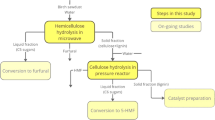
Abstract
The biological valorization of cork depends mainly on the weakening of cell wall recalcitrance. As a typical softwood, Chinese fir is more resistant to enzyme and microbial invasion than most biomass. This study compared the efficiency of two single-step pretreatments (dilute sulfuric acid pretreatment (DSA) and acidic sodium chlorite pretreatment (SC)) and their interaction effects on the pretreatment process. Two single pretreatments could selectively remove hemicellulose and some lignin from Chinese fir sawdust (CFS). Combined pretreatments resulted in a remarkable synergistic enhancement in delignification. The hemicellulose-removal-first strategy is favored by softwood delignification, the destruction of fiber morphology, and the redistribution of lignin. Using DSA-SC, more than 90% of lignin and hemicellulose were removed, and complete hydrolysis (99.3%) was obtained. Based on DSA-SC, the CFS biorefinery recovered 41.6% lignin and 52.8% hemicellulose sugar, and 306.1 g lactic acid was produced from 1000.0 g CFS. The integrated pretreatment process with a stepwise separation feature could be effectively applied in softwood destruction and provide more opportunities for softwood waste whole-component utilization.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Biorefinery is considered to be an effective means to address the problems caused by the excessive consumption of fossil fuels. Using lignocellulose biomass to produce biofuel or chemicals can not only reduce greenhouse gases but also conserve nonrenewable energy. Among the variety of lignocellulose biomass, forest residues are an important lignocellulose biomass source and have been widely used for the production of ethanol. However, wood lignocellulose has a relatively complex structure that is resistant to enzyme and microbial invasion, and among them, softwood is often considered the most recalcitrant type of wood lignocellulose [1]. Therefore, exploring an effective deconstruction pretreatment is key for softwood residue bioconversion.
In the last few decades, despite many pretreatment technologies having been developed to overcome the inherent recalcitrance of plant biomass for fermentable sugar production, only a few of them, including acid or SO2 preimpregnated steam explosion [2], alkaline or sulfite pulping-based pretreatment, and organosolv pretreatment [3, 4], have been proven to be partly effective for treating softwood (up to 90% or more hemicellulose or lignin removal). Among them, dilute acid pretreatment is one of the most representative chemical pretreatment methods and is effective in removing hemicellulose from cork, but its effect on cork lignin removal is far from satisfactory [5, 6]. When Li et al. pretreated camphor pine wood with dilute acid, the hemicellulose removal rate reached 99.4%, while the lignin removal rate was only 2.7% [7]. Additionally, the presence of lignin limits the increase in enzymatic hydrolysis yield. For example, Lim and Lee compared the effects of different acid species on coniferous wood chips at high temperatures, with enzymatic hydrolysis yields ranging from 35.6 to 61.2% despite the complete removal of hemicellulose [8]. In contrast, one researcher compared the effect of two dilute acid pretreatments on coniferous wood and found that the yield of enzymatic hydrolysis of coniferous wood after two dilute acid pretreatments was still below 50% [9].
Many other pretreatment technologies, including dilute alkali pretreatment [10], ionic liquid [11], and sodium chlorite treatment [12], have been shown to remove lignin more effectively than acid pretreatment. Franco et al. treated different coniferous raw materials using an alkaline sulfite/anthraquinone pretreatment combined with a fine grinding process. The delignification rate ranged from 25 to 50%, and the enzymatic hydrolysis yield could be increased from the original 20 to 70%, which could effectively overcome the recalcitrance of softwoods [11]. Gschwend et al. synthesized a new, low-cost ionic liquid in the treatment of Pinus sylvestris and found that the synthesized [DMBA] [HSO4] not only removed 66% of the lignin but also exhibited excellent enzymatic hydrolysis performance with 75% enzymatic yield [13]. Although alkali pretreatment and ionic liquids can leach more lignin from the raw material and improve the enzymatic hydrolysis yield of lignocellulose after pretreatment, there are still difficult challenges to overcome. For example, carbohydrate degradation during alkali pretreatment is serious, while lye recovery is difficult. Ionic liquids are generally expensive, the pretreatment process is long, and there is a lack of mature ionic liquid recovery technology. In contrast, chlorite pretreatment, which is widely used in industrial pulping, has the advantages of mild pretreatment conditions, exclusive lignin removal, and less degradation of polysaccharides [14]. The use of chlorite for the pretreatment of softwoods has been reported in the literature and has shown good delignification ability. Lauri et al. performed chlorite delignification on fir wood and almost completely eliminated lignin [15]. Some researchers have also used sodium chlorite pretreatment of Douglas fir for repeated lignin removal [16]. Additionally, several combined pretreatment techniques have been reported to target the recalcitrance of softwoods. Tang et al. also reported that they improved lignin removal and enzymatic hydrolysis yield (up to 97.7%) of larch by combining water preextraction with alkaline hydrogen peroxide [17]. Matsoukas et al. used acid-catalyzed organic solvent-vapor blast pretreatment for spruce without the enzymatic hydrolysis yield of spruce, which could be increased to 61% under the condition of considering the recovery of the hemicellulose fraction [3]. Combining two or more pretreatment methods can significantly improve the efficiency of the pretreatment process and is an emerging approach in the field of biorefining [18]. Nevertheless, the recalcitrant nature of the cork still affects the enzymatic effect.
