid start up and shutdown, ii) no need for agitation by fluidization which decreases the amount of particles in the obtained bio-oil yield and iii) uniform internal heating of the feedstock (Du et al. 2011; Borges et al. 2014). They also showed that in the presence of a catalyst like H-ZSM-5, there is an increase in the presence of moisture in liquid phase due to the production of water, but this reduced the higher heating level of the bio-oil obtained, but the overall quality of the bio-oil was improved due to a reduction in the amount of the number of species in the bio-oil. They concluded that the use of microwave absorbents in fat microwave-assisted pyrolysis is beneficial and improves the practical and consumer application of microwave pyrolysis (Borges et al. 2014). The same has also been confirmed by Hu et al. (2012).
Studies conducted by Du et al. (2011) show that 28.65 wt. % of bio-oil was obtained by the microwave pyrolysis of Chlorella sp. using char as a microwave absorber. The microwave power was set to a maximum of 750 W. The bio-oil obtained was characterized with low-oxygen content with aliphatic and aromatic hydrocarbons. Similar studies conducted on Chlorella sp. by Borges et al. (2014) and Fernandez et al. (2011) show that a bio-oil yield in the range of 41-57 wt % can be obtained at temperatures between 450 and 550 °C. Hu et al. (2012) conducted studies on the effect of microwave pyrolysis on Chlorella vulgaris which showed that bio-oil accounted for 21 to 39% of the total yield. For microwave powers of 1500 and 2250 W, respectively, the process was done at a temperature range of 650–800 °C. Table 6 shows the microwave-assisted pyrolysis.
Catalytic pyrolysis
Simply put, the pyrolysis of biomass in the presence of a catalyst is known as catalytic pyrolysis. Products formed under fast pyrolysis require further upgradation in order to improve the quality of products such as bio-oils. The currently most researched methods involve using supercritical water (Duan and Savage 2011) and catalytic hydro-deoxygenation (Guo et al. 2015). These methods are necessary for application of bio-oils in biofuel technology as the obtained oil is usually highly viscous with low-oxygen content and correspondingly low hydrogen.
Catalysts can help control the behaviour of the given feed during the pyrolysis reaction to give the products of required composition in the case of catalyst-assisted pyrolysis. For example, catalytic fast pyrolysis of maple wood using ZSM-5 zeolite catalysts yields lesser liquid product yield, while optimizing yield of deoxygenated aromatic pyrolysis products (Foster et al. 2012). Catalytic pyrolysis of macroalgae is conducted within a temperature range of 400–800 °C. Fixed bed setups are most commonly used for these experiments for algae feedstocks (Ma et al. 2019). However, studies have been conducted on use of fluidized bed reactors as well for catalytic or catalyst-assisted fast pyrolysis of biomass (Zhang et al. 2009; Aho et al. 2007).
(Ma et al. 2019) have shown that bio-oil obtained by catalytic pyrolysis contains a lesser number of compounds compared to bio-oil produced by non-catalytic pyrolysis. Another observation is the higher heating value (HHV) of bio-oil obtained from catalytic pyrolysis relative to that obtained from non-catalytic operation. This also corresponds with a reduction in oxygen percentage from elemental analysis of both the bio-oil samples. The comparative HHV values of bio-oil obtained from pyrolysis of different alga in the presence and absence of suitable catalysts are given in the following table. Table 7 shows a comparison of algal pyrolysis in catalyst-free and catalytic pyrolysis.
Perspectives
Algae, as a third-generation biofuel feedstock, are already seen as a viable raw material from which many products can be synthesized. Common by-product production includes biogas, ethanol, bio-hydrogen, syngas, bio-oil and biodiesel. Pyrolysis as a technology has seen lots of improvement to maximize the yield of bio-oil. Algae in its raw form are used extensively in wastewater purification of heavy metal-, dye-, phenolic- and textile-based effluents (Slaveykova and Wilkinson 2003; Zhang et al. 2019). There has been a considerable interest in using algal-based biochar as a potential adsorbent, due to its high surface area and pore distribution.
Future studies of algal pyrolysis deal with the upgradation of bio-oil quality. Factors that inhibit bio-oil usage such as phase separation from wet feedstock, high viscosity, high water content, thermal instability along with highly acidic natures are being combated by upgrading the bio-oil with catalysts. Hot vapour filtration, solvent addition and all enhance the quality of bio-oil, leading to further possibilities (Campanella and Harold 2012). Bio-oil is a substitute for light and heavy fuel oil. An example seen is in Finland, where the light oil market is attractive for burner development. Ease of handling, storage and combustion all make bio-oil preferred. Further research needs to be put in with good promotion and publicity plans. For long-term testing, fundamental research is required for optimization and development of commercial processes with strong collaboration with research and industry. Pro-active promotion of techniques will help in a long way in removing a negative image from the minds of industries.
Conclusion
Green macroalgae generate more biochar irrespective of method of pyrolysis when compared to green microalgae, which generate more bio-oil. The bio-oil obtained from microalgae has more potential to be used, due to lesser nitrogen content, and has a high calorific value when compared to the oil generated from macroalgae. Green algae are considered to be a better option than red or brown algae due to lesser water content, easier to harvest in a larger scale and being given better output per kilogram of raw feed used for conversion. Potential benefits of using algae as a third-generation biofuel are high and can be harnessed effectively.
