Abstract
This study applied dilute acid (DA) and sulfite pretreatment to overcome the recalcitrance of lignocelluloses (SPORL) to deconstruct earlywood and latewood cell walls of Douglas fir for fermentable sugars production through subsequent enzymatic hydrolysis. DA pretreatment removed almost all the hemicelluloses, while SPORL at initial pH = 4.5 (SP-B) removed significant amount of lignin between 20 and 25 %. But both are not sufficient for effective enzymatic saccharification. SPORL at low initial pH = 2 (SP-AB) combines the advantage of both DA and SPORL-B to achieve approximately 90 % hemicellulose removal and delignification of 10–20 %. As a result, SP-AB effectively removed recalcitrance and thereby significantly improved enzymatic saccharification compared with DA and SP-B. Results also showed that earlywood with significantly lower density produced less saccharification after DA pretreatment, suggesting that wood density does not contribute to recalcitrance. The thick cell wall of latewood did not limit chemical penetration in pretreatments. The high lignin content of earlywood limited the effectiveness of DA pretreatment for enzymatic saccharification, while hemicellulose limits the effectiveness of high pH pretreatment of SP-B. The higher hemicellulose content in the earlywood and latewood of heartwood reduced saccharification relative to the corresponding earlywood and latewood in the sapwood using DA and SP-AB.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Various types of biomass such as woody plants, herbaceous plants, grasses and agricultural residues have been studied as potential feedstocks for biofuel production. Woody biomass is an important feedstock that can be sustainably produced in large quantities—approximately 300 million tons annually in the US [1]. Woody biomass has several logistical advantages for transportation and storage [2]. However, woody biomass is very recalcitrant to biochemical conversion to sugars partially due to its strong physical structure and high lignin content. Unlike annual plant biomass, woody biomass has heartwood (juvenile) and sapwood (mature) cells with different chemical compositions [3]. Moreover, each growth ring contains wood cells that have progressively thickened walls as the growing season progresses which are commonly recognized as earlywood and latewood cells, depending upon many environmental factors [3, 4]. Understanding these cell wall structures and compositions is invaluable to overcome its recalcitrance [5, 6]. Each pretreatment method has its unique features in removing the recalcitrance of lignocellulloses. For example, acids hydrolyze hemicelluloses, alkalis solubilize lignin, sulfites facilitate hemicelluloses dissolution as well as remove lignin through sulfonation [7], and organo-solvents dissolve lignin [8, 9]. Therefore, it is of interest to evaluate the effectiveness of different pretreatment methods on removal of recalcitrance of earlywood and latewood that represent very different physical and chemical structures within individual growth rings.
Although there are significant variations in chemical composition between juvenile and mature wood, an early study suggested that cellulose enzymatic saccharification efficiency is not affected by wood maturity [10]. Furthermore, the variation in sugar yield within the sapwood was not significant among different rings. Earlywood and latewood are distinctly different in terms of density, chemical composition, and cell wall thickness [4]. Studies using the entire ring [10] obscured this difference due to averaging. It is important to examine whether or not this distinct difference between earlywood and latewood affect cellulose saccharification and sugar yield. Such an investigation can provide improved understanding of cell wall recalcitrance relative to yearly cell development. Furthermore, it may also reveal unique characteristics of a given pretreatment for removing a specific feature of cell wall recalcitrance.
In this study, strips of Douglas fir (Fig. 1) were cut from a wood disk taken from breast height, and then pretreated by dilute acid (DA) and sulfite pretreatment to overcome the recalcitrance of lignocellulloses (SPORL) [7]. SPORL was chosen because of its unique capability of removing woody biomass recalcitrance for efficient enzymatic saccharification, especially for softwood species [11–14]. SPORL was conducted at two pH levels to examine different levels of delignification and hemicellulose removal to improve enzymatic cellulose saccharification. Earlywood and latewood were separated for both the heartwood and sapwood after pretreatment. The differences in enzymatic saccharification efficiencies and sugar yields between the pretreated earlywood and latewood in the sapwood and heartwood were then evaluated.
Material and Methods
Materials
A commercial cellulase enzyme, CTec2, was provided by Novozymes of North America (Franklinton, NC, USA). The average activity of the cellulase was 147 filter paper unit (FPU)/mL as determined using a literature method [15]. All other chemicals were of ACS reagent grade purchased from Sigma-Aldrich (St. Louis, MO).
