Abstract
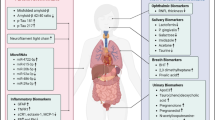
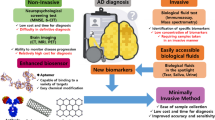
Alzheimer’s disease is a neurodegenerative disease, which affects intellectually is frequently observed in elderly generations and genetically linked individuals. The diagnosis of this disease is characterized by the presence of biomarkers such as amyloid beta (Aβ1–42), phosphorylated tau (P-tau), and total tau (t-tau) protein causing NFTs’ intercellularly interrupting synapse, which aids in transmitting the neuronal signals, thereby leading to memory loss and cognitive impairment failing to do basic tasks in daily life. Acquiring the diagnosis of this disease through the presence of biomarkers is categorized into two ways, invasive and noninvasive. The invasive way includes the collection of cerebrospinal fluid which is considered painful, but the advantage of this method is that it verifies the presence of the biomarkers accurately by taking into account its close association with the brain. This method is usually done at late stages for confirmation. The less painful or noninvasive ways include collecting samples from blood and ocular, olfactory, and oral fluids. These methods are in the preliminary stages of research that need further investigation to overcome methodological heterogeneity and discrepancy in accuracy and specificity.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Alzheimer’s disease
- Cognitive impairment
- Hippocampus atrophy
- Synapse
- Biomarkers
- Invasive
- Noninvasive
- Amyloid beta (Aβ1–42)
- Total tau (t-tau)
- Phosphorylated tau (P-tau)
- Neurofibrillary tangles (NFTs)
- Microsomal RNA
- Autoantibodies
- Isoprostanes
- ELISA
1 Introduction
Alzheimer’s disease (AD) causes considerable loss of forebrain cholinergic neurons, which undoubtedly facilitates dementia-causing components that include frequent manifestation of cell death, senile plaques, and neurofibrillary tangles in the neocortex, hippocampus, and basal nucleus of Meynert. Abetment of elements for AD diagnosis is the reduction in cerebral cortex and hippocampal acetylcholine neurotransmitter marked as one of the most significant abnormal alterations, and specific genes that undergo mutation such as amyloid precursor protein (APP), apolipoprotein (Apoε4), and presenilin 2 (PS 2) are known today, leading to β-amyloid protein buildup in cortex and hippocampus that are key areas for cognitive function and memory. Furthermore, forebrain cholinergic neurons appear to be directly disrupted by excessive glutamate receptor stimulation, mainly the NMDA receptor (Wenk 2003). The main attributes to be considered for a fluid biomarker are disease specificity and accuracy, appropriate results according to the disease’s level of infection, being noninvasive or unobtrusive concerning the retrieval of the fluid from the respective source, being convenient to employ on diverse individuals, and being effortless and cost-effective. The blood biomarkers satisfy most of these requirements, therefore counting it as a better source for knowing the disease advancement through the presence of biomarkers. Competently, the cerebrospinal fluid (CSF), although invasive due to the techniques involved in its collection, is of more importance considering the close association of CSF with the brain and also in furnishing the essential data regarding the chemical changes induced in the biological system happening in the brain during the preclinical stages of AD, while the other sources like blood, sweat, and tears give inefficient or inaccurate information about the disease progression. However, there are certain drawbacks when it comes to CSF because the mode of collection is invasive, extremely painful, and uncomfortable. On the other hand, if the mode of collection is blood, sweat, and tears, it is less painful and noninvasive, making these preferable over the former method (Ausó et al. 2020) and the detection of biomarkers of AD from these sources is characterized by the presence of Aβ1–42 (Aβ37, Aβ36, Aβ38, Aβ40), total tau, phospho-tau-181 (single or multiplex), multiple phosphorylated tau epitopes, or isoform-effectuated aggregation entailing extreme harm to neurons that are acquired using the analytical techniques like ELISA, INNO-BIA AlzBio3 Luminex-based technology (Innogenetics), quantitative real-time RT-PCR, LC/electrospray ionization MS, SELDI, and MALDI (Humpel 2011).
2 Biomarkers of AD
A biomarker is typically defined by substances (unnatural compounds, particular enzymes, cells, proteins, hormones, or (DNA, RNA) genetic material) or imaging findings that are utilized as attributes to identify the existence of a specific physiological condition and may aid in clinical diagnosis. Furthermore, a huge increase in research from 1980 to the present shows that the use of biomarkers is increasing for aiding the diagnosis and prognosis of AD.
2.1 Biomarkers Based on AD Stages
-
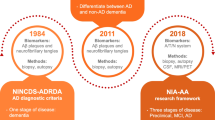
Stage 1: It includes people who may have a functional impairment but not cognitive impairment, which may only be determined through neuropsychological techniques. There is growing evidence that specific biomarkers, such as the presence of amyloid imaging and a altered CSF Aβ42 concentration, can indicate pathogenic alterations at an early preclinical period (Food and Administration 2018). The approval of AD treatment may be aided by early diagnosis based on biomarkers, which may have clinical advantages and result in better outcomes.
-
Stage 2: Although the patients have subtle cognitive alterations, and no functional deficits, they do not meet the requirements for dementia, which can be identified with the use of sensitive devices. The Food and Drug Administration (FDA) suggests that while diagnosing AD stage 2, sensitive neuropsychological tests and biomarker changes should be taken into consideration.
-
Stage 3: Pathophysiological biomarkers have been identified, and patients have begun to struggle with some measured everyday tasks. While the first two phases are preclinical, this stage of the disease is correlated with modest cognitive impairment.
-
Stages 4–6: Pathophysiological biomarkers are evident in the successive stages of mild, moderate, and severe Alzheimer’s disease dementia, with decreased cognitive impairment (Omar and Preddy 2020).
3 Invasive Biomarkers
The chief origin of noradrenaline in the brain is the locus coeruleus (LC) that is rich in neuromelanin is thought to influence attention and memory. Tau neurofibrillary tangles (NFTs) are first noticed in LC during the asymptomatic stage of AD before being found in other cerebral regions such as the entorhinal cortex and the neocortex. Tau clumps appear in the LC before the normal neuronal loss as AD progresses. Studies utilizing unbiased stereology have shown neuronal loss that is concentrated in the rostral/middle region of the LC, progressing from 30% in the prodromal stage to 55% when dementia is diagnosed, as well as an average drop in LC volume of 8.4% for each Braak stage advancement. In terms of function, this neuronal loss has been linked to cognitive degeneration and diminishing noradrenaline levels in the brain and hippocampus (Ausó et al. 2020).
Aβ42 is a 42-amino acid-long peptide associated with Aβ used as a biomarker for the diagnosis of AD. The concentration of Aβ42 in CSF is negatively proportional to the number of Aβ plaques implying that the reducing levels of Aβ42 and high concentration of Aβ plaques are observed in positron-emission tomography (PET) scans of CSF. The brain neuron contains tau protein in its axons. Escalated amounts of tau and phosphorylated tau are one of the main causes of AD (Gunes et al. 2022). As a result, if the patient responds well to treatment at the right time, the identification employed for in vivo imaging of early structural tissue modifications, like diminution in LC volume or metabolic changes, might aid in diagnosis and perhaps slows down the disease progression (Ausó et al. 2020).
3.1 CSF
The mean volume of CSF present in our brains’ ventricles, cranial and spinal subarachnoid spaces is 150 mL of which 25 mL is present in ventricles and the rest 125 mL in subarachnoid spaces. One of the most commonly known functions of CSF is its hydromechanical protection. Apart from this, CSF is necessary for brain growth regulation or turnover of brains’ ISF homeostasis and managing the function of neurons (Sakka et al. 2011). Obstruction in normal CSF circulation is thought to facilitate the cause of a wider range of advanced CNS pathologies like AD, one of the most commonly known neurodegenerative disorders (Simon and Iliff 2016).
Mild cognitive impairment (MCI) is an age-linked disorder that has obtained awareness among the scholarly community and common people. Most of the patients having MCI have inchoate AD, while others have benign MCI forms.
Neurodegeneration begins during the preclinical stage (2–3 decades prior to the clinical outset of the disease) due to an increase in tangle bundles at a certain threshold, and the first signs manifest (Blennow and Hampel 2003).
It is necessary to take into account proteins that are implicated in the abnormal breakdown of an APP as a peculiar biomarker for early diagnosis of AD. Specifically, the enzymes β-secretase 1 (BACE1) and presenilin 1 (PSEN1) are necessary for the breakdown of APP. PSEN1 and BACE1 activity levels were intensifying in the CSF of MCI individuals (García-Ayllón et al. 2013; Ewers et al. 2008). Furthermore, the Apoε4 gene has been linked to increased BACE1 (β-secretase 1) expression (Ewers et al. 2008). It was found that BACE1 activity was not elevated in MCI patients with persistent MCI, but only in those whose cognitive dysfunction declined to lead to later phases of dementia [10], making BACE1 not a strong candidate for being Apoε4 noncarrier even though it appears to be of more importance and is a potential early-stage biomarker to identify changes in the amyloidogenic pathway in Apoε4 carriers (Ewers et al. 2008).
Neuroinflammation and synaptic dysfunction are additional early AD features to emphasize, which consequently become distinct markers of these processes that may play a significant role that is closely linked to cognitive decline (Calsolaro and Edison 2016). Secretogranin II (SCG2), and chromogranin A (CHGA) are some proteins that take part in vesicular transport. The concentration of these proteins was found to be considerably more prominent in the CSF of patients with MCI, particularly whose condition is getting worse as AD pathology advances when compared to the healthy individuals (Duits et al. 2018). One research found that higher CHGA levels in the CSF of healthy elderly individuals suggested a subsequent decline in Aβ42 (Mattsson et al. 2013).
YKL-40 and visinin-like protein-1 are additional proteins that are vitally involved in inflammation (VILIP-1). In contrast to elderly people who are intellectually healthy, MCI and AD patients indicated a greater concentration of such compounds (Craig-Schapiro et al. 2010).