The potential of using green algal biomass for energy production is immense. Macroalgae and microalgae both can be used to get energy, in different forms, with macroalgae being used to produce biodiesel and then energy, while oil content in microalgae can be harnessed through thermochemical methods. A review of the methods of cultivation and harvest of the different types of green marine and freshwater algae shows the need to appropriately select the algal strain and conditions in order to maximize yield and hence ensure a proper supply is met. Different thermochemical methods have their own advantages and disadvantages. Pyrolysis is seen to be most enterprising, and advancements in the field of pyrolysis are seen with different bio-feedstocks of algae. The chemical distribution of compounds in algal species makes it ultimately a widely useful feed for various chemical operations.
References
Adamakis I-D, Lazaridis PA, Terzopoulou E, Torofias S, Valari M, Kalaitzi P, Rousonikolos V, Gkoutzikostas D, Zouboulis A, Zalidis G, Triantafyllidis KS (2018) Cultivation, characterization, and properties of Chlorella vulgaris microalgae with different lipid contents and effect on fast pyrolysis oil composition. Environ Sci Pollut Res Int 25:23018–23032. https://doi.org/10.1007/s11356-018-2368-5
Aho A, Kumar N, Eränen K, Salmi T, Hupa M, Murzin DY (2007) Catalytic pyrolysis of biomass in a fluidized bed reactor: influence of the acidity of h-beta zeolite. Process Saf Environ Prot 85:473–480. https://doi.org/10.1205/psep07012
Al Hattab M (2015) Microalgae harvesting methods for industrial production of biodiesel: critical review and comparative analysis. J Fundam Renew Energy Appl. https://doi.org/10.4172/2090-4541.1000154
Amin S (2009) Review on biofuel oil and gas production processes from microalgae. Energy Convers Manag 50(7):1834–1840. https://doi.org/10.1016/j.enconman.2009.03.001
Angin D (2013) Effect of pyrolysis temperature and heating rate on biochar obtained from pyrolysis of safflower seed press cake. Bioresour Technol 128:593–597. https://doi.org/10.1016/j.biortech.2012.10.150
Aysu T, Sanna A (2015) Nannochloropsis algae pyrolysis with ceria-based catalysts for production of high-quality bio-oils. Bioresour Technol 194:108–116. https://doi.org/10.1016/j.biortech.2015.07.027
Aysu T, Abd Rahman NA, Sanna A (2016) Catalytic pyrolysis of Tetraselmis and Isochrysis microalgae by nickel ceria based catalysts for hydrocarbon production. Energy 103:205–214. https://doi.org/10.1016/j.energy.2016.02.055
Babich IV, van der Hulst M, Lefferts L, Moulijn JA, O’Connor P, Seshan K (2011) Catalytic pyrolysis of microalgae to high-quality liquid bio-fuels. Biomass Bioenerg 35:3199–3207. https://doi.org/10.1016/j.biombioe.2011.04.043
Bae YJ, Ryu C, Jeon JK, Park J, Suh DJ, Suh YW, Park YK (2011) The characteristics of bio-oil produced from the pyrolysis of three marine macroalgae. Bioresour Technol 102(3):3512–3520. https://doi.org/10.1016/j.biortech.2010.11.023
Balaji M, Niju S (2019) Biochar-derived heterogeneous catalysts for biodiesel production. Environ Chem 1:1. https://doi.org/10.1007/s10311-019-00885-x
Balat M, Balat M, Kirtay E, Balat H (2009) Main routes for the thermo-conversion of biomass into fuels and chemicals. Part 1: pyrolysis systems. Energy Convers Manag 50:3147–3157. https://doi.org/10.1016/j.enconman.2009.08.014
Becker B, Marin B (2009) Streptophyte algae and the origin of embryophytes. Ann Bot 103:999–1004. https://doi.org/10.1093/aob/mcp044
Belotti G, De Caprariis B, De Filippis P, Scarsella M, Verdone N (2014) Effect of Chlorella vulgaris growing conditions on bio-oil production via fast pyrolysis. Biomass Bioenerg 61:187–195. https://doi.org/10.1016/j.biombioe.2013.12.011
Biller P, Ross AB (2011) Potential yields and properties of oil from the hydrothermal liquefaction of microalgae with different biochemical content. Bioresour Technol 102(1):215–225. https://doi.org/10.1016/j.biortech.2010.06.028
Biller P, Ross AB, Skill SC, Lea-Langton A, Balasundaram B, Hall C, Llewellyn CA (2012) Nutrient recycling of aqueous phase for microalgae cultivation from the hydrothermal liquefaction process. Algal Res 1(1):70–76. https://doi.org/10.1016/j.algal.2012.02.002
Bird MI, Wurster CM, de Paula Silva PH, Bass AM, de Nys R (2011) Algal biochar–production and properties. Bioresour Technol 102:1886–1891. https://doi.org/10.1016/j.biortech.2010.07.106
