Abstract
Neurological disorders are found to be influencing the peripheral tissues outside CNS. Recent developments in biomarkers for CNS have emerged with various diagnostic and therapeutic shortcomings. The role of central biomarkers including CSF-based and molecular imaging-based probes are still unclear for early diagnosis of major neurological diseases. Current trends show that early detection of neurodegenerative diseases with non-invasive methods is a major focus of researchers, and the development of biomarkers aiming peripheral tissues is in demand. Alzheimer’s and Parkinson’s diseases are known for the progressive loss in neural structures or functions, including the neural death. Various dysfunctions of metabolic and biochemical pathways are associated with early occurrence of neuro-disorders in peripheral tissues including skin, blood cells, and eyes. This article reviews the peripheral biomarkers explored for early detection of Alzheimer’s and Parkinson’s diseases including blood cells, skin fibroblast, proteomics, saliva, olfactory, stomach and colon, heart and peripheral nervous system, and others.

Graphical Abstract
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Despite various advancements in neurology in the last few decades, there are many mysteries in understanding the pathophysiology of several neurological diseases especially with the approach and possibilities of diagnosing and treating these abnormalities. Identifying novel biomarker for diagnosis, prognosis, and treatment is very important for neurodegenerative diseases (NDs). There are various key hurdles for identifying proximal biomarkers in neurological disorders including limited tissue availability from pathological site, requirement of lumbar puncture and cerebrospinal fluid (CSF) exploration, difficult to know biomarker sources in CNS disorders, low-quality clinical diagnosis, the complexity of brain tissue and functions, and absence of validation models. Peripheral biomarkers play an important role in solving these limitations and providing non-invasive alternate solutions for disease diagnosis. The exploration of biomarker models for early diagnosis is indeed an important goal for a better management of the neurological diseases. An ideal early diagnosis biomarker is supposed to be sensitive and specific to the initial neurological changes that occurs and should be able to differentiate among the diseased condition and normal aging conditions. Peripheral tissues are found to be indicators of cognitive and biological changes of brain and are supposed to differentiate the changes occurring during diseased conditions with normal conditions. This review article discuss about various peripheral biomarkers being discovered by researchers for early detection of Alzheimer’s disease (AD) and Parkinson’s disease (PD).
Alzheimer’s Disease
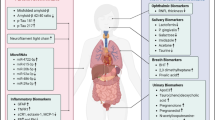
AD is considered the most common cause of dementia, and its medication costs around $172 billion per year in the USA alone. Twenty-four million people are supposed to be suffering from this disease presently, and the predication of it being four times is till year 2050 [1, 2]. There are three forms of AD. Mild cognitive impairment (MCI) is an early phase of AD and 40 to 60% is progressing toward AD. When AD occurs in young people of 30 years old and below, it is called as early onset or familial AD. It is associated with genes like amyloid β-protein precursor (AβPP), presenilin-1 (PS1), and presenilin-2 (PS2). The most common form is the last-onset AD and mostly affects elderly people of 65 years old and above [3, 4]. It is counted as incurable and fatal NDs. The clinical symptoms include progressively losing of memory, declining of learning and executive function. Histopathologically, AD is diagnosed by death of neurons in the hippocampus, cerebral cortex, and other areas of the brain, finding deposit of amyloid β (Aβ) plaques in extracellular and neurofibrillary tau tangles (NFT) in intracellular regions [5, 6]. Figure 1 provides an overview of the early symptoms and peripheral biomarkers for early detection of AD.
Blood Cells as Biomarkers
There are many reports finding abnormalities or early changes of red blood cells (RBCs), white blood cells (WBCs), and platelets in AD. Protein kinase C (PKC) plays an important role in synapse formation and is involve in the function of memory. Decreased PKC levels, its activity, and cellular localization have been found in the studies of AD patient brains [7]. PKC signaling pathways disrupts in AD patients and also in animal models [8].
In RBCs, alteration of PKC conformations is observed in both AD patients and healthy controls. Janoshazi et al. developed a method in 2006 which used fluorescence probe and spectrum to measure conformational changes of PKC. These conformational changes were not found in RBCs of PD patients who have no dementia [9]. Hye et al. in 2005 measured isoforms of glycogen synthase kinase-3 (GSK-3) protein and its activity in WBC of three groups which are divided as AD, MCI, and elderly normal control subjects (Fig. 2). In AD and MCI groups, GSK-3 activity was decreased but total GSK-3 protein was increased [10]. In the mitochondria of primary neurons, in transgenic mice which were expressing familial AD-linked forms of human APP, blocking GSK-3 activity by tau ablation can inhibit Aβ-induced transport deficits in anterograde axonal transport [11]. Soluble amyloid precursor protein alpha (sAPPalpha) also inhibits GSK-3 activity by blocking beta-site APP-converting enzyme 1 (BACE1). It can cause a reduction of tau and Aβ [12]. Investigation of GSK-3 activities might be a useful peripheral biomarker to diagnose early phase of AD.
Treatment with sAPPa decreases tau phosphorylation and increases GSK-3β (Ser9) phosphorylation in SH-SY5Y/BACE1 cells and HeLa/tau cells. SH-SY5Y/BACE1 cells (a, b) and HeLa/tau cells (c, d) were treated with sAPPa at indicated concentrations for 12 h. Cell lysates were prepared for WB analysis of both total and phosphorylated levels of tau and GSK-3β. a, c Phosphorylation status of tau was detected by anti-phospho-tau (p-tau (Thr231)) and PHF1 antibodies. Total tau (phosphorylated and non-phosphorylated) was detected by tau-46. b, d Phosphorylation status of GSK-3β (pGSK-3β (Ser9)) was detected by anti-phospho-GSK-3β (Ser9) antibody. e HeLa/tau cells were treated with BACE1 inhibitor LY2886721 at indicated concentrations (0, 12.5, 25, 50, 100, 200, and 400 nM) for 12 h and then cell lysates were prepared for WB analysis of both total and phosphorylated GSK-3β. These results indicated that the GSK-3β activity was dependent on BACE1 inhibition. In addition, BACE1 inhibitor dose-dependently induced inhibitory pGSK-3β (Ser9) phosphorylation in SH-SY5Y/BACE1 cells (date not shown). Densitometry analysis shows the band density ratio of p-tau (Thr231) to total tau and pGSK-3β (Ser9) to total GSK-3β as shown below each figure panel. WB results are representative of two independent experiments for pGSK-3β (Ser9) and total GSK-3β, and three experiments respectively for PHF1, p-tau (Thr231), and total tau. A t-test revealed a significant difference in pGSK-3β (Ser9) to total GSK-3β and p-tau to total tau ratios for both SH-SY5Y/BACE1 cells and human tau stable transfected Hela cells treated with either 6.25, 12.5, or 25 nM sAPPa compared with control (0 nM). In addition, a significant difference in pGSK-3β (Ser9) to total GSK-3β ratio was observed for HeLa/tau cells treated with either 200 or 400 nM BACE1 inhibitor compared with control (0 nM) (**P < 0.05; ***P < 0.001). Aβ40, 42 peptides were undetectable by Aβ ELISA of the conditioned media from either cell line with or without sAPPa treatment (data not shown). Republished figure with permission from [12]
Abnormalities on AD platelets are shown in Fig. 3 [13]. Tang et al. studied blood platelet levels targeting 22-kDa fragment in 10 age-matched healthy patients and 31 AD patients. In this study, both Aβ level and activation of BACE1 were increased but decreased APP ratios and decreased activation of ADAM10 were also observed [14]. Baskin group did the similar research and found a decreased APP ratio in AD patients who were normal in PD and hemorrhagic stroke (HS) patients [15]. APP isoform ratio of 120 to 110 kDa was significantly lower in carriers who have mutation for autosomal-dominant AD compared with non-carriers. In the caudate nucleus and precuneus, APP is inversely correlated with amyloid imaging [16]. Lower ratio of APP provides a potential peripheral biomarker for AD’s diagnosis, but further explorations are needed. Table 1 enlists the blood cells for peripheral biomarkers of AD.
Abnormalities on AD platelets. Some membrane (secretases, phospholipases), cytosolic (monoamine oxidase, cyclooxygenases), and mitochondrial activities (nitric oxide synthase, sodium potassium ATPase pump) are compromised in AD platelets. Alterations are evident in the APP processing itself, membrane fluidity, and cholesterol levels; in serotonin levels/uptake and intracellular Ca2+ levels; and in nitric oxide and peroxynitrite production. Abbreviations: PLCδ, phospholipase C δ; β, β-secretase; α, α-secretase; γ-secretase complex; APP forms, amyloid precursor protein forms; CaM, calmodulin; chl, cholesterol; MAO-B, monoamino-oxidase B; PLA2, phosholipase A2; COX-1, ciclooxigenase-1, COX-2, ciclooxigenase-2; 5HT, 5-hydroxytryptamine; NOS, nitric oxide synthase; NO nitric oxide; O2- superoxide anion; ONOO- peroxynitrite; Na+/K+-ATPase, sodium potassium ATPase pump; Ca2+, calcium. Adapted with permission from ref. [13]
MicroRNAs in the Plasma
MicroRNAs (miRNAs) are attractive molecules and considered one of the candidates for blood-based biomarkers in neurodegenerative diseases such as AD. MiRNAs are ~ 22 nt small non-coding RNAs that bind to the 3′-untranslated region of their target mRNAs to direct post-transcriptional repression of the target genes by forming the RNA-induced silencing complex, which leads to mRNA destabilization or translational inhibition [17, 18]. Some miRNAs are encapsulated in microvesicles, such as exosomes, and present as a relatively stable form in biofluid, including serum or plasma [19]. Downregulation of exosome miR-193b and upregulation of both exosomes miR-135a and miR-384 were observed in the serum of AD patients compared with normal patients. Among three miRNAs, miR-384 was the best candidate biomarker [20]. A significant upregulation of miR-455-3p expression in the serum, in the brains of AD patients, in the cerebral cortex of amyloid precursor protein transgenic mice, in the human and mouse neuroblastoma cells treated with the amyloid-β (1-42) peptide, revealed biomarker characteristics [21]. MiR-501-3p levels were downregulated in the serum of AD patients but remarkably upregulated in the brains of same donors and overexpression in the cultured cells (a human neuroblastoma cell line of SH-SY5Y cells). Serum-miR-501-3p is indicator that reflects pathological events occurring in AD patients’ brains [22]. Both miR-125b and miR-181c were downregulated in the serum of AD patients compared with that of normal controls [23]. MiR-125b is also decreased in the serum of AD patients as compared with non-inflammatory normal disease controls (NINDCs) (accuracy of 82%) [24]. Six candidate miRNAs (miR-9, miR-29a, miR-29b, miR-34a, miR-125b, and miR-146a) in plasma of AD patients and normal subjects were measured. Two plasma miR-34a and miR-146a levels in AD patients were significantly lower than in control subjects [25]. These miRNAs maybe useful for biomarker for AD diagnosis. Table 2 enlists the microRNAs as potential peripheral biomarker of AD.
Proteomics Approach for Biomarker
Amyloid plaques play a major role in the pathogenesis of AD. It is primarily composed of Aβ peptides which were derived from APP. In present time, diagnosis of AD is mainly based on clinical symptoms and neuroimaging. There is no gold standard central or peripheral biomarker to diagnose AD, and there is urgent need to develop promising biomarkers especially for the early-stage diagnosis. To know early stages of AD, measuring Aβ in plasma is an inexpensive as well as non-invasive method. But previous cross-section studies in humans show that plasma Aβ of AD patients is not much different from normal controls. More inconsistent plasma Aβ results were found in different mouse models. Longitudinal studies appear to be more promising than cross-section studies in both human and mouse models [26].