Interferon-induced protein-10 (IP-10) concentrations are elevated in the CSF of asymptomatic older generation who also showed increased concentrations of total tau and phospho-tau, which is another potential inflammatory marker (Bettcher et al. 2018).
Both MCI and AD patients continued to show increased concentrations of MCP-1, a low-molecular-weight cytokine associated with the inflammatory process (Dhiman et al. 2019).
Considering the existence of microRNAs (miRNAs), a large family of inherently occurring short noncoding RNAs that influence the amount of fully functional or mature miRNAs during the post transcriptional stage (Provost 2010), is also significant. The brain generates about 70% of the identified miRNAs, although some miRNA species are found in exosomes, making them promising prospects for use as biomarkers in clinical diagnosis. Only 40 of the 2000 human miRNAs that were discovered so far are highly expressed in the brain (Lukiw et al. 2013). The primary CSF biomarkers for clinical AD (Aβ42, total tau, and phospho-tau) tend to remain consistent which is helpful for diagnosis but are insufficient for monitoring the course of the disease. CSF miRNAs have the benefit of targeting significant pathological AD genes despite being collected in an invasive way, which is far from optimal. A small family of genes may be affected by a single miRNA’s ability to interfere with their expression. This is the situation regarding miRNA-125b (high in the case of AD), aiming at the cell cycle regulator CDKN2A and the enzymes 15-LOX and SYN-2. The possibility of using miRNAs as medicines in the subsequent years is more likely. Furthermore, the number of brain miRNAs was progressively reduced in areas of the brain that are susceptible to AD advancement (Mattsson et al. 2013), which may partly be explained by the dysregulation of particular miRNAs that may be associated with the etiopathogenesis of AD (Lukiw 2007) (Fig. 4.1).
Various biomarkers aid in detecting early morphological alterations of the brain observed in people already diagnosed with MCI comprising notable atrophy at different locations of the brain that include the LC or the hippocampus, and protein accumulation of amyloid plaques in extracellular Abeta plaques or intracellular tau prominences such as extracellular amyloid plaques or intracellular tau (accommodating NFTs). The cerebrospinal fluid of AD patients can be examined for indicators of degenerative processes linked to Alzheimer’s disease (AD), such as synaptic impairments, neuroinflammation, oxidative stress, or neuronal death. A new and foreshadowing tool for early AD diagnosis is the detection of miRNAs (Ausó et al. 2020).
3.1.1 Diagnostic Markers
Diagnostic markers are very important when it comes to the effective treatment of AD by using acetylcholinesterase inhibitors as they facilitate the detection to know the progression of the disease. There are two types of diagnostic markers. They are state and stage markers.
3.1.2 State Markers
The severity of the disease’s progress is mirrored by state markers. The total amount of tau protein is one illustration of a state marker representing the severity of neuronal deterioration and injury. There is a momentary rise in tau protein content in the CSF during acute diseases (e.g., ischemic stroke), and this rise is correlated with the extent of the infarct as determined by CT.
Additionally, disorders with severe neuronal degeneration, such as Creutzfeldt-Jakob disease, have the highest levels of increased tau protein concentrations in the CSF, whereas gradual increase is observed in AD patients, but patients with depression have no or marginal neuronal degeneration and have a normal concentration. In patients with MCI, state markers, such as tau protein, are likely to have a greater predictive value than stage markers, like hippocampal shrinkage on CT or MRI.
3.1.3 Stage Markers
They calculate the degeneration process. Hippocampal atrophy as evaluated by CT or MRI is one illustration of a stage marker. Stage markers also include clinical rating measures, which quantify the intensity of cognitive decline. Regardless of the phase, hippocampal atrophy (9–15% reduction in hippocampus volume) is less severe than elevated tau protein concentrations (210–290% increase).
3.1.4 Biomarkers in CSF for AD
Because the CSF and the brain’s extracellular space are directly associated, alterations in the brain’s biochemistry always have an impact on the CSF. AD pathology only affects the brain, so CSF is a natural place to detect AD biomarkers. The proportions of total tau protein, Aβ1–42 (the 42-amino acid version of Aβ), and phosphorylated tau protein in the CSF may potentially serve as biomarkers for these pathogenetic processes (Blennow and Hampel 2003).
3.1.4.1 Aβ1–42
APP undergoes consecutive cleavages by β- and γ-secretases to form profibrillary Aβ1–42, an essential constituent of senile plaques, chiefly composed of Aβ. Aβ42, an extensively soluble protein, gets accumulated on the walls of leptomeningeal structure and CAA. Generally, Aβ originates in our brain due to synapsis. Considerably, it gets dispersed through being ingested by microglial cells and astrocytes flowing across the BBB aided by solute transporters as well as PGP (D-glycoprotein) and LRP-1 (low-density lipoprotein receptor-related protein) that are dispersed through the perivascular glymphatic pathway. CSF levels of amyloid is elevated in humans, when a person is awake where glymphatic pathway activity and Aβ clearance are considerably reduced. Then, as sleep sets in, interstitial Aβ levels decrease as perivascular CSF-ISF exchange becomes active and interstitial Aβ clearance is accelerated. These results suggest that the physiological clearance of Aβ from the brain is significantly influenced by perivascular CSF-ISF exchange along the glymphatic pathway. This physiological clearance of Aβ through CSF-ISF exchange is reduced due to alterations in BBB Aβ dispersion, leading to the accumulation of Aβ protein resulting in neurodegeneration (Simon and Iliff 2016).
3.1.4.2 Tau Protein
Tau protein exists in the human brain in six different variants and has a vast group of phosphorylation sites (Fig. 4.2). There are 85 possible sites for serine (S), threonine (T), and tyrosine (Y) phosphorylation in the phosphorylated tau protein. The proline-rich domain of tau, on each side of the microtubule-binding domain, contains a large number of phosphorylated tau residues (Blennow and Hampel 2003). In the case of neurodegenerative disorders, they are present in abnormal amounts inside neurons and neuronal axis as NFTs.
The six human central nervous system (CNS) tau variants are shown (Fig. 4.3). Exons 2 and 3 (E2 and E3) code for two distinct 28-amino acid sequences located close to the tau’s N-terminal. Tau isoforms with 0 N are produced when E2 and E3 are absent, while tau isoforms with 1 N and 2 N are produced when E2 and E3 are present. The four incorrect-reoccurring microtubule-binding domains are represented by M1 through M4, with M2 being encoded by exon 10. When M2 is absent, 3R tau is produced, and when M2 is present, 4R tau is produced. The tau polypeptide’s central proline-rich region is highlighted. As a result, alternative splicing results in tau proteins with 352–441 amino acids (Fig. 4.3).
There are about 45 phosphorylation sites known, most of which are in the proline-rich domain and the areas surrounding the microtubule-binding domain.
The concentration of tau protein as a biomarker for AD in CSF was first reported in 1993 for which the ELISA method was used along with monoclonal antibodies. Later, the ELISA methods depending on monoclonal antibodies were developed that helped in identifying ensemble forms of tau protein autonomous of their phosphorylation and development. Tau phosphorylation is controlled by certain kinases and phosphatases. Tau hyperphosphorylation in disease is thought to be the outcome of an imbalance in tau kinase and phosphatase activity. GSK-3, cdk5, and AMPK are examples of proline-directed tau kinases. CK1, MARKs, PKA, and DYRK-1A are examples of non-proline-directed kinases. Tyrosine kinases include Syk, Fyn, and Abl. And also, several phosphatases include PP1, PP2A, and PP5 and dephosphorylated tau (Noble et al. 2013).
3.1.4.3 Phosphorylated Tau Protein
Phosphorylated tau protein is the latest biomarker for AD to be identified. For several tau protein phosphorylated homologs, such as threonine 181 and 231, threonine 181, threonine 231 and serine 235, threonine 199, threonine 231, and threonine 396 and 404, several techniques have been developed. The amount of phosphorylated tau protein in the CSF likely correlates with the level of phosphorylation of tau in the brain. But the amount of phosphorylated tau protein remains unchanged after an acute stroke with regard to the overall amount of tau protein. Moreover, despite a marginal increase in total tau protein concentrations, phosphorylated tau protein concentrations do not rise in Creutzfeldt-Jakob disease.
These results demonstrate that the concentration of phosphorylated tau protein in the CSF also depicts the phosphorylation state of tau and, consequently, the formation of tangles in AD, rather than just serving as a signal for neuronal damage like total tau protein (Blennow and Hampel 2003).
3.2 Association of Biological Biomarkers in CSF: The Diagnosis of AD in Different Cases
3.2.1 Role of Plasma Amyloid Proteins in Patients whose Diagnosis is Hereditary
At times, elevation in Aβ plasma concentration is noticed in patients with AD, especially those who are related and have pre-symptomatic carriers of mutations, making AD inheritable and genetically involved. It is observed in patients whose diagnosis is not hereditary and is not seen in spasmodic cases.
3.2.2 Role of Plasma Amyloid Proteins in Down Syndrome Patients who are Diagnosed with AD
Down syndrome (DS) patients ensure neuropathological alterations that are in commensuration with AD probably as a result of APP gene triplication and overexpression.
The rise in Aβ42 and Aβ40 plasma levels in DS patients is presumed as a primary cause in the facilitation of amyloidosis and the advancement of dementia.
A survey was done which included 30 individuals with DS where 21 of them did not develop dementia, but the eight patients developed. The cognitive and daily task performance of all 30 of them was evaluated along with blood samples every year for 5 years. During this delve, eight of them were diagnosed with dementia. The most unusual thing observed was that there were not any fluctuations in Aβ40 and Aβ42 plasma concentration compared to a healthy person. To straighten up, Aβ42 and Aβ40 are not convincing enough that they facilitate the advancement of AD (Frey et al. 2005).