Borges FC, Xie Q, Min M, Muniz LAÔR, Farenzena M, Trierweiler JO, Chen P, Ruan R (2014) Fast microwave-assisted pyrolysis of microalgae using microwave absorbent and HZSM-5 catalyst. Bioresour Technol 166:518–526. https://doi.org/10.1016/j.biortech.2014.05.100
Borowitzka M (1999) Commercial Production of Microalgae: ponds, Tanks Tubes and Fermenters. J Biotechnol 70(1–3):313–321. https://doi.org/10.1016/S0079-6352(99)80123-4
Brennan L, Owende P (2010) Biofuels from microalgae-A review of technologies for production, processing, and extractions of biofuels and co-products. Renew Sustain Energy Rev 14:557–577. https://doi.org/10.1016/j.rser.2009.10.009
British Petroleum Company (2018) BP statistical review of world energy. British Petroleum Co, London
Brown MR (1991) The amino-acid and sugar composition of 16 species of microalgae used in mariculture. J Exp Mar Bio Ecol 145:79–99. https://doi.org/10.1016/0022-0981(91)90007-J
Bruhn A, Dahl J, Nielsen HB, Nikolaisen L, Rasmussen MB, Markager S, Olesen B, Arias C, Jensen PD (2011) Bioenergy potential of Ulva lactuca: biomass yield, methane production and combustion. Bioresour Technol 102:2595–2604. https://doi.org/10.1016/j.biortech.2010.10.010
Budarin V, Zhao Y, Gronnow M, Shuttleworth P, Breeden S, Macquarrie D, Clark JH (2011) Microwave-mediated pyrolysis of macro-algae. Green Chem 13(9):2330. https://doi.org/10.1039/C1GC15560A
Burton T, Lyons H, Lerat Y, Stanley M, Rasmussen MB (2009) A review of the potential of marine algae as a source of biofuel in Ireland. Sustain, Energy Ireland
Campanella A, Harold MP (2012) Fast pyrolysis of microalgae in a falling solids reactor: effects of process variables and zeolite catalysts. Biomass Bioenerg 46:218–232. https://doi.org/10.1016/j.biombioe.2012.08.023
Capodaglio AG, Callegari A (2018) Feedstock and process influence on biodiesel produced from waste sewage sludge. J Environ Manag 216:176–182. https://doi.org/10.1016/j.jenvman.2017.03.089
Ceylan S, Goldfarb JL (2015) Green tide to green fuels: fTIR analysis and kinetic study of Ulva prolifera pyrolysis. Energy Convers Manag 101:263–270. https://doi.org/10.1016/j.enconman.2015.05.029
Chaiwong K, Kiatsiriroat T, Vorayos N, Thararax C (2012) Biochar production from freshwater algae by slow pyrolysis. Maejo Int J Sci Technol 6:186–195. https://doi.org/10.14456/mijst.2012.13
Chaiwong K, Kiatsiriroat T, Vorayos N, Thararax C (2013) Study of bio-oil and bio-char production from algae by slow pyrolysis. Biomass Bioenerg 56:600–606. https://doi.org/10.1016/j.biombioe.2013.05.035
Chang Z, Duan P, Xu Y (2015) Catalytic hydropyrolysis of microalgae: influence of operating variables on the formation and composition of bio-oil. Bioresour Technol 184:349–354. https://doi.org/10.1016/j.biortech.2014.08.014
Chen W-H, Lin B-J, Huang M-Y, Chang J-S (2015) Thermochemical conversion of microalgal biomass into biofuels: a review. Bioresour Technol 184:314–327. https://doi.org/10.1016/j.biortech.2014.11.050
Chiaramonti D, Prussi M, Buffi M, Rizzo AM, Pari L (2017) Review and experimental study on pyrolysis and hydrothermal liquefaction of microalgae for biofuel production. Appl Energy 185:963–972. https://doi.org/10.1016/j.apenergy.2015.12.001
Danquah MK, Gladman B, Moheimani N, Forde GM (2009) Microalgal growth characteristics and subsequent influence on dewatering efficiency. Chem Eng J 151:73–78. https://doi.org/10.1016/j.cej.2009.01.047
Darienko T, Gustavs L, Eggert A, Wolf W, Pröschold T (2015) Evaluating the species boundaries of green microalgae (Coccomyxa, Trebouxiophyceae, Chlorophyta) using integrative taxonomy and DNA barcoding with further implications for the species identification in environmental samples. PLoS One. https://doi.org/10.1371/journal.pone.0127838
Davy R, Gnatiuk N, Pettersson L, Bobylev L (2018) Climate change impacts on wind energy potential in the European domain with a focus on the Black Sea. Renew Sustain Energy Rev 81:1652–1659. https://doi.org/10.1016/j.rser.2017.05.253
de Pádua M, Fontoura PSG, Mathias AL (2004) Chemical composition of Ulvaria oxysperma (Kützing) bliding, Ulva lactuca (Linnaeus) and Ulva fascita (Delile), Brazilian. Arch Biol Technol 47:49–55. https://doi.org/10.1590/S1516-89132004000100007
Debiagi PEA, Trinchera M, Frassoldati A, Faravelli T, Vinu R, Ranzi E (2017) Algae characterization and multistep pyrolysis mechanism. J Anal Appl Pyrolysis 128:423–436. https://doi.org/10.1016/j.jaap.2017.08.007
Demirbas A (2010) Use of algae as biofuel sources. Energy Convers Manag 51:2738–2749. https://doi.org/10.1016/j.enconman.2010.06.010