Before and during early stages of AD, plasma Aβ 40 and Aβ 42 were elevated in some patients but decline on the late stage of disease. Aβ 40 and Aβ 42 are strongly correlated and increasing with age. Increased level of plasma Aβ 42 is directly related with mortality in AD patients [27]. Other studies showed that both an increase and no change can occur in plasma Aβ 42 level in normal-aging human without dementia [28,29,30,31]. Decreased Aβ 42 level in CSF is related with the AD and disease progression, but variation in human plasma Aβ is inconsistent [32], especially in sporadic AD.
Ray and group did a study to find significant inflammatory biomarkers in AD patients and non-demented control subjects which found 18 significant inflammatory biomarkers with nearly 90% accuracy in examining 120 cell signaling plasma protein. It can also differentiate between AD patients and non-demented control subjects. The baseline amounts of these proteins can also be distinguished MCI patients and other different dementia pathologies by 81% accuracy. There could be modified links which cause significant changing of cell signaling proteins. These links should be explored more in future [33]. Two research groups had shown a diagnostic accuracy of 60–70% [34, 35], and it could be a potential peripheral biomarker.
Hye et al. in 2006 did a case-control study with an idea to identify different proteins in the plasma of two groups, 50 AD patients and 50 elderly control subjects by using 2D gel electrophoresis and mass spectrometry. A group of 511 people including AD, other NDs, and elderly normal control subjects were used for validation. Image analysis showed disease cases with 80% specificity and 56% sensitivity while analysis of mass spectrometry showed proteins involved in disease pathology. Elevated complement factor H (CFH) precursor and alpha-2-macroglobulin (alpha-2M) are specific for AD and severity. To improve sensitivity and specificity, other assays would be necessary but CFH, alpha-2M, and other proteins are potential peripheral biomarkers for diagnosis of AD [36].
Liao’s group, using 2-DE, found that spot densities of six plasma proteins in AD group and non-demented group were different. Plasma level of alpha-1-antitrypsin and apolipoprotein J were tested again because of their involvement in amyloid plaque formation. Although plasma α1 antitrypsin was more elevated in the AD group (77% sensitivity and 88% specificity) than non-demented group, no difference in total apolipoprotein J concentration was found [37]. Plasma proteomic analyses identified oxidized form of alpha-1-antitrypsin in AD patients [38, 39].
In a study, inflammatory biomarkers, plasma IL-6 was increased and, plasma tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) was decreased in AD group (n = 40) compared with normal control group (n = 40). These two biomarkers were significantly correlated with cerebrospinal fluid levels [40]. Table 3 enlists various proteomic studies of peripheral biomarkers for the diagnosis of AD.
Eyes as Peripheral Biomarkers
Lens
In the lens of rats and monkeys, Frederikse and colleagues found Alzheimer’s precursor protein (beta APP) and A-beta [41]. In 2002, they also found that Aβ deposition, defect in fiber cell membrane which is as similar as found in human cataract in mouse models of Down syndrome-carrying human AbetaPP (hAbetaPP) gene [42]. More lens cataract with enhanced Aβ immune-reactivity is detected in transgenic mice with AD beta-amyloid rather than wild-type mice [43]. Goldstein and colleagues in 2003 first discovered Aβ1–40 and Aβ1–42 in lenses of both AD patients and control patients [44]. The concentration of Aβ in lens can compare with amount of concentration found in the brain. Also, concentration of Aβ1–40 in primary aqueous humor is comparable with CSF.
In AD patients, increased deposits of electron-dense Aβ immunoreactive aggregates within lens fiber-cell cytoplasm at supranuclear sub region of lens and supranuclear cataracts were found but not in the control subjects [44]. Juliet Moncaster and co-workers analyzed lenses obtained patients from Down syndrome (DS), AD and normal controls. Same result such as Aβ aggregates within fiber cell cytoplasmic and opacification with increased Aβ deposition at supranuclear region were reported. These findings are identical to the lens pathology in AD [45]. In AD and DS, accumulation of Aβ shows linking between lens and brain pathology [44, 45]. These findings revealed Aβ accumulation as a key pathogenic determinant linking lens and brain pathology in both DS and AD [44, 45]. In 2013, Kerbage et al. did the study to detect Aβ deposit in the lens. They used specific fluorescent signature of ligand which bound Aβ in the supranucleus region of lens. And then it was detected by a laser scanning device. Figure 4 shows fluorescence decay rates for bound (red) and unbound (green) compound to Aβ peptide. They found two important results. In both AD and control groups, the highest measurement of ligand bound fluorescence signal was obtained from deeper regions of the supranucleus (SN). A twofold differentiation factor between control groups and AD patients was also noted [46].
Fluorescence decay rates for bound (red) and unbound (green) compound No. 11 to Aβ peptide. Increase in fluorescence intensity (δI ∼ 2×) and decay rates of bound (τ = 2.4 ns) compound No. 11 vs. unbound (τ = 1.4 ns) to Aβ. Fluorescence lifetime image of aggregated Aβ peptide in color-coded representation. Figure as originally published in ref. [46]
Surprisingly, three reports showed opposite result. Michael and colleagues reported that no beta-amyloid in lenses was found in cortical cataracts of both control and donors with AD [47]. Ho et al. reported that β-amyloid, phospho-tau, and α-synuclein are present in different forms at lower levels or do not deposit in the eye [48]. Although participants with AD biomarkers positive (positron emission tomography-Pittsburgh compound B (PET-PiB) and CSF levels of Aβ42) have cataracts and cortical light scattering more advanced than biomarker-negative participants, no one showed significant statistical data progressing to AD [49].
Retinal Nerve Fiber Layer
Bearish et al. studied peripapillary retinal nerve fiber layer (RNFL) in nine AD patients and eight age-matched control subjects by using optical coherence tomography (OCT). They found reduced retinal blood flow with narrow veins and significant thinning of RNFL in the superior quadrant. But RNFL thickness in inferior, temporal, or nasal parts showed no significant differences [50].
Three research [51,52,53] also reported RNFL thinning in the superior and inferior quadrant. Figure 5 shows spectral domain OCT of the RNFL in a patient with AD. Gao et al. revealed that RNFL thinning in the superior quadrant as well as RNFL thickness at 12:00 position in the superior quadrant [54]. There is no relationship between RNFL thickness and mini-mental state examination score, but decreased thickness of RNFL in all quadrants is found in the AD group compared with the control group. These findings suggest that analysis for RNFL thickness may be useful in diagnosis of early stage of AD [55].
Spectral domain optical coherence tomography of the retinal nerve fiber layer (RNFL) in a patient with Alzheimer’s disease. The RNFL of the superior quadrant is reduced to the 0–5 percentiles for age (red), but the nasal, temporal, and inferior quadrants are normal (green). Adapted with permission from ref. [51]
Retinal Vessels
For retinal vascular abnormalities in AD, a study reported thinning of blood column diameter with decreased blood flow in major superior temporal venule. Laser Doppler device was used to measure these parameters [50]. Studying of the retina from AD transgenic mice revealed neuro-inflammation, elevated microvascular Aβ deposition, Aβ plaques, and hyperphosphorylated tau. Immunotherapy for Aβ showed retina plaques are clear but it worsens in retinal amyloid angiopathy. So, investigations such as non-invasive retinal imaging are good technique to know early diagnosis and severity of AD [56, 57]. Thirteen significant retinal vascular parameters were found in AD (n = 25) and healthy controls (n = 123). The method was that 19 vascular calibers were calculated by retinal photography in largest six arterioles and six venules (see more in [58]). Retinal photography together with vascular analysis could be a potential biomarker to detect early AD.
Retinal Ganglion Cells
Blanks et al. in 1989 showed degeneration of retinal ganglion cells (RGCs) in AD patients. The degeneration is unique due to finding of frothy and vacuolated appearance in the cytoplasm of RGCs, but no neurofibrillary tangles were found. In all examined AD patients, amyloid angiopathy is found but none of neuritic plaques is found in optic nerves or retinas [59]. In 1996, they found a decrease of 25% in total neurons in the ganglion cell layer of the central retina of AD patients (n = 9) compared with age-matched control subjects (n = 11) [60]. Retinal ganglion cell loss is also found in the retinas of the AD patients [61].
Williams et al. found that the dendritic integrity of RGCs was significantly decreased without RGC loss in Tg2576 mice in comparison with age-matched wild-type controls. RGC dendrites are confined to the inner plexiform layer of the retina, imaging techniques that focus on this layer, rather than the loss of RGCs, may provide a sensitive biomarker for monitoring neural damage in AD [62]. Another study by Lu et al. found that RGCs of the APP/PS1 double transgenic mice with AD group was comparatively much less than the control group [63]. Targeting RGCs combined with imaging techniques need to be explored more in future to diagnose early phase of AD [61,62,63].
Biomarkers for Skin Fibroblasts
Extracellular-Signal-Regulated Kinases (Erk1/2)
Zhao’s group in 2002 did a research study and found that abnormal phosphorylation of Erk1 and Erk2 was induced by bradykinin (BK) in cultured skin fibroblasts of AD patients compared with normal control subjects [64]. In 2004, Veeranna found that in the brain of AD patients, there is an association between phosphorylated Erk1, Erk2, and activated calpains. They found that in AD patients’ brain, intensities of phosphorylated Erk1 and Erk2 band is relatively increased but not in the brains of control subjects [65].
A research by Khan and Alkon in 2006 revealed that internally controlled AD-specific different phosphorylation ratio of Erk1 and Erk2 caused by bradykinin, a small nanopeptide which is PKC activator and natural inflammatory agonist [66]. It induced PKC-mediated Erk1 and Erk2 phosphorylation in two different types of human skin fibroblasts, fresh-taken skin fibroblasts, and banked skin fibroblasts. Skin fibroblasts samples were obtained by punch biopsy from total 264 cases including AD group, non-AD group, and age-matched control group. AD-Biomarker Index (AD-Index) was used to calculate quantitative imaging of the phosphorylated Erk1 and Erk2 bands. AD-Index positive was considered having AD and zero or negative as non-AD dementia. In 42 autopsy-confirmed cases out of total 264 cases, clinical diagnosis for AD had 78% sensitivity and 20% specificity while AD-biomarker diagnosis had 97% sensitivity and 100% specificity (overall accuracy was 98%). For validation, AD-biomarker diagnosis accuracy was 100% for dementia cases with duration no longer than 4 years while clinical diagnosis was only 52% accurate. The pathophysiologic relevance of AD-biomarker measurement was also tested by examining Aβ1–42-induced abnormal AD-biomarker phenotype [67]. Therefore, Erk1/2 phosphorylation of skin fibroblasts could have important clinical utility such as for increasing diagnostic certainty in early phase of the AD.
Calcium
Altered Ca2+ homeostasis is found in brain of the AD patients and in the peripheral tissue. The first report by Peterson et al. in 1985 demonstrated that decreased Ca2+ uptake is found in human skin fibroblasts of AD patients compared with age-matched controls [68].
In cultured skin fibroblasts from aging and AD patients, 70 and 81% decline of cytosolic-free calcium had been reported when compared with fibroblasts from age-matched controls and young adult donors [69]. In the fibroblast cells of aging patients and AD donors, various drugs treatment (serum, 3,4-diaminopyridine, N-formyl-methionyl-leucyl-phenylalanine, and bradykinin) can transiently elevate cytosolic-free calcium, but it is slower and lesser in young patients. Alteration of both dynamic and resting calcium homeostasis has been found in cultured skin fibroblasts from aging patients and AD donor [70]. There was more alteration of calcium homeostasis and mitochondrial functions in AD than normal aging [71]. In mitochondria of skin fibroblasts from histologically confirmed AD patients, calcium uptake is less than in mitochondria of fibroblasts from age matched controls. In mitochondria of AD patient, more calcium uptake than controls was seen after exposing with free radial.
