Abstract
The prognosis of patients with metastatic renal cell carcinoma has drastically improved due to the development of molecular-targeted drugs and their use in clinical practice. However, these drugs cause some diverse adverse reactions in patients and sometimes affect clinical outcomes of cancer therapy. Therefore, predictive markers are necessary to avoid severe adverse reactions, to establish novel and effective prevention methods, and to improve treatment outcomes. Some genetic factors involved in these adverse reactions have been reported; however, perspectives on each adverse response have not been integrated yet. In this review, genetic polymorphisms relating to molecular-targeted therapy-induced adverse reactions in patients with renal cell carcinoma are summarized in the points of pharmacokinetic and pharmacodynamic mechanisms. We also discuss about the relationship between systemic drug exposure and adverse drug reactions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
A number of novel drugs based on molecular targets relating to the progression of renal cell carcinoma (RCC) have been developed and used in clinical practice, drastically improving the prognosis of patients with metastatic RCC [1, 2]. However, specific adverse reactions which are not popular in the treatment with ordinal cytotoxic cancerous drugs are being reported [3,4,5]. A crucial issue in the safe and effective targeted chemotherapy is to identify mechanisms and predictive markers of adverse drug reactions.
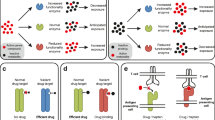
Some genetic factors of adverse drug reactions have been reported and broadly classified into pharmacokinetic and pharmacodynamics mechanisms. A part of molecular-targeted drugs are absorbed and distributed by various membrane transporters such as ATP-binding cassette (ABC) and solute carrier (SLC) transporters [6]. Moreover, almost all of these drugs are metabolized by cytochrome P-450s (CYPs). A large number of polymorphisms exist in the coding genes of factors involved in absorption, distribution, metabolism, and excretion (ADME) processes; these polymorphisms can affect the systemic and local concentrations of the drugs [7]. Polymorphisms in drug-targeted molecules such as vascular endothelial growth factor receptor (VEGFR) and FMS-like tyrosine kinase (FLT) 3 are associated with the efficacy and toxicity of the drugs [8]. Various reports on individual adverse reactions can be found; however, different perspectives on adverse responses have not been integrated yet, which is necessary for the development of preventive strategies against these adverse drug reactions and for their optimal usage in drug selection or dosage adjustment in clinical practice.
In this review, genetic factors relating to molecular-targeted therapy-induced adverse drug reactions in patients with RCC are summarized based on pharmacokinetic and pharmacodynamic mechanisms.
TKI-induced adverse reactions
Clinically, tyrosine kinase inhibitors (TKIs), mammalian target of rapamycin inhibitors (mTORi), and immune checkpoint inhibitors are used in RCC therapy. Several TKIs have been in use based on patient performance status; novel TKIs will continue to be developed [9, 10]. Molecular-targeted therapy-induced major adverse reactions recorded in leading clinical trials that evaluated the efficacy of first-line RCC therapy are shown in Table 1 [11,12,13,14,15,16]. Gastrointestinal toxicities such as diarrhea and fatigue are common reactions to TKIs. In addition, skin or mucosal toxicities such as hand–foot skin reaction, rash, and stomatitis are typical. Racial differences in the development of hand–foot skin reaction have been reported [17]. Liver injury is frequently induced by sunitinib and pazopanib. Hematological toxicities such as anemia, neutropenia, and thrombocytopenia are commonly observed events in sunitinib and pazopanib therapy; particularly sunitinib-induced hematological toxicity is likely to become severe, whereas sorafenib and axitinib are known to be less hematotoxic than other TKIs. Proteinuria and hypothyroidism are unique events in axitinib therapy. Interestingly, some reactions are well known to be associated with the efficacy of TKI cancer therapy [18, 19].
mTORi-induced adverse reactions
Oral everolimus and intravenous temsirolimus are mTORi used for the therapy of RCC. mTORi-induced adverse reactions differ from TKI-induced adverse reactions. Mucositis such as stomatitis is more frequently observed in the mTORi therapy. Skin disorders such as dry skin and paronychia are also reactions unique to these inhibitors. In addition, interstitial lung disease (ILD) is a critical reaction, and it is the key factor in the interruption of mTORi therapy, although its development is rare [20]. Racial differences in the development of ILD have been reported, with Asian patients being more likely to experience mTORi-induced ILD [21, 22]. Another unique adverse reaction to mTORi therapy is abnormality in lipid and glucose metabolism, which is known to occur at different frequencies comparing everolimus and temsirolimus therapy. Some mTORi-induced adverse reactions are also associated with therapeutic outcome [23,24,25].
