Abstract
Myoepithelial tumors in skin and soft tissue are uncommon but have been increasingly characterized over the past decade. Men and women are equally affected across all age groups and lesions arise most frequently on the extremities and limb girdles. Approximately 20 % of cases occur in pediatric patients, in whom they are frequently malignant. Similar to their salivary gland counterparts, myoepithelial tumors of soft tissue demonstrate heterogeneous morphologic and immunophenotypic features. Tumors are classified as mixed tumor/chondroid syringoma, myoepithelioma, and myoepithelial carcinoma; in soft tissue, tumors having at least moderate cytologic atypia are classified as malignant. Mixed tumor and myoepithelioma show a benign clinical course, with recurrence in up to 20 % (typically secondary to incomplete excision), and do not metastasize. In contrast, myoepithelial carcinoma shows more aggressive behavior with recurrence and metastasis in up to 40–50 % of cases. The majority of myoepithelial neoplasms typically coexpress epithelial antigens (cytokeratin and/or EMA) and S-100 protein; GFAP and p63 are frequently positive and a subset of malignant neoplasms lose INI1 expression. Up to 45 % of myoepitheliomas and myoepithelial carcinomas harbor EWSR1 gene rearrangements, unlike mixed tumor/chondroid syringoma which is characterized by PLAG1 gene rearrangement. While mixed tumor/chondroid syringoma are likely related to primary salivary myoepithelial tumors, soft tissue myoepithelioma and myoepithelial carcinoma appear to be pathologically distinct neoplasms.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Myoepithelial neoplasms arising in soft tissue and skin have been increasingly characterized by morphologic, immunohistochemical, and genetic means over the past 10–15 years. These tumors are now known to occur over a wide age range in a broad anatomic distribution (including visceral locations), though they most commonly arise as subcutaneous nodules on the extremities and proximal limb girdles [1–3]. Cutaneous lesions are typically confined to the dermis [2, 4, 5]. Men and women are affected equally, and approximately 20 % of cases occur in pediatric patients [2, 3]. While having many similarities to their salivary gland counterparts, soft tissue myoepithelial tumors are distinct in several ways, including histologic criteria for malignancy and their characteristic genetic aberrations.
Morphologic Range of Myoepithelial Neoplasms
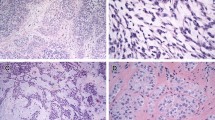
In contrast to myoepithelial neoplasms in the salivary gland, tumors that arise in soft tissue lack any known normal cellular counterpart (which likely contributed to their being initially under-recognized). Myoepithelial neoplasms of soft tissue show a wide range of cytologic and architectural features both within a given lesion and between different tumors. Though often well-circumscribed grossly, both benign and malignant tumors are unencapsulated and frequently have infiltrative margins. Tumors are typically characterized by multinodular or lobular growth of spindled, ovoid, or epithelioid cells arranged in variable growth patterns, namely reticular, trabecular, nested, and solid, embedded in a variably prominent myxoid and/or hyalinized or chondroid stroma (Fig. 1a–c). The cytoplasm ranges in appearance from eosinophilic to clear (similar to myoepithelial cells in salivary gland); occasional other morphologic appearances include tumor cells with copious vacuolated cytoplasm (formerly identified in so-called “parachordoma”), plasmacytoid cells with densely eosinophilic cytoplasm (“hyaline bodies”), or rhabdoid morphology [6]. Similar to pleomorphic adenoma/mixed tumor of the salivary gland, heterologous differentiation occurs in up to 15 % of cases (most frequently cartilaginous (Fig. 1d) and/or osseous [1–3, 7]; and less commonly squamous [1] or adipocytic [1, 7, 8]).