For this reason, many studies have been devoted to a deep investigation of the main limiting factors of softwood hydrolysis [19]. Compared to other biomasses, more carbon‒carbon linkages existed in softwood lignin, and a higher lignin content was observed. Mooney et al. deemed that the residue lignin was the main donor of steric hindrance limiting the enzymatic hydrolysis of four Douglas fir pulps [20]. Additionally, almost all lignin in softwood is linked to hemicellulose by covalent bonds, and researchers named this fraction the lignin-carbohydrate complex (LCC). LCC is regarded as an important obstacle to enzymatic hydrolysis and delignification [19, 21]. Therefore, compared to herb or hardwood, both hemicellulose and lignin were recommended to be removed before softwood hydrolysis due to the high lignified component and the natural special LCC structure [22, 23].
Chinese fir is a typical softwood in China and has been widely planted in many countries. Recently, along with its extensive application in the wood industry, vast processing residues have been generated every year. In this work, the potential of Chinese fir sawdust in biomass biorefineries was explored. Dilute sulfuric acid pretreatment, acidic sodium chlorite pretreatments, and their integrated pretreatment processes were introduced to deconstruct Chinese fir sawdust (CFS). The chemical and physical changes in substrates after various pretreatments were investigated. Moreover, the relationship between these changes and their effects on enzymatic hydrolysis was explored to provide well-established information on softwood destruction. Moreover, developing an integrated pretreatment process could provide more opportunities for softwood waste biorefineries.
2 Materials and Methods
2.1 Materials
The CFS used in this work was kindly provided by sawmills in Jiujiang City and was pretreated as described in previous works before use [24]. The cellulase enzyme Cellic@ Ctec2 solution with an activity of 215.3 FPU/mL used in the present work was obtained from Novozymes Investment Co. Ltd.
2.2 Strain and media
B. coagulans CC17A was used for SSF experiments on DSA-SC-treated material (cellulose content of 76.8%) at different substrate concentrations. The CC17A strain was obtained from the Laboratory of Biochemical Engineering, Nanjing Forestry University.
The medium used for B. coagulans CC17A culture included the following: (1) seed medium (20 g/L xylose, 1 g/L yeast extract, 1.5 g/L corn steep powder, 1 g/L NH4Cl, 0.2 g/L MgSO4, and 10 g/L CaCO3) and (2) cellulose simultaneous saccharification fermentation medium (pretreated solids as substrate with substrate concentrations of 40, 80 or 120 g/L; 2.5 g/L yeast extract; 1.2 g/L corn steep powder; 3 g/L (NH4)2SO4; 0.22 g/L KH2PO4; 0.4 g/L MgSO4·7H2O; 0.03 g/L MnSO4·H2O; 0.03 g/L FeSO4·7H2O; and 20 g/L CaCO3 with a final concentration of 20 mM, pH 5.5 phosphate buffer solution).
2.3 Dilute sulfuric acid pretreatment (DSA)
Dilute sulfuric acid pretreatment was carried out in a 120-mL glass tube using an oil bath for heating. Dry CFS (8.0 g) was soaked with 0.4% (w/v) sulfuric acid in a solid-to-liquid ratio of 1:10 at 160 °C for 60 min. After cooking, the obtained residues were separated by filtration and then extensively washed with deionized water until the filtrate pH was neutral.
2.4 Sodium chlorite pretreatment (SC)
Raw CFS (10.0 g) was first suspended in deionized water to form a 5% (g/g) solid slurry, and then, 1 mL of acetic acid and 20 g of sodium chlorite were added. The mixtures were then incubated at 75 °C for 40 min with gentle swirling. After pretreatment, the obtained residues were separated by filtration and then extensively washed with deionized water until the filtrate pH was neutral.
2.5 Combined pretreatments
Two combined pretreatments were designed: DSA followed by SC pretreatment (DSA-SC) and SC followed by DSA pretreatment (SC-DSA). For DSA-SC or SC-DSA, DSA was conducted as described in Section 2.3, and SC was performed as described in Section 2.4.
2.6 Enzymatic hydrolysis
Enzymatic hydrolysis was subjected to 30 mL of phosphate buffer (pH 5.5, 50 mM) at 3% (w/v) glucan, and the cellulase loading was 15 filter paper units (FPU) per gram of glucan. The hydrolysis experiment was conducted at 50 °C and 150 rpm on a shaker incubator for 48 h. Five hundred microliters of hydrolysis solution was taken at intervals, and then, the acquired supernatants were diluted and filtered through a 0.22-µm syringe filter for subsequent sugar analysis. The hydrolysis yield was calculated based on the glucose amount as a percentage of the theoretical maximum yield of glucose.
2.7 Simultaneous saccharification fermentation
Pretreated substrates (cellulose-rich) were collected after DSA-SC two-stage treatment and evaluated for their performance in simultaneous saccharification fermentation for lactic acid production. The experimental conditions for the simultaneous saccharification fermentation were as follows: 150 mL of simultaneous saccharification fermentation system; 4%, 8%, and 12% adiabatic solid concentrations; 12 FPU/g adiabatic solids of cellulase enzyme, nitrogen, and nutrients according to the B. coagulans fermentation medium components; 20 mM pH 5.5 phosphate buffer; 50 °C and 150 rpm shaker speed. B. coagulans CC17A was inoculated at 10% (v/v) and neutralized by adding calcium carbonate at a 1/2 molar ratio of the theoretical carbon source at the end of synchronous saccharification fermentation for up to 6 h. The concentrations of glucose, cellobiose, and lactic acid in the fermentation broth were measured by sampling at regular intervals.