Genetic factors associated with adverse reactions
TKI-induced diarrhea
Diarrhea is the most common adverse response to TKIs. Reported genetic polymorphisms are related with their pharmacokinetic mechanisms (Table 2). In a retrospective study, Chu et al. [26] reported that the T allele of 1236 T/C (rs1128503) and that of 3435 T/C (rs1045642) in the ABCB1 gene reduced the risk of sunitinib-induced diarrhea in Chinese patients as secondary endpoints. The TT genotype of 1236 T/C and that of 2677 G/T (rs2032582) in the ABCB1 gene are known to increase the clearance of sunitinib and its active metabolite [27]. In addition, Boudou-Rouquette et al. [28] emphasized that the T allele of − 2152 C/T (rs17868320) in the UDP-glucuronosyltransferase (UGT) 1A9 gene is associated with sorafenib-induced diarrhea, because this SNP is related with the higher hepatic expression of UGT1A9 and can increase the glucuronidation activity. Further, Bins et al. [29] reported the association between the G allele of 388 A/G (rs2306283) in the SLCO1B1 gene and development of sorafenib-induced diarrhea. Suttle et al. [30] reported that pazopanib-induced diarrhea showed a tendency of correlation with area under the curve (AUC) of pazopanib. On the other hand, no reports about the association between the development of TKI-induced diarrhea and pharmacodynamic factors based on genetic information can be found. Therefore, these findings suggested that TKI-induced diarrhea was associated with the activity or expression of transporters and conjugation enzymes affecting drug systemic exposure and distribution to local tissues. TKI-induced diarrhea can largely be explained by the genetic polymorphisms in the pharmacokinetic mechanisms.
TKI-induced hand–foot skin reaction
Several previous reports showed that hand–foot skin reaction was related to genetic polymorphisms of both pharmacokinetic and pharmacodynamics mechanisms. The TTT haplotype of rs1045642, rs1128503, and rs2032582 in the ABCB1 gene was associated with the development of hand–foot skin reaction due to increased systemic exposure [31, 32]. In addition, it was reported that carriers of the AA genotype of 421 C/A (rs2231142) in the ABCG2 gene developed hand–foot skin reaction more frequently. In this report, higher systemic exposure because of lower expression of breast cancer resistant protein (BCRP) with occurrence of the A allele of rs2231142 in the ABCG2 gene was a significant cause of frequent hand–foot skin reaction [33]. On the one hand, an association between systemic exposure to sunitinib and development of hand–foot skin reaction is controversial. Mizuno et al. [34] showed the lack of association between AUC of sunitinib and development of hand–foot skin reaction in secondary evaluations in a small-sample study. Noda et al. [35] also reported no significant association between severity of hand–foot skin reaction and plasma trough concentration of sunitinib and its metabolite. However, some studies have found that sorafenib concentrations were significantly correlated to the grade of hand–foot skin reaction [36, 37]. Genetic variants of the UGT1A9 gene were found to be associated with AUC of sorafenib and grade of hand–foot skin reaction [28, 37,38,39]. The severity of pazopanib-induced hand–foot skin reaction was also correlated to AUC of pazopanib [30]. Therefore, sorafenib- or pazopanib-induced hand–foot skin reaction may be associated with their systemic exposure of these drugs, and genetic variants of transporters may affect the local accumulation of TKIs.