Myoepithelial neoplasms are frequently characterized by multinodular or lobular architecture (a) and consist of spindled, ovoid, or epithelioid cells arranged in variable growth patterns, namely reticular (b), trabecular or nested/solid (c), embedded in a variably myxoid, hyalinized or chondroid stroma. Cartilaginous and/or osseous metaplasia may be present (d)
Myoepithelial neoplasms in skin and soft tissue are classified as mixed tumor/chondroid syringoma, myoepithelioma, and myoepithelial carcinoma. Mixed tumors/chondroid syringoma show tubuloductal differentiation, appearing similar to their salivary gland counterparts (Fig. 2a). Myoepithelioma is comprised predominantly of spindled or ovoid myoepithelial cells, which have nuclei with little to no atypia, fine chromatin, and inconspicuous to small nucleoli (Fig. 3a). In skin and soft tissue sites, malignancy in myoepithelial neoplasms is defined strictly by cytologic atypia, in contrast to their salivary gland counterparts in which malignancy is based on architectural features (i.e. invasive growth), frequently being associated with a benign precursor [9]. Tumors classified as myoepithelial carcinoma have moderate-to-severe atypia with vesicular nuclei and prominent nucleoli (Fig. 3b); cytologic atypia has been shown to be the sole predictor of aggressive behavior in soft tissue [2]. Though not reliably predictive of aggressive behavior, high mitotic rates and necrosis are common features of myoepithelial carcinoma [2]. Myoepithelial carcinomas are thus graded (largely on a subjective basis) as low, intermediate, and high based on the degree of cytologic atypia and additional presence of mitoses and necrosis. That being said, as with salivary myoepithelial carcinomas, there are no clearly defined or validated criteria for grading. Unlike salivary gland neoplasms, areas of morphologically benign myoepithelioma or mixed tumor are only infrequently present within a myoepithelial carcinoma, being reported in less than 1 % of soft tissue tumors of this type [2, 3].
Myoepithelial Carcinomas in Pediatric Patients
Approximately 20 % of myoepithelial tumors occur in children, approximately 65 % of which are malignant. Pediatric tumors also most frequently arise in the extremities and trunk. In a series of 29 cases of myoepithelial carcinoma of soft tissue, the majority of tumors (27/29; 93 %) were comprised of a heterogeneous tumor cell population, and epithelioid morphology predominated in 93 % of cases [3]. Ten percent of cases had an undifferentiated round cell component (Fig. 4). For the 23 patients with available follow-up, 9 (39 %) patients developed local recurrence (8 patients with marginal excision, 1 with widely negative margins), 12 (52 %) developed distant metastases, and 10 (43 %) patients died of disease. Sites of metastasis were lungs/pleura, lymph node, bone, soft tissue, liver, brain, skin, and soft tissue. Subsequent unpublished personal experience has confirmed these findings.
Cutaneous Syncytial Myoepithelioma
Cutaneous syncytial myoepithelioma is a recently described morphologic variant characterized by mainly intradermal syncytial growth of ovoid, spindled or histiocytoid cells with eosinophilic cytoplasm [7, 8] (Fig. 5). Tumors have a distinctive immunophenotype which is described below. These tumors more frequently affect men, occur over a wide age range, and most commonly arise on the extremities. Tumors lack nuclear atypia and show an indolent course, with infrequent local recurrence and no reported metastases [7].
Immunohistochemical Features
The majority of myoepithelial neoplasms typically coexpress epithelial antigens (cytokeratin and/or EMA) and S-100 protein. Broad-spectrum cytokeratin (pan-keratin, AE1/AE3, Cam5.2; Fig. 6a) is positive in 93–100 % of cases [1–3, 10], while the extent of EMA positivity is lower (19–66 %) [1–3]. S-100 protein is frequently positive (72–100 %) [1–3, 10] (Fig. 6b). GFAP staining is variable, ranging from 27 to 54 % [1–3]. Of the myogenic markers, calponin is most frequently positive (86–100 %) [2, 3]; SMA positivity ranges from 36 to 64 % [2, 3, 10], HHF-35 ranges from 20 to 60 % [1, 3], and desmin is infrequently positive (0–20 %) [1–4]. Rates of positivity for p63 range from 7 to 45 % [2, 3, 11] (Fig. 6c), which is lower than that seen in salivary neoplasms in which p63 positivity is seen in all cases of pleomorphic adenoma and any carcinoma having a component of myoepithelial cells [12, 13]. SMARCB1/INI1 expression is lost in a subset of myoepitheliomas and myoepithelial carcinomas (9–22 % [3, 14]) (Fig. 6d) likely owing to functional loss of material on chromosome 22q (see below).