Demirbaş A (2001) Biomass resource facilities and biomass conversion processing for fuels and chemicals. Energy Convers Manag 42:1357–1378. https://doi.org/10.1016/S0196-8904(00)00137-0
Demirbaş A (2008) Production of biodiesel from algae oils. energy sources, part a: recovery, utilization, and environmental effects. Energy Source Part A 31(2):163–168. https://doi.org/10.1080/15567030701521775
Domozych DS, Ciancia M, Fangel JU, Mikkelsen MD, Ulvskov P, Willats WGT (2012) The cell walls of green algae: a journey through evolution and diversity. Front. Plant Sci. https://doi.org/10.3389/fpls.2012.00082
Du Z, Li Y, Wang X, Wan Y, Chen Q, Wang C, Lin X, Liu Y, Chen P, Ruan R (2011) Microwave-assisted pyrolysis of microalgae for biofuel production. Bioresour Technol 102:4890–4896. https://doi.org/10.1016/j.biortech.2011.01.055
Duan P, Savage PE (2011) Upgrading of crude algal bio-oil in supercritical water. Bioresour Technol 102:1899–1906. https://doi.org/10.1016/j.biortech.2010.08.013
Duran S, Kumar P, Sandhu SS (2018) A review on microalgae strains, cultivation, harvesting. biodiesel conversion and engine implementation. Biofuels. https://doi.org/10.1080/17597269.2018.1457314
Ebadi A, Ebadi A (2017) Gasification of Algal Biomass (Cladophora glomerata L.) with CO 2/H 2 O/O 2 in a Circulating Fluidized Bed. Environ Technol 40:1–21. https://doi.org/10.1080/09593330.2017.1406538
Edzwald JK (1995) Principles and Applications of Dissolved Air Flotation. Water Sci Technol 31(3–4):1–23. https://doi.org/10.1016/0273-1223(95)00200-7
Elliott DC (1988) Relation of reaction time and temperature to chemical composition of pyrolysis oils 1988:55–65. https://doi.org/10.1021/bk-1988-0376.ch006
Elliott DC, Hart TR, Schmidt AJ, Neuenschwander GG, Rotness LJ, Olarte MV, Holladay JE (2013) Process Development for Hydrothermal Liquefaction of Algae Feedstocks in a Continuous-Flow Reactor. Algal Res 2(4):445–454. https://doi.org/10.1016/j.algal.2013.08.005
Fathy AA (2007) Evaluation of nutritional composition of some attached and drifted marine algae from Alexandria, Egypt, 2007
Fernandez Y, Arenillas A, Angel J (2011) Microwave heating applied to pyrolysis. In: Advances in induction and microwave heating of mineral and organic materials. InTech, 2011. https://doi.org/10.5772/13548
Finkelman RB, Tian L (2018) The health impacts of coal use in China. Int Geol Rev 60:579–589. https://doi.org/10.1080/00206814.2017.1335624
Finkelman RB, Orem W, Castranova V, Tatu CA, Belkin HE, Zheng B, Lerch HE, Maharaj SV, Bates AL (2002) Health impacts of coal and coal use: possible solutions. Int J Coal Geol 50:425–443. https://doi.org/10.1016/S0166-5162(02)00125-8
Foster AJ, Jae J, Cheng YT, Huber GW, Lobo RF (2012) Optimizing the aromatic yield and distribution from catalytic fast pyrolysis of biomass over ZSM-5. Appl Catal A Gen 423–424:154–161. https://doi.org/10.1016/j.apcata.2012.02.030
Fotiadis M, Polemis ML (2018) The role of sustainability-related strategies on the biofuel industry: trends, prospects and challenges. Bus Strateg Environ 27:757–772. https://doi.org/10.1002/bse.2029
Francavilla M, Manara P, Kamaterou P, Monteleone M, Zabaniotou A (2015) Cascade Approach of Red Macroalgae Gracilaria gracilis Sustainable Valorization by Extraction of Phycobiliproteins and Pyrolysis of Residue. Bioresour Technol 184:305–313. https://doi.org/10.1016/j.biortech.2014.10.147
Garcia Alba L, Torri C, Samorì C, van der Spek J, Fabbri D, Kersten SRA, Brilman DWF (2011) Hydrothermal treatment (HTT) of microalgae: evaluation of the process as conversion method in an algae biorefinery concept. Energy Fuels 26(1):642–657. https://doi.org/10.1021/ef201415s
Golberg A, Liberzon A, Vitkin E, Yakhini Z (2018) Design and analysis of offshore macroalgae biorefineries. Methods Mol, Biol
Gong X, Zhang B, Zhang Y, Huang Y, Xu M (2014a) Investigation on pyrolysis of low lipid microalgae Chlorella vulgaris and Dunaliella salina. Energy Fuels 2014:95–103. https://doi.org/10.1021/ef401500z
Gong X, Zhang B, Zhang Y, Huang Y, Xu M (2014b) Investigation on pyrolysis of low lipid microalgae chlorella vulgaris and dunaliella salina. Energy Fuels 28(1):95–103. https://doi.org/10.1021/ef401500z
Gonzalez-Fernandez C, Ballesteros M (2013) Microalgae autoflocculation: an alternative to high-energy consuming harvesting methods. J Appl Phycol 25:991–999. https://doi.org/10.1007/s10811-012-9957-3
Guo Q, Wu M, Wang K, Zhang L, Xu X (2015) Catalytic hydrodeoxygenation of algae bio-oil over bimetallic Ni-Cu/ZrO2 catalysts. Ind Eng Chem Res 54:890–899. https://doi.org/10.1021/ie5042935