A few factors in pharmacodynamic mechanisms of hand–foot skin reaction have been reported. Several reports focused on VEGF, VEGFR, and FLT3, which are targets of TKIs [40,41,42]. Mutations in the 5′ UTR or 3′ UTR such as rs2010963 in the VEGF gene can modify the potential binding sites of transcription factors, resulting in lower expressions of VEGF [43, 44]. Moreover, because 1192 G/A (rs2305948) and 1719 A/T (rs1870377) in the VEGFR2 gene affect the VEGF binding domain, these polymorphisms may have a differential effect on VEGF ligand binding and its downstream signaling through VEGFR2 [45]. Overall, patients with weaker signaling in the VEGF/kinase insert domain-containing receptor (KDR) pathway may more frequently develop hand–foot skin reaction; however, further information is needed for confirmation.
An association between development of hand–foot skin reaction and SNPs in cytokine-related factors such as tumor necrosis factor (TNF)-α and signal transducer and activator of transcription (STAT) 3 has been recently suggested [38, 46]; thus, indirect factors may contribute to the mechanism of hand–foot skin reaction. Therefore, hand–foot skin reaction is likely to involve integrated mechanisms including pharmacokinetic, pharmacodynamic, and indirect factors.
Sorafenib-induced skin rash
Skin rash is an adverse reaction involving immunological mechanisms, unlike hand–foot skin reaction. An association between sorafenib-induced skin rash and human leukocyte antigen (HLA)-A*24 has been reported in a small Japanese population. HLA-A*24 is known to be associated with phenytoin and lamotrigine-induced Stevens–Johnson syndrome (SJS) or toxic epidermal necrolysis; this can be relevant to allergic responses induced by different drugs. On the other hand, Tsuchiya et al. [47] reported that patients with the CC genotype of − 24 C/T (rs717620) in the ABCC2 gene were at a significantly higher risk of skin rash than those with the CT genotype. Carriers of the C allele of − 24 C/T in the ABCC2 gene show a higher export function of the multidrug resistance-associated protein 2 (MRP-2) than carriers of the T allele [48, 49]. Therefore, patients with C allele may experience lower plasma concentrations of sorafenib, because MRP-2 mediates the biliary excretion of sorafenib [50]. On the one hand, Fukudo et al. reported a lack of association between sorafenib plasma concentration and severe (> grade 2) skin rash. Relationship between pharmacokinetic factors and sorafenib-induced skin rash remained to be examined further.
Sunitinib-induced mucositis
Some reports investigated about the pharmacokinetic mechanisms in sunitinib-induced stomatitis. Diekstra et al. reported the associations between development of stomatitis and SNPs in ABCB1; they also reported that ligand-activated nuclear receptor (NR)1/3 genes affect the expression of CYP3A4 [41, 51]. Interestingly, polymorphisms in the ABCB1 gene influence the concentration of P-glycoprotein substrates in saliva [52]. Therefore, TKI-induced stomatitis can be related to the drug concentration in the oral cavity, but not to the systemic concentration. It is also reported that SNPs in NR1/3 and CYP1A1 genes are associated with the development of stomatitis [31, 41]. Carriers of the G allele of 4889 A/G (rs1048493) in the CYP1A1 gene have a higher catalytic activity of CYP1A1 [53, 54]. An association between systemic plasma concentration and development of sunitinib-induced stomatitis is generally accepted.
Watanabe et al. [55] reported that sunitinib-induced stomatitis more frequently develops in carriers of STAT3 genetic polymorphisms. TKI-induced mucositis may be related to immune system function; however, further studies are required for confirmation.
TKI-induced hypertension
Sunitinib-induced hypertension is reported to be associated with 6986 A/G (rs776746) in the CYP3A5 gene and rs2231142 in the ABCG2 gene, and these SNPs affect the systemic concentration of sunitinib [41]. Moreover, sorafenib-induced hypertension is reported to be associated with rs1045642 in the ABCB1 gene [42]. It has been suggested that rs776746 in the CYP3A5 gene can be a dose reduction marker of sunitinib, because rs776746 A allele carriers have higher concentrations of sunitinib [56]. Furthermore, carriers of the ABCG2 rs2231142 AA genotype have higher AUC of substrate drugs than carriers of the CC genotype [57, 58]. In addition, rs4646437 G/A in the CYP3A4 gene was reported to be associated with sunitinib-induced hypertension [59]. The A allele of rs4646437 is associated with a high plasma concentration of substrate drugs [60] due to altered splicing of primary transcripts [61]. Therefore, carriers of the rs4646437 A allele have increased drug exposure with stronger inhibition of VEGFR in patients taking sunitinib [59]. An association between TKI-induced hypertension and high systemic exposure to TKI has been reported [34, 37, 62].