A panel including cytokeratin, EMA, S-100 protein, and GFAP will identify myoepithelial differentiation in the majority of cases. Notably, cutaneous syncytial myoepithelioma has a distinct immunophenotype of EMA and S-100 positivity but infrequent cytokeratin staining [7]. PLAG1 immunostaining is positive in 58–100 % of mixed tumors [15, 16] (Fig. 2b), but is negative in myoepitheliomas lacking ductal differentiation [16]. Similarly, the majority of salivary pleomorphic adenomas are positive for PLAG1 [17, 18] and frequent positivity is observed in 77 % of carcinomas ex pleomorphic adenoma in salivary gland, including those classified as the myoepithelial carcinoma subtype [19].
Genetics
Up to 45 % of myoepithelial tumors arising in skin, soft tissue, and bone harbor EWSR1 gene rearrangement (and rarely alternate FUS rearrangement) [20–23]. Documented fusion partners thus far include POU5F1 (6p21), PBX1 (1q23), ZNF444 (19q23), ATF1 (12q13) and PBX3 (9q33) [22, 24, 25]. Homozygous deletion of the SMARCB1 gene has recently been reported in 3/5 cases of myoepithelial carcinoma lacking EWSR1 gene rearrangement [26]. The significance of the gene fusion products is currently unknown, but may be associated with specific morphologic appearances. In the large series by Antonescu et al. [22], myoepithelial tumors with EWSR1-POU5F1 fusion had the distinctive appearance of nested epithelioid cells with clear cytoplasm (Fig. 7), and tumors with EWSR1-PBX1 fusion had predominant sclerotic stroma in which bland-appearing spindle cells were embedded (Fig. 7). EWSR1-rearrangement occurs in up to 44 % of cutaneous myoepithelial tumors [25] (including cutaneous syncytial myoepithelioma [7]). While EWSR1 rearrangement is present in hyalinizing clear cell carcinoma [24], EWSR1 rearrangement has not been identified to date in other salivary neoplasms, including myoepithelial tumors [27, 28] (see Addendum).
Mixed tumors show PLAG1 gene rearrangement in 37–72 % of cases [15, 29], suggesting a genetic relationship to their salivary gland counterparts, as up to 88 % of salivary pleomorphic adenoma [30] and up to 63–75 % of carcinoma ex pleomorphic adenoma have PLAG1 gene alterations [19, 30].
Behavior
Mixed tumors and myoepitheliomas of soft tissue typically show a benign clinical course [2, 4]. Up to 18 % of myoepitheliomas are known to recur, and the risk appears higher with incomplete resection [2]. Distant metastasis of morphologically benign myoepithelial neoplasms is rare [2]. Myoepithelial carcinomas show more aggressive behavior, with a recurrence rate of 39–42 % and distant metastasis in 32–52 % of affected patients [2, 3]. Commonly reported sites of metastasis are lung, bone, lymph node, and soft tissue [1–3]. All large series have reported some frequency of disease-related death (13–43 %) [2, 3, 10], although correlation with histologic grade is unreliable.
Conclusion
Myoepithelial neoplasms of skin and soft tissue are similar in many respects to their salivary gland counterparts, but differ in that cytologic atypia is the chief criterion for malignancy and that EWSR1 translocation is frequent in soft tissue myoepithelioma and myoepithelial carcinoma. Mixed tumor/chondroid syringoma, having ductal differentiation and PLAG1 gene rearrangement, is likely closely related to its salivary gland counterpart. Morphologic variants of myoepithelial neoplasm are increasingly being recognized, including cutaneous syncytial myoepithelioma and the clear cell morphology characteristic of tumors harboring EWSR1-POU5F1 fusion. Myoepithelial differentiation can be supported by an immunohistochemical panel including cytokeratin, EMA, S-100, and GFAP; staining for p63 and INI1 can also be helpful.