A freshly cut Douglas fir wood disk taken from the breast height of a tree in the US Pacific Northwest were provided by Weyerhaeuser NR Company (Federal Way, WA). The disk was fairly circular. Several strips (12.7 × 6.35 × 127 mm) were cut out from the disk along the east to west diameter direction determined based on radius (the largest radius towards the South). As shown in Fig. 1, the latewood (the dark color fraction in each ring) and early wood as well as the sapwood and heartwood (reddish colored rings in the untreated sample) were clearly distinguishable from color even after pretreatment.
DA and SPORL Pretreatments
Experiments were carried out according to the schematic diagram shown in Fig. 2. One DA and two SPORL pretreatments with (SP-AB) or without (SP-B) sulfuric acid were conducted. Each pretreatment used four Douglas fir strips of approximately 28 g in oven dry (od) weight to react with a dilute acid or a sulfite solution with a fixed liquor-to-wood ratio of L/W = 4:1 at 180 °C for 20 min. In SP-AB pretreatment, the sulfuric acid and concentration was A = 0.4 % (v/v) or 2.2 wt% and sodium bisulfite charge on od wood was B = 10.0 wt%. These chemical loadings and pretreatment temperature of 180 °C were found optimal for Douglas fir-based on our recent laboratory optimization study [16]. However, a shorter pretreatment duration of 20 min and a higher liquor-to-wood ratio of 4:1 than those used previously [16, 17] are to avoid over pretreatment so that differences between pretreated earlywood and latewood and among three pretreatments can be clearly observed. The same sodium bisulfite loading of 10 % was used in SP-B but without acid and the same acid loading of 2.2 wt% was used in DA pretreatment but without sulfite, so that comparisons among these three pretreatment can be made under the same conditions. The use of wood strips without separating earlywood and latewood for pretreatment is to resemble practical settings for woody biomass pretreatment and ensure identical chemistry was applied to earlywood and latewood. All pretreatments were carried out in three 20-mL tube reactors placed in an oil bath and rotated at 5 rpm.
The wood strips remained intact after pretreatment (Fig. 1). The pretreatment spent liquor was separated using a screen and stored at 4 °C for subsequent analysis of sugar and sugar degradation products (inhibitors). One set of pretreated wood strips was used for Transmission Electron Microscope (TEM) analysis. The other two sets of pretreated strips were used for enzymatic hydrolysis after manual separations of the earlywood and latewood fractions of individual growth rings using a chisel (Fig. 2). For wood strips from the same pretreatment, all the earlywood and latewood sections from the sapwood were respectively combined to make two samples of Sapwood Earlywood (SE) and Sapwood Latewood (SL). Similarly, all the earlywood and latewood sections from the heartwood were respectively combined to make two samples of Heartwood Earlywood (HE) and Heartwood Latewood (HL). A total of 12 samples from three different pretreatments were produced. The average mass fractions of SE, SL, HE and HL in the original wood disk were measured in replicate using two similar strips and used for mass balance analysis (Table 1). The rational for combining earlywood or latewood materials from different rings are to accumulate enough material for enzymatic hydrolysis. Furthermore, the differences between earlywood and latewood in terms of chemical composition and wood density are much larger than the variations among different rings [3, 4]. Each pretreated solid sample was separately refined using a low speed for 2 min in a Waring commercial blender (Model 31BL92, Dynamics Corporation of America, New Hartford, CT). Approximately 200 mL water was added to facilitate refining. The size-reduced solids were dewatered to an approximately 30 % solids by vacuum filtration using a Buchner funnel. The pretreatment solids yield was determined from the wet weight and moisture content of the collected solids. The resultant solids were later used for chemical compositional analysis and enzymatic hydrolysis.
