Abstract
Epidermal growth factor (EGF) has been proposed as a candidate biomarker for cognitive impairment in Parkinson’s disease (PD). We aimed to assess the relationship between serum EGF and cognitive functions in early, drug-naive PD patients and evaluate the predictive value of EGF on cognitive functions in a 2-year follow-up study. Serum EGF was measured in 65 early, drug-naive PD patients, that underwent a comprehensive neuropsychological battery. Motor symptoms were assessed by means of the Unified Parkinson’s Disease Rating Scale, Part III (UPDRS-III). Neuropsychological evaluation was repeated after 2 years. Spearman’s rank correlation was used to assess the relationship between serum EGF levels and neuropsychological variables. Linear regression analysis was used to evaluate the relationship between EGF and neuropsychological scores as well as other variables (age, gender, UPDRS-III, levodopa equivalent dose, and type of treatment at follow-up) potentially affecting cognitive performance. Variation over time in cognitive scores was analyzed using repeated-measures ANOVA. At baseline, EGF was the only significant variable associated with performance on semantic fluency (R 2 = 0.131; p = 0.005). EGF levels (p = 0.025), together with UPDRS-III (p = 0.009) and age (p = 0.011), were associated with performance on frontal assessment battery (R 2 = 0.260). At 2-year follow-up, EGF was the only significant variable to predict performance on semantic fluency (R 2 = 0.147; p = 0.025) and color naming task of Stroop color-word test (R 2 = 0.121; p = 0.044). Serum EGF levels are related to frontal and temporal cognitive functions in early, drug-naive PD patients and predict performance on frontal and posterior cognitive functions at 2-year follow-up. EGF is proposed as a potential serum biomarker for early cognitive impairment in PD.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cognitive impairment in Parkinson’s disease (PD) patients without dementia can occur in the early stages of disease and may be associated with subtle changes in cognitive function that are not apparent to the patients, their families, or clinicians. Twenty-five percent of patients who have been newly diagnosed with PD may present some cognitive dysfunction [1, 2]. Cognitive function may decline over time, with many patients eventually developing dementia [3]. However, some PD patients may exhibit early cognitive deficits that do not evolve into PD dementia or do so over an extended period [3, 4], but may be associated with subtle functional impairments [5]. The development of cognitive impairment is a significant transition for patients and families, yet its occurrence is unpredictable.
Epidermal growth factor (EGF) acts as a neurotrophic factor on dopaminergic nigrostriatal neurons in animal models of PD [6–8]. EGF mediates dopamine-induced proliferation of adult precursor cells in the mammalian subventricular zone [9], and increases hippocampal neurogenesis in slice cultures and damaged rodent brains [10, 11]. Expression of EGF/EGF receptor is widespread in the neocortex and limbic cortex, cerebellum, hippocampus and the midbrain. EGFR has been recently suggested to have a central role in neurometabolic aging and neurodegeneration [12]. Nevertheless, one of the best-known roles of EGFR in the brain is the maintenance of memory pattern formation in the hippocampus [13]. Moreover, a region-specific neurodegeneration involving the frontal cortex occurs in EGFR-null mice, supporting a role of EGF pathway in the maintenance of neuronal survival in this specific area [14, 15]. Recently, Chen-Plotkin et al. [16] screened more than 100 plasma proteins in a cohort of advanced PD patients and found that low plasma levels of EGF predicted cognitive decline assessed by means of the Mattis Dementia Rating Scale after a median follow-up of 21 months.
In the present study, we aimed to assess the relationship between serum EGF levels and cognitive functions in a cohort of drug-naive PD patients and to evaluate the predictive value of EGF levels on neuropsychological functions, with particular regard to frontal and temporal functions, after a 2-year follow-up.