2.8 Component analysis
The concentrations of glucose and other monosaccharides (arabinose, galactose, xylose, and mannose) in the water phase were determined as described in the work of Tang [17].
where m0 is the mass of the component (glucan, hemicellulose, or lignin) in the raw material; m1 is the mass of the component (glucan, hemicellulose, or lignin) in the pretreated material; mglu2 is the mass of glucose obtained in the enzyme hydrolysate of the DSA-SC sample; and mglu3, mman3, mgal3, mxyl3, and mara3 represent the mass of glucose, mannose, galactose, xylose, and arabinose in the liquid of the first step of DSA-SC pretreatment, respectively.
2.9 Scanning electron microscopy (SEM)
The surface morphologies of solid materials were detected by using a scanning electron microscope (FEI Quanta 400 instrument, HITACHI, Japan). The surface images were captured at magnifications of 600 and 3000 times to reveal their macro and micro appearances.
2.10 FTIR-ATR spectroscopic analysis
A Spectrum One FTIR system (Themo Scientific, USA, Thermo Nicolet 360) was used to characterize the changes in the functional groups of the samples with different pretreatments. FTIR spectra were obtained by 64 scans from 4000 to 400 cm−1 at 2 cm−1 resolution. A 2-mg dried sample was flaked with 200 mg KBr before FTIR analysis.
2.11 Enzyme adsorption on pretreated materials
The enzyme adsorption was determined under the same conditions as the enzymatic hydrolysis at 4 °C [25]. After incubation, the samples were centrifuged, and the enzyme concentration of the supernatant was determined by Bradford assay.
where Cini is the initial enzyme concentration of the supernatant before incubation and Csuper is the enzyme concentration in the supernatant after incubation.
2.12 Determination of cellulose accessibility
Cellulose accessibility determined the adsorption capacity of direct red dye (DR28) on biomass [26]. Briefly, the experiment was carried out at 1% (w/v) biomass substrate with a series of increasing direct red dye concentrations (0.00, 0.05, 0.10, 1.00, 2.00, 3.00, 4.00 g/L), and the mixtures were incubated at 50 °C and 150 rpm for 24 h. After incubation, the unadsorbed DR28 was determined by measuring the absorbance at 498 nm. A Langmuir nonlinear regression was used to evaluate the maximum adsorption capacity of DR28, which was interpreted as the accessibility of the substrate.
3 Results and discussion
3.1 Chemical composition changes after various pretreatments
To selectively remove hemicellulose and lignin in CFS, dilute sulfuric acid and sodium chlorite pretreatments (DSA and SC) were first employed in this study. As shown in Table 1, glucan recovery remained at 79.5–85.9% after various pretreatments, which indicated that all pretreatment processes resulted in a slight glucose loss. SC treatment alone had a prominent selectivity for lignin removal and less of an effect on hemicellulose removal. There were no obvious changes in the cellulose and hemicellulose contents of pretreated CFS, but the lignin content significantly decreased from 36.5 to 22.1%. For the DSA pretreatment, a large portion of hemicellulose (91.1%) was removed along with minor cellulose and lignin removal. After DSA, the cellulose, hemicellulose, and lignin contents of the DSA sample were 48.5%, 2.7%, and 42.5%, respectively. This result was consistent with previous conclusions that DSA is an effective method for hemicellulose removal in industrial applications [27, 28]. Two single pretreatments did not have a good effect on the simultaneous removal of lignin and hemicellulose. However, there seemed to be a complement in lignin and hemicellulose removal. Thus, the combined process was further used for the pretreatment of CFS.
Two different sequential two-stage pretreatment approaches (SC-DSA and DSA-SC) were investigated for their effectiveness in delignification and hemicellulose removal (Table 1). From a logical point of view, if there is no interaction influence between SC and DSA, the recovery of any component and total solid could be estimated as the product of the recovery of each separation process. Therefore, the theoretical hemicellulose removal, lignin removal, and dextran recovery for the combined pretreatment were calculated to be 92.9%, 61.4%, and 71.5%, respectively. Moreover, the total solid recovery should be 56.6% theoretical. However, as shown in Table 1, the decrease in solid recovery confirmed that an interaction effect existed in CFS pretreatment. Glucan recovery did not decrease obviously with the supplementation of the second pretreatment step, whether DSA or SC. This result indicated that two-stage pretreatment did not result in more glucan loss, which is an important criterion for pretreatment technology. Moreover, close to 95% hemicellulose removal indicated that the combined pretreatment had no significant effect on it. However, it should be noted that combining SC and DSA demonstrated a synergistic effect for improving lignin removal. More importantly, the order of combined pretreatment was critical for lignin removal. Approximately 71.2% delignification was found after SC-DSA, which increased lignin removal from 15.5 to 36.9% in the DSA process. By comparison, lignin removal increased to 93.6% when the order of the combined pretreatments was reversed (DSA-SC). In particular, 92.4% of lignin removal in the 2nd SC was observed, which increased by 38.1% more than in a single SC. In softwood, lignin and hemicellulose can form lignin-carbohydrate complexes, and the lignin in spruce is linked to hemicellulose mainly through covalent bonds [19]. Thus, it was hypothesized that the presence of hemicellulose in CFS hinders the permeation of sodium chlorite to attack lignin in lignin-carbohydrate complexes. When hemicellulose was first removed during the DSA process and the structure of the LCC complex was destroyed, sodium chlorite could more easily react with lignin, and the delignification was greatly improved. It was concluded that sequential component removal was good for softwood pretreatment and that the hemicellulose-removal-first strategy contributed to better delignification.