3.2.3 Role of Sex Differentiation Linked to AD Biomarkers’ Prominence and Intellectual Depreciation
There is a pretentious rife of AD in women because of the disease’s commonness and acuteness. It has been revealed in earlier autopsy research that women affected with AD are more vulnerable to clinical implications of AD. By investigating whether sex influences the existing correlation between CSF biomarker levels and consequences of brain aging, Mary E.I. Koran et al. expanded this research work broader. In this investigation, individuals with normal intellect (n = 348), MCI (n = 565), and AD (n = 185) were chosen in addition to the people selected from ADNI, employing multivariate degeneration models. They obtained an analysis that impacts baseline levels of CSF biomarker Aβ42 and an ensemble of tau on cross-sectional, extensive brain aging as an aftermath. They also procured a substantial interlinkage betwixt sex and Aβ42 on the progression of hippocampal atrophy (p = 0.002), extensive memory loss (p = 0.017), and cognitive impairment (p = 0.025) analogously; they also noticed a substantial interaction betwixt sex and overall tau protein on extensive hippocampal atrophy (p = 0.008) and cognitive decline (p = 0.034). Sexual diversity is shown to be prominent in people with MCI whose literacy rate is low.
3.2.3.1 Influence of Sex on Aβ42
After the initial assessment, participants were monitored for an average of 2.5 years (range: 0–9 years). Initial memory performance (t (1198) = 1.58, p = 0.11), executive function performance (t (1198) = 0.29, p = 0.77), hippocampus volume (t (1082) = 0.93, p = 0.35, and t (1082) = 0.12, p = 0.90, respectively), or sex did not influence Aβ42. Throughout cumulative comparisons, there existed a correlation between sex and Aβ42 where three of the five manifested brains aging during evaluation included cognitive performance and left hippocampal shrinkage (t (2739) = 3.07, p = 0.002, and t (4154) = 2.39, p = 0.02). In all cases, the female sex was linked to deteriorating outcomes when there was a low Aβ42 level. Low CSF Aβ42 is a sign of higher brain Aβ42. Concerning right hippocampus atrophy, sex did not interact with Aβ42(t (2739) = 1.54, p = 0.12).
3.2.3.2 Influence of Sex on Tau
After an initial assessment, sex is not associated with tau concerning cognitive decline (t (1198) = 0.51, p = 0.61), right hippocampus volume (t (1082) = 0.49, p = 0.62) or left hippocampal volume (t (1082) = 0.44, p = 0.66), and memory decline (t (1198) = 0.22, p = 0.83). Throughout cumulative comparisons, the association of tau with sex was observed which included left hippocampus shrinkage and cognitive decline (t(2739)=−2.61, p=0.009) and (t (4132) =−2.11, p = 0.03, respectively). When there was an elevated amount of total tau, the female sex was linked to deteriorating executive function and hippocampus atrophy. But sex did not interfere with the ensemble tau level associated with overall cognitive performance (t (4154) = 1.20, p = 0.23) or right hippocampus atrophy (t (2739) = 0.56, p = 0.58) (Koran et al. 2017).
3.2.4 Role of CSF Abundance and Clearance Associated with the Prominence of AD Biomarkers and Other Factors
The BBB Aβ efflux pathways in the geriatric brain are hampered, which may lead to amyloid aggregation and later neuronal death. This dysfunction causes the downregulation of PGP and LRP-1 that aid in CSF production. However, as age progresses, the expression of the choroid plexus (CP) Aβ efflux transporter escalates indicating that the CP and BCSFB could serve a contributing role in amyloid clearance from the ventricular CSF and concomitant ISF. With senescence, AD symptoms show deposition of amyloid within the CP and reduced CSF secretion by the CPs; therefore, the effectiveness of amyloid clearance with the bulk reabsorption of CSF via arachnoid villi and along cranial nerve sheaths minimizes the rate of CSF turnover.
The brains of elderly people also exhibit disrupted glymphatic pathway function, which includes altered perivascular CSF-ISF exchange and impeded clearance of interstitial amyloid β. Uncertainty exists regarding the possibility that decreased CSF secretion by the CP is contributing to the perivascular CSF flow through the aging brain slowing.
Glymphatic pathway function was slowed when CSF secretion was experimentally disrupted by providing carbonic anhydrase inhibitor acetazolamide, which infers that lowered CSF secretion at the CP could be a contributor to disrupted lymphatic pathway function in the aging brain. But perivascular AQP4 that takes part in CSF production whose localization was also substantially diminished in the aging brain was attributed to a delayed perivascular CSF inflow into and through the brain parenchyma. It has not yet been formally ascertained whether perivascular AQP4 location loss stimulates amyloid aggregation in the aging brain. Nevertheless, the ability of Aβ deposits that lead to AQP4 mislocalization, be it in the form of senile plaques or CAA, indicates the existence of a growing pathogenic cycle, with reactive astrogliosis obstructing glymphatic pathway function as well as Aβ clearance, leading to amyloid deposition and furthermore neuroinflammation, resulting in disruption of the glymphatic pathway (Simon and Iliff 2016).
4 Noninvasive Biomarkers
There is a need for the development of early noninvasive diagnostic techniques for AD; even though the established neuroimaging techniques show promising results, they are expensive and cause radiation. Collection of CSF fluid needs lumbar puncture, so the diagnosis/analysis of peripheral fluids is a good alternative which includes blood, urine, and ocular, oral, and olfactory fluids (Gleerup et al. 2019).
4.1 Oral
Studies suggest that saliva is one of the potential sources of a noninvasive biomarker for AD diagnosis; biomarkers could be produced directly in salivary glands/diffused from blood. Degeneration of neurons or nerve cells is observed in AD; it is also observed that this causes damage to nerve cells related to autonomic nervous system (ANS) and blood. ANS regulates the production of saliva via three major salivary glands (parotid, sublingual, submandibular); thus, there will be alteration in the production and composition of saliva. Various studies have identified and determined Aβ40, Aβ42, P-tau, and t-tau in the saliva of patients diagnosed with AD (Tvarijonaviciute et al. 2020).
4.1.1 Beta Amyloid
The pathological hallmarks of AD include Aβ accumulation and tau hyperphosphorylation. Pareja et al. reported a statistical increase of Aβ42 in the saliva of people with mild AD and no visible change in people with severe AD (Bermejo-Pareja et al. 2010). Later, studies by Sabbagh et al. reported a 2.45-fold rise in levels of Aβ42 at all stages of AD (mild to moderate) (Liang and Lu 2019).
4.1.2 Tau
AD is marked by tau protein hyperphosphorylation and aggregation; t-tau (total tau) represents the intensity of neuronal damage, and P-tau represents the phosphorylated state of tau, which has been assessed in various studies. Origin of salivary tau remains unknown; there are numerous possibilities which include the possibility of salivary tau protein release from nerves that innervate salivary glands as they are proximal to CNS via cranial nerves. Another possibility includes the secretion of tau by acinal epithelial cells of salivary glands. The P-tau/t-tau ratio was shown to be significantly higher in AD participants compared to cognitively healthy older subjects, particularly at the S396 phosphorylation site.
4.1.3 Acetylcholinesterase (AChE)
Acetylcholine (ACh) cholinergic neurotransmitter is metabolized to its subsequent components, i.e., choline and acetic acid, by AChE. Cholinergic neurons, which are essential for memory and learning, are damaged at an early stage of AD. Because of differences in sample methodologies and potential nonlinear changes in enzyme activity correlated with progression of the disease, data on changes in AchE activity linked with AD in peripheral bodily fluids have been inconsistent (Tvarijonaviciute et al. 2020). Balchitiari et al. state that despite the fact that salivary AChE activity in persons with AD was lower than in the control group, there were no significant changes (Bakhtiari et al. 2017).
4.1.4 Lactoferrin
According to a few studies and evidence reported, bacterial/viral infections in the brain may cause or exacerbate the pathophysiology of AD. Because of its antibacterial protein composition, saliva constitutes one of the body’s first lines of defense. Lactoferrin is an Aβ-binding glycoprotein, a foremost antimicrobial peptide in saliva. Chero et al. reported decreased levels of saliva lactoferrin. Decreased levels of saliva lactoferrin increase the risk by greater than 77% of converting into amnestic MCI and AD dementia (Carro et al. 2017). The accuracy of salivary lactoferrin diagnosis was found to be greater than that obtained from CSF biomarkers (T-tau, CSF Aβ) (Liang and Lu 2019).
4.1.5 Others
Reports have proposed a relation between high salivary sugar levels and progress of AD. Therefore, salivary sugar levels (trehalose) may serve as a potential biomarker of AD (Lukiw 2007) (Fig. 4.4).
4.2 Eye
4.2.1 Retinal Biomarkers
4.2.1.1 Retinal Nerve Fiber Layer (RNFL) and Optic Nerve
Hinton et al. presented the first histopathological evidence of retinal abnormalities in AD, i.e., widespread ganglion cell losses, retinal nerve fiber layer (RNFL) thinning, and optic nerve degeneration in comparison to healthy controls (Hinton et al. 1986).
Blanks et al. (1996) provide the basis for the usage of retinal imaging as a potential biomarker for AD. In vivo assessments of the nerve fiber layer and the optic nerve are often done using scanning laser ophthalmoscopy (SLO) and optic coherence tomography (OCT), with SLO being preferable because it uses a confocal scanning laser system that enables accurate depth resolution. SLO was used by Kergoat et al. (2001) and Danesh-Meyer et al. (2006) for identifying RNFL thinning in retina’s peripapillary and macula areas (Lim et al. 2016).
Berisha et al. observed RNFL thinning in AD; these assessments are supported by various studies in patients with MCI and AD, which showed that ganglion cell complex (including ganglion cell bodies, their dendrites, and RNFL) at macula is a significantly better indicator than RNFL thickness. In addition, this method is used in order to differ AD and MCI from healthy controls (Berisha et al. 2007).
4.2.1.2 Retinal Blood Flow and Vasculature
Researchers have theorized about the possible value of evaluating the blood volume of the eye as a biomarker of AD due to the resemblances between the blood vessels of the brain and the eye. Common vascular issues linked with AD include decreased blood vessel density, decreased blood flow, vasoconstriction, and inadequate Aβ clearance.