Treating with U-74500A, the antioxidant drug or deferoxamine, iron chelator, prevents free radial, inducing increased calcium uptake of control mitochondria and it only protects partially in Alzheimer’s mitochondria. It showed that in the mitochondria of Alzheimer’s fibroblast cells, calcium transport processes is impaired and sensitivity for oxygenic free radicals is increased [72]. But one research group in 1992 reported that ionic calcium levels in cytoplasm of Alzheimer’s fibroblast cell have no pathological or diagnostic value [73]. Because of this result, several research groups focuses on different methods for Ca2+ to be used as a peripheral biomarker. Etcheberrigaray et al. in 1994 reported that K+ channel blocker TEA can increase intracellular Ca2+ in normal skin fibroblasts but response to TEA stimulation is low in cells of few familial AD cases and sporadic AD cases [74].
Low-dose Bradykinin can induce intracellular Ca2+ release by IP3 generation, activating phospholipase C and evokes enhanced Ca2+ signaling in AD fibroblasts [75, 76]. Because of these findings, response of Ca2+ in skin fibroblasts after stimulation with TEA or bradykinin was measured by using standard Ca2+ fluorescence imaging techniques. The biochemical response was reported as the ratio of percent response after TEA stimulation and bradykinin stimulation [75, 77]. Targeting Ca+ homeostasis and Ca+ signaling in the skin fibroblast could be a potential peripheral biomarker for AD. Table 4 enlists the skin fibroblasts for peripheral biomarkers of AD.
Parkinson’s Disease
PD is a chronic progressive and second most common ND. It is a chronic and progressive movement disorder and mostly occurs in elderly people of 60 years and above. It is characterized by a significant reduction of dopaminergic neurons in the substantia nigra together with physical signs (rigidity of muscle on passive movement, instability in posture, resting tremor and akinesia). Currently, there are no definitive diagnosis criteria or biomarkers [78, 79]. Clinical signs and symptoms and post mortem examination are the criteria to diagnose PD. Specific, sensitive, and economical central or peripheral biomarkers are needed to diagnose early stage of disease, severity, differential diagnosis, prognosis, and treatment of PD. In the following sections. peripheral biomarkers explored for early diagnosis of PD are discussed. Figure 6 provides an overview of the early symptoms and peripheral biomarkers for early detection of PD.
α-Synuclein in Blood
Histologically, PD is characterized by the presence of inclusions known as Lewy bodies, which is a composition of α-synuclein, in the intracytoplasmic, degeneration, and reduction of dopaminergic neurons in the substantia nigra of brain. It is also found in other parts of the brain such as the thalamus, hippocampus, neocortex, and cerebellum [80, 81]. It is an unfolded protein, it is soluble, and polymerization of α-synuclein becomes fibrils. Accumulation of fibrils found inclusions known as glial cytoplasmic inclusions, Lewy neuritis, and bodies. It is a highly acidic, heat-stable amino acid compound (140 amino acid; 14 kDa) [82,83,84]. Its filaments are found in many NDs. The role of α-synuclein in blood and peripheral tissue in PD is discussed.
Initially, α-synuclein was considered an intracellular protein, but identification in both CSF and plasma is the evidence that it is secreted from cells. An effort for α-synuclein in CSF as a diagnostic biomarker have been underway and found that total α-synuclein in CSF is lower in PD patients than healthy control subjects [85, 86]. Figure 7 shows CSF α-synuclein concentrations in study groups [85]. α-Synuclein protein (alphaS) aggregates from a monomer to assemblies such as oligomers, protofibrils, and mature fibrils. While the alphaS concentration in CSF is significantly decreased in patients with PD, alphaS oligomer concentration was elevated in CSF and in the blood of PD patients. It showed that alphaS in the CSF and in the blood could be a peripheral biomarker for PD [87].
CSF α-synuclein concentrations in study groups. a Scatter plot for CSF α-synuclein values from patients with early Parkinson’s disease and healthy subjects, as measured by two different and independently operated ELISA systems (ELISA 1 and 2). Concentrations measured by ELISA 1 and 2 correlated positively by Pearson’s analysis (0.229; p = 0.01). b Graphs in whisker-box plot format summarize results for the total CSF α-synuclein concentration (pg/ml) shown in (a). HC, healthy controls; PD, Parkinson’s disease. Adapted with permission from ref. [85]
Three research groups tried to measure total α-synuclein [88], phosphorylated α-synuclein [89], and oligomeric forms of α-synuclein [90]. Total α-synuclein was measured by Tinsley and colleagues. They developed a new ELISA specifically for quantifying α-synuclein in human plasma using a novel α-synuclein-specific antibody that has very high sensitivity and signal. Although the study was done on small cohort of patients and control subjects, it is promising and has potential as peripheral biomarker [88].
Alpha synuclein can be phosphorylated at serine129 (P-S129) by kinase(s). The presence of highly phosphorylated α-synuclein in Lewy bodies suggests that it has an important pathological role. In healthy brain, only 4% phosphorylated alpha-synuclein is found while approximately 90% has been observed in the brains of PD patients. Phosphorylated alpha-synuclein is toxic to dopaminergic cells. Significant elevation of P-S129 α-synuclein leads to accumulation and increased cell death [91,92,93].
One research group developed a novel ELISA method that detects only oligomeric α-synuclein soluble aggregates. They found significantly elevated plasma levels of α-synuclein oligomeric forms (P = 0.002) which were obtained from 34 PD patients compared with 27 control subjects (specificity of 0.852 and sensitivity of 0.529) [90]. The mean plasma level of phospho-α-synuclein was higher than the controls (P = 0.053). It is not different for oligo-α-synuclein, total α-synuclein, or oligo-phospho-α-synuclein from 38 PD patients and 30 healthy control participants [89]. Studying for various forms of α-synuclein is still underway, although the results are promising and have potential to be the peripheral biomarkers.
α-Synuclein in Peripheral Tissue
Skin Samples
Michell et al. in 2005 reported that a low rate of α-synuclein (19%) was detected in skin biopsy samples of 16 PD patients and concluded that diagnosis of PD and its severity is not related with α-synuclein level. The results showed that skin α-synuclein would not be an appropriate diagnostic biomarker for PD [94]. But phosphorylated α-synuclein was found in the cervical skin site of all patients with Idiopathic PD (IPD) but not in the controls. This result significantly distinguishes IPD from other forms of parkinsonism. It may be useful as a biomarker especially for differential diagnosis [95].
Saliva and Salivary Gland
A research group tried to find biomarker for PD in saliva. The result showed significantly low α-synuclein level and a specific protein in the saliva of PD patients. The measurement of α-synuclein in the saliva have more advantage than other biological fluids such as plasma and blood because it can be obtained easily and with less contamination than blood [96].
Studying tissue sections of submandibular glands from patients with PD (n = 9), Lewy body disease (n = 3), multiple system atrophy (n = 2), and control subjects (n = 19) using α-synuclein immunohistochemistry revealed that Lewy body pathology in all PD patients but not in patients with multiple system atrophy and controls [97].
In another study, they used the same salivary gland with a different method. From submandibular gland of living patients, needle core biopsies are obtained and found a Lewy-type α-synucleinopathy in 75% of cases [98] (Fig. 8). α-Nuclein was detected in the labial salivary glands of both in PD cases (60%) and in control cases (30%) [99]. Saliva and salivary gland alone is not a diagnosis biomarker but in combination with other tests can be a potential biomarker.
Photomicrographs of needle core tissue from submandibular gland biopsies of subjects with Parkinson’s disease. Sections were stained with an immunohistochemical method for phosphorylated α-synuclein (see “Methods”). Only structures immunoreactive for phosphorylated α-synuclein (black) and morphologically consistent with nervous tissue were considered to represent a positive biopsy finding. Sections were counterstained with neutral red. a Typical needle core tissue sample. b Single immunoreactive nerve fiber within a nerve fascicle running in connective tissue stroma adjacent to glandular parenchyma. c Single immunoreactive nerve fiber within a stromal nerve fascicle. d Immunoreactive puncta within the adventitia of a small artery in the stroma. e Several immunoreactive nerve fibers running beneath duct epithelium. f Immunoreactive nerve fibers interweaving among parenchymal serous gland cells. g Non-specific staining (black) of hair follicle within a fragment of skin. h Non-specific staining (black) of the edges of muscle fibers. Adapted with permission from ref. [98]
Olfactory
In neurological disorders including PD, a common feature is the olfactory dysfunction. Duda et al. [100] used antibodies to detect α- and β-synucleins. They found abnormal dystrophic neurites in the olfactory epithelium of both, PD patients and controls. In the olfactory mucosa, the most abundant synuclein is the α-synuclein. It is most significantly found in olfactory receptor neurons [100]. Lewy-type α-synucleinopathy (LTS) involvement in the olfactory bulb occurs in an early stage of PD. Beach and colleagues in 2009 reported that in PD, sensitivity is 95% and specificity is 91% compared with elderly control [101]. But one report in the same year found that in the olfactory epithelium of both PD patients and healthy control subjects, the expression or distribution of α-synuclein showed no histochemical differences [102].
Stomach and Colon
Lebouvier’s group, in 2008 paper reported that 80% of ascending colon biopsies from submucosa of PD patients showed positive staining for phosphorylated α-synuclein-immunoreactive neurites [103].
In 2010, they found Lewy pathology in the colonic biopsies of PD patients (72%) which are obtained from submucosal plexus of ascending and descending colon but not in the control subjects [104]. Pouclet and co-workers in 2012 demonstrated that 55% of patients with PD have Lewy pathology in the mucosa and submucosa but not in the control subjects [105]. Lewy neurites were found by same group in the colonic biopsies from submucosa of rectal, ascending and descending colon in PD patients (23 to 65%) but not in controls [106].
Biopsies taken from distal sigmoid colon by flexible sigmoidoscopy from patients with early stage of PD (n = 7), control subjects (n = 23), and subjects with inflammatory bowel disease (n = 23). All PD patients (100%) showed α-synuclein staining in nerve fibers in colonic submucosa but not in the control samples as shown in Fig. 9 [107]. α-Synuclein inclusions were also detected in the gastric mucosa [108]. In vivo evidence of α-synuclein in peripheral tissue with underlying PD may be useful and it can be a safe tool providing development of peripheral biomarkers.
αSYN immunohistochemistry of colon at × 40 magnification (a, c, e) and × 120 magnification (b, d, f). Nerve fiber staining was seen in PD subjects (a, b). Some subjects with inflammatory bowel disease showed a pattern of immunostaining in round cells of unknown origin (c, d). Controls and most subjects with inflammatory bowel disease showed no α-synuclein immunostaining (e, f). Scale bar in (e) and (f) represents 50 and 20 μm and applies to (a, c, e) and (b, d, f), respectively. Adapted with permission from ref. [107]
Heart and Peripheral Nervous System
Both sympathetic nerve and heart tissue from 11 PD patients and 7 incidental Lewy body disease patients (iLBD) were examined. Results showed that Lewy bodies and α-synuclein were found in the neurites in PD patients (9 out of 11) and 7 iLBD patients (100%) [109].
Phosphorylated α-synuclein aggregates were found in the cardiac sympathetic nervous system in 90% of iLBD cases and 60% of PD cases, but no phosphorylated α-synuclein is found in control subjects [110]. α-Synuclein positive neurites were also found in epicardium in PD cases (n = 14) and iLBD cases (n = 11), but not in normal controls (n = 4) [111].
Biopsies from gastric myenteric and submucosal plexuses were taken from five autopsy cases. The brains of the autopsy cases also staged for Parkinson-associated synucleinopath and immunocytochemistry was done. In neurons of the submucosal Meissner plexus, α-synuclein immunoreactive inclusions were observed [112].
Biopsies from pharyngeal sensory nerves from autopsy bodies of PD (n = 10) and controls (n = 4) was taken by Mu et al. to detect Lewy pathology and immunohistochemistry for phosphorylated α-synuclein. Axonal α-synuclein aggregates were found 100% in PD cases, but no α-synuclein aggregates were found in control cases [113]. Table 5 enlists α-synuclein in peripheral tissue.