Regarding compositional changes, the percentage of dextran content did not change much after the two single pretreatments, while the combined pretreatment significantly increased the percentage of dextran content, decreased the hemicellulose and lignin content, and increased the purity of cellulose (Table 1). In particular, especially after DSA-SC, the lignin content in the CFS residual solids decreased from 36.5 to 5.3%, while the dextran content increased from 42 to 79.8%, reaching the highest level. It should be noted that hemicellulose removal by DSA pretreatment followed by combined SC pretreatment not only yielded good results for hemicellulose removal (93.8%) and lignin (93.6%) but also a very high cellulose purity close to 80% and a low lignin composition of 5.3%. This is the very high cellulose purity obtained in the currently known reported articles on the pretreatment of softwoods. Kumar et al. obtained a maximum glucan content of 57.0%, including 40.1% acid-insoluble lignin, after pretreatment of six yellow fir species with steam at 200 °C, 5 min, and 4% SO2 [2]. Matsoukas et al. used a newly established hybrid organic solvent-vapor blast pretreatment of spruce yielded pretreated solids with high cellulose (72% w/w) and low lignin (up to 79.4% w/w in delignification) content [3].
3.2 Structure and functional group changes of pretreated substrate
The morphological changes in different pretreated samples were studied via SEM images (Fig. 1). A complete and compact, ordered fiber cell wall structure was observed for the untreated CFS. Its surface was smooth (Fig. 1B, A). After DSA or SC treatment alone, both of the pretreated samples still maintained an overall ordered fiber structure, but many ordered holes were generated on the surface (Fig. 1B and C). The SEM images at higher magnification showed that DSA produced a rougher surface than SC, and these deep pores were often trapped in collapsed and fractured holes, allowing cellulose accessibility and accelerating enzymatic hydrolysis [29]. In comparison, the pore structure of the SC samples was shallow and small, and there was no obvious damage or rupturing. SC likely had a lower impact on structural disruption than DSA. Regardless of SC-DSA and DSA-SC, the two-step pretreatments produced more fragmentation, resulting in cell wall structures that were no longer straight and were more disorganized, indicating severe damage. When the fiber-organized structure was destroyed and more irregular cracks and fragments were generated, the enzymolysis could be enhanced due to the increased fiber exposure [30]. As noted for DSA-SC, irregular fragments were generated on the surface of DSA-SC, and many aggregated spheres were also clearly observed on the surface. Previous literature reported that these droplets were likely to be lignin deposits on the surface, as observed in some pretreated lignocellulosic samples [31, 32]. Integrating these results with those of the compositional changes of the pretreated CFS, a more comprehensive view was provided that the order of two-stage pretreatment not only influenced the amount of lignin removal but also exhibited a difference concerning the subcellular dissolution of the lignin fraction. It is worth noting that DSA-SC, along with the highest lignin removal, could transfer the residual lignin from the interior cell wall to the exterior surface, which eliminated the negative effect of lignin and facilitated enzymatic hydrolysis.
Scanning electron microscopy (SEM) images of different samples: a, untreated Chinese fir sawdust (CFS); b, CFS pretreated with DSA (0.4% w/v H2SO4, 1 g (substrate):8 mL acid, 160 °C, 60 min); c, CFS pretreated SC (10% w/v sodium chlorite solution, 1 g (substrate):20 mL solution, 75 °C, 40 min); d, CFS pretreated with SC-DSA (SC + DSA); e, CFS pretreated with DSA-SC (DSA + SC). 1 was magnified 600 times; 2 was magnified 3000 times
The chemical fingerprinting of untreated and pretreated CFS was characterized by FTIR, and their spectra are shown in Fig. 2. The band at approximately 1740 cm−1 represents the alkyl ester of the acetyl group in hemicellulose, and the diminishing of this peak is indicative of hemicellulose removal after pretreatment. Softwood is known for its high recalcitrance, mainly due to its high content of guaiacyl [33]. The peak near 1510–1505 cm−1 has been shown to be related to the guaiacyl ring, which was present in the raw sawdust and the DSA sample. Comparatively, a significant reduction in this peak after DSA-SC is attributed to the high removal of lignin. Moreover, it was found that the peak near 898 cm−1 was significantly increased by DSA-SC when compared to the peak intensity observed in the other samples, which was related to amorphous cellulose [34]. The exposure of amorphous cellulose would be helpful to enhance the enzymatic hydrolysis efficiency.