Patients and methods
We enrolled de novo, drug-naive patients with parkinsonism consecutively referred to the Department of Neurological Sciences of the University “Federico II” of Naples. Inclusion criteria were: (1) the presence of parkinsonian syndrome according to United Kingdom Parkinson’s Disease Society Brain Bank Diagnostic Criteria [17] (bradykinesia associated to tremor or rigidity or postural instability), (2) disease duration <2 years, and (3) no history of present or past therapy with pro-dopaminergic agents. Exclusion criteria were the presence of: (1) clinical signs satisfying the criteria of possible atypical parkinsonisms, (2) secondary or iatrogenic parkinsonism, (3) familial parkinsonism, (4) dementia according to recent consensus criteria [18], and major depressive disorder according to DSM-IV criteria. Additional criteria for inclusion were lack of significant cerebral lesions at the MRI and/or CT or severe concomitant disease that might explain the presence of cognitive disturbances or focal brain metabolic alterations. None of the patients were treated with anticholinergic agents, choline esterase inhibitors, antidepressants, or other centrally acting drugs.
Patients underwent clinical re-evaluation 1 year later to assess the diagnosis of PD according to both exclusion and supportive criteria of the United Kingdom Parkinson’s Disease Society Brain Bank Diagnostic Criteria for Parkinson’s Disease [17]. All participants gave written informed consent, and the study was approved by the local ethics committee.
Clinical and neuropsychological evaluation
Motor symptoms were assessed by means of the UPDRS (section III = UPDRS-III) scale, and global cognitive function by mini-mental state examination (MMSE). At baseline, all patients underwent a comprehensive neuropsychological battery that includes immediate and delayed recall of the Rey auditory verbal learning test, and delayed recall of the Rey-Osterrieth complex figure test to measure memory, Benton judgment of line orientation test, constructional apraxia test, and clock drawing test to assess visuospatial abilities. Attention/executive functions were evaluated by frontal assessment battery (FAB), phonological and semantic fluency task, copy task of Rey-Osterrieth complex figure test, Corsi’s block test, verbal span test, Trail making test: part B minus Part A (TMT:B−A), and interference task of Stroop color-word test. This latter test also includes two non-executive tasks (reading and colors naming task) that were entered in statistical analysis. This neuropsychological battery was also administered after a 2-year follow-up.
EGF measurement
Venous blood samples were drawn at baseline visit in the morning after an overnight fast to evaluate IGF-I levels. Blood samples were centrifuged and frozen (−20 °C). To reduce analytical variance, the samples were batch-analyzed using the same assay lot. EGF concentrations were measured in serum by immunoassay (Quantikine® Human EGF Immunoassay, R&D Systems, Minneapolis, MN, USA). The WHO EGF International Reference Reagent 91/530 was evaluated in this assay. The mean intra-assay CV, determined by assaying the EGF concentration in three samples in replicates of 20, is reported by the manufacturer to be 2.0 %. The mean inter-assay CV, determined by assaying three samples replicates of 20, has been determined to be 4.6 %. The minimal detectable (MDD) concentration of EGF is <0.7 pg/ml. Sera from 60 age-matched (36 M, 24 F; mean age 58.8 ± 7.2 years) healthy controls recruited among spouses, relatives, or friends of patients or through local advertising were analyzed to obtain reference values for EGF. Neuropsychological examination was not performed on these healthy controls.
Statistical analysis
Spearman’s rank correlation coefficient was used to evaluate the correlations between baseline EGF levels and clinical and neuropsychological variables at baseline and at follow-up. Neuropsychological variables that were found to be significantly related to EGF by means of Spearman’s rank correlation test were used as dependent variables in linear regression analyses, and EGF, age, gender, and UPDRS were used as independent variables to assess whether these factors affected cognitive performance. A stepwise approach was employed. For linear regression analyses at follow-up, levodopa equivalent dose [19] and treatment with dopamine agonists (yes/no) or levodopa (yes/no) were also used as independent variables. All analyses were performed using SPSS software (version 19.0, SPSS Inc., Chicago, IL, USA). Variation over time in cognitive scores was analyzed using repeated-measures ANOVA. In order to identify the effect of baseline EGF on cognitive functions, the PD population was stratified into two groups using the median EGF value (≤638 and >638 pg/ml).