Other Biomarkers from Blood
DJ-1
DJ-1 mutations are associated with PD, both familial and sporadic types. DJ-1 ELISA kit was used to detect DJ-1 in serum of both PD patients and control subjects. Two research groups in 2003 and 2008 reported that secreted DJ-1 level is not only different in two groups but also there is no association with age and severity of PD. Moreover, there is no correlation with level of oxidative stress [114, 115].
In 2010, Hong’s group did a research in PD patients (n = 117), AD patients (n = 50), and healthy controls (n = 50) by using highly sensitive Luminex technology. They found that contamination of blood in CSF also influenced the level of DJ-1. When several major confounders were controlled to control blood contamination, the result showed that DJ-1 is decreased in PD vs. controls or AD. There was no correlation between severity of PD and level of DJ-1 [116].
In the same year, one research group reported the similar result. There was no correlation between severity of PD and levels of DJ-1. DJ-1 level was decreased (more than 95% in RBCs, 1 to 4% in platelets and 1% or less than 1% in WBCs and plasma). It showed that platelet contamination and hemolysis could be significant factors affecting DJ-1 level in serum or plasma and blood. The results showed that DJ-1 in blood is not specific biomarker to diagnose, to know progression and severity of PD [117].
After this setback, researchers are trying to focus on the total DJ-1 and its isoforms. In a study of a group of 119 people including control subjects, AD patients and patients with different PD stages revealed 7 DJ-1 isoforms, 4 major post-translationally modified (PTMs), and no significance in the level of total DJ-1 in blood samples of control subjects and PD patients. Four major PTMs are methionine oxidation, cysteine oxidation, phosphorylation, and 4-hydroxy-2-nonenal (HNE) adduction. Among four PTMs, only HNE-modified DJ-1 isoforms is decreased in control subjects, AD patients, and early-stage PD patients while it is increased in late-stage PD patients. A validation study for 114 people was done, and the result was confirmed. Because PTM isoforms are candidate biomarkers only for late-stage PD [118], more studies are needed to identify PTM isoforms to diagnose early-stage PD.
Uric Acid
Serum urate is found to reduce the risk of PD. It is derived from purine and has potent antioxidant action. Church and Ward’s group in 1994 reported that in the brain of PD patients, at substantia nigra region, uric acid (54%) and dopamine (85%) levels were significantly reduced [119]. Davis and colleagues did the hypothesis among men. From 1965 to 1968, they measured serum uric acid level in 7968 men. From that group of study, 92 men suffered IPD. Forty percent reduction in IPD incidence was found in men whose uric acid concentration was above the median at the time of measurement [120]. Two groups found that serum uric acid level is inversely related with PD [121, 122] and gout is inversely related with risk of PD [123]. In men, a higher dietary urate index and high plasma urate concentration were correlated with lower risk of PD [124]. But one report revealed that in women, urate is not strongly associated with lower risk of PD [125]. Urate has neuro-protectant, antioxidant, metal-complexing properties, and higher urate level is related with lower risk of PD. Urate/uric acid can be identified as a promising biomarker of the PD [126, 127].
Epidermal Growth Factor
In most of the PD patients, cognitive impairment is developed together with PD. From a group of 70 PD patients, baseline levels of different proteins (n = 102) in the plasma determined by using a bead-based immunoassay. Cognitive status at baseline and cognitive status at annual follow-up were determined by Mattis Dementia Rating Scale-2 (DRS). Among 11 proteins identified, epidermal growth factor (EGF) was the best candidate. If baseline cognitive test scores are poor, it is associated with low levels of EGF. For people with intact baseline cognition, cognitive decline to DRS is eightfold greater risk at follow-up. Separate study of 113 PD patients was also done using another technical platform to know relation between levels of the top biomarker and cognitive performance. They found that a weaker result than previous 70 PD patients study group, but there was significant correlation between cognitive performance and EGF plasma level [128].
Same study to assess cognitive functions and EGF in serum was done in a group of 65 drug-naive PD patients. Baseline and 2-year follow-up study showed that cognitive functions of the frontal and temporal portion of PD patients are correlated with serum EGF levels and performance on posterior and frontal cognitive function. Although EGF is a candidate biomarker for early cognitive impairment in PD, further studies are needed to be a diagnosis marker for early stage of PD [129].
Apolipoprotein A-I
The role of biomarkers may improve diagnosis for NDs, but none of the biomarker is a definitive biomarker to diagnose early stage of PD. Many studies have shown decreased levels of apolipoprotein A-I (ApoA-I) in CSF and serum in PD patients compared with healthy controls [130,131,132,133].
To identify proteins which were associated with PD, 96 proteins were measured by multiplex immunoassay in a group of 152 PD patients. Second study was to identify top candidate biomarkers and its plasma levels in 187 PD patients by ELISA method. Third study is to confirm the association between top biomarker and PD in the group of 134 people who are at risk but asymptomatic. To evaluate the association of dopaminergic system integrity and plasma protein levels, they used dopamine transporter (DAT) imaging, and the best candidate protein biomarker is apolipoprotein A1. Early onset of PD is correlated with low levels of ApoA1 [130, 131]. DAT deficit in putamen is also related with lower plasma levels of ApoA1 [130]. Figure 10 describes ApoA1 as a biomarker for age at PD onset. A higher ApoA1 level has relative protection against risk of PD, and drug such as statin can modify Apo-A1 level. Continuous use of statin is associated with decreased PD risk [134] while stopping using of statin is associated with PD [135].
Apolipoprotein A1 (ApoA1) as a biomarker for age at Parkinson’s disease (PD) onset. a Low ApoA1 levels are predictive of early age at PD onset in the discovery cohort. Survival curves of time to PD onset are shown here for the lowest tertile of ApoA1 plasma levels (red line), middle tertile (yellow line), and highest tertile (green line), as measured by multiplex immunoassay. Outcomes for age at PD onset between the different tertiles were significantly different (p < 0.001, hazard ratio = 0.742, 95% confidence interval = 0.606–0.909), after adjustment for age at plasma draw and gender. b Number of individuals (N) and ApoA1 plasma values (median and full range in mg/ml) for each tertile are listed. ***p value < 0.001. c High-density lipoprotein (HDL) levels (mg/dl) are shown on the y-axis; ApoA1 levels (mg/ml) are shown on the x-axis. HDL levels correlate well with immunoassay ApoA1 values, as reflected by an R2 of 0.79. d In contrast with HDL levels, total cholesterol levels do not correlate with ApoA1 levels. Total cholesterol levels (mg/dl) are shown on the y-axis; ApoA1 levels (mg/ml) are shown on the x-axis. Adapted with permission from ref. [130]
MicroRNAs
MicroRNAs (miRNAs) are non-protein coding transcripts that post-transcriptionally regulate gene expression during development. RNAs encapsulated in exosome-like microvesicles in serum were extracted analyzed by quantitative reverse transcription polymerase chain reaction (qRT-PCR). Results showed a downregulation of miR-19b and upregulation of miR-195 and miR-24 in PD patients when compared with normal patients [136]. Sixteen serum miRNAs were measured in PD patients and control subjects. Four miRNAs (miR-29c, miR-146a, miR-214, and miR-221) were significantly downregulated in PD patients, and miR-221 is the most promising biomarker [137]. Other study showed that four serum miRNAs (miR-141, miR-214, miR-146b-5p, and miR-193a-3p) were significantly decreased [138]. Analysis of peripheral blood from PD patients and control subjects identified six differentially expressed miRNAs. MiR-1, miR-22, and miR-29 expression levels allowed distinguishing non-treated PD from healthy subjects while miR-16-2, miR-26a2, and miR30a differentiated treated PD patients from untreated patients [139]. Studying of plasma by another research group identified seven overexpressed miRNAs (miR-181c, miR-331-5p, miR-193a-3p, miR-196b, miR-454, miR-125a-3p, and miR-137) [140]. These microRNAs may represent novel biomarkers for the early detection and diagnosis of PD. Table 6 enlists the MicroRNAs as potential peripheral biomarker for PD,
Eyes as Potential Biomarker
As unmyelinated axons from retina are directly synapsing into the central nervous system, retina is considered a prominent environment to study the neurological diseases. Pathologic changes are identified in the RNFL of the eye and associated with neurological conditions. Because of it, eyes are the unique environment to find a surrogate biomarker for early stages of neurodegeneration.
OCT is a non-invasive tool used for measuring tissue at micro meter resolution [141]. The parapapillary RNFL represents axons of the ganglion cells and a thinning of this nerve in PD was first reported by Inzelberg’s group in 2004 (Fig. 11) [143]. Later several other research groups found the same result [144,144,145,147].
Retinal nerve fiber layer analysis by OCT in a patient with PD and a healthy control. a Healthy control. b Parkinson’s disease patient. The OCT report compares current analysis with the baseline evaluation (5 years prior). The graphic on the right side of each report shows marked changes (thinning) in the RNFL. Compared with the healthy control, the patient shows progressive RNFL loss in the superior, nasal, and inferior sectors of the optic nerve, marked with an asterisk. Republished figure with permission from [142]
Both eyes from 42 untreated newly diagnosed PD patients and 40 healthy controls were examined by using spectral-domain OCT. They found that the mean RNFL thickness is less in PD patients when compared with healthy controls. Selective thinning of the RNFL was observed in temporal region of both PD patients and controls [148]. Jimenez’s group in 2014 did the same research in larger group. They found that peripapillary RNFL thickness gradually decreased while evolution and severity of PD progress [149, 150]. Retinal dopamine loss, foveal dysfunction, and visual hallucination (VH) are noted in PD patients [150]. Although one paper in 2011 reported that there was no difference of RNFL thickness in PD patients (n = 51) and healthy control subjects (n = 25) [151], other research groups demonstrated the gradual diminishing of RNFL thickness over time together with validating OCT as potential research to find a diagnosis biomarker.
Discussion
During the early research, it was believed that neurological diseases are associated with brain only, but further, through the discovery of various biomarkers, it is proved to be also affecting the peripheral tissues. For AD, amyloid beta, the main pathological hallmark, is found in the brain as well as peripheral tissues. Decreased PKC level, alteration of PKC conformation in RBCs [7, 8], increased GSK-3 protein in WBCs [10], and reduced APP ratios in platelets [14] were found in the AD patients. Finding plasma Aβ in cross-sectional studies of human and mouse showed no significance, but longitudinal studies were more promising [26]. Other studies showed both increasing and decreasing of Aβ in AD patients [29,30,31]. Moreover, changes in human plasma Aβ is inconsistent, especially in sporadic AD [32].
CFH precursor and alpha-2-macroglobulin (alpha-2M) elevation are specific for AD and correlated with disease severity. But alternative assays would be necessary to improve sensitivity and specificity [36]. Alpha-1-antitrypsin and apolipoprotein J were involved in AD amyloid plaque formation. By validation with either ELISA or Western blot, plasma concentration of Alpha-1-antitrypsin showed a difference between AD patients and controls but no difference for apolipoprotein J [38, 39]. Other plasma proteins were also investigated as potential biomarkers (see more in [152]), but targeting plasma proteins as peripheral biomarkers are not promising at the current moment.
Aβ deposition is found in supranuclear region of the lens together with Aβ aggregates in cytoplasm of the lens fiber cell [45, 46]. But other researchers reported that no Aβ and only lower levels in different forms are present in the lens of AD patients and in controls with cortical cataract [47, 48]. So different groups reported different results, and more research are needed to use lens as a peripheral biomarker.
Narrow retinal veins, thinning of the major superior temporal venule blood column diameter, and thinning of RNFL at superior quadrant are found in AD patients [50,51,52,53,54]. Results for RNFL, retina artioles, and veins are promising, and further investigations are needed. Degeneration of RGCs [59], reduced number of the RGCs, and reduced dendritic integrity of RGCs were found in both AD patients and transgenic mice [61,62,63]. Decreased numbers of neurons in the ganglion cell layer (GCL) of the central retina (fovea/foveola/parafoveal retina) in the eyes of AD patients were also observed [153]. Targeting RGCs combined with imaging techniques could be a potential peripheral biomarker for AD in the future [61,62,63, 153].