3.3 Improving enzymatic hydrolysis of pretreated sawdust properly
To evaluate pretreatment efficiency for hydrolysis, the enzymatic hydrolysis yield of various pretreated samples is illustrated in Fig. 3. As expected, the hydrolysis yield of raw CFS was only 8.8% at 48 h. Both hemicellulose and lignin in CFS are major obstacles to enzymatic hydrolysis, especially for softwood [35]. SC demonstrated better delignification (54.3%) than hemicellulose removal (21.3%). However, more than half of the delignification effect did not significantly enhance enzymatic hydrolysis. In contrast, when DSA removed most of the hemicelluloses, the hydrolysis yield of CFS pretreated by DSA reached 27.3%, almost twice as high as that of the SC-pretreated CFS. It seems that removing hemicellulose by DSA has a greater positive effect on enzymatic hydrolysis than lignin removal [36]. For one-step pretreatments, the relatively low enzymatic digestibility correlated nicely with their overall ordered fiber structure. The above results could account for the greater number of collapsed and fractured fibers discovered in the DSA sample than in the SC sample. This result may be due to the disruption of the LLC structure by the removal of hemicellulose, which is speculated to be a potential reason for the superiority of DSA over SC. Therefore, when using a combined design, SC-DSA and DSA-SC, pretreated CFS gave the highest hydrolysis yield (99.0%) by DSA-SC, followed by SC-DSA (53.1%). Among the known experiments on hydrolysis of recalcitrant cork, the present experiment has achieved a fairly good hydrolysis effect. Xiao et al. treated Metasequoia using different pretreatments while increasing the substrate enzymatic hydrolysis yield from the original 3.56 to 71.6% [37]. Matsakas et al. treated spruce using an acid-catalyzed organic solvent-vapor blast pretreatment to increase the enzymatic hydrolysis yield of spruce to 61% [3]. This significant improvement could be attributed to the removal of most of the lignin and hemicellulose (> 70%) and the destruction of the fiber-organized structure, which was supported by the SEM results. In corn stover pretreatment, An et al. noted that the sequence of the two-step process has a great influence on enzymatic digestion [38] and that priority should be given to removing hemicellulose in the combined pretreatment process [1, 39]. Our results confirmed that first removing hemicellulose contributed to more effective delignification, which is critical for improving the enzymatic digestibility of CFS. Moreover, taking into account the lignin droplets found in pretreated CFS by DSA-SC, it was reasonable to assume that the factors affecting the enzymatic hydrolysis might not only include chemical composition and substrate fiber morphology but also may include the redistribution of lignin [40].
To further elucidate the mechanism behind the pretreatment effect, the enzyme adsorption ratio and cellulose accessibility of the different pretreated samples were determined as evaluation parameters for enzymatic hydrolysis. Generally, enzyme–substrate interactions are correlated with the enzyme adsorption capacity. Lignin and hemicellulose have been proven to nonproductively bind to cellulase [41,42,43]. Overall, the decrease in the enzyme adsorption ratio favors enhanced enzymatic hydrolysis (Table 2). For all three pretreatments (DSA, SC-DSA, and DSA-SC), all hemicellulose removal rates of up to 90% were observed, and thus, their decrease in enzyme adsorption rate mainly depends on the removal of lignin. These results suggested that the decrease in lignin in the CFS might contribute more to nonproductive binding and yield enhancement. However, an exception was observed in that a lower enzyme adsorption ratio by SC did not bring a better yield than that by DSA. This could explain the higher cellulose accessibility of DSA than SC. Cellulose accessibility is an important evaluation index for evaluating the major physical barriers faced by cellulose [26]. Thus, a widely used Simons’ staining method of direct red dye (DR28) was applied to evaluate the cellulose accessibility of wet lignocellulose [44]. For lignocellulose saccharification, hemicellulose and lignin were recommended to first be removed for cellulose accessibility [36, 44]. In this study, the removal of hemicellulose was more important for the increase in cellulose accessibility than delignification. Furthermore, when the mass of hemicellulose was removed, further delignification by SC demonstrated a remarkable increase in cellulose accessibility (from 121.09 to 238.9 mg/g). The largest accessibility was found in the DSA-SC sample, which corresponded to the highest enzymatic hydrolysis yield. SC exhibited lower enzyme nonproductive binding and unexpectedly lower hydrolysis yield. This indicated that the trend of the enzyme adsorption ratio did not always coincide with the hydrolysis performance and the cellulose accessibility, which is related to substrate morphology and the redistribution of lignin and hemicellulose and might be a more important factor for enzymatic hydrolysis [42, 45].
3.4 SSF of DSA-SC pretreatment materials and overall mass balance
To evaluate the potential of DSA-SC in biorefineries, simultaneous saccharification and fermentation (SSF) experiments were performed for pretreated samples using B. coagulans CC17A. The lactic production under different substrate concentrations is shown in Fig. 4. The lactic acid concentrations after 48 h of SSF reached 27.5, 52.1, and 71.2 g/L when using 4%, 8%, and 12% substrate (w/v absolute dry solids mass), respectively. The high conversion rate of cellulose into lactic acid (> 70%) suggested that DSA-SC is an effective pretreatment technology for softwood biorefineries with good hydrolysis and fermentation performance. Moreover, a mass balance based on 100.0 g of dry CFS was conducted. As shown in Fig. 5, after the first pretreatment step, the soluble fraction contained mainly glucose (4.1 g), mannose (5.6 g), xylose (2.9 g), galactose (1.0 g), and arabinose (0.4 g), yielding a total of approximately 14 g of monosaccharides. These monosaccharides from hemicellulose were relatively pure and were further converted into chemicals by chemical conversion or fermentation. In the first step of pretreatment, the hemicellulose fraction could be selectively and completely separated from the biomass. The second pretreatment step resulted in a high lignin removal of 92.4%. The soluble fraction mainly contained soluble lignin, and a total of 15.2 g of lignin was recovered by multiple ethanol precipitation. According to the design, relatively pure lignin was obtained in the second step, which could be applied in the macromolecular industry [46]. Finally, when using the pretreated solids as substrate, a total of 30.6 g lactic acid was produced by SSF. The overall balance of the CFS biorefinery showed that 41.6% of lignin and 52.8% of hemicellulose were recovered with good selectivity by the DSA-SC process, and 306.1 g lactic acid could be produced from 1000.0 g CFS. The layer-layer separation during two-step pretreatment paves the way for a multipurpose project for Chinese fir sawdust.