Transmission Electron Microscope
TEM images of ultra-thin cross-sections of Douglas fir wood strips were obtained using a JEOL 2011 TEM with Gatan 4 k × 4 k digital camera operating at 200 kV. Sections were cut using a Leica UCT ultra-microtome with a diamond knife from prepared wood slices. These wood slices consisted of fiber bundles with width of 1–2 mm and length of 6–7 mm. The slices were prepared from the control and treated earlywood samples by cutting along the fiber length. These fiber bundle slices were dehydrated with a serial concentration of acetone, and then infiltrated with the resin of Epon-Araldite followed by embedding in BEEM capsules. Eight BEEM capsules were made with control and treated earlywood fiber bundles, respectively. Each capsule included one or two fiber bundles, and each bundle contained five to eight fibers. At least ten sections with a thickness of 100–110 nm were cut-off along the axis orientation of fiber bundles within the embedding capsule under the ultra-microtome.
As the cutting was performed, the ultra-thin sections would fall into a distilled-water slot. They were then collected on formvar-carbon-coated copper grids (200 and 100 mesh sizes). In order to improve the contrast of the images, chemical staining of the polysaccharides in the cross-sections was carried out. The grids were post-stained with 5 % aqueous uranyl acetate (10 min) and lead citrate (10 min). All the sections were examined in the TEM and the images were taken.
Chemical Compositional Analysis
The chemical compositions of the untreated and pretreated biomass substrates were analyzed by the Analytical and Microscopy Laboratory of the Forest Products Laboratory as described previously [18]. All the substrates were ground using a Wiley mill (model #2, Arthur Thomas Co., Philadelphia, PA, USA) to pass a 40-mesh (∼0.35 mm) screen. The milled samples were hydrolyzed using sulfuric acid of 72 % (v/v) at 30 °C for 1 h and 3.6 % (v/v) at 120 °C for 1 h. The hydrolysate was then analyzed for carbohydrates using high-performance anion exchange chromatography with pulsed amperometric detection (HPAEC-PAD). The Klason lignin content was measured gravimetrically after washing and drying the solid residues from the acid hydrolysis. The pretreatment spent liquor was also analyzed for fermentation inhibitors such as furan using the same HPLC with UV detection previously described [18]. For rapid analysis, glucose in the enzymatic hydrolysate was measured using a commercial glucose analyzer (YSI 2700S, YSI Inc., Yellow Springs, OH, USA). Duplicate analyses were carried out and averaged for reporting.
Enzymatic Hydrolysis
All enzymatic hydrolysis experiments were performed using Novozymes CTec2 at 2 % (w/v) solids loading in a 50-mM sodium acetate buffer (pH 5.5) in a flask on a shaker (Thermo Fisher Scientific, Model 4450, Waltham, MA, USA) at 50 °C and 200 rpm. Our previous studies indicated that an elevated pH of approximately 5.5 can significantly reduce nonproductive cellulase binding to bound lignin remained in solid substrate to enhance enzymatic saccharification [19, 20]. CTec2 loading was 22.5 FPU/g glucan, higher compared with our previous studies using Douglas fir [14, 17] due to the milder pretreatments applied. All enzymatic hydrolysis experiments were conducted in duplicate. Standard deviations were used to represent error bars in plots.
Results and Discussion
Wood Moisture, Density, and Chemical Composition
Variations in moisture content between earlywood and latewood were observed for both heartwood and sapwood (Table 1). The differences in cell wall thickness and porosity (or density) contribute to this difference. Significant difference in moisture content between sapwood and heartwood is partly due to the fact that the wood was harvested under wet conditions. Water occurs in three forms in the living wood: bound by hydration in the cell walls, as a gel in the protoplasmic materials of the cells, and as free water in the cell cavities [21]. The first and third forms of water are common in heartwood. But sapwood is the “living” wood that transports water from the roots to the leaves. Water easily binds to cellulose microfibrils in the cell wall making the wood soft and pliable.
It is well known that earlywood and latewood is distinguished by cell wall thickness and local density [4]. The earlywood has thinner cell walls and lower local density while the latewood has thicker cell walls and higher local density. To illustrate this distinction for the present Douglas fir wood sample, Ring 14 (14th growth ring) in the sapwood was analyzed by an in house wood optical densitometer [22]. The results indicate that wood density varied from as low as 0.15 g/cm3 in earlywood to 1.1 g/cm3 for latewood (Fig. 3). A transition from earlywood to latewood characterized by cell wall thickness can be clearly seen in the density profile (the middle insert image in Fig. 3). We divided the entire ring into different segments based on measured density and cell diameter. Quantitative information about each segment is listed in Table 2. The separation between earlywood and latewood was typically made at the initial transition zone (T1). The percentage of mass in earlywood based on this separation is 27 %, approximately equal to measured average mass percentage of earlywood of 28 %. This results in an estimated density for the separated earlywood and latewood fractions used for enzymatic hydrolysis as 0.30 and 0.85 g/cm3, respectively.