Erk1/2 phosphorylation in skin fibroblasts has higher sensitivity and specificity for AD than clinical diagnosis [64,65,66,67]. Dynamic and resting calcium homeostasis is altered in skin fibroblasts of aged and AD donors [70]. Alzheimer’s fibroblast mitochondria are found to be impairing calcium transport process and increasing the sensitivity to oxygenic free radicals [72]. Targeting Ca+ homeostasis and Ca+ signaling in the skin fibroblast showed promising results but still need more tests to be used as a peripheral biomarker.
AlphaS are found in both CSF and blood with different forms such as α-synuclein, phosphorylated α-synuclein, and oligomeric forms of α-synuclein. Reports showed different results. Studying for various forms of alpha-synuclein is still underway, but the results are promising [87,88,89,90]. α-Synuclein results in skin samples are not promising. One research group [94] reported that α-synuclein in skin samples has no diagnosis value while another research group [95] reported that it can distinguish between IPD and other forms of parkinsonism.
Low α-synuclein level and a specific protein was detected in saliva of Parkinson’s patients with an extinction coefficient of 27.25 M cm−1 [96]. Targeting salivary gland alone is not a diagnosis biomarker but in combination with other tests can be a potential biomarker [97,98,99]. Although α-synuclein is most abundant synuclein in olfactory mucosa, reports for α-synuclein in olfactory mucosa are controversial, showing positive and negative results [100,101,102].
α-Synuclein is found in submucosa of descending, ascending, rectal, and sigmoid colon [104,105,106,107,108]. Lewy bodies and α-synuclein are found in heart tissue, epicardium, cardiac sympathetic nervous system, submucosal Meissner plexus, and pharyngeal sensory nerves. The results are promising, but all tests are invasive and stressful for patients [109,110,111,112,113].
Total DJ-1 or α-synuclein in plasma alone is not useful as biomarkers for PD diagnosis or progression [117], but only one PTM isoform of DJ-1 is a candidate biomarker to know late stage of PD [118].
UA is decreased in nigrostriatal dopamine neurons in parkinsonian patients [119] and high serum levels of uric acid were linked with significant decrease in risk of PD. Urate/uric acid can be identified as a promising biomarker of the PD [121, 122, 125, 126]. Significant relationship between EGF and cognitive impairment in PD was observed [128, 129]. Low-plasma Apo-A1 is associated with earlier PD onset and greater putaminal DAT deficit [130, 131]. The thickness of the RNFL in PD, as evaluated by OCT, gradually diminishes if the evolution and severity of PD progress [149, 150], but one research showed no difference [151]. The availability of different studies with the same biomarkers shows sometime conflicting results, and the cause of these discrepancies can be related to limitations in standardization of these biomarkers, variation in laboratory conditions, interpretation of experimental data, procedure for acquisition and analysis of samples, varying severities of abnormalities conferring different prognoses, the selection of study group, period of observation or prediction, and many other factors [154]. With the advancements in researches of peripheral biomarkers for early detection of NDs, although the developments are not perfectly reliable and sensitive, further studies will explore the potentials.
Conclusion
The present review article comprehensively highlighted the significant research models being explored for early detection of Alzheimer’s and Parkinson’s diseases as peripheral biomarkers. It can be conferred that there are very few definitive peripheral biomarker present till the current research scenario. As reliable diagnosis of neurodegenerative diseases are still based on clinical symptoms, post-mortem result, and immature molecular imaging due to limited capabilities of central biomarkers, the discovery of early, sensitive, specific, and economically effective peripheral biomarker for definite diagnosis, prognosis, and treatment of Alzheimer’s and Parkinson’s diseases is the requirement of the near future.
References
Reitz C, Mayeux R (2014) Alzheimer disease: epidemiology, diagnostic criteria, risk factors and biomarkers. Biochem Pharmacol 88(4):640–651. https://doi.org/10.1016/j.bcp.2013.12.024
Brookmeyer R, Johnson E, Ziegler-Graham K, Arrighi HM (2007) Forecasting the global burden of Alzheimer’s disease. Alzheimers Dement 3(3):186–191. https://doi.org/10.1016/j.jalz.2007.04.381
Gatz M, Reynolds CA, Fratiglioni L, Johansson B, Mortimer JA, Berg S, Fiske A, Pedersen NL (2006) Role of genes and environments for explaining Alzheimer disease. Arch Gen Psychiatry 63(2):168–174. https://doi.org/10.1001/archpsyc.63.2.168
Petersen RC, Stevens JC, Ganguli M, Tangalos EG, Cummings JL, DeKosky ST (2001) Practice parameter: early detection of dementia: mild cognitive impairment (an evidence-based review). Report of the Quality Standards Subcommittee of the American Academy of Neurology. Arch Neurology 56(9):1133–1142
Tiraboschi P, Hansen LA, Thal LJ, Corey-Bloom J (2004) The importance of neuritic plaques and tangles to the development and evolution of AD. Neurology 62(11):1984–1989
Brookmeyer R, Gray S, Kawas C (1998) Projections of Alzheimer’s disease in the United States and the public health impact of delaying disease onset. Am J Public Health 88(9):1337–1342
Pascale A, Amadio M, Govoni S, Battaini F (2007) The aging brain, a key target for the future: the protein kinase C involvement. Pharmacol Res 55(6):560–569. https://doi.org/10.1016/j.phrs.2007.04.013
Alkon DL, Sun MK, Nelson TJ (2007) PKC signaling deficits: a mechanistic hypothesis for the origins of Alzheimer’s disease. Trends Pharmacol Sci 28(2):51–60. https://doi.org/10.1016/j.tips.2006.12.002
Janoshazi A, Sellal F, Marescaux C, Danion JM, Warter JM, de Barry J (2006) Alteration of protein kinase C conformation in red blood cells: a potential marker for Alzheimer’s disease but not for Parkinson’s disease. Neurobiol Aging 27(2):245–251. https://doi.org/10.1016/j.neurobiolaging.2005.02.009
Hye A, Kerr F, Archer N, Foy C, Poppe M, Brown R, Hamilton G, Powell J et al (2005) Glycogen synthase kinase-3 is increased in white cells early in Alzheimer’s disease. Neurosci Lett 373(1):1–4. https://doi.org/10.1016/j.neulet.2004.10.031
Vossel KA, Xu JC, Fomenko V, Miyamoto T, Suberbielle E, Knox JA, Ho K, Kim DH et al (2015) Tau reduction prevents Abeta-induced axonal transport deficits by blocking activation of GSK3beta. J Cell Biol 209(3):419–433. https://doi.org/10.1083/jcb.201407065
Deng J, Habib A, Obregon DF, Barger SW, Giunta B, Wang YJ, Hou H, Sawmiller D et al (2015) Soluble amyloid precursor protein alpha inhibits tau phosphorylation through modulation of GSK3beta signaling pathway. J Neurochem 135(3):630–637. https://doi.org/10.1111/jnc.13351
Catricala S, Torti M, Ricevuti G (2012) Alzheimer disease and platelets: how’s that relevant. Immunity & Ageing 9(1):20. https://doi.org/10.1186/1742-4933-9-20
Tang K, Hynan LS, Baskin F, Rosenberg RN (2006) Platelet amyloid precursor protein processing: a bio-marker for Alzheimer’s disease. J Neurol Sci 240(1-2):53–58. https://doi.org/10.1016/j.jns.2005.09.002
Baskin F, Rosenberg RN, Iyer L, Hynan L, Cullum CM (2000) Platelet APP isoform ratios correlate with declining cognition in AD. Neurology 54(10):1907–1909
Chatterjee P, Gupta VB, Fagan AM, Jasielec MS, Xiong C, Sohrabi HR, Dhaliwal S, Taddei K et al (2015) Decreased platelet APP isoform ratios in autosomal dominant Alzheimer’s disease: baseline data from a DIAN cohort subset. Curr Alzheimer Res 12(2):157–164
Bartel DP (2004) MicroRNAs: genomics, biogenesis, mechanism, and function. Cell 116(2):281–297
Bartel DP (2009) MicroRNAs: target recognition and regulatory functions. Cell 136(2):215–233. https://doi.org/10.1016/j.cell.2009.01.002
Mitchell PS, Parkin RK, Kroh EM, Fritz BR, Wyman SK, Pogosova-Agadjanyan EL, Peterson A, Noteboom J et al (2008) Circulating microRNAs as stable blood-based markers for cancer detection. Proc Natl Acad Sci USA 105(30):10513–10518. https://doi.org/10.1073/pnas.0804549105
Yang TT, Liu CG, Gao SC, Zhang Y, Wang PC (2018) The serum exosome derived microRNA-135a, −193b, and −384 were potential Alzheimer’s disease biomarkers. Biomed Environ Sci 31(2):87–96. https://doi.org/10.3967/bes2018.011
Kumar S, Vijayan M, Reddy PH (2017) MicroRNA-455-3p as a potential peripheral biomarker for Alzheimer’s disease. Hum Mol Genet 26(19):3808–3822. https://doi.org/10.1093/hmg/ddx267
Hara N, Kikuchi M, Miyashita A, Hatsuta H, Saito Y, Kasuga K, Murayama S, Ikeuchi T et al (2017) Serum microRNA miR-501-3p as a potential biomarker related to the progression of Alzheimer’s disease. Acta Neuropathol Commun 5(1):10. https://doi.org/10.1186/s40478-017-0414-z
Tan L, Yu JT, Liu QY, Tan MS, Zhang W, Hu N, Wang YL, Sun L et al (2014) Circulating miR-125b as a biomarker of Alzheimer’s disease. J Neurol Sci 336(1-2):52–56. https://doi.org/10.1016/j.jns.2013.10.002
Galimberti D, Villa C, Fenoglio C, Serpente M, Ghezzi L, Cioffi SM, Arighi A, Fumagalli G et al (2014) Circulating miRNAs as potential biomarkers in Alzheimer’s disease. J Alzheimer’s Dis 42(4):1261–1267. https://doi.org/10.3233/jad-140756
Kiko T, Nakagawa K, Tsuduki T, Furukawa K, Arai H, Miyazawa T (2014) MicroRNAs in plasma and cerebrospinal fluid as potential markers for Alzheimer’s disease. J Alzheimer’s Dis 39(2):253–259. https://doi.org/10.3233/jad-130932
Oh ES, Troncoso JC, Fangmark Tucker SM (2008) Maximizing the potential of plasma amyloid-beta as a diagnostic biomarker for Alzheimer’s disease. Neuromol Med 10(3):195–207. https://doi.org/10.1007/s12017-008-8035-0
Mayeux R, Honig LS, Tang MX, Manly J, Stern Y, Schupf N, Mehta PD (2003) Plasma A[beta]40 and A[beta]42 and Alzheimer’s disease: relation to age, mortality, and risk. Neurology 61(9):1185–1190