Berisha et al.’s (2007) study showed reduced venous blood flow and substantial narrowing of retinal veins in comparison to healthy controls by using Doppler imaging device (Berisha et al. 2007). Cheung et al. (2014), using fundus photography and automated vessel segmentation software, showed (1) a decrease in retinal vessel caliber and (2) a decrease in fractal dimension (a measurement of the overall branching structure of the retinal vascular tree). (3) There is no change in the angle subtended between both daughter vessels at each bifurcation, which is the vessel branching angle. (4) An increase in vascular tortuosity, or the average percentage of curliness/“non-straightness” of retinal vessels, is also seen.
Thus, vascular measures may be beneficial for screening, but the question is whether age, atherosclerosis, and vascular related problems can be distinguished from AD-related alteration; moreover, retinal vascular parameters that can distinguish MCI from AD are yet unclear (Cheung et al. 2015).
4.2.1.3 Intraretinal Tau and Amyloid Deposition
Ratnayaka et al.’s studies confirmed that retinal ganglion cells express APP in the inner nuclear layer of the retina and retinal pigment epithelium; these retinal ganglion cells also express an enzyme which is capable of producing Aβ, i.e., β-secretase (Ratnayaka et al. 2015). AD deposits (tagging): first successful demonstration of noninvasive in vivo visualization of curcumin-bound fluorescent Aβ in the bitransgenic mice retina, which possesses the mutated genes that regulate presenilin-1 (PS-1) and APP.
The CSIRO in Australia presented extensive preliminary results in humans using curcumin contrast in the patients with proven brain deposits using (Pittsburg compound B) PiB-PET imaging.
However, other studies have not observed the same results, suggesting that P-tau could be a potential biomarker compared to Aβ; presence of P-tau is demonstrated using SLO imaging of FBS [(trans, trans)-1-fluoro-2,5-bis-(3-hydroxycarbonyl-4-hydroxy) styryl benzene] bound tau in the retina of P310S human tau mouse line.
Despite being hopeful, HO et al. (2014) were unable to locate Aβ- or P-tau deposits in the retina of AD donors. The authors conclude that AD hallmarks do not accumulate in the eye like that of the brain, despite the possibility that using different assays, such as limited retinal cross sections instead of whole mounts and paraffin-embedded sections rather than frozen tissues, may reduce the sensitivity of these proteins’ identification (Ho et al. 2013).
AD deposits (visualizing): cross polarizers are newly developed imaging technology that can discriminate between controls and ex vivo human volunteer retinas with AD (Campbell et al. 2015; Hamel et al. 2016). By using the fibrillary arrangement of Aβ, this technique produces distinct modifications to birefringence that may be quantified using Mueller matrix polarimetry (Campbell et al. 2015; Hamel et al. 2016).
4.2.1.4 Choroidal Thickness
The choroid, which is placed between the retina and the outermost layer of the eye, is specifically responsible for the blood flow to the retina. Modern OCT devices include enhanced depth imaging (EDI) capabilities that use longer wavelengths in the 1060 nm range (Wong et al. 2011), allowing for deeper penetration into the eye’s layers and viewing of the choroid (Wong et al. 2011).
According to Gharbiya et al. (2014), studies of AD participants (n = 21) had choroid that was 30% thinner than that of healthy controls (n = 21) directly below and within 1 mm of the fovea, and RNFL thinning was not observed; this indicates that choroidal thinning in AD occurred before RNFL alterations (Gharbiya et al. 2014). The major challenge is that choroidal thinning endures substantial diurnal variations (Kinoshita et al., 2016). Moreover, choroidal thinning is a symptom of several other conditions, including uveitis, chronic obstructive pulmonary disease, myopia, and aging (Barteselli et al., 2012) (Kinoshita et al. 2017; Shah et al. 2012).
4.2.2 Nonretinal Biomarker
4.2.2.1 Pupillary Reactions
The dilator muscles and iris sphincter work in harmony to balance forces that affect pupil size and pupillary response to light. The former is innervated by parasympathetic cholinergic receptors emerging from the Edinger-Westphal nucleus, while the other is innervated by postganglionic sympathetic noradrenaline receptors emerging from the superior cervical ganglion.
Together, these findings demonstrate that high-speed video pupillography pupil flash responses, which directly evaluate the integrity of the cholinergic system in the nervous system, may be a promising biomarker. It is unclear if using AD drugs such as cholinesterase inhibitors can minimize this outcome. Indeed, further research is needed to determine the dependability and reproducibility of these growing technologies (Lim et al. 2016).
4.2.2.2 Crystalline Lens
The lens is a transparent biconvex structure in most land vertebrate eyes; the crystalline lens, together with the cornea, aqueous and vitreous humors, is responsible for focusing incident light from the outside world towards the retina.
The crystalline lens is primarily composed of crystalline proteins in higher concentration, giving the ability to examine protein aggregation, which contributes to cataract formation (van Wijngaarden et al. 2017). Studies have identified Aβ deposits in crystalline lenses of rats, rabbits, monkeys, and transgenic mice. The first report of an AD-specific cataract in humans was made by Goldstein et al. (2003), who found deposits in the cytoplasm of the equatorial supranuclear/deep cortical lens fiber cells (Goldstein et al. 2003). This AD cataract does not impede vision and is difficult to detect during a regular eye examination until full dilatation is achieved; studies have failed to detect Aβ in human lenses using either confocal Raman spectroscopy or standard immune histochemistry methods (Lim et al. 2016). Kerbage et al. (2013) performed two small clinical studies on people with AD; researchers used in vivo imaging of lens. The researchers used a fluorescent ligand (aftobetin hydrochloride) with Aβ affinity and exhibited ocular penetration after topical application of an ointment (Kerbage et al. 2013).
Using in vivo laser fluorescence spectroscopy, the researchers were able to identify supranuclear amyloid in the lenses of the majority of AD patients with 95% specificity and 85% sensitivity in a group of 20 AD and 20 healthy control subjects (Kerbage et al. 2015).
The clinical prediction was more accurate using this approach than AβPET imaging (florbetapir F18). If this strategy is duplicated in a bigger study with a diverse range of people with variable disease severity, it may show promise as a biomarker for AD. The disadvantage of this strategy is that it will be ineffective in those who have had cataract surgery (van Wijngaarden et al. 2017).
4.2.2.3 Eye Movements
Due to suboptimal eye movements, AD patients are known to have difficulty in reading; they demonstrate decreased eye movement velocity, failure to concentrate on a target, and failure to track a moving target; these errors are observed due to damage to cortex and brain stem neural generators. Further studies are needed to establish a distinct AD signature, eye movements, as a possible biomarker (Lim et al. 2016).
4.2.3 Potential Future Biomarkers
4.2.3.1 Ocular Fluid Biomarkers
Since the 1990s, proteomics has been utilized for extensive protein analysis in both health and diseased conditions, including AD. Aqueous humor samples were subjected to proteomic analysis for presence of the AD-related peptides. Tear fluid, which is simple to obtain, has recently been demonstrated to be viable clinically (Kalló et al. 2016) and has demonstrated promise in the diagnosis of neurological illnesses like glaucoma (Kalló et al. 2016; Tezel 2013).
miRNA has attracted a lot of interest as a subclass of noncoding RNA regulatory molecules that may regulate gene expression at the post transcriptional stage by interacting with the untranslated region of mRNAs that they target. Numerous potential miRNAs have already been shown to play a part in controlling the production of amyloid, including hsa-miR-106, -153, -101, -29, and -107 (Kumar et al. 2013). The viability of miRNA molecules obtained from tears as a fluid biomarker is further investigated (Kumar et al. 2013).
4.2.3.2 Corneal Nerve Imaging
The three layers of corneal nerves are found between the Bowman’s layer and the basal epithelium (subbasal nerve plexus), the Bowman’s layer and the anterior stroma (subepithelial nerve plexus), and the stroma (stromal nerve plexus) (Lim et al. 2016).
The nerve growth factor plays an important role in the growth and maintenance of neuronal cells in the cornea, central nervous system, and peripheral nervous system. Cholinergic neurodegeneration in AD has been connected to impaired retrograde nerve growth factor (NGF) transport (Rocco et al. 2018). The viability of the corneal nerve may serve as an AD biomarker. Given the direct imaging capabilities of corneal nerves, a functional measure of axonal transport may be more effective in identifying AD. The various ocular parts shown in Fig. 4.5 can serve as a source of AD biomarkers.
4.3 Urine
Urine is now a popular source of illness indicators due to its noninvasive nature. Its composition comprises cellular components, metabolic substances, and proteins derived from glomerular filtration. Urogenital tract secretion/renal tube secretion reflects the pathophysiological and metabolic condition of an individual (Bălașa et al. 2020). Studies have reported the presence of protein biomarkers in urine, which include (AD7c-NTP) AD-associated NTP; neural thread protein (NTP) levels are increased in CSF of people with AD, and thus, it has been considered as a possible AD biomarker, detected by using monoclonal antibody assay. Studies by L. Ma et al. showed a rise in urinary levels of NTP in patients with MCI and AD. As the specificity is unclear, further research is needed to distinguish AD from other types of dementia (Ma et al. 2015).
Oxidative stress is one of the important factors in AD; research into the molecules associated with this brain damage may provide possible early AD indicators in urine (Bălașa et al. 2020). Isoprostane 8,12-iso-iPF (2-alpha)-VI, a free amino acid product of lipid peroxidation, was shown to significantly increase in several studies. This finding may imply a pathological transition from MCI to AD dementia (García-Blanco et al. 2018; Praticò et al. 2002).