References
Kilpatrick SE, Hitchcock MG, Kraus MD, et al. Mixed tumors and myoepitheliomas of soft tissue: a clinicopathologic study of 19 cases with a unifying concept. Am J Surg Pathol. 1997;21:13–22.
Hornick JL, Fletcher CD. Myoepithelial tumors of soft tissue: a clinicopathologic and immunohistochemical study of 101 cases with evaluation of prognostic parameters. Am J Surg Pathol. 2003;27:1183–96.
Gleason BC, Fletcher CD. Myoepithelial carcinoma of soft tissue in children: an aggressive neoplasm analyzed in a series of 29 cases. Am J Surg Pathol. 2007;31:1813–24.
Mentzel T, Requena L, Kaddu S, et al. Cutaneous myoepithelial neoplasms: clinicopathologic and immunohistochemical study of 20 cases suggesting a continuous spectrum ranging from benign mixed tumor of the skin to cutaneous myoepithelioma and myoepithelial carcinoma. J Cutan Pathol. 2003;30:294–302.
Kutzner H, Mentzel T, Kaddu S, et al. Cutaneous myoepithelioma: an under-recognized cutaneous neoplasm composed of myoepithelial cells. Am J Surg Pathol. 2001;25:348–55.
Thway K, Bown N, Miah A, et al. Rhabdoid variant of myoepithelial carcinoma, with EWSR1 rearrangement: expanding the spectrum of EWSR1-rearranged myoepithelial tumors. Head Neck Pathol. 2014. doi:10.1007/s12105-014-0556-2.
Jo VY, Antonescu CR, Zhang L, et al. Cutaneous syncytial myoepithelioma: clinicopathologic characterization in a series of 38 cases. Am J Surg Pathol. 2013;37:710–8.
Hornick JL, Fletcher CD. Cutaneous myoepithelioma: a clinicopathologic and immunohistochemical study of 14 cases. Hum Pathol. 2004;35:14–24.
Savera AT, Sloman A, Huvos AG, et al. Myoepithelial carcinoma of the salivary glands: a clinicopathologic study of 25 patients. Am J Surg Pathol. 2000;24:761–74.
Michal M, Miettinen M. Myoepitheliomas of the skin and soft tissues. Report of 12 cases. Virchows Arch. 1999;434:393–400.
Jo VY, Fletcher CD. p63 immunohistochemical staining is limited in soft tissue tumors. Am J Clin Pathol. 2011;136:762–6.
Bilal H, Handra-Luca A, Bertrand JC, et al. p63 is expressed in basal and myoepithelial cells of human normal and tumor salivary gland tissues. J Histochem Cytochem. 2003;51:133–9.
Genelhu MC, Gobbi H, Soares FA, et al. Immunohistochemical expression of p63 in pleomorphic adenomas and carcinomas ex-pleomorphic adenomas of salivary glands. Oral Oncol. 2006;42:154–60.
Hornick JL, Dal Cin P, Fletcher CD. Loss of INI1 expression is characteristic of both conventional and proximal-type epithelioid sarcoma. Am J Surg Pathol. 2009;33:542–50.
Bahrami A, Dalton JD, Krane JF, et al. A subset of cutaneous and soft tissue mixed tumors are genetically linked to their salivary gland counterpart. Genes Chromosomes Cancer. 2012;51:140–8.
Matsuyama A, Hisaoka M, Hashimoto H. PLAG1 expression in mesenchymal tumors: an immunohistochemical study with special emphasis on the pathogenetical distinction between soft tissue myoepithelioma and pleomorphic adenoma of the salivary gland. Pathol Int. 2012;62:1–7.
Matsuyama A, Hisaoka M, Nagao Y, et al. Aberrant PLAG1 expression in pleomorphic adenomas of the salivary gland: a molecular genetic and immunohistochemical study. Virchows Arch. 2011;458:583–92.