Mass balance for DSA-SC pretreatment and enzymatic hydrolysis. The first-step pretreatment conditions: 0.4% (w/w) H2SO4, 1:10 w/v ratio (dry substrate: dilute H2SO4), 160 °C for 60 min. The second-step pretreatment conditions: 10% (w/w) sodium chlorite solution, 1:20 w/v ratio (dry substrate: sodium chlorite solution), 75 °C for 40 min
4 Conclusion
Two pretreatments and their sequential combination were introduced to investigate their effect on CFS deconstruction. Neither of the pretreatments alone produced simultaneous removal of lignin and hemicellulose from the CFS. However, combining SC and DSA demonstrated a remarkable synergistic effect for improving lignin removal. After DSA-SC, 93.8% of hemicellulose and 93.6% lignin were removed, and the pretreated substrate had a high cellulose purity of close to 80% and a low lignin content of 5.3%. Moreover, the residual lignin in the CFS was found to transfer from the interior cell wall to the exterior surface. Pretreating CFS with DSA-SC gave the highest hydrolysis yield (99.0%), which increased by 45.9% and 71.7% compared with SC-DSA and DSA, respectively. Furthermore, lactic acid production reached 71.2 g/L at 12% substrate after 48 h of SSF using B. coagulans CC17A. An overall balance for the CFS biorefinery showed that 41.6% lignin and 52.8% hemicellulose sugar were recovered and obtained with good selectivity, and 306.1 g lactic acid was produced from 1000.0 g CFS by this stepwise separation using DSA-SC. DSA-SC ladder separation is a potential pretreatment method for recalcitrant softwood biorefineries due to its high efficiency and good selectivity.
Data availability
The data supporting the findings of the article is available by corresponding authors after any responsible request.
Abbreviations
- CFS:
-
Chinese fir Sawdust
- DSA:
-
Dilute sulfuric acid pretreatment
- SC:
-
Sodium chlorite pretreatment
- DSA-SC:
-
Dilute sulfuric acid combined with sodium chlorite pretreatment
- SC-DSA:
-
Sodium chlorite combined with dilute sulfuric acid pretreatment
- SSF:
-
Simultaneous saccharification and fermentation
References
Pielhop T, Reinhard C, Hecht C et al (2017) Application potential of a carbocation scavenger in autohydrolysis and dilute acid pretreatment to overcome high softwood recalcitrance. Biomass Bioenerg 105:164–173. https://doi.org/10.1016/j.biombioe.2017.07.005
Kumar L, Chandra R, Chung PA, Saddler J (2010) Can the same steam pretreatment conditions be used for most softwoods to achieve good, enzymatic hydrolysis and sugar yields? Bioresour Technol 101:7827–7833. https://doi.org/10.1016/j.biortech.2010.05.023
Matsakas L, Raghavendran V, Yakimenko O et al (2019) Lignin-first biomass fractionation using a hybrid organosolv – steam explosion pretreatment technology improves the saccharification and fermentability of spruce biomass. Bioresour Technol 273:521–528. https://doi.org/10.1016/j.biortech.2018.11.055
Satari B, Karimi K, Molaverdi M (2018) Structural features influential to enzymatic hydrolysis of cellulose-solvent-based pretreated pinewood and elmwood for ethanol production. Bioprocess Biosyst Eng 41:249–264. https://doi.org/10.1007/s00449-017-1863-2
Shuai L, Yang Q, Zhu JY et al (2010) Comparative study of SPORL and dilute-acid pretreatments of spruce for cellulosic ethanol production. Bioresour Technol 101:3106–3114. https://doi.org/10.1016/j.biortech.2009.12.044
Behera S, Arora R, Nandhagopal N, Kumar S (2014) Importance of chemical pretreatment for bioconversion of lignocellulosic biomass. Renew Sustain Energy Rev 36:91–106. https://doi.org/10.1016/j.rser.2014.04.047
Li X, Luo X, Li K et al (2012) Effects of SPORL and dilute acid pretreatment on substrate morphology, cell physical and chemical wall structures, and subsequent enzymatic hydrolysis of lodgepole pine. Appl Biochem Biotechnol 168:1556–1567. https://doi.org/10.1007/s12010-012-9878-2
Lim W, Lee J (2013) Influence of pretreatment condition on the fermentable sugar production and enzymatic hydrolysis of dilute acid-pretreated mixed softwood. Bioresour Technol 140:306–311. https://doi.org/10.1016/j.biortech.2013.04.103
Galbe M, Zacchi G (2002) A review of the production of ethanol from softwood. Appl Microbiol Biotechnol 59:618–628. https://doi.org/10.1007/s00253-002-1058-9
Singh R, Shukla A, Tiwari S, Srivastava M (2014) A review on delignification of lignocellulosic biomass for enhancement of ethanol production potential. Renew Sustain Energy Rev 32:713–728. https://doi.org/10.1016/j.rser.2014.01.051