Polymorphisms related to the VEGF/KDR pathway are also associated with TKI-induced hypertension [40, 63]. It is considered that these SNP carriers have reduced signaling in the VEGF/KDR pathway. Moreover, Diekstra et al. [64] also reported an association between hypertension and polymorphisms in the IL-8 gene. The effect of SNPs in the IL8 gene is little known; however, these SNPs are expected to affect the protein expression of IL8 [65,66,67]. It also remains unclear how the IL8 protein may relate to sunitinib-induced hypertension; IL8 may directly or indirectly influence the VEGFR pathway [68, 69].
TKI-induced liver injury
Pazopanib-induced hyperbilirubinemia was associated with UGT1A1*28 (rs8175347) [29, 70]. Bilirubin is metabolized by UGT1A1 for the biliary elimination, and UGT1A1 activity is strongly inhibited by pazopanib. Because the UGT1A1 genetic variant TA7 is known to cause reduced expression of UGT1A1 [71], its carriers may be susceptible to the inhibitory effects of pazopanib. This UGT1A1 TA-repeat polymorphism has also been reported to associate with hyperbilirubinemia induced by several drugs [72,73,74]. Low et al. [75] reported that the ABCG2 rs2231142 variant was associated with sunitinib-induced hepatic transaminase (AST and ALT) increase. In addition, some studies found that plasma concentrations of sorafenib or pazopanib show a tendency of correlation with ALT increase [30, 37]. Interestingly, Xu et al. [76] reported that the rs2858996/rs707889 polymorphisms in the HFE gene may associate with the reversible ALT elevation in pazopanib-treated patients. HFE, the hemochromatosis gene, encodes a membrane protein that regulates iron homeostasis. Genetic mutations in this gene result in hereditary hemochromatosis, an iron storage disorder. Other HFE-associated syndromes such as nonalcoholic steatohepatitis result in liver injury because of aberrant iron metabolism and oxidative stress [77, 78]. Furthermore, HFE and VEGFR-2 share several hypoxia-induced transcriptional regulators, particularly hypoxia inducible factor (HIF)-1α; the inhibition of VEGF signaling may reduce induction of HFE [79]. Xu et al. [80] also reported that HLA-B057:01 confers higher risk of ALT elevation in patients receiving pazopanib. Recent pharmacogenetic studies of hepatotoxicity have identified strong associations between HLA polymorphisms and various drug-induced ALT elevations [81,82,83,84,85].
Liver injury is a complex condition that cannot be justified by individual mechanisms. Hyperbilirubinemia may be related to pharmacokinetic differences in bilirubin metabolism inhibition by TKIs between UGT1A1 genetic variant carriers; ALT elevation may be associated with the factors in pharmacokinetic and pharmacodynamic mechanisms including immune components such as HLA and iron storage homeostasis.
TKI-induced thrombocytopenia
Some reports have suggested that TKI-induced thrombocytopenia is associated with pharmacokinetic factors. Studies have shown an association between sunitinib-induced thrombocytopenia and rs2231142 in the ABCG2 gene in Japanese and Korean patients [33, 75]. Carriers of the ABCG2 rs2231142 C allele are known to have higher AUC of sunitinib [34]. In addition, studies have suggested associations between plasma trough level of sunitinib and platelet counts, and between AUC of sunitinib and development of thrombocytopenia [34, 35]. Therefore, TKI-induced thrombocytopenia may be a hematological toxicity dependent on systemic drug exposure. Moreover, Bins et al. [29] showed an association between 521 C/T (rs4149056) in the SLCO1B1 gene and sorafenib-induced thrombocytopenia. Some TKIs including nilotinib, pazopanib, sorafenib, and sunitinib are substrates of OATP1B1 encoded by the SLCO1B1 gene [86, 87] with rs4149056 T allele carriers showing higher concentration of the substrates [88]. These findings support the hypothesis that TKI-induced thrombocytopenia is dependent on systemic drug exposure.