Sundelof J, Giedraitis V, Irizarry MC, Sundstrom J, Ingelsson E, Ronnemaa E, Arnlov J, Gunnarsson MD et al (2008) Plasma beta amyloid and the risk of Alzheimer disease and dementia in elderly men: a prospective, population-based cohort study. Arch Neurol 65(2):256–263. https://doi.org/10.1001/archneurol.2007.57
van Oijen M, Hofman A, Soares HD, Koudstaal PJ, Breteler MM (2006) Plasma Abeta(1-40) and Abeta(1-42) and the risk of dementia: a prospective case-cohort study. Lancet Neurol 5(8):655–660. https://doi.org/10.1016/s1474-4422(06)70501-4
Fukumoto H, Tennis M, Locascio JJ, Hyman BT, Growdon JH, Irizarry MC (2003) Age but not diagnosis is the main predictor of plasma amyloid beta-protein levels. Arch Neurol 60(7):958–964. https://doi.org/10.1001/archneur.60.7.958
Tamaoka A, Fukushima T, Sawamura N, Ishikawa K, Oguni E, Komatsuzaki Y, Shoji S (1996) Amyloid beta protein in plasma from patients with sporadic Alzheimer’s disease. J Neurol Sci 141(1-2):65–68
Irizarry MC (2004) Biomarkers of Alzheimer disease in plasma. NeuroRx 1(2):226–234. https://doi.org/10.1602/neurorx.1.2.226
Ray S, Britschgi M, Herbert C, Takeda-Uchimura Y, Boxer A, Blennow K, Friedman LF, Galasko DR et al (2007) Classification and prediction of clinical Alzheimer’s diagnosis based on plasma signaling proteins. Nat Med 13(11):1359–1362. https://doi.org/10.1038/nm1653
Marksteiner J, Kemmler G, Weiss EM, Knaus G, Ullrich C, Mechtcheriakov S, Oberbauer H, Auffinger S et al (2011) Five out of 16 plasma signaling proteins are enhanced in plasma of patients with mild cognitive impairment and Alzheimer’s disease. Neurobiol Aging 32(3):539–540. https://doi.org/10.1016/j.neurobiolaging.2009.03.011
Soares HD, Chen Y, Sabbagh M, Roher A, Schrijvers E, Breteler M (2009) Identifying early markers of Alzheimer’s disease using quantitative multiplex proteomic immunoassay panels. Ann N Y Acad Sci 1180:56–67. https://doi.org/10.1111/j.1749-6632.2009.05066.x
Hye A, Lynham S, Thambisetty M, Causevic M, Campbell J, Byers HL, Hooper C, Rijsdijk F et al (2006) Proteome-based plasma biomarkers for Alzheimer’s disease. Brain 129(Pt 11):3042–3050. https://doi.org/10.1093/brain/awl279
Liao PC, Yu L, Kuo CC, Lin C, Kuo YM (2007) Proteomics analysis of plasma for potential biomarkers in the diagnosis of Alzheimer’s disease. Proteomics Clin Appl 1(5):506–512. https://doi.org/10.1002/prca.200600684
Yu HL, Chertkow HM, Bergman H, Schipper HM (2003) Aberrant profiles of native and oxidized glycoproteins in Alzheimer plasma. Proteomics 3(11):2240–2248. https://doi.org/10.1002/pmic.200300475
Choi J, Malakowsky CA, Talent JM, Conrad CC, Gracy RW (2002) Identification of oxidized plasma proteins in Alzheimer’s disease. Biochem Biophys Res Commun 293(5):1566–1570. https://doi.org/10.1016/s0006-291x(02)00420-5
Wu YY, Hsu JL, Wang HC, Wu SJ, Hong CJ, Cheng IH (2015) Alterations of the neuroinflammatory markers IL-6 and TRAIL in Alzheimer’s disease. Dement Geriatr Cogn Dis Extra 5(3):424–434. https://doi.org/10.1159/000439214
Frederikse PH, Garland D, Zigler JS Jr, Piatigorsky J (1996) Oxidative stress increases production of beta-amyloid precursor protein and beta-amyloid (Abeta) in mammalian lenses, and Abeta has toxic effects on lens epithelial cells. J Biol Chem 271(17):10169–10174
Frederikse PH, Ren XO (2002) Lens defects and age-related fiber cell degeneration in a mouse model of increased AbetaPP gene dosage in Down syndrome. Am J Pathol 161(6):1985–1990
Melov S, Wolf N, Strozyk D, Doctrow SR, Bush AI (2005) Mice transgenic for Alzheimer disease beta-amyloid develop lens cataracts that are rescued by antioxidant treatment. Free Radic Biol Med 38(2):258–261. https://doi.org/10.1016/j.freeradbiomed.2004.10.023
Goldstein LE, Muffat JA, Cherny RA, Moir RD, Ericsson MH, Huang X, Mavros C, Coccia JA et al (2003) Cytosolic beta-amyloid deposition and supranuclear cataracts in lenses from people with Alzheimer’s disease. Lancet 361(9365):1258–1265. https://doi.org/10.1016/s0140-6736(03)12981-9
Moncaster JA, Pineda R, Moir RD, Lu S, Burton MA, Ghosh JG, Ericsson M, Soscia SJ et al (2010) Alzheimer’s disease amyloid-beta links lens and brain pathology in Down syndrome. PLoS One 5(5):e10659. https://doi.org/10.1371/journal.pone.0010659
Kerbage C, Sadowsky CH, Jennings D, Cagle GD, Hartung PD (2013) Alzheimer’s disease diagnosis by detecting exogenous fluorescent signal of ligand bound to Beta amyloid in the lens of human eye: an exploratory study. Front Neurol 4:62. https://doi.org/10.3389/fneur.2013.00062
Michael R, Rosandic J, Montenegro GA, Lobato E, Tresserra F, Barraquer RI, Vrensen GF (2013) Absence of beta-amyloid in cortical cataracts of donors with and without Alzheimer’s disease. Exp Eye Res 106:5–13. https://doi.org/10.1016/j.exer.2012.10.012
Ho CY, Troncoso JC, Knox D, Stark W, Eberhart CG (2014) Beta-amyloid, phospho-tau and alpha-synuclein deposits similar to those in the brain are not identified in the eyes of Alzheimer’s and Parkinson’s disease patients. Brain Pathol 24(1):25–32. https://doi.org/10.1111/bpa.12070
Bei L, Shui YB, Bai F, Nelson SK, Van Stavern GP, Beebe DC (2015) A test of lens opacity as an indicator of preclinical Alzheimer disease. Exp Eye Res 140:117–123. https://doi.org/10.1016/j.exer.2015.03.010
Berisha F, Feke GT, Trempe CL, McMeel JW, Schepens CL (2007) Retinal abnormalities in early Alzheimer’s disease. Invest Ophthalmol Vis Sci 48(5):2285–2289. https://doi.org/10.1167/iovs.06-1029
Kirbas S, Turkyilmaz K, Anlar O, Tufekci A, Durmus M (2013) Retinal nerve fiber layer thickness in patients with Alzheimer disease. J Neuroophthalmol 33(1):58–61. https://doi.org/10.1097/WNO.0b013e318267fd5f
Lu Y, Li Z, Zhang X, Ming B, Jia J, Wang R, Ma D (2010) Retinal nerve fiber layer structure abnormalities in early Alzheimer’s disease: evidence in optical coherence tomography. Neurosci Lett 480(1):69–72. https://doi.org/10.1016/j.neulet.2010.06.006
Chi Y, Wang YH, Yang L (2010) The investigation of retinal nerve fiber loss in Alzheimer’s disease. Zhonghua Yan Ke Za Zhi 46(2):134–139
Gao L, Liu Y, Li X, Bai Q, Liu P (2015) Abnormal retinal nerve fiber layer thickness and macula lutea in patients with mild cognitive impairment and Alzheimer’s disease. Arch Gerontol Geriatr 60(1):162–167. https://doi.org/10.1016/j.archger.2014.10.011
Gunes A, Demirci S, Tok L, Tok O, Demirci S (2015) Evaluation of retinal nerve fiber layer thickness in Alzheimer disease using spectral-domain optical coherence tomography. Turk J Med Sci 45(5):1094–1097
Perez SE, Lumayag S, Kovacs B, Mufson EJ, Xu S (2009) Beta-amyloid deposition and functional impairment in the retina of the APPswe/PS1DeltaE9 transgenic mouse model of Alzheimer’s disease. Invest Ophthalmol Vis Sci 50(2):793–800. https://doi.org/10.1167/iovs.08-2384
Liu B, Rasool S, Yang Z, Glabe CG, Schreiber SS, Ge J, Tan Z (2009) Amyloid-peptide vaccinations reduce {beta}-amyloid plaques but exacerbate vascular deposition and inflammation in the retina of Alzheimer’s transgenic mice. Am J Pathol 175(5):2099–2110. https://doi.org/10.2353/ajpath.2009.090159
Frost S, Kanagasingam Y, Sohrabi H, Vignarajan J, Bourgeat P, Salvado O, Villemagne V, Rowe CC et al (2013) Retinal vascular biomarkers for early detection and monitoring of Alzheimer’s disease. Transl Psychiatry 3:e233. https://doi.org/10.1038/tp.2012.150
Blanks JC, Hinton DR, Sadun AA, Miller CA (1989) Retinal ganglion cell degeneration in Alzheimer’s disease. Brain Res 501(2):364–372
Blanks JC, Schmidt SY, Torigoe Y, Porrello KV, Hinton DR, Blanks RH (1996) Retinal pathology in Alzheimer’s disease. II. Regional neuron loss and glial changes in GCL. Neurobiol Aging 17(3):385–395
Sadun AA, Bassi CJ (1990) Optic nerve damage in Alzheimer’s disease. Ophthalmology 97(1):9–17
Williams PA, Thirgood RA, Oliphant H, Frizzati A, Littlewood E, Votruba M, Good MA, Williams J et al (2013) Retinal ganglion cell dendritic degeneration in a mouse model of Alzheimer’s disease. Neurobiol Aging 34(7):1799–1806. https://doi.org/10.1016/j.neurobiolaging.2013.01.006
Lu Y, Tang N, Wang R (2012) Retinal ganglion cell loss on APP/PS1 transgenic mice with Alzheimer’s disease. Zhonghua Yan Ke Za Zhi 48(9):836–841
Zhao WQ, Ravindranath L, Mohamed AS, Zohar O, Chen GH, Lyketsos CG, Etcheberrigaray R, Alkon DL (2002) MAP kinase signaling cascade dysfunction specific to Alzheimer’s disease in fibroblasts. Neurobiol Dis 11(1):166–183
Veeranna, Kaji T, Boland B, Odrljin T, Mohan P, Basavarajappa BS, Peterhoff C, Cataldo A et al (2004) Calpain mediates calcium-induced activation of the Erk1,2 MAPK pathway and cytoskeletal phosphorylation in neurons: relevance to Alzheimer’s disease. Am J Pathol 165(3):795–805. https://doi.org/10.1016/s0002-9440(10)63342-1
Khan TK, Alkon DL (2006) An internally controlled peripheral biomarker for Alzheimer’s disease: Erk1 and Erk2 responses to the inflammatory signal bradykinin. Proc Natl Acad Sci U S A 103(35):13203–13207. https://doi.org/10.1073/pnas.0605411103
Khan TK, Alkon DL (2010) Early diagnostic accuracy and pathophysiologic relevance of an autopsy-confirmed Alzheimer’s disease peripheral biomarker. Neurobiol Aging 31(6):889–900. https://doi.org/10.1016/j.neurobiolaging.2008.07.010
Peterson C, Gibson GE, Blass JP (1985) Altered calcium uptake in cultured skin fibroblasts from patients with Alzheimer’s disease. N Engl J Med 312(16):1063–1065. https://doi.org/10.1056/nejm198504183121618
Peterson C, Ratan RR, Shelanski ML, Goldman JE (1986) Cytosolic free calcium and cell spreading decrease in fibroblasts from aged and Alzheimer donors. Proc Natl Acad Sci U S A 83(20):7999–8001
Peterson C, Ratan RR, Shelanski ML, Goldman JE (1988) Altered response of fibroblasts from aged and Alzheimer donors to drugs that elevate cytosolic free calcium. Neurobiol Aging 9(3):261–266