Results
We enrolled 65 de novo, drug-naive patients (39 M, 26 F; mean age 59.7 ± 8.3 years). Mean (±SD) age at onset was 58.5 ± 8.3 years and mean (±SD) UPDRS was 14.5 ± 6.7. At 2-year follow-up, none of the patients showed any clinical features suggestive of atypical or secondary parkinsonism and all had a positive response to dopaminergic treatment, confirming the diagnosis of idiopathic PD. Forty PD patients agreed to repeat neuropsychological evaluation at follow-up. Twenty-five patients did not undergo neuropsychological follow-up after 2 years due to missing their appointments or refusing to repeat the tests. However, their demographic and clinical features (sex, age, age at onset, UPDRS-III score) were not different from those of the patients completing the follow-up.
Mean (±SD) serum EGF levels were increased (646.5 ± 196.3 vs. 336 ± 153.4 pg/ml; p < 0.001) as compared to healthy controls. Mean (±SD) MMSE score was 27.2 ± 1.8. Table 1 shows mean (±SD) scores obtained by PD patients at baseline neuropsychological evaluation and number of patients with altered performance on specific neuropsychological tests according to Italian normative values. As shown in Table 1, PD patients at baseline presented major difficulties in frontal/executive tasks (FAB, Rey-Osterrieth complex figure test-copy and delayed recall, Stroop test) and visuospatial task (Benton judgment of line orientation test).
At baseline, Spearman’s rank correlation analysis showed that EGF was related with immediate recall of Rey auditory verbal learning test (ρ = 0.274, p = 0.029), semantic fluency (ρ = 0.261, p = 0.02), constructional apraxia test (ρ = 0.282, p = 0.024), FAB (ρ = 0.291, p = 0.02), color naming task of Stroop color-word test (ρ = 0.270, p = 0.033), and TMT:B−A (ρ = −0.292, p = 0.021). Complete correlation results are shown in the Web table. EGF levels were not related to the MMSE score. By means of linear regression analysis we found that EGF was the only significant variable associated with semantic fluency (R 2 = 0.131; p = 0.005). EGF levels (p = 0.025), together with UPDRS-III (p = 0.009) and age (p = 0.011), were associated with performance on FAB (R 2 = 0.260).
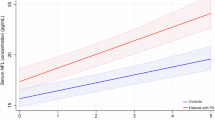
At 2-year follow-up, mean (±SD) levodopa equivalent dose was 293 ± 131 mg/day; 67.6 % of patients were treated with dopamine agonists and 32.4 % with levodopa. Spearman’s rank correlation analysis showed that EGF was related with Corsi’s block span (ρ = 0.387, p = 0.014), semantic fluency (ρ = 0.327, p = 0.04) (Fig. 1), and color naming task of Stroop color-word test (ρ = 0.341, p = 0.031) (Fig. 2). By means of linear regression analysis, we found that EGF was the only significant variable to predict semantic fluency (R 2 = 0.147; p = 0.025) and color naming task of Stroop color-word test (R 2 = 0.121; p = 0.044).
Repeated-measures ANOVA revealed that the time factor significantly affected cognitive functions (p < 0.001); in particular, the following tests showed a significant worsening over time (Table 2): constructional apraxia test (p < 0.01), copy task (p < 0.001) and delayed recall (p = 0.012) of Rey-Osterrieth complex figure test, and TMT:B−A (p < 0.001). With regard to group effect, patients with baseline EGF ≤638 pg/ml showed significantly lower scores at semantic fluency (p = 0.022), colors naming task of Stroop test (p = 0.005), and interference task of Stroop test (p = 0.013) as compared to patients with baseline EGF >638 pg/ml at both evaluations (Table 2). There was no significant interaction between time and group on any neuropsychological measure.