Haarhoff J, Maritz Rykaart E (1995) Rational design of packed saturators. Water Sci Technol 31:179–190. https://doi.org/10.1016/0273-1223(95)00216-A
Harman-Ware AE, Morgan T, Wilson M, Crocker M, Zhang J, Liu K, Stork J, Debolt S (2013) Microalgae as a renewable fuel source: fast pyrolysis of Scenedesmus sp. Renew. Energy. 60:625–632. https://doi.org/10.1016/j.renene.2013.06.016
Heimann K, Huerlimann R (2015) Microalgal classification: major classes and genera of commercial micro algal species. Major classes and genera of commercial microalgal species. In: Handbook of Marine Microalgae: Biotechnology Advances. Elsevier Inc., pp. 25–41. https://doi.org/10.1016/b978-0-12-800776-1.00003-0
Hoogwijk M, Faaij A, van den Broek R, oran Berndes G, Gielen D, Turkenburg W (2003) Exploration of the ranges of the global potential of biomass for energy. Biomass Bioenerg 25:119–133. https://doi.org/10.1016/S0961-9534(02)00191-5
Horiuchi J-I, Ohba I, Tada K, Kobayashi M, Kanno T, Kishimoto M (2003) Effective cell harvesting of the halotolerant microalga Dunaliella tertiolecta with pH control. J Biosci Bioeng 95:412–415. https://doi.org/10.1016/S1389-1723(03)80078-6
Hu Z, Ma X, Chen C (2012) A study on experimental characteristic of microwave-assisted pyrolysis of microalgae. Bioresour Technol 107:487–493. https://doi.org/10.1016/j.biortech.2011.12.095
Hu Z, Zheng Y, Yan F, Xiao B, Liu S (2013) Bio-oil production through pyrolysis of blue-green algae blooms (BGAB): product distribution and bio-oil characterization. Energy 52:119–125. https://doi.org/10.1016/j.energy.2013.01.059
Hubacek K, Guan D, Barua A (2007) Changing lifestyles and consumption patterns in developing countries: a scenario analysis for China and India. Futures 39:1084–1096. https://doi.org/10.1016/j.futures.2007.03.010
IEA (2018) World Energy Outlook 2018. IEA, Paris
Jakab E (2015) Analytical techniques as a tool to understand the reaction mechanism. In: Recent advances in thermochemical conversion of biomass. Elsevier Inc., pp. 75–108. https://doi.org/10.1016/b978-0-444-63289-0.00003-x
Jena U, Das KC, Kastner JR (2011) Effect of Operating Conditions of Thermochemical Liquefaction on Biocrude Production from Spirulina platensis. Bioresou Technol 102(10):6221–6229. https://doi.org/10.1016/j.biortech.2011.02.057
Kabir E, Kumar P, Kumar S, Adelodun AA, Kim KH (2018) Solar energy: potential and future prospects. Renew Sustain Energy Rev 82:894–900. https://doi.org/10.1016/j.rser.2017.09.094
Kaur R, Gera P, Jha MK (2015) Study on effects of different operating parameters on the pyrolysis of biomass: a review. J Biofuels Bioenergy 1:135. https://doi.org/10.5958/2454-8618.2015.00015.2
Kebelmann K, Hornung A, Karsten U, Griffiths G (2013) Intermediate pyrolysis and product identification by TGA and Py-GC/MS of green microalgae and their extracted protein and lipid components. Biomass Bioenerg 49:38–48. https://doi.org/10.1016/j.biombioe.2012.12.006
Kim SS, Ly HV, Choi GH, Kim J, Woo HC (2012) Pyrolysis Characteristics and Kinetics of the Alga Saccharina japonica. Bioresour Technol 123:445–451. https://doi.org/10.1016/j.biortech.2012.07.097
Kim SS, Ly HV, Kim J, Choi GH, Woo HC (2013) Thermogravimetric characteristics and pyrolysis kinetics of Alga Sagarssum sp. Biomass Bioresour Technol 139:242–248. https://doi.org/10.1016/j.biortech.2013.03.192
Kirubakaran V, Sivaramakrishnan V, Nalini R, Sekar T, Premalatha M, Subramanian P (2009) A review on gasification of biomass. Renew Sustain Energy Rev 13(1):179–186. https://doi.org/10.1016/j.rser.2007.07.001
Knuckey RM, Brown MR, Robert R, Frampton DMF (2006) Production of microalgal concentrates by flocculation and their assessment as aquaculture feeds. Aquac Eng 35:300–313. https://doi.org/10.1016/j.aquaeng.2006.04.001
Kocer AT, Inan B, Ozcimen D (2018) A comparison of bioethanol and biochar production from various algal biomass samples and sweet sorghum energy crop. Environ Res Technol 1:43–50
Kornprobst J-M (2014) Chlorophyceae (Green Algae) and marine spermatophyta. In: Encyclopedia of Marine Natural Products. Wiley, pp 1–28. https://doi.org/10.1002/9783527335855.marprod012
Lane DJ, Ashman PJ, Zevenhoven M, Hupa M, van Eyk PJ, de Nys R, Lewis DM (2013) Combustion behavior of algal biomass: carbon release, nitrogen release, and char reactivity. Energy Fuels 28(1):41–51. https://doi.org/10.1021/ef4014983
Lin KC, Lin YC, Hsiao YH (2014) Microwave plasma studies of spirulina algae pyrolysis with relevance to hydrogen production. Energy 64:567–574. https://doi.org/10.1016/j.energy.2013.09.055