Peterson C, Goldman JE (1986) Alterations in calcium content and biochemical processes in cultured skin fibroblasts from aged and Alzheimer donors. Proc Natl Acad Sci U S A 83(8):2758–2762
Kumar U, Dunlop DM, Richardson JS (1994) Mitochondria from Alzheimer’s fibroblasts show decreased uptake of calcium and increased sensitivity to free radicals. Life Sci 54(24):1855–1860
Borden LA, Maxfield FR, Goldman JE, Shelanski ML (1992) Resting [Ca2+]i and [Ca2+]i transients are similar in fibroblasts from normal and Alzheimer’s donors. Neurobiol Aging 13(1):33–38
Etcheberrigaray R, Ito E, Kim CS, Alkon DL (1994) Soluble beta-amyloid induction of Alzheimer’s phenotype for human fibroblast K+ channels. Science 264(5156):276–279
Hirashima N, Etcheberrigaray R, Bergamaschi S, Racchi M, Battaini F, Binetti G, Govoni S, Alkon DL (1996) Calcium responses in human fibroblasts: a diagnostic molecular profile for Alzheimer’s disease. Neurobiol Aging 17(4):549–555
Ito E, Oka K, Etcheberrigaray R, Nelson TJ, McPhie DL, Tofel-Grehl B, Gibson GE, Alkon DL (1994) Internal Ca2+ mobilization is altered in fibroblasts from patients with Alzheimer disease. Proc Natl Acad Sci U S A 91(2):534–538
Etcheberrigaray R, Hirashima N, Nee L, Prince J, Govoni S, Racchi M, Tanzi RE, Alkon DL (1998) Calcium responses in fibroblasts from asymptomatic members of Alzheimer’s disease families. Neurobiol Dis 5(1):37–45. https://doi.org/10.1006/nbdi.1998.0176
Sharma S, Moon CS, Khogali A, Haidous A, Chabenne A, Ojo C, Jelebinkov M, Kurdi Y et al (2013) Biomarkers in Parkinson’s disease (recent update). Neurochem Int 63(3):201–229. https://doi.org/10.1016/j.neuint.2013.06.005
Louis ED, Bennett DA (2007) Mild Parkinsonian signs: an overview of an emerging concept. Mov Disord 22(12):1681–1688. https://doi.org/10.1002/mds.21433
Beach TG, Adler CH, Lue L, Sue LI, Bachalakuri J, Henry-Watson J, Sasse J, Boyer S et al (2009) Unified staging system for Lewy body disorders: correlation with nigrostriatal degeneration, cognitive impairment and motor dysfunction. Acta Neuropathol 117(6):613–634. https://doi.org/10.1007/s00401-009-0538-8
Grundemann J, Schlaudraff F, Haeckel O, Liss B (2008) Elevated alpha-synuclein mRNA levels in individual UV-laser-microdissected dopaminergic substantia nigra neurons in idiopathic Parkinson’s disease. Nucleic Acids Res 36(7):e38. https://doi.org/10.1093/nar/gkn084
Wu KP, Kim S, Fela DA, Baum J (2008) Characterization of conformational and dynamic properties of natively unfolded human and mouse alpha-synuclein ensembles by NMR: implication for aggregation. J Mol Biol 378(5):1104–1115. https://doi.org/10.1016/j.jmb.2008.03.017
Bonini NM, Giasson BI (2005) Snaring the function of alpha-synuclein. Cell 123(3):359–361. https://doi.org/10.1016/j.cell.2005.10.017
Maiti NC, Apetri MM, Zagorski MG, Carey PR, Anderson VE (2004) Raman spectroscopic characterization of secondary structure in natively unfolded proteins: alpha-synuclein. J Am Chem Soc 126(8):2399–2408. https://doi.org/10.1021/ja0356176
Mollenhauer B, Trautmann E, Taylor P, Manninger P, Sixel-Doring F, Ebentheuer J, Trenkwalder C, Schlossmacher MG (2013) Total CSF alpha-synuclein is lower in de novo Parkinson patients than in healthy subjects. Neurosci Lett 532:44–48. https://doi.org/10.1016/j.neulet.2012.11.004
Kang JH, Irwin DJ, Chen-Plotkin AS, Siderowf A, Caspell C, Coffey CS, Waligorska T, Taylor P et al (2013) Association of cerebrospinal fluid beta-amyloid 1-42, T-tau, P-tau181, and alpha-synuclein levels with clinical features of drug-naive patients with early Parkinson disease. JAMA Neurol 70(10):1277–1287. https://doi.org/10.1001/jamaneurol.2013.3861
Ono K, Yamada M (2014) Alpha-Synuclein in blood and cerebrospinal fluid of patients with alpha-synucleinopathy. Rinsho Byori 62(3):241–245
Tinsley RB, Kotschet K, Modesto D, Ng H, Wang Y, Nagley P, Shaw G, Horne MK (2010) Sensitive and specific detection of alpha-synuclein in human plasma. J Neurosci Res 88(12):2693–2700. https://doi.org/10.1002/jnr.22417
Foulds PG, Mitchell JD, Parker A, Turner R, Green G, Diggle P, Hasegawa M, Taylor M et al (2011) Phosphorylated alpha-synuclein can be detected in blood plasma and is potentially a useful biomarker for Parkinson’s disease. Faseb J 25(12):4127–4137. https://doi.org/10.1096/fj.10-179192
El-Agnaf OM, Salem SA, Paleologou KE, Curran MD, Gibson MJ, Court JA, Schlossmacher MG, Allsop D (2006) Detection of oligomeric forms of alpha-synuclein protein in human plasma as a potential biomarker for Parkinson’s disease. Faseb J 20(3):419–425. https://doi.org/10.1096/fj.03-1449com
Chau KY, Ching HL, Schapira AH, Cooper JM (2009) Relationship between alpha synuclein phosphorylation, proteasomal inhibition and cell death: relevance to Parkinson’s disease pathogenesis. J Neurochem 110(3):1005–1013
Fujiwara H, Hasegawa M, Dohmae N, Kawashima A, Masliah E, Goldberg MS, Shen J, Takio K et al (2002) α-Synuclein is phosphorylated in synucleinopathy lesions. Nat Cell Biol 4(2):160
Muntane G, Ferrer I, Martinez-Vicente M (2012) α-Synuclein phosphorylation and truncation are normal events in the adult human brain. Neuroscience 200:106–119
Michell AW, Luheshi LM, Barker RA (2005) Skin and platelet alpha-synuclein as peripheral biomarkers of Parkinson’s disease. Neurosci Lett 381(3):294–298. https://doi.org/10.1016/j.neulet.2005.02.030
Donadio V, Incensi A, Leta V, Giannoccaro MP, Scaglione C, Martinelli P, Capellari S, Avoni P et al (2014) Skin nerve alpha-synuclein deposits: a biomarker for idiopathic Parkinson disease. Neurology 82(15):1362–1369. https://doi.org/10.1212/wnl.0000000000000316
Al-Nimer MS, Mshatat SF, Abdulla HI (2014) Saliva alpha-synuclein and a high extinction coefficient protein: a novel approach in assessment biomarkers of Parkinson’s disease. N Am J Med Sci 6(12):633–637. https://doi.org/10.4103/1947-2714.147980
Del Tredici K, Hawkes CH, Ghebremedhin E, Braak H (2010) Lewy pathology in the submandibular gland of individuals with incidental Lewy body disease and sporadic Parkinson’s disease. Acta Neuropathol 119(6):703–713. https://doi.org/10.1007/s00401-010-0665-2
Adler CH, Dugger BN, Hinni ML, Lott DG, Driver-Dunckley E, Hidalgo J, Henry-Watson J, Serrano G et al (2014) Submandibular gland needle biopsy for the diagnosis of Parkinson disease. Neurology 82(10):858–864. https://doi.org/10.1212/WNL.0000000000000204
Cersosimo MG, Perandones C, Micheli FE, Raina GB, Beron AM, Nasswetter G, Radrizzani M, Benarroch EE (2011) Alpha-synuclein immunoreactivity in minor salivary gland biopsies of Parkinson’s disease patients. Mov Disord 26(1):188–190. https://doi.org/10.1002/mds.23344
Duda JE, Shah U, Arnold SE, Lee VM, Trojanowski JQ (1999) The expression of alpha-, beta-, and gamma-synucleins in olfactory mucosa from patients with and without neurodegenerative diseases. Exp Neurol 160(2):515–522. https://doi.org/10.1006/exnr.1999.7228
Beach TG, White CL III, Hladik CL, Sabbagh MN, Connor DJ, Shill HA, Sue LI, Sasse J et al (2009) Olfactory bulb alpha-synucleinopathy has high specificity and sensitivity for Lewy body disorders. Acta Neuropathol 117(2):169–174. https://doi.org/10.1007/s00401-008-0450-7
Witt M, Bormann K, Gudziol V, Pehlke K, Barth K, Minovi A, Hahner A, Reichmann H et al (2009) Biopsies of olfactory epithelium in patients with Parkinson’s disease. Mov Disord 24(6):906–914. https://doi.org/10.1002/mds.22464
Lebouvier T, Chaumette T, Damier P, Coron E, Touchefeu Y, Vrignaud S, Naveilhan P, Galmiche JP et al (2008) Pathological lesions in colonic biopsies during Parkinson’s disease. Gut 57(12):1741–1743. https://doi.org/10.1136/gut.2008.162503
Lebouvier T, Neunlist M, Bruley des Varannes S, Coron E, Drouard A, N’Guyen JM, Chaumette T, Tasselli M et al (2010) Colonic biopsies to assess the neuropathology of Parkinson’s disease and its relationship with symptoms. PLoS One 5(9):e12728. https://doi.org/10.1371/journal.pone.0012728
Pouclet H, Lebouvier T, Coron E, Bruley des Varannes S, Neunlist M, Derkinderen P (2012) A comparison between colonic submucosa and mucosa to detect Lewy pathology in Parkinson’s disease. Neurogastroenterol Motil 24(4):e202–e205. https://doi.org/10.1111/j.1365-2982.2012.01887.x
Pouclet H, Lebouvier T, Coron E, Bruley des Varannes S, Rouaud T, Roy M, Neunlist M, Derkinderen P (2012) A comparison between rectal and colonic biopsies to detect Lewy pathology in Parkinson’s disease. Neurobiol Dis 45(1):305–309. https://doi.org/10.1016/j.nbd.2011.08.014
Shannon KM, Keshavarzian A, Mutlu E, Dodiya HB, Daian D, Jaglin JA, Kordower JH (2012) Alpha-synuclein in colonic submucosa in early untreated Parkinson’s disease. Mov Disord 27(6):709–715. https://doi.org/10.1002/mds.23838
Sanchez-Ferro A, Rabano A, Catalan MJ, Rodriguez-Valcarcel FC, Diez SF, Herreros-Rodriguez J, Garcia-Cobos E, Alvarez-Santullano MM et al (2014) In vivo gastric detection of alpha-synuclein inclusions in Parkinson’s disease. Mov Disord. https://doi.org/10.1002/mds.25988
Iwanaga K, Wakabayashi K, Yoshimoto M, Tomita I, Satoh H, Takashima H, Satoh A, Seto M et al (1999) Lewy body-type degeneration in cardiac plexus in Parkinson’s and incidental Lewy body diseases. Neurology 52(6):1269–1271
Orimo S, Uchihara T, Nakamura A, Mori F, Kakita A, Wakabayashi K, Takahashi H (2008) Axonal alpha-synuclein aggregates herald centripetal degeneration of cardiac sympathetic nerve in Parkinson’s disease. Brain 131(Pt 3):642–650. https://doi.org/10.1093/brain/awm302
Fujishiro H, Frigerio R, Burnett M, Klos KJ, Josephs KA, Delledonne A, Parisi JE, Ahlskog JE et al (2008) Cardiac sympathetic denervation correlates with clinical and pathologic stages of Parkinson’s disease. Mov Disord 23(8):1085–1092. https://doi.org/10.1002/mds.21989
Braak H, de Vos RA, Bohl J, Del Tredici K (2006) Gastric alpha-synuclein immunoreactive inclusions in Meissner’s and Auerbach’s plexuses in cases staged for Parkinson’s disease-related brain pathology. Neurosci Lett 396(1):67–72. https://doi.org/10.1016/j.neulet.2005.11.012