4.4 Olfactory
Postmortem investigations have revealed pathological abnormalities in AD, such as the development of neurofibrillary tangles in the anterior olfactory nucleus and olfactory bulb regions crucial in olfactory information processing. Braak staging is used for early AD pathology in entorhinal and trans-entorhinal areas (Murphy 2019). Based on it, studies found 90% of tau and 9% of Aβ in patients with AD (loss of smell) than in healthy individuals (Attems and Jellinger 2006). The basic hallmarks of AD are also observed in the nasal secretions, neuritic plaques, neuropil threads, and neurofibrillary tangles that were found to be in olfactory bulbs of AD patients in a study conducted in 1987 (Ohm and Braak 1987). Moon J et al. (2016) reported increased levels of microRNA-206 by performing an intranasal biopsy of the olfactory epithelia in AD patients compared to healthy controls. Preliminary research on oral, ocular, olfactory, and urine biofluid potential noninvasive biomarkers is still ongoing, which requires additional study to address methodological variability and difference in accuracy and specificity (Moon et al. 2016).
4.5 Blood
Compared to other bodily fluids like CSF, blood is the most easily accessible biological sample. It offers affordable clinical diagnostic or screening procedures and is even practical for achieving repeatable outcomes in clinical trials. Biofluid blood has been established as a biomarker in the diagnosis and study of cancer and cardiovascular disease; as a result, it may function as a crucial component in the early detection of AD (O’Bryant et al. 2017). Aβ is considered as a potential blood biomarker due to its ability to pass through the BBB and its presence in the prodromic stage of AD (Omar and Preddy 2020).
4.5.1 Aβ
The results on Aβ42 and Aβ40 levels in the plasma of AD patients throughout the first decade of the 2000s were inconsistent and contrary. Mayeux et al. discovered elevated plasma Aβ42 levels, however not plasma Aβ40 levels, in AD patients. When compared to people with low plasma Aβ42, the chance of developing AD was more than twice as high in individuals with high plasma Aβ42. In further research, van Oijen et al. observed that high plasma Aβ40 concentration is linked to an improved risk of dementia (Preische et al. 2019). However, other studies discovered that a lesser plasma Aβ42/Aβ40 ratio is linked to higher cognitive decline among older adults. This discrepancy results from the clinical evaluation stage or the combination of different kinds of dementia.
Trials based on immunological precipitation-mass spectrometry-based assays were conducted, which demonstrated a considerable decrease in both Aβ40 and Aβ42 concentrations in plasma, to obtain reliability and precision in the analysis of biomarkers (Nakamura et al. 2018; Ovod et al. 2017). A novel ELISA approach was used to examine in vivo levels of Aβ oligomers vs. Aβ monomers in both plasma and brain tissues of individuals with sporadic and familial AD, which revealed a strict association between the Aβ oligomers and amount of Aβ42; however, the concentration of plasma Aβ is not clear yet.
4.5.2 Tau
Studies have indicated that p-tau is a late-stage biomarker of AD (Omar and Preddy 2020), and because t-tau levels are so low in MCI and/or AD collated to CSF, it is essentially undetectable in these conditions. Increased t-tau levels are seen in the plasma of AD patients compared to healthy controls and MCI using an ultrasensitive approach created by Zetlerberg et al.; however, it was unable to differentiate among patients with stable MCI and MCI that acquired AD (Zetterberg et al. 2013). Tatebe et al. developed a novel ultrasensitive immune assay for P-tau 181 quantification, which showed increased levels of p-tau 181 in patients with AD (Tatebe et al. 2017).
4.5.3 Isoprostanes
Increased plasma levels of isoprostanes (F2-IsoP) are observed in patients with AD (Praticò et al. 2000); it is considered as an unspecific biomarker for AD due to increased levels of isoprostanes in other diseases associated with oxidative stress (Montine et al. 2002).
4.5.4 Post translational Modification (PTM)-Plasma
PTMs are generally modifications (non-coded DNA) to the structure/composition of proteins that generate neo-epitopes (novel and distinct protein components); modifications include isomerization, methylation, acetylation, nitrosylation, etc. (Karsdal et al. 2010). Few neo-epitopes are discovered and utilized as biochemical markers to track disease severity. Aβ1–40, Aβ1–42, and phosphorylated tau serve as post translational modified protein species; however, they are not established as a biomarker of AD in the blood largely as a result of technical reasons (Thambisetty and Lovestone 2010; Mayeux and Schupf 2011). Pathologically, AD is an interesting disease for PTM-based biomarker development due to the formation of Aβ, P-tau, and other proteins linked to neuronal pathologies such as DNA binding/protein 43 and alpha-synuclein.
4.5.5 Autoantibodies
Autoantibodies are established to be present in AD, but it is unclear whether they are more likely to be defensive or destructive in terms of pathology. However, given that many of them are present in both blood and CSF, their potential as biomarkers for AD is of significant notice (Colasanti et al. 2010).
Autoantibodies against Aβ have drawn a lot of interest, and there are signs that they may have diagnostic value (Gustaw-Rothenberg et al. 2010). They exist in both forms: antigen-bound and free antibodies. It is still unclear how beneficial measuring these autoantibodies is for AD diagnosis and/or prognosis, even though the instruments to do so have been developed (Maftei et al. 2012). Serum autoantibody profiling was utilized in a study by Nagele and colleagues, who discovered that autoantibodies are prevalent in serum from early to advanced stages of AD. In a panel of ten autoantibodies, they also found remarkable specificity and sensitivity (90%) for AD diagnosis. It is yet unclear, nevertheless, whether these features are more prominent in higher AD populations and are unique to AD compared to other dementias. Hence, before using this strategy, there is still a lot of validation to be done (Nagele et al. 2011).
4.5.6 MicroRNA
As compared to autoantibodies, here is a strong confirmation that changes in microRNA levels are connected to various aspects of AD pathology (Geekiyanage et al. 2012); as a result, efforts are being made to measure changes in the concentration of specific microRNAs in blood as AD biomarkers. The theory that blood microRNAs might act as biomarkers for AD and/or MCI has some value, although there are currently only a few studies employing a limited number of individuals. Compared to AD, MCI had greater variation in microRNA levels. The lack of overlap between two studies and the study’s comparatively small sample sizes point to the need for additional research to fully comprehend the utility of these blood-based AD biomarkers (Sheinerman et al. 2012).
4.5.7 Blood-Derived Genetic Markers
It is well known that Apoε4 alleles increase the risk of AD; they are considered as genetic biomarkers for early-onset types of AD. Epigenetic studies on the Apoε4 allele have suggested that this gene may have a role in AD. More significantly, similar modifications were seen in corresponding blood lymphocytes as well as brain tissues (Wang et al. 2008). Another study found that in comparison to healthy control subjects, AD patients expressed hypermethylated promoter regions of the Apoε4 gene (Kaddurah-Daouk et al. 2011). The fact that this study only examined brain samples emphasizes the necessity for additional analysis of epigenetic modifications in blood-derived samples along with larger patient populations (Patel et al. 2011). However, these results indicate that abnormal gene methylation may have a major effect on AD, and if it can be found in blood-derived cells like lymphocytes, it might potentially be an AD biomarker (Henriksen et al. 2014).
4.5.8 Transcriptome
Several separate organizations have described the possible utility of blood-based gene expression profiling in the diagnosis of brain diseases (Sharp et al. 2006; Burczynski and Dorner 2006; Gladkevich et al. 2004). The transcriptome, or collection of messenger RNAs in a specific cell or tissue type, represents essential information for determining the final expression of the proteome that is used to develop a blood-based signature to distinguish AD patients from asymptomatic control subjects (Henriksen et al. 2014).
Two transcriptome-based strategies, one employing a 96-gene set and the other a 136-gene strategy, have been reported. Both methods produced useful diagnostic results, by comparing AD patients with control subjects who were of similar age. The study by Rye and colleagues, which showed a connection to CSF biomarker levels, emphasized the potential of these strategies (Rye et al. 2011). Interestingly, the study by Booij and colleagues reported a distinction between AD and patients with Parkinson’s (Booij et al. 2011). These investigations may aid in the development of novel ideas about the AD pathophysiology and may explicate various physiological phenomena, such as inflammatory processes, involved in the onset of the disease. Even so, there are still critical data gaps for example, validation in a large clinical group as the AD Neuroimaging Initiative or the Australian Imaging Biomarkers and Lifestyle research (Fehlbaum-Beurdeley et al. 2012).
4.5.9 Markers of Inflammation
Amyloid deposition in the AD brain triggers several reactive inflammatory responses, including astrocytosis, microgliosis, an increase in proinflammatory cytokines, acute-phase reactions, and complement activation (Weiner and Selkoe 2002). Because most of these proteins have difficulty crossing the BBB, it is difficult to determine if the buildup of cytokines and acute-phase reactants in brain are similarly reflected in serum or plasma. Alternatively, AD may be associated with a more widespread immune dysregulation that is detectable in plasma. The measurement of immune mediators in AD serum or plasma is a topic of significant debate in the literature. While various cytokines such as IL-12, and interferon remain unchanged, inflammatory substances such as C-reactive protein, interleukin (IL)-1, tumor necrosis factor, IL-6, IL-6 receptor complex, 1-antichymotrypsin, and transforming growth factor show variable changes among studies (Teunissen et al. 2002).
For instance, IL-6 has been widely studied in AD. A receptor complex made up of the IL-6 receptor subunit and glycoprotein 130 mediates the effects of IL-6, a cytokine involved in inflammation, acute-phase reactions, and cell proliferation. Some studies have found elevated IL-6 levels in AD plasma and serum (Panossian et al. 2003; Kalman et al. 1997; Licastro et al. 2000; Maes et al. 1999; Singh and Guthikonda 1997; Tarkowski et al. 1999), while other cohorts did not find this to be the case (Angelis et al. 1998; Blum-Degen et al. 1995; Chao et al. 1994; van Duijn et al. 1990). Different plasma collection techniques, assay methodologies, assay sensitivity, limited sample numbers, varied patient demographics, impact of disease severity, age, and comorbid inflammatory condition are few confounding issues in this research. To determine if IL-6 can be an effective biomarker for AD, more studies are needed (Lönneborg 2008).