Luo Z, Wang S, Liao Y, Zhou J, Gu Y, Cen K (2004) Research on biomass fast pyrolysis for liquid fuel. Biomass Bioenerg 26:455–462. https://doi.org/10.1016/j.biombioe.2003.04.001
Luque R, Menéndez JA, Arenillas A, Cot J (2012) Microwave-Assisted Pyrolysis of Biomass Feedstocks: the Way Forward? Energy Environ Sci 5(2):5481–5488. https://doi.org/10.1039/C1EE02450G
Ma C, Geng J, Zhang D, Ning X (2019) Non-catalytic and catalytic pyrolysis of Ulva prolifera macroalgae for production of quality bio-oil. J Energy Inst. https://doi.org/10.1016/j.joei.2019.03.001
Menéndez JA, Arenillas A, Fidalgo B, Fernández Y, Zubizarreta L, Calvo EG, Bermúdez JM (2010) Microwave heating processes involving carbon materials. Fuel Process Technol 91:1–8. https://doi.org/10.1016/j.fuproc.2009.08.021
Miao X, Wu Q (2004a) High Yield Bio-Oil Production from Fast Pyrolysis by Metabolic Controlling of Chlorella Protothecoides. J Biotechnol 110(1):85–93. https://doi.org/10.1016/j.jbiotec.2004.01.013
Miao X, Wu Q (2004b) High yield bio-oil production from fast pyrolysis by metabolic controlling of Chlorella protothecoides. J Biotechnol 110:85–93. https://doi.org/10.1016/j.jbiotec.2004.01.013
Milledge JJ, Heaven S (2013) A Review of the Harvesting of Micro-Algae for Biofuel Production. Rev Environ Sci Biotechnol 12:165–178. https://doi.org/10.1007/s11157-012-9301-z
Min M, Wang L, Li Y, Mohr M, Hu B, Chen P, Ruan R (2011) Cultivating Chlorella sp. in a Pilot-Scale Photobioreactor Using Centrate Wastewater for Microalgae Biomass Production and Wastewater Nutrient Removal. Appl Biochem Biotechnol 165(1):123–137. https://doi.org/10.1007/s12010-011-9238-7
Mine I, Menzel D, Okuda K (2008) Morphogenesis in Giant-Celled Algae. Int. Rev. Cell Mol. Biol. 266:37–83. https://doi.org/10.1016/S1937-6448(07)66002-X
Minowa T, Yokoyama S, Kishimoto M, Okakura T (1995) Oil Production from Algal Cells of Dunaliella tertiolecta by Direct Thermochemical Liquefaction. Fuel 74(12):1735–1738. https://doi.org/10.1016/0016-2361(95)80001-X
Mohn F (1988) Harvesting of micro-algal biomass. In: Borowitzka LJ, Borowitzka MA (eds) Micro-algal biotechnology. Cambridge University Press, Cambridge. https://doi.org/10.1002/jctb.280470214
Molina Grima E, Belarbi EH, Acién Fernández FG, Robles Medina A, Chisti Y (2003) Recovery of microalgal biomass and metabolites: process options and economics. Biotechnol Adv 20:491–515. https://doi.org/10.1016/S0734-9750(02)00050-2
Mooney-McAuley KM, Edwards MD, Champenois J, Gorman E (2016) Best Practice Guidelines for Seaweed Cultivation and Analysis. Public Output report of the EnAlgae project, Swansea, p 36
Mwangi JK, Lee WJ, Whang LM, Wu TS, Chen WH, Chang JS, Chen CY, Chen CL (2015) Microalgae oil: algae cultivation and harvest, algae residue torrefaction and diesel engine emissions tests. Aerosol Air Qual Res 15:81–98. https://doi.org/10.4209/aaqr.2014.10.0268
Neveux N, Yuen AK, Jazrawi C, Magnusson M, Haynes BS, Masters AF, Montoya A, Paul NA, Maschmeyer T, de Nys R (2014) Biocrude Yield and Productivity from the Hydrothermal Liquefaction of Marine and Freshwater Green Macroalgae. Bioresour Technol 155:334–341. https://doi.org/10.1016/j.biortech.2013.12.083
Norouzi O, Jafarian S, Safari F, Tavasoli A, Nejati B (2016) Promotion of hydrogen-rich gas and phenolic-rich bio-oil production from green macroalgae Cladophora glomerata via pyrolysis over its bio-char. Bioresour Technol 219:643–651. https://doi.org/10.1016/j.biortech.2016.08.017
Pattiya A (2017) 1—Fast pyrolysis. In: Direct thermochemical liquefaction for energy applications. Elsevier, pp 3–28. https://doi.org/10.1016/b978-0-08-101029-7.00001-1
Peng L, Fu D, Chu H, Wang Z, Qi H (2019) Biofuel production from microalgae: a review. Environ Chem. https://doi.org/10.1007/s10311-019-00939-0
Petrusevski B, van Breemen AN, Alaerts GJ, Bolier G (1995) Tangential flow filtration: a method to concentrate freshwater algae. Water Res 29:1419–1424. https://doi.org/10.1016/0043-1354(94)00269-D
Pillai U (2015) Drivers of cost reduction in solar photovoltaics. Energy Econ 50:286. https://doi.org/10.1016/j.eneco.2015.05.015
Pourkarimi S, Hallajisani A, Alizadehdakhel A, Nouralishahi A (2019a) Biofuel production through micro- and macroalgae pyrolysis: a review of pyrolysis methods and process parameters. J Anal Appl Pyrol. https://doi.org/10.1016/j.jaap.2019.04.015
Pourkarimi S, Hallajisani A, Alizadehdakhel A, Nouralishahi A (2019b) Biofuel production through micro- and macroalgae pyrolysis – A review of pyrolysis methods and process parameters, J Appl Pyrolysis Anal https://doi.org/10.1016/j.jaap.2019.04.015