Sunitinib-induced leukopenia
Leukopenia is a type of hematological toxicity; therefore, the occurrence of leukopenia is considered to associate with systemic concentration of TKIs. However, some factors in pharmacodynamic mechanism are also reported. van Erp et al. [31] reported that sunitinib-induced leukopenia is associated with rs1048943 in the CYP1A1 gene and the CAG haplotype (rs2307424, rs2307418, and rs4073054) in the NR1/3 gene, but not with SNPs in the VEGFR genes.
Sunitinib is likely to be a substrate of CYP1A1 and is known to be an inducer of CYP1A1 protein mediated by aryl hydrocarbon receptor activation [89, 90]. Lu et al. found that Caucasians with the rs1048943 GG genotype in the CYP1A1 gene might have an increased risk of acute lymphoid leukemia and chronic myelogenous leukemia [91, 92]. This SNP results in increased catalytic activity and higher mRNA level of CYP1A1, leading to enhanced DNA adduct formation [93]. These DNA adducts are responsible for causing mutations in tumor suppressor genes and oncogenes; thus, trigger uncontrolled hematopoietic cell proliferation and reduced differentiation and decreased apoptosis of malignant hematopoietic blast cells [54]. It is not yet clear if these mechanisms are associated with sunitinib-induced leukopenia; however, CYP1A1 variants may be a factor of pharmacodynamic mechanism if the above mechanism involves sunitinib-induced leukopenia. NR1/3 is well known to regulate the expression of CYP3A4. Although the CAG haplotype in the NR1/3 gene is likely to lead to a higher concentration of sunitinib [94], this mechanism remains to be clarified.
Some studies have found that sunitinib-induced leukopenia is associated with FLT3 variants [26, 31]. The importance of the FLT3 receptor has been described with respect to the development of several subtypes of leukemia, wherein FLT3 is frequently overexpressed and/or mutated [95, 96]. The functional effect of 738 C/T (rs1933437) in the FLT3 gene is not yet clarified; however, its protein product may be altered because of amino acid substitution.
mTORi-induced adverse reactions
Associations between mTORi-induced adverse reactions in RCC therapy and genetic polymorphisms related to pharmacokinetic or pharmacodynamic factors are yet to be elucidated. However, the association between everolimus-induced adverse reactions in patients with breast cancer and genetic polymorphisms was reported [97]. It is reported that polymorphisms in mTOR pathway-related factors are associated with everolimus-induced leucopenia, hyperglycemia, and pneumonitis; however, data in patients with RCC have not been reported. de Velasco et al. [98] reported a lack of association between adverse reactions to everolimus or temsirolimus and some genetic polymorphisms such as CYP3A4, CYP3A5, and ABCB1. de Wit et al. found that patients with everolimus-induced severe stomatitis (grade 3) had higher AUC and trough concentration than patients with non-severe stomatitis (grade 0–2); however, the development of stomatitis (any grade) was not associated with AUC or trough concentration. Thus, mTORi-induced adverse reactions may be not influenced by pharmacokinetic genetic factors.
Conclusion and perspectives
Understanding the mechanism of adverse reactions and identifying genetic markers have become increasingly important because of spiraling medical costs and development of different molecular-targeted drugs. The application of genetic engineering techniques to medical research, such as genome-wide association studies, is showing good progress. Therefore, mechanistic analysis of targeted therapy based on genetic information is also necessary. Although a lot of retrospective or secondary analytic data have accumulated, there continues to be a lack of reports evaluating clinical outcome by using genetic information while controlling or avoiding adverse reactions in prospective studies. This review is aimed at encouraging the practical use of genetic information for the management of molecular-targeted drug-induced adverse drug reactions.
References
Barata PC, Rini BI. Treatment of renal cell carcinoma: current status and future directions. CA Cancer J Clin. 2017;67(6):507–24. https://doi.org/10.3322/caac.21411.
Sanchez-Gastaldo A, Kempf E, Gonzalez Del Alba A, Duran I. Systemic treatment of renal cell cancer: a comprehensive review. Cancer Treat Rev. 2017;60:77–89. https://doi.org/10.1016/j.ctrv.2017.08.010.