Significant differences in chemical composition among different wood fractions were also observed (Table 1). Earlywood has higher lignin and xylan and lower glucan and mannan content than latewood in both heartwood and sapwood, in agreement with a previous study using spruce [3]. Earlywood also has a lower total hemicellulose content than latewood (Table 1).
Effect of Pretreatment on Cell Wall Modification
Wood cell walls are architecturally complex and containing many cross-linked polysaccharide networks, glycosylated proteins, and lignin. Various pretreatment strategies can be tailored to depolymerize the cell wall structure in very specific ways. Acid is very effective in hydrolyzing hemicelluloses at high temperatures. Sulfite is known to be capable of effectively removing lignin even under acidic conditions. TEM images of the cross section of an earlywood cell from sapwood were taken to examine the effects of the three different pretreatments reported here. DA pretreatment caused delamination in the secondary cell wall with high hemicellulose content (comparing Fig. 4a with b) with an opening of approximately 500 nm, significantly greater than typical enzyme molecular size of 51 Å [23]. This delamination is considered desirable for creating accessible surface to cellulase critical to enzymatic hydrolysis [24]. During the course of cell wall digestion by cellulase, hydrolysis occurs preferentially on the interior (lumen) of the cell walls. The outer surface or primary wall played a very small role in cellulose hydrolysis [25–27]. Despite the quantitative dimensions of the openings shown in the TEM image (Fig. 4b) may be influenced by factors other than pretreatment such as sample preparation; nevertheless, the images did show a more open structure of the DA pretreated sample than its corresponding untreated sample (Fig. 4a).
Lignin is highly concentrated in the compound middle lamella and then decreases in the secondary layer [28]. The SP-B pretreatment produced significant delignification due to sulfite and slightly high initial pH of around 4.5. The opening of the middle lamella, due to removal of lignin can be seen from Fig. 4c. When the initial pH is reduced to approximately 2.0 with the application of sulfuric acid with sodium bisulfite in SP-AB pretreatment, the cross section resemble a combination of that DA (Fig. 4b) and SP-B (Fig. 4c), i.e., some level of delamination in the secondary cell wall and lignin removal from the middle lamella as shown in Fig. 4d. However, delamination and delignification was not as pronounced as that observed from DA and SP-B pretreatment, respectively.
Effect of Pretreatment on Cell Wall Chemical Composition
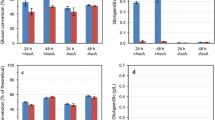
The observed qualitative effects of different pretreatments on cell wall modification can be quantitatively substantiated by examining the change in cell wall composition. As shown in Fig. 5a, b, DA removed almost all of the hemicelluloses represented by xylan and mannan but only a very small fraction of lignin due to lignin condensation. SP-B only removed 50–70 % of the hemicelluloses but solubilized 20–25 % of the lignin. SP-AB removed approximately 90 % of the hemicelluloses and 10–20 % of the lignin (Table 3).
The differences in carbohydrate degradation by different pretreatments were also obvious from the measured glucose and furan concentrations in the pretreatment spent liquor (Table 4). The glucose concentration in the liquor was 12.7 g/L for DA, much higher than the 1.1 and 6.0 g/L in the SP-B and SP-AB spent liquor, respectively. Furfural and HMF concentrations were 2.6 and 3.9 g/L for DA, significantly higher than 0.14 and 0.19 g/L in SP-B and 0.52 and 0.56 g/L in SP-AB.
The results in Fig. 5a also show some very interesting results. Under the strong acidic condition of DA, essentially all of hemicelluloses were removed from all the samples. However, under the less acidic conditions of SP-B, hemicellulose removal was considerably higher in latewood (70 %) than in earlywood (50 %). This effect was also observed from SP-AB pretreated samples but not as pronounced. This is likely due to the higher lignin content in the earlywood samples, i.e., approximately 5 percentage points higher than the latewood samples for both the heartwood and sapwood (Table 1), which resulted in better protection of carbohydrate from degradation, i.e., higher recalcitrance [29].