Qi B, Chen X, Wan Y (2010) Pretreatment of wheat straw by nonionic surfactant-assisted dilute acid for enhancing enzymatic hydrolysis and ethanol production. Bioresour Technol 101:4875–4883. https://doi.org/10.1016/j.biortech.2010.01.063
Ishizawa CI, Jeoh T, Adney WS et al (2009) Can delignification decrease cellulose digestibility in acid pretreated corn stover? Cellulose 16:677–686. https://doi.org/10.1007/s10570-009-9313-1
Gschwend FJV, Chambon CL, Biedka M et al (2019) Quantitative glucose release from softwood after pretreatment with low-cost ionic liquids. Green Chem 21:692–773. https://doi.org/10.1039/C8GC02155D
Wi SG, Kim HJ, Mahadevan SA et al (2009) The potential value of the seaweed Ceylon moss (Gelidium amansii) as an alternative bioenergy resource. Bioresour Technol 100:6658–6660. https://doi.org/10.1016/j.biortech.2009.07.017
Senila L, Senila M, Varaticeanu C et al (2015) Autohydrolysis pretreatment and delignification of silver fir wood to obtain fermentable sugars for bioethanol production. Energy Sources Part A Recover Util Environ Eff 37:1890–1895. https://doi.org/10.1080/15567036.2012.658139
Ono Y, Takeuchi M, Isogai A (2022) Changes in neutral sugar composition, molar mass and molar mass distribution, and solid-state structures of birch and Douglas fir by repeated sodium chlorite delignification. Cellulose 29:2119–2129. https://doi.org/10.1007/s10570-022-04448-2
Tang S, Liu W, Huang C et al (2019) Improving the enzymatic hydrolysis of larch by coupling water pre- extraction with alkaline hydrogen peroxide post-treatment and adding enzyme cocktail. Bioresour Technol 285:121322. https://doi.org/10.1016/j.biortech.2019.121322
Duan Y, Ma Y, Zhao X et al (2018) Real-time adsorption and action of expansin on cellulose. Biotechnol Biofuels 11:317. https://doi.org/10.1186/s13068-018-1318-2
Várnai A, Siika-aho M, Viikari L (2010) Restriction of the enzymatic hydrolysis of steam-pretreated spruce by lignin and hemicellulose. Enzyme Microb Technol 46:185–193. https://doi.org/10.1016/j.enzmictec.2009.12.013
Mooney CA, Mansfield SD, Touhy MG, Saddler JN (1998) The effect of initial pore volume and lignin content on the enzymatic hydrolysis of softwoods. Bioresour Technol 64:113–119. https://doi.org/10.1016/S0960-8524(97)00181-8
Tarasov D, Leitch M, Fatehi P (2018) Lignin-carbohydrate complexes: properties, applications, analyses, and methods of extraction: a review. Biotechnol Biofuels 11:1–28. https://doi.org/10.1186/s13068-018-1262-1
Von Freiesleben P, Spodsberg N, Stenbæk A et al (2018) Boosting of enzymatic softwood saccharification by fungal GH5 and GH26 endomannanases. Biotechnol Biofuels 11:194. https://doi.org/10.1186/s13068-018-1184-y
Zhang C, Lei X, Scott CT et al (2014) Comparison of dilute acid and sulfite pretreatment for enzymatic saccharification of earlywood and latewood of Douglas fir. Bioenergy Res 7:362–370. https://doi.org/10.1007/s12155-013-9376-6
Ouyang S, Shi J, Qiao H et al (2021) The key role of delignification in overcoming the inherent recalcitrance of Chinese fir for biorefining. Bioresour Technol 319:124154. https://doi.org/10.1016/j.biortech.2020.124154
Lai C, Tu M, Li M, Yu S (2014) Remarkable solvent and extractable lignin effects on enzymatic digestibility of organosolv pretreated hardwood. Bioresour Technol 156:92–99. https://doi.org/10.1016/j.biortech.2014.01.030
Wiman M, Dienes D, Hansen MAT et al (2012) Cellulose accessibility determines the rate of enzymatic hydrolysis of steam-pretreated spruce. Bioresour Technol 126:208–215. https://doi.org/10.1016/j.biortech.2012.08.082
Rajendran K, Drielak E, Sudarshan Varma V et al (2018) Updates on the pretreatment of lignocellulosic feedstocks for bioenergy production–a review. Biomass Convers Biorefinery 8:471–483. https://doi.org/10.1007/s13399-017-0269-3
Zheng Y, Zhao J, Xu F, Li Y (2014) Pretreatment of lignocellulosic biomass for enhanced biogas production. Prog Energy Combust Sci 42:35–53. https://doi.org/10.1016/j.pecs.2014.01.001
Zhang H, Fan Z, Li J, Han L (2019) A comparative study on enzyme adsorption and hydrolytic performance of different scale corn stover by two-step kinetics. Bioresour Technol 282:384–389. https://doi.org/10.1016/j.biortech.2019.03.005
Ravindran R, Jaiswal S, Abu-Ghannam N, Jaiswal AK (2017) Two-step sequential pretreatment for the enhanced enzymatic hydrolysis of coffee spent waste. Bioresour Technol 239:276–284. https://doi.org/10.1016/j.biortech.2017.05.049
He J, Huang C, Lai C et al (2018) Elucidation of structure-inhibition relationship of monosaccharides derived pseudo-lignin in enzymatic hydrolysis. Ind Crops Prod 113:368–375. https://doi.org/10.1016/j.indcrop.2018.01.046