Li Y, Gao ZH, Qu XJ. The adverse effects of sorafenib in patients with advanced cancers. Basic Clin Pharmacol Toxicol. 2015;116(3):216–21. https://doi.org/10.1111/bcpt.12365.
Frampton JE. Pazopanib: a review in advanced renal cell carcinoma. Targeted Oncol. 2017;12(4):543–54. https://doi.org/10.1007/s11523-017-0511-8.
Cohen RB, Oudard S. Antiangiogenic therapy for advanced renal cell carcinoma: management of treatment-related toxicities. Invest New Drugs. 2012;30(5):2066–79. https://doi.org/10.1007/s10637-012-9796-8.
Josephs DH, Fisher DS, Spicer J, Flanagan RJ. Clinical pharmacokinetics of tyrosine kinase inhibitors: implications for therapeutic drug monitoring. Ther Drug Monit. 2013;35(5):562–87. https://doi.org/10.1097/FTD.0b013e318292b931.
Neul C, Schaeffeler E, Sparreboom A, Laufer S, Schwab M, Nies AT. Impact of membrane drug transporters on resistance to small-molecule tyrosine kinase inhibitors. Trends Pharmacol Sci. 2016;37(11):904–32. https://doi.org/10.1016/j.tips.2016.08.003.
Vaziri SA, Kim J, Ganapathi MK, Ganapathi R. Vascular endothelial growth factor polymorphisms: role in response and toxicity of tyrosine kinase inhibitors. Current Oncol Rep. 2010;12(2):102–8. https://doi.org/10.1007/s11912-010-0085-4.
Song M. Recent developments in small molecule therapies for renal cell carcinoma. Eur J Med Chem. 2017. https://doi.org/10.1016/j.ejmech.2017.08.007.
Subramanian P, Haas Md NB. Recent advances in localized RCC: a focus on VEGF and immuno-oncology therapies. Urol Oncol. 2017. https://doi.org/10.1016/j.urolonc.2017.09.008.
Escudier B, Eisen T, Stadler WM, Szczylik C, Oudard S, Siebels M, et al. Sorafenib in advanced clear-cell renal-cell carcinoma. New Engl J Med. 2007;356(2):125–34. https://doi.org/10.1056/NEJMoa060655.
Motzer RJ, Hutson TE, Tomczak P, Michaelson MD, Bukowski RM, Oudard S, et al. Overall survival and updated results for sunitinib compared with interferon alfa in patients with metastatic renal cell carcinoma. J Clin Oncol. 2009;27(22):3584–90. https://doi.org/10.1200/jco.2008.20.1293.
Hutson TE, Lesovoy V, Al-Shukri S, Stus VP, Lipatov ON, Bair AH, et al. Axitinib versus sorafenib as first-line therapy in patients with metastatic renal-cell carcinoma: a randomised open-label phase 3 trial. Lancet Oncol. 2013;14(13):1287–94. https://doi.org/10.1016/s1470-2045(13)70465-0.
Sternberg CN, Davis ID, Mardiak J, Szczylik C, Lee E, Wagstaff J, et al. Pazopanib in Locally advanced or metastatic renal cell carcinoma: results of a randomized phase III trial. J Clin Oncol. 2010;28(6):1061–8. https://doi.org/10.1200/jco.2009.23.9764.
Motzer RJ, Escudier B, Oudard S, Hutson TE, Porta C, Bracarda S, et al. Efficacy of everolimus in advanced renal cell carcinoma: a double-blind, randomised, placebo-controlled phase III trial. Lancet (Lond, Engl). 2008;372(9637):449–56. https://doi.org/10.1016/s0140-6736(08)61039-9.
Hudes G, Carducci M, Tomczak P, Dutcher J, Figlin R, Kapoor A, et al. Temsirolimus, interferon alfa, or both for advanced renal-cell carcinoma. New Engl J Med. 2007;356(22):2271–81. https://doi.org/10.1056/NEJMoa066838.