Discussion
This study set out to assess whether EGF levels are related to cognitive functions in early drug-naive patients and to evaluate if EGF may predict neuropsychological performance after a 2-year follow-up. We found that EGF levels are increased in de novo, drug-naive PD patients as compared to healthy controls, suggesting a role for EGF as an early biomarker of PD. The pathophysiological changes underlying the relatively increased EGF levels in early PD are unknown. Interestingly, increased levels of EGF have also been found in Alzheimer’s disease (AD), although not allowing to distinguish AD from other dementias [20, 21]. Since EGF has recognized neuroprotective properties, we could speculate that elevated EGF levels in early PD may indicate a primarily activated system combating increased cell loss. According to this hypothesis, the failure of such a compensatory system indicated by relatively low levels of EGF in some patients could contribute to the pathogenesis of cognitive decline.
At baseline, we found an association between EGF levels and cognitive tasks evaluating verbal memory, visuospatial abilities, and attention/executive functions, indicating that higher EGF levels may predict a better performance on these tasks. Indeed, low EGF levels were found to be associated with poor performance on semantic fluency task by means of linear regression analysis. Several studies investigating verbal fluency task indicate that semantic word retrieval is mediated by inferior frontal and temporal brain areas, whereas the prefrontal cortex seems to play an additional important role during phonemic processing [22, 23]. Our results may suggest that low EGF levels are associated with altered cognitive functions mediated by frontal and temporal lobes.
At 2-year follow-up, we found that EGF levels were positively related to different cognitive tasks evaluating both attention/executive functions and color naming. After regression analysis, we found that EGF levels predict performance on semantic fluency task and color naming of Stroop test independently from other variables potentially affecting cognitive functions. Impaired color perception may manifest as slowing of color naming [24], and central deficits of color processing have been generally associated with lesions affecting the occipital and posterior basal temporal lobes [25]. It is also known that semantic fluency is related to inferior frontal and temporal brain areas [22, 23]. Thus, our results suggest that baseline EGF levels may predict alterations of cognitive functions mediated by posterior (temporal and occipital) and frontal cerebral areas.
Results of repeated-measures ANOVA are consistent with regression analysis, since performance on both semantic fluency and color naming of Stroop test were found to be significantly worse in patients with lower EGF values than in patients with higher EGF values at baseline, and this significant difference persisted at 2-year follow-up. Moreover, ANOVA showed that performance on interference task of Stroop test was also worse in patients with lower EGF values during 2 years of follow-up. This finding supports the hypothesis that alterations of executive functions mediated by frontal areas are related to low EGF levels.
A relation between cognitive performance and EGF in PD has recently been proposed by Chen-Plotkin et al. [16]. They screened more than 100 plasma proteins in a cohort of 70 PD patients with a median disease duration of 7 years and found that low EGF levels not only correlated with poor scores of the DRS at baseline but also predicted an eight-fold risk of cognitive decline to dementia-range of the DRS after a median follow-up of 21 months [16]. Our findings are in line with this previous study with regard to the positive relation between EGF levels and cognitive performance. However, unlike the previous study, we assessed patients in the early disease stages and explored the relationship between EGF and different cognitive domains by means of a comprehensive neuropsychological battery, thus suggesting a potential role for EGF as an early marker of cognitive dysfunction in PD. As in previous study, we correlated baseline serum EGF levels with cognitive functions at baseline and at follow-up, since our aim was to identify a possible biomarker predicting cognitive impairment. However, longitudinal measurement of EGF levels in future studies could also be useful in assessing the relationships with changes in cognitive functions.
EGF acts as a neurotrophic factor on dopaminergic nigrostriatal neurons and hippocampal neurons [6–8, 10, 11]. Moreover, in cell cultures, EGF stimulates the release of soluble non-amyloidogenic fragments of amyloid precursor protein [26]. EGF receptors are normally expressed by hippocampal, striatal, and cerebral cortical neurons in human brain [27, 28], and are reported to be reduced in the prefrontal cortex and the striatum of PD patients [6]. Although it is not known whether increased EGF expression in PD, such as in AD, contributes to the pathological process or represents an attempt at repair, our results, together with experimental evidence supporting a neurotrophic, neuroprotective role for EGF, are consistent with the second possibility.