Enzymatic Saccharification
The substrate cellulose enzymatic digestibility (SED), defined as the percentage of substrate glucan enzymatically saccharified to glucose, varied with pretreatment methods (Fig. 6). DA pretreatment produced the largest variations in SEDs among different wood samples. It is interesting to note that the SED of latewood is higher than earlywood for both heartwood and sapwood despite that the density of latewood is significantly higher than that of earlywood (Fig. 3 and Table 3). This suggests that wood density does not affect recalcitrance. Chemical penetration does not appear to be limited by the thickness of the cell wall. The difference in SED between earlywood and latewood may be more directly relevant to the lignin content (Table 1). Earlywood in both the heartwood and sapwood has higher lignin content resulting in lower SED compared to latewood. When comparing heartwood with sapwood, the higher lignin and hemicellulose content of the heartwood resulted in lower SED for both earlywood and latewood.
SP-B pretreated samples produced the lowest SED despite the highest delignification among the three pretreatments. This is because significant amounts of hemicelluloses remained in the pretreated solids (Fig. 5a). Previously, hemicellulose removal was found to be directly proportional cellulose saccharification [30, 31]. The results in Fig. 6 also indicate that the significant difference in hemicellulose removal between earlywood (50 %) and latewood (70 %) by SP-B did not affect SED. This suggests that 70 % hemicellulose removal is not sufficient to improve SED. As a result, SED among different SP-B pretreated wood samples are approximately the same of 25 %.
SP-AB pretreated substrates have highest SEDs among for all substrates. SP-AB produced 10–20 % delignification as well as approximately 90 % hemicellulose removal, which significantly open the pore structure of the cell wall [25]. With improved delignification, it appears that SED of earlywood is higher compared to latewood (Fig. 6) because of its slightly lower hemicelluloses content despite its higher lignin content. Again, the SEDs of sapwood are higher than those of heartwood for both earlywood and latewood possibly due to the relatively higher lignin content in heartwood.
The effect of wood lignin on SED was further examined in Fig. 7. DA pretreatment is very sensitive to wood lignin content. SED decreases as wood lignin content increases. However, SEDs of SP-B pretreated substrates do not appear to be dependent on wood lignin content because of significant delignification that removed the lignin barrier to cellulose accessibility. It appears that 20 % delignification (Fig. 5b) is sufficient to remove the lignin barrier. Compared with more than 70 % removal of hemicellulose to improve SED discussed above and to be discussed at the end of this section, the barrier of hemicellulose to improve SED is higher than lignin, in agreement with our previous study [6]. Despite lacking complete knowledge of the cell wall structure, recent studies indicate that hemicelluloses, such as xylan, can be divided into fractions coated on cellulose fibrils and fractions interconnected with cellulose fibrils [32]. It is also known that hemicellulose has a “fast fraction” (80–95 %) that can be easily hydrolyzed and another “slow fraction” (5–20 %) that is difficult to be hydrolyzed [17, 30]. Earlier investigations showed that near complete removal of hemicelluloses, approximately equal to the amount of fast hemicelluloses, is necessary to achieve good enzymatic saccharification [14, 30]. Therefore, the amount of fast hemicellulose may be used to represent the hemicellulose-barrier to improved enzymatic saccharification.
Conclusions
Wood density does not appear to contribute to wood recalcitrance and enzymatic saccharification. The thick cell wall is not a factor limiting chemical penetrations in three aqueous chemical pretreatments studied. Cell wall chemical composition, especially lignin and hemicellulose content, contribute to cell wall recalcitrance. It appears the recalcitrance barrier contributed by hemicelluloses is equivalent to the amount of “fast fraction” hemicelluloses in the given feedstock, while the recalcitrance barriers contributed by lignin is equivalent to approximately 20 % of wood lignin for very recalcitrant softwoods with high lignin content such as Douglas fir. Different pretreatment processes have unique characteristics to remove specific feature of cell wall recalcitrance. SPORL pretreatment at initial solution pH = 2 combines acid hydrolysis and sulfite delignification through sulfonation can effectively remove the recalcitrance of woody biomass even for earlywood of Douglas fir with lignin content 32 %. While DA can effectively fractionate hemicelluloses, it produced low enzymatic cellulose saccharification due of lack of lignin removal. SPORL pretreatment at high initial pH = 4.5 cannot efficiently fractionate hemicelluloses which result in low cellulose saccharification even with delignification.