Lee SH, Bang YJ, Mainwaring P, Ng C, Chang JW, Kwong P, et al. Sunitinib in metastatic renal cell carcinoma: an ethnic Asian subpopulation analysis for safety and efficacy. Asia Pac J Clin Oncol. 2014;10(3):237–45. https://doi.org/10.1111/ajco.12163.
Poprach A, Pavlik T, Melichar B, Puzanov I, Dusek L, Bortlicek Z, et al. Skin toxicity and efficacy of sunitinib and sorafenib in metastatic renal cell carcinoma: a national registry-based study. Ann Oncol Off J Eur Soc Med Oncol/ESMO. 2012;23(12):3137–43. https://doi.org/10.1093/annonc/mds145.
Rautiola J, Donskov F, Peltola K, Joensuu H, Bono P. Sunitinib-induced hypertension, neutropaenia and thrombocytopaenia as predictors of good prognosis in patients with metastatic renal cell carcinoma. BJU Int. 2014. https://doi.org/10.1111/bju.12940.
Willemsen AE, Grutters JC, Gerritsen WR, van Erp NP, van Herpen CM, Tol J. mTOR inhibitor-induced interstitial lung disease in cancer patients: comprehensive review and a practical management algorithm. Int J Cancer J Int Cancer. 2016;138(10):2312–21. https://doi.org/10.1002/ijc.29887.
Tsukamoto T, Shinohara N, Tsuchiya N, Hamamoto Y, Maruoka M, Fujimoto H, et al. Phase III trial of everolimus in metastatic renal cell carcinoma: subgroup analysis of Japanese patients from RECORD-1. Jpn J Clin Oncol. 2011;41(1):17–24. https://doi.org/10.1093/jjco/hyq166.
Noguchi S, Masuda N, Iwata H, Mukai H, Horiguchi J, Puttawibul P, et al. Efficacy of everolimus with exemestane versus exemestane alone in Asian patients with HER2-negative, hormone-receptor-positive breast cancer in BOLERO-2. Breast Cancer. 2014;21(6):703–14. https://doi.org/10.1007/s12282-013-0444-8.
Atkinson BJ, Cauley DH, Ng C, Millikan RE, Xiao L, Corn P, et al. Mammalian target of rapamycin (mTOR) inhibitor-associated non-infectious pneumonitis in patients with renal cell cancer: predictors, management, and outcomes. BJU Int. 2014;113(3):376–82. https://doi.org/10.1111/bju.12420.
Penttila P, Donskov F, Rautiola J, Peltola K, Laukka M, Bono P. Everolimus-induced pneumonitis associates with favourable outcome in patients with metastatic renal cell carcinoma. Eur J Cancer. 2017;81:9–16. https://doi.org/10.1016/j.ejca.2017.05.004.
Conteduca V, Santoni M, Medri M, Scarpi E, Burattini L, Lolli C, et al. Correlation of stomatitis and cutaneous toxicity with clinical outcome in patients with metastatic renal-cell carcinoma treated with everolimus. Clin Genitourin Cancer. 2016;14(5):426–31. https://doi.org/10.1016/j.clgc.2016.02.012.
Chu YH, Li H, Tan HS, Koh V, Lai J, Phyo WM, et al. Association of ABCB1 and FLT3 polymorphisms with toxicities and survival in Asian patients receiving sunitinib for renal cell carcinoma. PLoS ONE. 2015;10(8):e0134102. https://doi.org/10.1371/journal.pone.0134102.
Diekstra MH, Klumpen HJ, Lolkema MP, Yu H, Kloth JS, Gelderblom H, et al. Association analysis of genetic polymorphisms in genes related to sunitinib pharmacokinetics, specifically clearance of sunitinib and SU12662. Clin Pharmacol Ther. 2014;96(1):81–9. https://doi.org/10.1038/clpt.2014.47.
Boudou-Rouquette P, Narjoz C, Golmard JL, Thomas-Schoemann A, Mir O, Taieb F, et al. Early sorafenib-induced toxicity is associated with drug exposure and UGTIA9 genetic polymorphism in patients with solid tumors: a preliminary study. PLoS ONE. 2012;7(8):e42875. https://doi.org/10.1371/journal.pone.0042875.