Reay D, Ratcliff GA (1973) Removal of Fine Particles from Water by Dispersed Air Flotation: effects of Bubble Size and Particle Size on Collection Efficiency. Can J Chem Eng 51(2):178–185. https://doi.org/10.1002/cjce.5450510207
Renewable Global Statistics Report (2018) https://www.ren21.net/wp-content/uploads/2019/08/Full-Report-2018.pdf
Renewable Global Statistics Report (2018) Retrived from https://www.ren21.net/wp-content/uploads/2019/08/Full-Report-2018.pdf
Roslee AN, Munajat NF (2018) Comparative study on pyrolysis behavior and kinetics of two macroalgae biomass (Ulva cf.flexuosa and hy.edulis) using thermogravimetric analysis. J. Teknol. 80:123–130. https://doi.org/10.11113/jt.v80.11454
Rossignol N, Vandanjon L, Jaouen P, Quemeneur F (1999) Membrane Technology for the Continuous Separation Microalgae/Culture Medium: compared Performances of Cross-Flow Microfiltration and Ultrafiltration. Aquacult Eng 20:191–208. https://doi.org/10.1016/S0144-8609(99)00018-7
Rushton A (1996) Solid Liquid Filtration and Separation Technology. Wiley, Weinheim, pp 1–32
Salehizadeh H, Shojaosadati S (2001) Extracellular Biopolymeric Flocculants: recent Trends and Biotechnological Importance. Biotechnol Adv 19:371–385. https://doi.org/10.1016/S0734-9750(01)00071-4
Schlenk D, Batlet G, King C, Stauber J, Adams M, Simpson S, Maher W, Oris J (2007) Effects of Light on Microalgae Concentrations and Selenium Uptake in Bivalves Exposed to Selenium-Amended Sediments. Arch Environ Contam Toxicol 53:365–370. https://doi.org/10.1007/s00244-006-0248-3
Schlesinger A, Eisenstadt D, Bar-Gil A, Carmely H, Einbinder S, Gressel J (2012) Inexpensive Non-Toxic Flocculation of Microalgae Contradicts Theories: overcoming a Major Hurdle to Bulk Algal Production. Biotechnol Adv 30:1023–1030. https://doi.org/10.1016/j.biotechadv.2012.01.011
Schuck S (2006) Biomass as an energy source. Int J Environ Stud 63:823–835. https://doi.org/10.1080/00207230601047222
Shammas NK (2005) Coagulation and flocculation. In: Physicochemical treatment processes, Handbook of environmental engineering. The Humana Press, Totowa, New Jersey, pp 103–139
Sharma KK, Garg S, Li Y, Malekizadeh A, Schenk PM (2013) Critical Analysis of Current Microalgae Dewatering Techniques. Biofuels. 4:397–407. https://doi.org/10.4155/BFS.13.25
Shelef G, Sukenik A, Green M (1965) Microalgae harvesting and processing: a literature review. A subcontract report, 1965. https://doi.org/10.2172/6204677
Sim TS, Goh A, Becker EW (1988) Comparison of centrifugation, dissolved air flotation and drum filtration techniques for harvesting sewage-grown algae. Biomass. 16:51–62. https://doi.org/10.1016/0144-4565(88)90015-7
Singh A, Nigam PS, Murphy JD (2011) Mechanism and challenges in commercialisation of algal biofuels. Bioresour Technol 102:26–34. https://doi.org/10.1016/j.biortech.2010.06.057
Slaveykova VI, Wilkinson KJ (2003) Effect of pH on Pb biouptake by the freshwater alga Chlorella kesslerii. Environ Chem 1(3):185–189. https://doi.org/10.1007/s10311-003-0041-8
Smith RW, Miettinen M (2006) Microorganisms in flotation and flocculation: future technology or laboratory curiosity? Miner Eng 19:548–553. https://doi.org/10.1016/j.mineng.2005.09.007
Sovacool BK, Walter G (2019) Internationalizing the political economy of hydroelectricity: security, development and sustainability in hydropower states. Rev Int Polit Econ 26:49–79. https://doi.org/10.1080/09692290.2018.1511449
Spellman FR (2008) Handbook of water and wastewater treatment plant operations, 2nd edn. CRC Press, Boca Raton
Starks TL, Shubert LE, Trainor FR (1981) Ecology of soil algae: a review. Phycologia 20:65–80. https://doi.org/10.2216/i0031-8884-20-1-65.1
Stauffer E, Dolan JA, Newman R (2008) Chemistry and physics of fire and liquid fuels. In: Fire debris analysis. Elsevier, pp 85–129. https://doi.org/10.1016/b978-012663971-1.50008-7
Sudhakar K, Mamat R, Samykano M, Azmi WH, Ishak WFW, Yusaf T (2018) An overview of marine macroalgae as bioresource. Renew Sustain Energy Rev 91:165–179. https://doi.org/10.1016/j.rser.2018.03.100
Swer S, Singh OP (2004) Status of water quality in coal mining areas of Meghalaya, India
Taher H, Al-Zuhair S, Al-Marzouqi A, Haik Y, Farid M (2011) A Review of Enzymatic Transesterification of Microalgal Oil-Based Biodiesel Using Supercritical Technology. Enzyme Res. https://doi.org/10.4061/2011/468292
Tarleton S, Wakeman R (2006) Solid/liquid separation: equipment selection and process design. Elsevier Science, Sydney