Mu L, Sobotka S, Chen J, Su H, Sanders I, Nyirenda T, Adler CH, Shill HA et al (2013) Parkinson disease affects peripheral sensory nerves in the pharynx. J Neuropathol Exp Neurol 72(7):614–623. https://doi.org/10.1097/NEN.0b013e3182965886
Maita C, Tsuji S, Yabe I, Hamada S, Ogata A, Maita H, Iguchi-Ariga SM, Sasaki H et al (2008) Secretion of DJ-1 into the serum of patients with Parkinson’s disease. Neurosci Lett 431(1):86–89. https://doi.org/10.1016/j.neulet.2007.11.027
Bonifati V, Rizzu P, van Baren MJ, Schaap O, Breedveld GJ, Krieger E, Dekker MC, Squitieri F et al (2003) Mutations in the DJ-1 gene associated with autosomal recessive early-onset parkinsonism. Science 299(5604):256–259. https://doi.org/10.1126/science.1077209
Hong Z, Shi M, Chung KA, Quinn JF, Peskind ER, Galasko D, Jankovic J, Zabetian CP et al (2010) DJ-1 and alpha-synuclein in human cerebrospinal fluid as biomarkers of Parkinson’s disease. Brain 133(Pt 3):713–726. https://doi.org/10.1093/brain/awq008
Shi M, Zabetian CP, Hancock AM, Ginghina C, Hong Z, Yearout D, Chung KA, Quinn JF et al (2010) Significance and confounders of peripheral DJ-1 and alpha-synuclein in Parkinson’s disease. Neurosci Lett 480(1):78–82. https://doi.org/10.1016/j.neulet.2010.06.009
Lin X, Cook TJ, Zabetian CP, Leverenz JB, Peskind ER, Hu SC, Cain KC, Pan C et al (2012) DJ-1 isoforms in whole blood as potential biomarkers of Parkinson disease. Sci Rep 2:954. https://doi.org/10.1038/srep00954
Church WH, Ward VL (1994) Uric acid is reduced in the substantia nigra in Parkinson’s disease: effect on dopamine oxidation. Brain Res Bull 33(4):419–425
Davis JW, Grandinetti A, Waslien CI, Ross GW, White LR, Morens DM (1996) Observations on serum uric acid levels and the risk of idiopathic Parkinson’s disease. Am J Epidemiol 144(5):480–484
Weisskopf MG, O’Reilly E, Chen H, Schwarzschild MA, Ascherio A (2007) Plasma urate and risk of Parkinson’s disease. Am J Epidemiol 166(5):561–567. https://doi.org/10.1093/aje/kwm127
de Lau LM, Koudstaal PJ, Hofman A, Breteler MM (2005) Serum uric acid levels and the risk of Parkinson disease. Ann Neurol 58(5):797–800. https://doi.org/10.1002/ana.20663
De Vera M, Rahman MM, Rankin J, Kopec J, Gao X, Choi H (2008) Gout and the risk of Parkinson’s disease: a cohort study. Arthritis Rheum 59(11):1549–1554. https://doi.org/10.1002/art.24193
Gao X, Chen H, Choi HK, Curhan G, Schwarzschild MA, Ascherio A (2008) Diet, urate, and Parkinson’s disease risk in men. Am J Epidemiol 167(7):831–838. https://doi.org/10.1093/aje/kwm385
O’Reilly EJ, Gao X, Weisskopf MG, Chen H, Schwarzschild MA, Spiegelman D, Ascherio A (2010) Plasma urate and Parkinson’s disease in women. Am J Epidemiol 172(6):666–670. https://doi.org/10.1093/aje/kwq195
Constantinescu R, Zetterberg H (2011) Urate as a marker of development and progression in Parkinson’s disease. Drugs Today (Barc) 47(5):369–380. https://doi.org/10.1358/dot.2011.47.5.1591834
Cipriani S, Chen X, Schwarzschild MA (2010) Urate: a novel biomarker of Parkinson’s disease risk, diagnosis and prognosis. Biomark Med 4(5):701–712. https://doi.org/10.2217/bmm.10.94
Chen-Plotkin AS, Hu WT, Siderowf A, Weintraub D, Goldmann Gross R, Hurtig HI, Xie SX, Arnold SE et al (2011) Plasma epidermal growth factor levels predict cognitive decline in Parkinson disease. Ann Neurol 69(4):655–663. https://doi.org/10.1002/ana.22271
Pellecchia MT, Santangelo G, Picillo M, Pivonello R, Longo K, Pivonello C, Vitale C, Amboni M et al (2013) Serum epidermal growth factor predicts cognitive functions in early, drug-naive Parkinson’s disease patients. J Neurol 260(2):438–444. https://doi.org/10.1007/s00415-012-6648-6
Qiang JK, Wong YC, Siderowf A, Hurtig HI, Xie SX, Lee VM, Trojanowski JQ, Yearout D et al (2013) Plasma apolipoprotein A1 as a biomarker for Parkinson disease. Ann Neurol 74(1):119–127. https://doi.org/10.1002/ana.23872
Zhang X, Yin X, Yu H, Liu X, Yang F, Yao J, Jin H, Yang P (2012) Quantitative proteomic analysis of serum proteins in patients with Parkinson’s disease using an isobaric tag for relative and absolute quantification labeling, two-dimensional liquid chromatography, and tandem mass spectrometry. Analyst 137(2):490–495. https://doi.org/10.1039/c1an15551b
Wang ES, Sun Y, Guo JG, Gao X, Hu JW, Zhou L, Hu J, Jiang CC (2010) Tetranectin and apolipoprotein A-I in cerebrospinal fluid as potential biomarkers for Parkinson’s disease. Acta Neurol Scand 122(5):350–359. https://doi.org/10.1111/j.1600-0404.2009.01318.x
Yin GN, Lee HW, Cho JY, Suk K (2009) Neuronal pentraxin receptor in cerebrospinal fluid as a potential biomarker for neurodegenerative diseases. Brain Res 1265:158–170. https://doi.org/10.1016/j.brainres.2009.01.058
Gao X, Simon KC, Schwarzschild MA, Ascherio A (2012) Prospective study of statin use and risk of Parkinson disease. Arch Neurol 69(3):380–384. https://doi.org/10.1001/archneurol.2011.1060
Lee YC, Lin CH, Wu RM, Lin MS, Lin JW, Chang CH, Lai MS (2013) Discontinuation of statin therapy associates with Parkinson disease: a population-based study. Neurology 81(5):410–416. https://doi.org/10.1212/WNL.0b013e31829d873c
Cao XY, Lu JM, Zhao ZQ, Li MC, Lu T, An XS, Xue LJ (2017) MicroRNA biomarkers of Parkinson’s disease in serum exosome-like microvesicles. Neurosci Lett 644:94–99. https://doi.org/10.1016/j.neulet.2017.02.045
Ma W, Li Y, Wang C, Xu F, Wang M, Liu Y (2016) Serum miR-221 serves as a biomarker for Parkinson’s disease. Cell Biochem Funct 34(7):511–515. https://doi.org/10.1002/cbf.3224
Dong H, Wang C, Lu S, Yu C, Huang L, Feng W, Xu H, Chen X et al (2016) A panel of four decreased serum microRNAs as a novel biomarker for early Parkinson’s disease. Biomarkers 21(2):129–137. https://doi.org/10.3109/1354750x.2015.1118544
Margis R, Margis R, Rieder CR (2011) Identification of blood microRNAs associated to Parkinson’s disease. J Biotechnol 152(3):96–101. https://doi.org/10.1016/j.jbiotec.2011.01.023
Cardo LF, Coto E, de Mena L, Ribacoba R, Moris G, Menendez M, Alvarez V (2013) Profile of microRNAs in the plasma of Parkinson’s disease patients and healthy controls. J Neurol 260(5):1420–1422. https://doi.org/10.1007/s00415-013-6900-8
Greenberg BM, Frohman E (2010) Optical coherence tomography as a potential readout in clinical trials. Ther Adv Neurol Disord 3(3):153–160. https://doi.org/10.1177/1756285610368890
Satue M, Rodrigo MJ, Obis J, Vilades E, Gracia H, Otin S, Fuertes MI, Alarcia R, Crespo JA, Polo V, Larrosa JM, Pablo LE, Garcia-Martin E (2017) Evaluation of progressive visual dysfunction and retinal degeneration in patients with parkinson's disease. Invest Ophthalmol Vis Sci 58(2):1151–1157. https://doi.org/10.1167/iovs.16-20460. http://iovs.arvojournals.org/article.aspx?articleid=2604719
Inzelberg R, Ramirez JA, Nisipeanu P, Ophir A (2004) Retinal nerve fiber layer thinning in Parkinson disease. Vision Res 44(24):2793–2797. https://doi.org/10.1016/j.visres.2004.06.009
Hajee ME, March WF, Lazzaro DR, Wolintz AH, Shrier EM, Glazman S, Bodis-Wollner IG (2009) Inner retinal layer thinning in Parkinson disease. Arch Ophthalmol 127(6):737–741. https://doi.org/10.1001/archophthalmol.2009.106
Altintas O, Iseri P, Ozkan B, Caglar Y (2008) Correlation between retinal morphological and functional findings and clinical severity in Parkinson’s disease. Doc Ophthalmol 116(2):137–146. https://doi.org/10.1007/s10633-007-9091-8
Yavas GF, Yilmaz O, Kusbeci T, Ozturk F (2007) The effect of levodopa and dopamine agonists on optic nerve head in Parkinson disease. Eur J Ophthalmol 17(5):812–816
Wojtkowski M, Srinivasan V, Fujimoto JG, Ko T, Schuman JS, Kowalczyk A, Duker JS (2005) Three-dimensional retinal imaging with high-speed ultrahigh-resolution optical coherence tomography. Ophthalmology 112(10):1734–1746. https://doi.org/10.1016/j.ophtha.2005.05.023
Tian T, Zhu XH, Liu YH (2011) Potential role of retina as a biomarker for progression of Parkinson’s disease. Int J Ophthalmol 4(4):433–438. https://doi.org/10.3980/j.issn.2222-3959.2011.04.21
Jimenez B, Ascaso FJ, Cristobal JA, Lopez del Val J (2014) Development of a prediction formula of Parkinson disease severity by optical coherence tomography. Mov Disord 29(1):68–74. https://doi.org/10.1002/mds.25747
Lee JY, Ahn J, Kim TW, Jeon BS (2014) Optical coherence tomography in Parkinson’s disease: is the retina a biomarker? J Parkinsons Dis 4(2):197–204. https://doi.org/10.3233/jpd-130306
Archibald NK, Clarke MP, Mosimann UP, Burn DJ (2011) Retinal thickness in Parkinson’s disease. Parkinsonism Relat Disord 17(6):431–436. https://doi.org/10.1016/j.parkreldis.2011.03.004
Hye A, Riddoch-Contreras J, Baird AL, Ashton NJ, Bazenet C, Leung R, Westman E, Simmons A et al (2014) Plasma proteins predict conversion to dementia from prodromal disease. Alzheimers Dement 10(6):799–807.e792. https://doi.org/10.1016/j.jalz.2014.05.1749
Blanks JC, Torigoe Y, Hinton DR, Blanks RH (1996) Retinal pathology in Alzheimer’s disease. I. Ganglion cell loss in foveal/parafoveal retina. Neurobiol Aging 17(3):377–384
Menéndez-González M (2014) The many questions on the use of biomarkers for neurodegenerative diseases in clinical practice. Front Aging Neurosci 6:45
Funding
The authors (BG and PP) acknowledges The Lee Kong Chian School of Medicine, Nanyang Technological University MOE Start-Up Grant and MOE Tier-1 grants (2014-T1-001-229-1T1-04/14 and L0421160-1T1-06/15), Singapore.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Research Involving Human Participants and/or Animals
Not applicable
Informed Consent
Not applicable
Conflict of Interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Htike, T.T., Mishra, S., Kumar, S. et al. Peripheral Biomarkers for Early Detection of Alzheimer’s and Parkinson’s Diseases. Mol Neurobiol 56, 2256–2277 (2019). https://doi.org/10.1007/s12035-018-1151-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-018-1151-4