4.6 Biomarkers Through Noninvasive Diagnostic Methods
4.6.1 Cognitive Biomarkers
The current noninvasive AD diagnostic criteria are based on a person’s medical history, cognitive and neuropsychological state, and clinical rating score obtained from the Mini-Mental State Examination (MMSE), the Wechsler Memory Scale (WMS), Clinical Dementia Rating (CDR), and Logical Memory (LM) test (Lopez et al. 2011). The MMSE (0–30) score classifies a person as having mild dementia if their score falls between 20 and 24; moderate dementia if their score falls between 13 and 20; and severe dementia if their score falls below 12 (Folstein et al. 1975).
4.6.2 Imaging Biomarkers
A second line of diagnostic criteria for the diagnosis of AD has been imaging of the brain, including positron-emission tomography (PET), magnetic resonance imaging (MRI), and functional magnetic resonance imaging (fMRI) (Omar and Preddy 2020). Current guidelines follow structural imaging in a scientific setting, such as magnetic resonance imaging (MRI) or computed tomography (CT), which is primarily necessary for the examination of patients presenting with a cognitive/dementia disease (Sheikh-Bahaei et al. 2017). MRI is utilized as a structural and functional imaging tool to examine the visualization of AD-related cortical atrophy and alterations in brain connectivity (Scheltens et al. 1992).
The functional connectivity of the brain was also shown by fMRI investigations, including abnormalities in the hippocampus (Wang et al. 2006). Positron-emission tomography (PET) is a sophisticated imaging technique that detects Aβ-related metabolic activity and plaque deposition in AD by labeling substances with transient positron-emitting radionuclides (Kadir et al. 2012). PiB and fluoro-2-deoxy-D-glucose (FDG), two extensively used PET tracers, show good sensitivity and specificity in imaging biomarkers of plaque-associated amyloid progression in humans, particularly in the initial phases (Cohen and Klunk 2014).
5 Conclusion
Aβ42, P-tau, and t-tau have already been identified as CSF biomarkers for AD. They are well known for their great diagnostic specificity. Several more candidates in noninvasive biological fluids are currently being studied for their potential therapeutic utility in early AD diagnosis and prognosis. So far, a growing number of molecules have been found, with NFL showing the most promise in both CSF and blood, lactoferrin in saliva, and Aβ42 and p-tau in plasma.
However, most current AD fluidic biomarkers are obtained through a single study, or there is a substantial inconsistency in outcomes obtained from different studies, including pre-analytical sample process standardization and analytical method validation. Other criteria such as our age, presence of multiple medical conditions, and disease diagnosis/stage must also be thoroughly considered.
These restrictions are slightly relieved by advanced imaging techniques that enable the detection of structural and functional biomarkers associated with AD offering information that is simple to understand and highly accurate for identifying the disease stage of AD. In this context, recently developed biosensors are showing promise as rapid, affordable, and straightforward alternatives for diagnosing AD, even in its early stages.
These techniques are powerful analytical methods with elevated sensitivity and selectivity, which can be used to detect AD biomarkers in physiological fluids such as blood, urine, and tears.
Combining more than one of the aforementioned indicators, particularly fluidic molecular examination and imaging studies, is now commonly accepted as the best strategy to accurately diagnose AD and disease stage progression. Depending on the case being assessed, several biomarkers and procedures may be combined.
Abbreviations
- 15-LOX:
-
15-Lipoxygenase
- AChE:
-
Acetylcholine esterase
- AD:
-
Alzheimer’s disease
- ADNI:
-
Alzheimer’s disease neuroimaging initiative
- AMPK:
-
5′ Adenosine monophosphate-activated protein kinase
- Apoε4:
-
Apolipoprotein-E
- APP:
-
Amyloid precursor protein
- Aβ:
-
Beta-amyloid
- BBB:
-
Blood-brain barrier
- BCSFB:
-
Blood-cerebrospinal fluid barrier
- CAA:
-
Cerebral amyloid angiopathy
- cdk5:
-
Cyclin-dependent kinase 5
- CDR:
-
Clinical dementia rating
- CHGA:
-
Chromogranin A
- CK 1:
-
Casein kinase 1
- CNS:
-
Central nervous system
- CSF:
-
Cerebrospinal fluid
- CSF-ISF exchange:
-
Cerebrospinal fluid and interstitial fluid exchange
- CT:
-
Computerized tomography
- DNA:
-
Deoxyribonucleic acid
- DS:
-
Down syndrome
- DYRK-1A:
-
Dual-specificity tyrosine-phosphorylation-regulated kinase 1A
- ELISA:
-
Enzyme-linked immunosorbent assay
- FDG:
-
Fluoro-2-deoxy-D-glucose
- FMRI:
-
Functional magnetic resonance imaging
- GSK-3:
-
Glycogen synthase kinase-3
- IL:
-
Interleukin
- IP-10:
-
Interferon-induced protein 10
- LC:
-
Locus coeruleus
- LM:
-
Logical memory
- LRP-1:
-
Low-density lipoprotein receptor-related protein
- Lys C:
-
Lysozyme C
- MALDI-MS:
-
Matrix-assisted laser desorption/ionization-mass spectroscopy
- MARKS:
-
Microtubule affinity-regulating kinases
- MCI:
-
Mild cognitive impairment
- MCP-1:
-
Monocyte chemoattractant protein 1
- miRNA:
-
Microsomal RNA
- MMSE:
-
Mini-mental state examination
- MRI:
-
Magnetic resonance imaging
- MS:
-
Mass spectroscopy
- Multiplex iTRAQ:
-
Multiplexed isobaric tagging technology for relative quantitation
- NCANP:
-
Neurocan core protein
- NFTs:
-
Neurofibrillary tangles
- NGF:
-
Nerve growth factor
- NMDA:
-
N-methyl-D-aspartate
- NPTX1:
-
Neuronal pentraxin 1
- NRXNs:
-
Neurexins
- NTP:
-
Neuronal thread protein
- OCT:
-
Optical coherence tomography
- PET:
-
Positron-emission tomography
- PGP-D:
-
D-glycol protein
- PiB:
-
Pittsburgh compound B
- PKA:
-
Cyclic AMP-dependent protein kinase A
- PP1:
-
Protein phosphate-1
- PP2A:
-
Protein phosphate-2A
- PP5:
-
Protein phosphate-5
- PS 2:
-
Presenilin
- P-tau:
-
Phosphorylated tau
- RNA:
-
Ribonucleic acid
- RNFL:
-
Retinal nerve fiber layer
- RT-PCR:
-
Real-time reverse transcriptase-polymerase chain reaction
- SCG2:
-
Secretogranin 2
- SELDI:
-
Surface-enhanced laser desorption/ionization time-of-flight mass spectroscopy
- SYN-2:
-
Synaptic protein synapsin-2
- t-tau:
-
Total tau
- VILIP-1:
-
Visinin-like protein 1
- WMS:
-
Wechsler memory scale
- β2M:
-
β2-Macroglobulin
References
Angelis P, Scharf S, Mander A, Vajda F, Christophidis N (1998) Serum interleukin-6 and interleukin-6 soluble receptor in Alzheimer’s disease. Neurosci Lett 244(2):106–108
Attems J, Jellinger KA (2006) Olfactory tau pathology in Alzheimer disease and mild cognitive impairment. Clin Neuropathol 25(6):265
Ausó E, Gómez-Vicente V, Esquiva G (2020) Biomarkers for Alzheimer’s disease early diagnosis. J Pers Med 10(3):114
Bakhtiari S, Moghadam NB, Ehsani M, Mortazavi H, Sabour S, Bakhshi M (2017) Can salivary acetylcholinesterase be a diagnostic biomarker for Alzheimer? J Clin Diagn Res 11(1):ZC58
Bălașa AF, Chircov C, Grumezescu AM (2020) Body fluid biomarkers for Alzheimer’s disease—an up-to-date overview. Biomedicine 8(10):421
Berisha F, Feke GT, Trempe CL, McMeel JW, Schepens CL (2007) Retinal abnormalities in early Alzheimer’s disease. Invest Ophthalmol Vis Sci 48(5):2285–2289
Bermejo-Pareja F, Antequera D, Vargas T, Molina JA, Carro E (2010) Saliva levels of Abeta1-42 as potential biomarker of Alzheimer’s disease: a pilot study. BMC Neurol 10(1):1–7
Bettcher BM, Johnson SC, Fitch R, Casaletto KB, Heffernan KS, Asthana S et al (2018) Cerebrospinal fluid and plasma levels of inflammation differentially relate to CNS markers of Alzheimer’s disease pathology and neuronal damage. J Alzheimers Dis 62(1):385–397
Blennow K, Hampel H (2003) CSF markers for incipient Alzheimer’s disease. Lancet Neurol 2(10):605–613
Blum-Degen D, Müller T, Kuhn W, Gerlach M, Przuntek H, Riederer P (1995) Interleukin-1 beta and interleukin-6 are elevated in the cerebrospinal fluid of Alzheimer’s and de novo Parkinson’s disease patients. Neurosci Lett 202(1–2):17–20
Booij BB, Lindahl T, Wetterberg P, Skaane NV, Sæbø S, Feten G et al (2011) A gene expression pattern in blood for the early detection of Alzheimer’s disease. J Alzheimers Dis 23(1):109–119
Burczynski ME, Dorner AJ (2006) Transcriptional profiling of peripheral blood cells in clinical pharmacogenomic studies. Pharmacogenomics 7:187–202
Calsolaro V, Edison P (2016) Neuroinflammation in Alzheimer’s disease: current evidence and future directions. Alzheimers Dement 12(6):719–732