References
Perlack RD, Stokes BJ: DOE. 2011. U.S. billion-ton update: biomass supply for a bioenergy and bioproducts industry. In. Oak Ridge: Oakridge National Laboratory; 2011
Zhu JY, Pan XJ (2010) Woody biomass pretreatment for cellulosic ethanol production: technology and energy consumption evaluation. Bioresour Technol 101:4992–5002
Bertaud F, Holmbom B (2004) Chemical composition of earlywood and latewood in Norway spruce heartwood, sapwood and transition zone wood. Wood Sci Technol 38(4):245–256
Zhu JY, Scott CT, Scallon KL, Myers GC (2007) Effects of plantation density on wood density and anatomical properties of red pine (Pinus resinosa Ait). Wood Fiber Sci 39(3):502–512
Mansfield SD, Mooney C, Saddler JN (1999) Substrate and enzyme characteristics that limit cellulose hydrolysis. Biotechnol Prog 15:804–816
Leu SY, Zhu JY (2013) Substrate-related factors affecting enzymatic saccharification of lignocelluloses: our recent understanding. Bioenerg Res 6(2):405–415
Zhu JY, Pan XJ, Wang GS, Gleisner R (2009) Sulfite pretreatment (SPORL) for robust enzymatic saccharification of spruce and red pine. Bioresour Technol 100(8):2411–2418
Pan XJ, Arato C, Gilkes N, Gregg D, Mabee W, Pye K, Xiao ZZ, Zhang X, Saddler J (2005) Biorefining of softwoods using ethanol organosolv pulping: preliminary evaluation of process streams for manufacture of fuel-grade ethanol and co-products. Biotechnol Bioeng 90(4):473–481
Iakovlev M, van Heiningen A (2012) Efficient fractionation of spruce by SO2–ethanol–water treatment: closed mass balances for carbohydrates and sulfur. ChemSusChem 5(8):1625–1637
DeMartini JD, Wyman CE (2011) Changes in composition and sugar release across the annual rings of Populus wood and implications on recalcitrance. Bioresour Technol 102(2):1352–1358
Zhu JY, Gleisner R, Scott CT, Luo XL, Tian S (2011) High titer ethanol production from simultaneous enzymatic saccharification and fermentation of aspen at high solids: a comparison between SPORL and dilute acid pretreatments. Bioresour Technol 102(19):8921–8929
Tian S, Luo XL, Yang XS, Zhu JY (2010) Robust cellulosic ethanol production from SPORL-pretreated lodgepole pine using an adapted strain S. cerevisiae without detoxification. Bioresour Technol 101:8678–8685
Lan TQ, Gleisner R, Zhu JY, Dien BS, Hector RE (2013) High titer ethanol production from SPORL-pretreated lodgepole pine by simultaneous enzymatic saccharification and combined fermentation. Bioresour Technol 127:291–297
Leu S-Y, Gleisner R, Zhu JY, Sessions J, Marrs G: Robust enzymatic saccharification of a Douglas-fir forest harvest residue by SPORL. biomass and bioenergy (submitted) 2013.
Wood TM, Bhat M: Methods for measuring cellulase activities. In: In: Colowick SP, Kaplan NO, editors Methods in Enzymology, Vol 160, Biomass (Part A, Cellulose and Hemicellulose) Vol editors: Wood WA, Kellogg ST New York: Academic, Inc, p 87–112. 1988: 87–112.
Zhang C, Houtman CJ, Zhu JY: Using low temperature to balance enzymatic saccharification and furan formation in SPORL pretreatment of Douglas-fir. AIChE J (submitted) 2013.
Zhang C, Houtman CJ, Zhu JY: Using low temperature to balance enzymatic saccharification and furan formation in SPORL pretreatment of Douglas-fir. Bioresource Technology (submitted) 2013.