Thangalazhy-Gopakumar S, Adhikari S, Gupta RB (2012) Catalytic pyrolysis of biomass over H +ZSM-5 under hydrogen pressure. In: Energy and fuels, pp 5300–5306. https://doi.org/10.1021/ef3008213
Torri C, Samorì C, Adamiano A, Fabbri D, Faraloni C, Torzillo G (2011) Preliminary investigation on the production of fuels and bio-char from Chlamydomonas reinhardtii biomass residue after bio-hydrogen production. Bioresour Technol 102:8707–8713. https://doi.org/10.1016/j.biortech.2011.01.064
Tripathi M, Sahu JN, Ganesan P (2016) Effect of process parameters on production of biochar from biomass waste through pyrolysis: a review. Renew Sustain Energy Rev 55:467–481. https://doi.org/10.1016/j.rser.2015.10.122
Vandamme D, Pontes SCV, Goiris K, Foubert I, Pinoy LJJ, Muylaert K (2011) Evaluation of electro-coagulation-flocculation for harvesting marine and freshwater microalgae. Biotechnol Bioeng 108:2320–2329. https://doi.org/10.1002/bit.23199
Vandna P, Ravindra S, Pankaj G, Rakesh P (2015) Microalgae as emerging source of energy: a review. Res J Chem Sci 5:63–68
Vardon DR, Sharma BK, Blazina GV, Rajagopalan K, Strathmann TJ (2012) Thermochemical conversion of raw and defatted algal biomass via hydrothermal liquefaction and slow pyrolysis. Bioresour Technol 109:178–187. https://doi.org/10.1016/j.biortech.2012.01.008
Vassilev SS, Vassileva CG (2016) Composition, properties and challenges of algae biomass for biofuel application: an overview. Fuel 181:1–33. https://doi.org/10.1016/j.fuel.2016.04.106
Wang S, Wang Q, Jiang X, Han X, Ji H (2013) Compositional analysis of bio-oil derived from pyrolysis of seaweed. Energy Convers Manag 68:273–280. https://doi.org/10.1016/j.enconman.2013.01.014
Ward AJ, Lewis DM, Green FB (2014) Anaerobic Digestion of Algae Biomass: a Review. Algal Res 5:204–214. https://doi.org/10.1016/j.algal.2014.02.001
White JE, Catallo WJ, Legendre BL (2011) Biomass pyrolysis kinetics: a comparative critical review with relevant agricultural residue case studies. J Anal Appl Pyrolysis 91:1–33. https://doi.org/10.1016/j.jaap.2011.01.004
Wichard T, Charrier B, Mineur F, Bothwell JH, De Clerck O, Coates JC (2015) The green seaweed Ulva: a model system to study morphogenesis. Front. Plant Sci. https://doi.org/10.3389/fpls.2015.00072
World Coal Association (2019)
World Wind Energy Association (2018) Statistics, 2018 estimation (cited on Dec 12 2019)
Worstell J (2014) Adiabatic fixed-bed reactors, 1st Edition Practical Guides in Chemical Engineering ISBN 9780128013069
Biochar Production: Fast and Slow Pyrolysis
Yu KL, Lau BF, Show PL, Ong HC, Ling TC, Chen WH, Ng EP, Chang JS (2017) Recent developments on algal biochar production and characterization. Bioresour Technol 246:2–11. https://doi.org/10.1016/j.biortech.2017.08.009
Yu KL, Show PL, Ong HC, Ling TC, Chen WH, Salleh MAM (2018) Biochar production from microalgae cultivation through pyrolysis as a sustainable carbon sequestration and biorefinery approach. Clean Technol Environ Policy. 20:2047–2055. https://doi.org/10.1007/s10098-018-1521-7
Zhang H, Xiao R, Wang D, Zhong Z, Song M, Pan Q, He G (2009) Catalytic fast pyrolysis of biomass in a fluidized bed with fresh and spent fluidized catalytic cracking (FCC) catalysts. Energy Fuels 23:6199–6206. https://doi.org/10.1021/ef900720m
Zhang R, Li L, Tong D, Hu C (2016) Microwave-enhanced pyrolysis of natural algae from water blooms. Bioresour Technol 212:311–317. https://doi.org/10.1016/j.biortech.2016.04.053
Zhang C, Wang X, Ma Z, Luan Z, Wang Y, Wang Z, Wang L (2019) Removal of phenolic substances from wastewater by algae: A review. Environ Chem 1:1. https://doi.org/10.1007/s10311-019-00953-2
Zhao H, Yan HX, Liu M, Sun BB, Zhang Y, Dong SS, Qi LB, Qin S (2013) Production of bio-oil from fast pyrolysis of macroalgae Enteromorpha prolifera powder in a free-fall reactor, Energy Sources, Part A Recover. Util Environ Eff 35:859–867. https://doi.org/10.1080/15567036.2012.680000
Acknowledgements
This work was supported by SSN College of Engineering, under the aegis of Shiv Nadar University, Republic of India. This work was also funded for experimental studies with algal pyrolysis by the above-mentioned institution. The authors also thank Dr P Senthil Kumar for his help during the manuscript preparation.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Aravind, S., Kumar, P.S., Kumar, N.S. et al. Conversion of green algal biomass into bioenergy by pyrolysis. A review. Environ Chem Lett 18, 829–849 (2020). https://doi.org/10.1007/s10311-020-00990-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10311-020-00990-2