The pathophysiology of cognitive impairment in PD is complex, involving multiple neurotransmitter systems and diffuse neurodegeneration, and there remain significant gaps in our knowledge of cognitively impaired, non-demented PD patients. A large prospective study has shown that cognitive deficits in tasks related to posterior cortical areas are associated with the development of dementia [4]. Interestingly, we found that low EGF levels at baseline predicted poor performance on cognitive tasks related to temporal and occipital cortical areas at 2-year follow-up. Further follow-up study of our cohort of de novo patients is ongoing to assess the predictive value of EGF on the development of dementia.
Our results suggest that EGF is a potential serum biomarker for cognitive impairment in PD. If confirmed by further studies, the measurement of EGF might be useful both as a clinical diagnostic tool and in the design of trials aimed at preserving cognition in PD.
References
Muslimovic D, Post B, Speelman JD, Schmand B (2005) Cognitive profile of patients with newly diagnosed Parkinson disease. Neurology 65:1239–1245
Aarsland D, Bronnick K, Fladby T (2011) Mild cognitive impairment in Parkinson’s disease. Curr Opin Neurol Neurosci Rep 11:371–378
Aarsland D, Andersen K, Larsen JP et al (2001) Risk of dementia in Parkinson’s disease: a community-based, prospective study. Neurology 56:730–736
Williams-Gray CH, Evans JR, Goris A et al (2009) The distinct cognitive syndromes of Parkinson’s disease: 5-year follow-up of the CamPaIGN cohort. Brain 132:2958–2969
Klepac N, Trkulja V, Realja M, Babic T (2008) Is quality of life in non-demented Parkinson’s disease patients related to cognitive performance? A clinic-based cross-sectional study. Eur J Neurol 15:128–133
Iwakura Y, Piao Y, Mizuno M et al (2005) Influences of dopaminergic lesion on epidermal growth factor-ErbB signals in Parkinson’s disease and its model: neurotrophic implication in nigrostriatal neurons. J Neurochem 93:974–983
Ventrella LL (1993) Effect of intracerebroventricular infusion of epidermal growth factor in rats’ hemitransected in the nigro-striatal pathway. J Neurosurg Sci 37:1–8
Pezzoli G, Zecchinelli A, Ricciardi S et al (1991) Intraventricular infusion of epidermal growth factor restores dopaminergic pathway in hemiparkinsonian rats. Mov Disord 6:281–287
O’Keeffe GC, Tyers P, Aarsland D et al (2009) Dopamine-induced proliferation of adult neural precursor cells in the mammalian subventricular zone is mediated through EGF. Proc Natl Acad Sci USA 106:8754–8759
Raineteau O, Rietschin L, Gradwohl G et al (2004) Neurogenesis in hippocampal slice cultures. Mol Cell Neurosci 26:241–250
Collombet JM, Beracochea D, Liscia P et al (2011) Long-term effects of cytokine treatment on cognitive behavioural recovery and neuronal regeneration in soman-poisoned mice. Behav Brain Res 221:261–270
Siddiqui A, Fang M, Ni B, Daoyuan L, Martin B, Maudsley S (2012) Central role of the EGF receptor in neurometabolic aging. Int J Endocrinol [Epub 2012 Jun 17]
Oyagi A, Moriguchi S, Nitta A et al (2011) Heparin-binding EGF-like growth factor is required for synaptic plasticity and memory formation. Brain Res 1419:97–104
Sibilia M, Steinbach JP, Aguzzi A, Stingl L, Wagner EF (1998) A strain-independent postnatal neurodegeneration in mice lacking the EGF receptor. EMBO J 17:719–731
Wagner B, Natarajan S, Grunaug S, Kroismayr R, Wagner EF, Sibilia M (2006) Neuronal survival depends on EGFR signalling in cortical but not midbrain astrocytes. EMBO J 25:752–762
Chen-Plotkin AS, Hu WT, Siderowf A et al (2011) Plasma epidermal growth factor levels predict cognitive decline in Parkinson disease. Ann Neurol 69:655–663