Campbell MC, De Vries D, Emptage L, Cookson C, Kisilak M, Bueno JM, Avila FJ (2015) Polarization properties of amyloid beta in the retina of the eye as a biomarker of Alzheimer’s disease. In: Bio-optics: design and application. Optica Publishing Group, p BM3A-4
Carro E, Bartolomé F, Bermejo-Pareja F, Villarejo-Galende A, Molina JA, Ortiz P et al (2017) Early diagnosis of mild cognitive impairment and Alzheimer’s disease based on salivary lactoferrin. Alzheimer’s Dement 8:131–138
Chao CC, Ala TA, Hu S, Crossley KB, Sherman RE, Peterson PK, Frey WH 2nd (1994) Serum cytokine levels in patients with Alzheimer’s disease. Clin Diagn Lab Immunol 1(4):433
Cheung CYL, Ong YT, Hilal S, Ikram MK, Low S, Ong YL et al (2015) Retinal ganglion cell analysis using high-definition optical coherence tomography in patients with mild cognitive impairment and Alzheimer’s disease. J Alzheimers Dis 45(1):45–56
Cohen AD, Klunk WE (2014) Early detection of Alzheimer’s disease using PiB and FDG PET. Neurobiol Dis 72:117–122
Colasanti T, Barbati C, Rosano G, Malorni W, Ortona E (2010) Autoantibodies in patients with Alzheimer’s disease: pathogenetic role and potential use as biomarkers of disease progression. Autoimmun Rev 9(12):807–811
Craig-Schapiro R, Perrin RJ, Roe CM, Xiong C, Carter D, Cairns NJ et al (2010) YKL-40: a novel prognostic fluid biomarker for preclinical Alzheimer’s disease. Biol Psychiatry 68(10):903–912
Dhiman K, Blennow K, Zetterberg H, Martins RN, Gupta VB (2019) Cerebrospinal fluid biomarkers for understanding multiple aspects of Alzheimer’s disease pathogenesis. Cell Mol Life Sci 76:1833–1863
Duits FH, Brinkmalm G, Teunissen CE, Brinkmalm A, Scheltens P, Van der Flier WM et al (2018) Synaptic proteins in CSF as potential novel biomarkers for prognosis in prodromal Alzheimer’s disease. Alzheimers Res Ther 10(1):1–9
Ewers M, Zhong Z, Bürger K, Wallin A, Blennow K, Teipel SJ et al (2008) Increased CSF-BACE 1 activity is associated with ApoE-ε4 genotype in subjects with mild cognitive impairment and Alzheimer’s disease. Brain 131(5):1252–1258
Fehlbaum-Beurdeley P, Sol O, Désiré L, Touchon J, Dantoine T, Vercelletto M et al (2012) Validation of AclarusDx™, a blood-based transcriptomic signature for the diagnosis of Alzheimer’s disease. J Alzheimers Dis 32(1):169–181
Folstein MF, Folstein SE, McHugh PR (1975) “Mini-mental state”: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 12(3):189–198
Food U, Administration D (2018) Early Alzheimer’s disease: developing drugs for treatment–guidance for industry. https://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM596728.pdf. Accessed 10 Mar 2023
Frey H, Mattila K, Korolainen M et al (2005) Problems associated with biological markers of Alzheimer’s disease. Neurochem Res 30:1501–1510
García-Ayllón MS, Campanari ML, Brinkmalm G, Rábano A, Alom J, Saura CA et al (2013) CSF Presenilin-1 complexes are increased in Alzheimer’s disease. Acta Neuropathol Commun 1(1):1–13
García-Blanco A, Peña-Bautista C, Oger C, Vigor C, Galano JM, Durand T et al (2018) Reliable determination of new lipid peroxidation compounds as potential early Alzheimer disease biomarkers. Talanta 184:193–201
Geekiyanage H, Jicha GA, Nelson PT, Chan C (2012) Blood serum miRNA: non-invasive biomarkers for Alzheimer’s disease. Exp Neurol 235(2):491–496
Gharbiya M, Trebbastoni A, Parisi F, Manganiello S, Cruciani F, D’Antonio F et al (2014) Choroidal thinning as a new finding in Alzheimer’s disease: evidence from enhanced depth imaging spectral domain optical coherence tomography. J Alzheimers Dis 40(4):907–917
Gladkevich A, Kauffman HF, Korf J (2004) Lymphocytes as a neural probe: potential for studying psychiatric disorders. Prog Neuro-Psychopharmacol Biol Psychiatry 28(3):559–576
Gleerup HS, Hasselbalch SG, Simonsen AH (2019) Biomarkers for Alzheimer’s disease in saliva: a systematic review. Dis Markers 2019:1
Goldstein LE, Muffat JA, Cherny RA, Moir RD, Ericsson MH, Huang X et al (2003) Cytosolic β-amyloid deposition and supranuclear cataracts in lenses from people with Alzheimer’s disease. Lancet 361(9365):1258–1265
Gunes S, Aizawa Y, Sugashi T, Sugimoto M, Rodrigues PP (2022) Biomarkers for Alzheimer’s disease in the current state: a narrative review. Int J Mol Sci 23(9):4962
Gustaw-Rothenberg KA, Siedlak SL, Bonda DJ, Lerner A, Tabaton M, Perry G, Smith MA (2010) Dissociated amyloid-β antibody levels as a serum biomarker for the progression of Alzheimer’s disease: a population-based study. Exp Gerontol 45(1):47–52
Hamel MT, Emptage L, DeVries D, Oliveros C, Chow T, Shah N et al (2016) Polarization properties of amyloid deposits in the retinas of an animal model of Alzheimer’s disease differ in those with and without cognitive impairment. Invest Ophthalmol Vis Sci 57(12):2216–2216
Henriksen K, O’Bryant SE, Hampel H, Trojanowski JQ, Montine TJ, Jeromin A et al (2014) The future of blood-based biomarkers for Alzheimer’s disease. Alzheimers Dement 10(1):115–131
Hinton DR, Sadun AA, Blanks JC, Miller CA (1986) Optic-nerve degeneration in Alzheimer’s disease. N Engl J Med 315(8):485–487
Ho M, Liu DT, Chan VC, Lam DS (2013) Choroidal thickness measurement in myopic eyes by enhanced depth optical coherence tomography. Ophthalmology 120(9):1909–1914
Humpel C (2011) Identifying and validating biomarkers for Alzheimer’s disease. Trends Biotechnol 29(1):26–32
Kaddurah-Daouk R, Rozen S, Matson W, Han X, Hulette CM, Burke JR et al (2011) Metabolomic changes in autopsy-confirmed Alzheimer’s disease. Alzheimers Dement 7(3):309–317
Kadir A, Almkvist O, Forsberg A, Wall A, Engler H, Långström B, Nordberg A (2012) Dynamic changes in PET amyloid and FDG imaging at different stages of Alzheimer’s disease. Neurobiol Aging 33(1):198–1e1
Kalló G, Emri M, Varga Z, Ujhelyi B, Tőzsér J, Csutak A, Csősz É (2016) Changes in the chemical barrier composition of tears in Alzheimer’s disease reveal potential tear diagnostic biomarkers. PLoS One 11(6):e0158000
Kalman J, Juhasz A, Laird G, Dickens P, Jardanhazy T, Rimanoczy A et al (1997) Serum interleukin-6 levels correlate with the severity of dementia in down syndrome and in Alzheimer’s disease. Acta Neurol Scand 96(4):236–240
Karsdal MA, Henriksen K, Leeming DJ, Woodworth T, Vassiliadis E, Bay-Jensen AC (2010) Novel combinations of post-translational modification (PTM) neo-epitopes provide tissue-specific biochemical markers—are they the cause or the consequence of the disease? Clin Biochem 43(10–11):793–804
Kerbage C, Sadowsky CH, Jennings D, Cagle GD, Hartung PD (2013) Alzheimer’s disease diagnosis by detecting exogenous fluorescent signal of ligand bound to Beta amyloid in the lens of human eye: an exploratory study. Front Neurol 4:62
Kerbage C, Sadowsky CH, Tariot PN, Agronin M, Alva G, Turner FD et al (2015) Detection of amyloid β signature in the lens and its correlation in the brain to aid in the diagnosis of Alzheimer’s disease. Am J Alzheimers Dis Other Dement 30(8):738–745
Kinoshita T, Mitamura Y, Shinomiya K, Egawa M, Iwata A, Fujihara A et al (2017) Diurnal variations in luminal and stromal areas of choroid in normal eyes. Br J Ophthalmol 101(3):360–364
Koran MEI, Wagener M, Hohman TJ, Alzheimer’s Neuroimaging Initiative (2017) Sex differences in the association between AD biomarkers and cognitive decline. Brain Imaging Behav 11(1):205–213
Kumar P, Dezso Z, MacKenzie C, Oestreicher J, Agoulnik S, Byrne M et al (2013) Circulating miRNA biomarkers for Alzheimer’s disease. PLoS One 8(7):e69807
Liang D, Lu H (2019) Salivary biological biomarkers for Alzheimer’s disease. Arch Oral Biol 105:5–12
Licastro F, Pedrini S, Caputo L, Annoni G, Davis LJ, Ferri C et al (2000) Increased plasma levels of interleukin-1, interleukin-6 and α-1-antichymotrypsin in patients with Alzheimer’s disease: peripheral inflammation or signals from the brain? J Neuroimmunol 103(1):97–102
Lim JK, Li QX, He Z, Vingrys AJ, Wong VH, Currier N et al (2016) The eye as a biomarker for Alzheimer’s disease. Front Neurosci 10:536
Lönneborg A (2008) Biomarkers for Alzheimer disease in cerebrospinal fluid, urine, and blood. Mol Diagn Ther 12:307–320
Lopez OL, McDade E, Riverol M, Becker JT (2011) Evolution of the diagnostic criteria for degenerative and cognitive disorders. Curr Opin Neurol 24(6):532–541
Lukiw WJ (2007) Micro-RNA speciation in fetal, adult and Alzheimer’s disease hippocampus. Neuroreport 18(3):297–300
Lukiw WJ, Andreeva TV, Grigorenko AP, Rogaev EI (2013) Studying micro RNA function and dysfunction in Alzheimer’s disease. Front Genet 3:327