Rotellini M, Palomba A, Baroni G, et al. Diagnostic utility of PLAG1 immunohistochemical determination in salivary gland tumors. Appl Immunohistochem Mol Morphol. 2014;22:390–4.
Bahrami A, Dalton JD, Shivakumar B, et al. PLAG1 alteration in carcinoma ex pleomorphic adenoma: immunohistochemical and fluorescence in situ hybridization studies of 22 cases. Head Neck Pathol. 2012;6:328–35.
Brandal P, Panagopoulos I, Bjerkehagen B, et al. Detection of a t(1;22)(q23;q12) translocation leading to an EWSR1-PBX1 fusion gene in a myoepithelioma. Genes Chromosomes Cancer. 2008;47:558–64.
Brandal P, Panagopoulos I, Bjerkehagen B, et al. t(19;22)(q13;q12) Translocation leading to the novel fusion gene EWSR1-ZNF444 in soft tissue myoepithelial carcinoma. Genes Chromosomes Cancer. 2009;48:1051–6.
Antonescu CR, Zhang L, Chang NE, et al. EWSR1-POU5F1 fusion in soft tissue myoepithelial tumors. A molecular analysis of sixty-six cases, including soft tissue, bone, and visceral lesions, showing common involvement of the EWSR1 gene. Genes Chromosomes Cancer. 2010;49:1114–24.
Agaram NP, Chen HW, Zhang L, et al. EWSR1-PBX3: a novel gene fusion in myoepithelial tumors. Genes Chromosomes Cancer. 2015;54:63–71.
Antonescu CR, Katabi N, Zhang L, et al. EWSR1-ATF1 fusion is a novel and consistent finding in hyalinizing clear-cell carcinoma of salivary gland. Genes Chromosomes Cancer. 2011;50:559–70.
Flucke U, Palmedo G, Blankenhorn N, et al. EWSR1 gene rearrangement occurs in a subset of cutaneous myoepithelial tumors: a study of 18 cases. Mod Pathol. 2011;24:1444–50.
Le Loarer F, Zhang L, Fletcher CD, et al. Consistent SMARCB1 homozygous deletions in epithelioid sarcoma and in a subset of myoepithelial carcinomas can be reliably detected by FISH in archival material. Genes Chromosomes Cancer. 2014;53:475–86.
Shah AA, LeGallo RD, van Zante A, et al. EWSR1 genetic rearrangements in salivary gland tumors: a specific and very common feature of hyalinizing clear cell carcinoma. Am J Surg Pathol. 2013;37:571–8.
Bastaki JM, Purgina BM, Dacic S, et al. Secretory myoepithelial carcinoma: a histologic and molecular survey and a proposed nomenclature for mucin producing signet ring tumors. Head Neck Pathol. 2014;8:250–60.
Antonescu CR, Zhang L, Shao SY, et al. Frequent PLAG1 gene rearrangements in skin and soft tissue myoepithelioma with ductal differentiation. Genes Chromosomes Cancer. 2013;52:675–82.
Martins C, Fonseca I, Roque L, et al. PLAG1 gene alterations in salivary gland pleomorphic adenoma and carcinoma ex-pleomorphic adenoma: a combined study using chromosome banding, in situ hybridization and immunocytochemistry. Mod Pathol. 2005;18:1048–55.
Author information
Authors and Affiliations
Corresponding author
Addendum
Addendum
Since the time of submission and acceptance of this manuscript, a series ov clear cell myoepithelial carcinomas arising in salivary gland and showing quite frequent EWSR1 gene rearrangement has been published—see Skalova et al., Am J Surg Pathol. 2015;39:338–348.
Rights and permissions
About this article
Cite this article
Jo, V.Y., Fletcher, C.D.M. Myoepithelial Neoplasms of Soft Tissue: An Updated Review of the Clinicopathologic, Immunophenotypic, and Genetic Features. Head and Neck Pathol 9, 32–38 (2015). https://doi.org/10.1007/s12105-015-0618-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12105-015-0618-0