Wang K, Yang C, Xin Xu, Lai C, Daihui Zhang QY (2022) 2-Naphthol modification alleviated the inhibition of ethanol organosolv lignin on enzymatic hydrolysis. Renew Energy 200:767–776. https://doi.org/10.1016/j.renene.2022.10.053
Cannella D, Sveding PV, Jørgensen H (2014) PEI detoxification of pretreated spruce for high solids ethanol fermentation. Appl Energy 132:394–403. https://doi.org/10.1016/j.apenergy.2014.07.038
Meng X, Sun Q, Kosa M et al (2016) Physicochemical structural changes of poplar and switchgrass during biomass pretreatment and enzymatic hydrolysis. ACS Sustain Chem Eng 4:4563–4572. https://doi.org/10.1021/acssuschemeng.6b00603
Zhang C, Houtman CJ, Zhu JY (2014) Using low temperature to balance enzymatic saccharification and furan formation during SPORL pretreatment of Douglas-fir. Process Biochem 49:466–473. https://doi.org/10.1016/j.procbio.2013.12.017
Leu SY, Zhu JY (2013) Substrate-related factors affecting enzymatic saccharification of lignocelluloses: our recent understanding. Bioenergy Res 6:405–415. https://doi.org/10.1007/s12155-012-9276-1
Xiao K, Yuan Y, Xiao N et al (2017) Enzymatic saccharification responses of Eichhornia crassipes, sugarcane bagasse and Metasequoia glyptostroboides to two oxidation pretreatments for biofuel production. Ind Crops Prod 107:22–29. https://doi.org/10.1016/j.indcrop.2017.05.017
An S, Li W, Liu Q et al (2019) Combined dilute hydrochloric acid and alkaline wet oxidation pretreatment to improve sugar recovery of corn stover. Bioresour Technol 271:283–288. https://doi.org/10.1016/j.biortech.2018.09.126
Sun S, Sun S, Cao X, Sun R (2016) The role of pretreatment in improving the enzymatic hydrolysis of lignocellulosic materials. Bioresour Technol 199:49–58. https://doi.org/10.1016/j.biortech.2015.08.061
Liu W, Chen W, Hou Q et al (2017) Bioresource Technology Surface lignin change pertaining to the integrated process of dilute acid pre-extraction and mechanical refining of poplar wood chips and its impact on enzymatic hydrolysis. Bioresour Technol 228:125–132. https://doi.org/10.1016/j.biortech.2016.12.063
Hou S, Shen B, Zhang D et al (2022) Understanding of promoting enzymatic hydrolysis of combined hydrothermal and deep eutectic solvent pretreated poplars by Tween 80. Bioresour Technol 362:127825. https://doi.org/10.1016/j.biortech.2022.127825
Macaskill JJ, Suckling ID, Lloyd JA, Manley-harris M (2018) Unravelling the e ff ect of pretreatment severity on the balance of cellulose accessibility and substrate composition on enzymatic digestibility of steam- pretreated softwood. Biomass Bioenerg 109:284–290. https://doi.org/10.1016/j.biombioe.2017.12.018
Pan X, Xie D, Gilkes N (2005) Strategies to enhance the enzymatic hydrolysis of pretreated softwood with high residual lignin content. Appl Biochem Biotechnol 121:1069–1079
Lai C, Yang B, He J et al (2018) Enhanced enzymatic digestibility of mixed wood sawdust by lignin modification with naphthol derivatives during dilute acid pretreatment. Bioresour Technol 269:18–24. https://doi.org/10.1016/j.biortech.2018.08.086
Tong W, Fang H, Song K et al (2023) Modified acid pretreatment to alter physicochemical properties of biomass for full cellulose/hemicellulose utilization. Carbohydr Polym 299:120182. https://doi.org/10.1016/j.carbpol.2022.120182
Jiang Y, Liu X, Yang S et al (2019) Combining organosolv pretreatment with mechanical grinding of sugarcane bagasse for the preparation of nanofibrillated cellulose in a novel green approach. BioResources 14:313–335
Funding
The authors acknowledge support from the National Key Research and Development Program of China (2018YFA0902200).
Author information
Authors and Affiliations
Contributions
Jiaming Fu: investigation, data curation, formal analysis, visualization, writing—original draft. Shuiping Ouyang: conceptualization, investigation, data curation, visualization, writing—original draft, resources. Zijie Wang: investigation, resources. Hui Qiao: formal analysis, investigation, methodology. Zhaojuan Zheng: writing—review and editing. Jia Ouyang: writing—review and editing, supervision, funding acquisition.
Corresponding author
Ethics declarations
Ethics approval
Since this study did not recruit human and/or animal subjects, this section does not apply.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Fu, J., Ouyang, S., Wang, Z. et al. Enhancing enzymatic hydrolysis of Chinese fir sawdust by using the synergistic effect of dilute sulfuric acid and sodium chlorite pretreatment. Biomass Conv. Bioref. 14, 16159–16169 (2024). https://doi.org/10.1007/s13399-023-03788-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13399-023-03788-8