Ma L, Chen J, Wang R, Han Y, Zhang J, Dong W et al (2015) The level of Alzheimer-associated neuronal thread protein in urine may be an important biomarker of mild cognitive impairment. J Clin Neurosci 22(4):649–652
Maes M, DeVos N, Wauters A, Demedts P, Maurits V, Neels H et al (1999) Inflammatory markers in younger vs elderly normal volunteers and in patients with Alzheimer’s disease. J Psychiatr Res 33(5):397–405
Maftei M, Thurm F, Leirer VM, von Arnim CA, Elbert T, Przybylski M et al (2012) Antigen-bound and free β-amyloid autoantibodies in serum of healthy adults. PLoS One 7(9):e44516
Mattsson N, Insel P, Nosheny R, Zetterberg H, Trojanowski JQ, Shaw LM et al (2013) CSF protein biomarkers predicting longitudinal reduction of CSF β-amyloid42 in cognitively healthy elders. Transl Psychiatry 3(8):e293–e293
Mayeux R, Schupf N (2011) Blood-based biomarkers for Alzheimer’s disease: plasma Aβ40 and Aβ42, and genetic variants. Neurobiol Aging 32:S10–S19
Montine TJ, Quinn JF, Milatovic D, Silbert LC, Dang T, Sanchez S et al (2002) Peripheral F2-isoprostanes and F4-neuroprostanes are not increased in Alzheimer’s disease. Ann Neurol 52(2):175–179
Moon J, Lee ST, Kong IG, Byun JI, Sunwoo JS, Shin JW et al (2016) Early diagnosis of Alzheimer’s disease from elevated olfactory mucosal miR-206 level. Sci Rep 6(1):1–9
Murphy C (2019) Olfactory and other sensory impairments in Alzheimer disease. Nat Rev Neurol 15(1):11–24
Nagele E, Han M, DeMarshall C, Belinka B, Nagele R (2011) Diagnosis of Alzheimer’s disease based on disease-specific autoantibody profiles in human sera. PLoS One 6(8):e23112
Nakamura A, Kaneko N, Villemagne VL, Kato T, Doecke J, Doré V et al (2018) High performance plasma amyloid-β biomarkers for Alzheimer’s disease. Nature 554(7691):249–254
Noble W, Hanger DP, Miller CC, Lovestone S (2013) The importance of tau phosphorylation for neurodegenerative diseases. Front Neurol 4:83
O’Bryant SE, Mielke MM, Rissman RA, Lista S, Vanderstichele H, Zetterberg H et al (2017) Blood-based biomarkers in Alzheimer disease: current state of the science and a novel collaborative paradigm for advancing from discovery to clinic. Alzheimers Dement 13(1):45–58
Ohm TG, Braak H (1987) Olfactory bulb changes in Alzheimer’s disease. Acta Neuropathol 73(4):365–369
Omar SH, Preddy J (2020) Advantages and pitfalls in fluid biomarkers for diagnosis of Alzheimer’s disease. J Pers Med 10(3):63
Ovod V, Ramsey KN, Mawuenyega KG, Bollinger JG, Hicks T, Schneider T et al (2017) Amyloid β concentrations and stable isotope labeling kinetics of human plasma specific to central nervous system amyloidosis. Alzheimers Dement 13(8):841–849
Panossian LA, Porter VR, Valenzuela HF, Zhu X, Reback E, Masterman D et al (2003) Telomere shortening in T cells correlates with Alzheimer’s disease status. Neurobiol Aging 24(1):77–84
Patel S, Shah RJ, Coleman P, Sabbagh M (2011) Potential peripheral biomarkers for the diagnosis of Alzheimer’s disease. Int J Alzheimers Dis 2011:572495
Praticò D, Clark CM, Lee VMY, Trojanowski JQ, Rokach J, FitzGerald GA (2000) Increased 8, 12-iso-iPF2α-VI in Alzheimer’s disease: correlation of a noninvasive index of lipid peroxidation with disease severity. Ann Neurol 48(5):809–812
Praticò D, Clark CM, Liun F, Lee VYM, Trojanowski JQ (2002) Increase of brain oxidative stress in mild cognitive impairment: a possible predictor of Alzheimer disease. Arch Neurol 59(6):972–976
Preische O, Schultz SA, Apel A, Kuhle J, Kaeser SA, Barro C et al (2019) Serum neurofilament dynamics predicts neurodegeneration and clinical progression in presymptomatic Alzheimer’s disease. Nat Med 25(2):277–283
Provost P (2010) Interpretation and applicability of microRNA data to the context of Alzheimer’s and age-related diseases. Aging (Albany NY) 2(3):166
Ratnayaka JA, Serpell LC, Lotery AJ (2015) Dementia of the eye: the role of amyloid beta in retinal degeneration. Eye 29(8):1013–1026
Rocco ML, Soligo M, Manni L, Aloe L (2018) Nerve growth factor: early studies and recent clinical trials. Curr Neuropharmacol 16(10):1455–1465
Rye P, Booij BB, Grave G, Lindahl T, Kristiansen L, Andersen HM et al (2011) A novel blood test for the early detection of Alzheimer’s disease. J Alzheimers Dis 23(1):121–129
Sakka L, Coll G, Chazal J (2011) Anatomy and physiology of cerebrospinal fluid. Eur Ann Otorhinolaryngol Head Neck Dis 128(6):309–316
Scheltens P, Leys D, Barkhof F, Huglo D, Weinstein HC, Vermersch P et al (1992) Atrophy of medial temporal lobes on MRI in “probable” Alzheimer’s disease and normal ageing: diagnostic value and neuropsychological correlates. J Neurol Neurosurg Psychiatry 55(10):967–972
Shah RD, Randleman JB, Grossniklaus HE (2012) Spontaneous corneal clearing after Descemet’s stripping without endothelial replacement. Ophthalmology 119(2):256–260
Sharp FR, Xu H, Lit L, Walker W, Apperson M, Gilbert DL et al (2006) The future of genomic profiling of neurological diseases using blood. Arch Neurol 63(11):1529–1536
Sheikh-Bahaei N, Sajjadi SA, Manavaki R, Gillard JH (2017) Imaging biomarkers in Alzheimer’s disease: a practical guide for clinicians. J Alzheimers Disease Rep 1(1):71–88
Sheinerman KS, Tsivinsky VG, Crawford F, Mullan MJ, Abdullah L, Umansky SR (2012) Plasma microRNA biomarkers for detection of mild cognitive impairment. Aging 4(9):590–605
Simon MJ, Iliff JJ (2016) Regulation of cerebrospinal fluid (CSF) flow in neurodegenerative, neurovascular and neuroinflammatory disease. Biochim Biophys Acta 1862(3):442–451
Singh VK, Guthikonda P (1997) Circulating cytokines in Alzheimer’s disease. J Psychiatr Res 31(6):657–660
Tarkowski E, Blennow K, Wallin A, Tarkowski A (1999) Intracerebral production of tumor necrosis factor-[alpha], a local neuroprotective agent, in Alzheimer disease and vascular dementia. J Clin Immunol 19(4):223
Tatebe H, Kasai T, Ohmichi T, Kishi Y, Kakeya T, Waragai M et al (2017) Quantification of plasma phosphorylated tau to use as a biomarker for brain Alzheimer pathology: pilot case-control studies including patients with Alzheimer’s disease and down syndrome. Mol Neurodegener 12:1–11
Teunissen CE, De Vente J, Steinbusch HWM, De Bruijn C (2002) Biochemical markers related to Alzheimer’s dementia in serum and cerebrospinal fluid. Neurobiol Aging 23(4):485–508
Tezel G (2013) A proteomics view of the molecular mechanisms and biomarkers of glaucomatous neurodegeneration. Prog Retin Eye Res 35:18–43
Thambisetty M, Lovestone S (2010) Blood-based biomarkers of Alzheimer’s disease: challenging but feasible. Biomark Med 4(1):65–79
Tvarijonaviciute A, Zamora C, Ceron JJ, Bravo-Cantero AF, Pardo-Marin L, Valverde S, Lopez-Jornet P (2020) Salivary biomarkers in Alzheimer’s disease. Clin Oral Investig 24:3437–3444
van Duijn CM, Hofman A, Nagelkerken L (1990) Serum levels of interleukin-6 are not elevated in patients with Alzheimer’s disease. Neurosci Lett 108(3):350–354
van Wijngaarden P, Hadoux X, Alwan M, Keel S, Dirani M (2017) Emerging ocular biomarkers of Alzheimer disease. Clin Exp Ophthalmol 45(1):54–61
Wang L, Zang Y, He Y, Liang M, Zhang X, Tian L et al (2006) Changes in hippocampal connectivity in the early stages of Alzheimer’s disease: evidence from resting state fMRI. NeuroImage 31(2):496–504
Wang SC, Oelze B, Schumacher A (2008) Age-specific epigenetic drift in late-onset Alzheimer’s disease. PLoS One 3(7):e2698
Weiner HL, Selkoe DJ (2002) Inflammation and therapeutic vaccination in CNS diseases. Nature 420(6917):879–884
Wenk GL (2003) Neuropathologic changes in Alzheimer’s disease. J Clin Psychiatry 64(Suppl 9):7–10
Wong IY, Koizumi H, Lai WW (2011) Enhanced depth imaging optical coherence tomography. Ophthalmic Surg Lasers Imaging 42 Suppl:S75–S84
Zetterberg H, Andreasson U, Hansson O, Wu G, Sankaranarayanan S, Andersson ME et al (2008) Elevated cerebrospinal fluid BACE1 activity in incipient Alzheimer disease. Arch Neurol 65(8):1102–1107
Zetterberg H, Wilson D, Andreasson U, Minthon L, Blennow K, Randall J, Hansson O (2013) Plasma tau levels in Alzheimer’s disease. Alzheimers Res Ther 5(2):1–3
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2023 The Author(s), under exclusive license to Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Gaddam, M., Motamarri, E.R., Sharma, A. (2023). Biomarkers for Alzheimer’s Disease. In: Sharma, A., Modi, G.P. (eds) Natural Product-based Synthetic Drug Molecules in Alzheimer's Disease. Springer, Singapore. https://doi.org/10.1007/978-981-99-6038-5_4
Download citation
DOI: https://doi.org/10.1007/978-981-99-6038-5_4
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-99-6037-8
Online ISBN: 978-981-99-6038-5
eBook Packages: MedicineMedicine (R0)