Hughes AJ, Daniel SE, Kilford L, Lees AJ (1992) Accuracy of clinical diagnosis of idiopathic Parkinson’s disease: a clinic-pathological study of 100 cases. J Neurol Neurosurg Psychiatry 55:181–184
Emre M, Aarsland D, Brown R et al (2007) Clinical diagnostic criteria for dementia associated with Parkinson’s disease. Mov Disord 22:1689–1707
Tomlinson CL, Stowe R, Patel S et al (2010) Systematic review of levodopa dose equivalency reporting in Parkinson’s disease. Mov Disord 25:2649–2653
Bjorkqvist M, Ohlsson M, Minthon L, Hansson O (2012) Evaluation of a previously suggested plasma biomarker panel to identify Alzheimer’s disease. PLoS One 7:e29868
Marksteiner J, Kemmler G, Weiss EM et al (2011) Five out of 16 plasma signaling proteins are enhanced in plasma of patients with mild cognitive impairment and Alzheimer’s disease. Neurobiol Aging 32:539–540
Henry JD, Crawford JR (2004) A meta-analytic review of verbal fluency performance following focal cortical lesions. Neuropsychology 18:284–295
Costafreda SG, Fu CH, Lee L et al (2006) A systematic review and quantitative appraisal of fMRI studies of verbal fluency: role of the left inferior frontal gyrus. Hum Brain Mapp 27:799–810
Banaschewski T, Ruppert S, Tannock R et al (2006) Colour perception in ADHD. J Child Psychol Psychiatry 47:568–572
Simmons WK, Ramjee V, Beauchamp MS et al (2007) A common neural substrate for perceiving and knowing about color. Neuropsychologia 45:2802–2810
Slack BE, Breu J, Muchnicki L, Wurtman RJ (1997) Rapid stimulation of amyloid precursor protein by epidermal growth factor: role of protein kinase C. Biochem J 327:245–249
Werner MH, Nanney LB, Stoscheck CM, King LE (1988) Localization of immunoreactive epidermal growth factor receptors in human nervous system. J Histochem Cytochem 36:81–86
Wiedermann CJ, Jelesof NJ, Pert CB, Braunsteiner H (1988) Neuromodulation by polypeptide growth factors: preliminary results on the distribution of epidermal growth factor receptors in adult brain. Wien Klin Wochenschr 100:760–763
Dubois B, Slachevsky A, Litvan I, Pillon B (2000) The FAB: a frontal assessment battery at bedside. Neurology 55:1621–1626
Carlesimo GA, Caltagirone C, Gainotti G (1996) The Mental Deterioration Battery: normative data, diagnostic reliability and qualitative analyses of cognitive impairment. The Group for the Standardization of the Mental Deterioration Battery. Eur Neurol 36:378–384
Caffarra P, Vezzadini G, Dieci F et al (2002) Rey-Osterrieth complex figure: normative values in an Italian population sample. Neurol Sci 22:443–447
Barbarotto R, Laiacona M, Frosio R et al (1998) A normative study on visual reaction times and two Stroop colour word tests. Ital J Neurol Sci 19:161–170
Spinniler H, Tognoni G (1987) Standardizzazione e taratura italiana di una batteria di test neuropsicologici. Ital J Neurol Sci Suppl 6
Giovagnoli AR, Del Pesce M, Mascheroni S, Simoncelli M, Laiacona M, Capitani E (1996) Trail making test: normative values from 287 normal adult controls. Ital J Neurol Sci 17:305–309
Mondini S, Mapelli D, Vestri A, Bisiacchi P (2003) L’Esame Neuropsicologico Breve. Raffaello Cortina Editore, Milano
Benton AL, Varney NR, Hamsher KD (1978) Visuospatial judgment: a clinical test. Arch Neurol 35:364–367
Conflicts of interest
The authors declare that they have no conflicts of interest.
Ethical standards
All human studies must state that they have been approved by the appropriate ethics committee and have therefore been performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Pellecchia, M.T., Santangelo, G., Picillo, M. et al. Serum epidermal growth factor predicts cognitive functions in early, drug-naive Parkinson’s disease patients. J Neurol 260, 438–444 (2013). https://doi.org/10.1007/s00415-012-6648-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-012-6648-6