Luo X, Gleisner R, Tian S, Negron J, Horn E, Pan XJ, Zhu JY (2010) Evaluation of mountain beetle infested lodgepole pine for cellulosic ethanol production by SPORL pretreatment. Ind Eng Chem Res 49(17):8258–8266
Lou H, Zhu JY, Lan TQ, Lai H, Qiu X (2013) pH-induced lignin surface modification to reduce nonspecific cellulase binding and enhance enzymatic saccharification of lignocelluloses. ChemSusChem 6(5):919–927
Lan TQ, Lou H, Zhu JY (2013) Enzymatic saccharification of lignocelluloses should be conducted at elevated pH 5.2–6.2. Bioenerg Res 6(2):476–485
F. B, Graystone JA: Wood coatings: theory and practice. Elsevier; 2009.
Vahey DW, Zhu JY, Scott CT: Method for characterizing the density and cross-section morphology of trees. US Patent 2011, 7, 945, 098 B2.
Cowling EB, Kirk TK (1976) Properties of cellulose and lignocellulosic materials as substrates for enzymatic conversion processes. Biotechnol bioeng symp 6:95–123
Donohoe BS, Selig MJ, Viamajala S, Vinzant TB, Adney WS, Himmel ME (2009) Detecting cellulase penetration into corn stover cell walls by immuno-electron microscopy. Biotechnol Bioeng 103(3):480–489
Wang QQ, He Z, Zhu Z, Zhang Y-HP, Ni Y, Luo XL, Zhu JY (2012) Evaluations of cellulose accessibilities of lignocelluloses by solute exclusion and protein adsorption techniques. Biotechnol Bioeng 109(2):381–389
Luo X, Zhu JY (2011) Effects of drying-induced fiber hornification on enzymatic saccharification of lignocelluloses. Enzyme Microb Technol 48(1):92–99
Ding SY, Liu YS, Zeng Y, Himmel ME, Baker JO, Bayer EA (2012) How does plant cell wall nanoscale architecture correlate with enzymatic digestibility? Science 338(6110):1055–1060
Panshin AJ, de Zeeuw C (1980) Textbook of wood technology 4th edn. McGraw-Hill, New York
Wang ZJ, Zhu JY, Gleisner R, Chen KF (2012) Ethanol production form poplar wood the rough enzymatic saccharification and fermentation by dilute acid and SPORL pretreatments. Fuel 95:606–614
Zhu W, Houtman CJ, Zhu JY, Gleisner R, Chen KF (2012) Quantitative predictions of bioconversion of aspen by dilute acid and SPORL pretreatments using a unified combined hydrolysis factor (CHF). Process Biochem 47:785–791
Zhang DS, Yang Q, Zhu JY, Pan XJ (2013) Sulfite (SPORL) pretreatment of switchgrass for enzymatic saccharification. Bioresour Technol 129:127–134
Bromley JR, Busse-Wicher M, Tryfona T, Mortimer JC, Zhang Z, Brown DM, Dupree P (2013) GUX1 and GUX2 glucuronyltransferases decorate distinct domains of glucuronoxylan with different substitution patterns. Plant J 74(3):423–434
Acknowledgments
This work, as part of the Northwest Advanced Renewables Alliance (NARA), was funded by the Agriculture and Food Research Initiative Competitive Grant No. 2011-68005-30416 from the USDA National Institute of Food and Agriculture (NIFA). We would also like to acknowledge Novozymes North America for their constant support by complementary providing cellulase enzymes. We would also like to thank Fred Matt of USDA Forest Products Laboratory for conducting detailed substrate chemical composition analysis. We also would like to acknowledge Gevan Marrs of Weyerhaeuser NR Company for providing the Douglas wood disk for the study. The financial support from USDA NIFA and the Chinese Scholarship Council made the visiting appointment of Zhang at the USDA Forest Products Laboratory possible.
Author information
Authors and Affiliations
Corresponding author
Additional information
This work was conducted while Zhang was a visiting student at the USDA Forest Products Laboratory and on official government time of Zhu and Scott.
Rights and permissions
About this article
Cite this article
Zhang, C., Lei, X., Scott, C.T. et al. Comparison of Dilute Acid and Sulfite Pretreatment for Enzymatic Saccharification of Earlywood and Latewood of Douglas fir. Bioenerg. Res. 7, 362–370 (2014). https://doi.org/10.1007/s12155-013-9376-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12155-013-9376-6