Bins S, Lenting A, El Bouazzaoui S, van Doorn L, Oomen-de Hoop E, Eskens FA, et al. Polymorphisms in SLCO1B1 and UGT1A1 are associated with sorafenib-induced toxicity. Pharmacogenomics. 2016;17(14):1483–90. https://doi.org/10.2217/pgs-2016-0063.
Suttle AB, Ball HA, Molimard M, Hutson TE, Carpenter C, Rajagopalan D, et al. Relationships between pazopanib exposure and clinical safety and efficacy in patients with advanced renal cell carcinoma. Br J Cancer. 2014;111(10):1909–16. https://doi.org/10.1038/bjc.2014.503.
van Erp NP, Eechoute K, van der Veldt AA, Haanen JB, Reyners AK, Mathijssen RH, et al. Pharmacogenetic pathway analysis for determination of sunitinib-induced toxicity. J Clin Oncol. 2009;27(26):4406–12. https://doi.org/10.1200/JCO.2008.21.7679.
Numakura K, Tsuchiya N, Kagaya H, Takahashi M, Tsuruta H, Inoue T, et al. Clinical effects of single nucleotide polymorphisms on drug-related genes in Japanese metastatic renal cell carcinoma patients treated with sunitinib. Anticancer Drugs. 2017;28(1):97–103. https://doi.org/10.1097/cad.0000000000000425.
Kim HR, Park HS, Kwon WS, Lee JH, Tanigawara Y, Lim SM, et al. Pharmacogenetic determinants associated with sunitinib-induced toxicity and ethnic difference in Korean metastatic renal cell carcinoma patients. Cancer Chemother Pharmacol. 2013;72(4):825–35. https://doi.org/10.1007/s00280-013-2258-y.
Mizuno T, Fukudo M, Terada T, Kamba T, Nakamura E, Ogawa O, et al. Impact of genetic variation in breast cancer resistance protein (BCRP/ABCG2) on sunitinib pharmacokinetics. Drug Metab Pharmacokinet. 2012;27(6):631–9.
Noda S, Otsuji T, Baba M, Yoshida T, Kageyama S, Okamoto K, et al. Assessment of sunitinib-induced toxicities and clinical outcomes based on therapeutic drug monitoring of sunitinib for patients with renal cell carcinoma. Clin Genitourin Cancer. 2015;13(4):350–8. https://doi.org/10.1016/j.clgc.2015.01.007.
Fukudo M, Ito T, Mizuno T, Shinsako K, Hatano E, Uemoto S, et al. Exposure-toxicity relationship of sorafenib in Japanese patients with renal cell carcinoma and hepatocellular carcinoma. Clin Pharmacokinet. 2014;53(2):185–96. https://doi.org/10.1007/s40262-013-0108-z.
Mai H, Huang J, Zhang Y, Qu N, Qu H, Mei GH, et al. In-vivo relation between plasma concentration of sorafenib and its safety in Chinese patients with metastatic renal cell carcinoma: a single-center clinical study. Oncotarget. 2017;8(26):43458–69. https://doi.org/10.18632/oncotarget.16465.
Lee JH, Chung YH, Kim JA, Shim JH, Lee D, Lee HC, et al. Genetic predisposition of hand-foot skin reaction after sorafenib therapy in patients with hepatocellular carcinoma. Cancer. 2013;119(1):136–42. https://doi.org/10.1002/cncr.27705.
Peer CJ, Sissung TM, Kim A, Jain L, Woo S, Gardner ER, et al. Sorafenib is an inhibitor of UGT1A1 but is metabolized by UGT1A9: implications of genetic variants on pharmacokinetics and hyperbilirubinemia. Clin Cancer Res. 2012;18(7):2099–107. https://doi.org/10.1158/1078-0432.CCR-11-2484.
Jain L, Sissung TM, Danesi R, Kohn EC, Dahut WL, Kummar S, et al. Hypertension and hand-foot skin reactions related to VEGFR2 genotype and improved clinical outcome following bevacizumab and sorafenib. J Exp Clin Cancer Res CR. 2010;29:95. https://doi.org/10.1186/1756-9966-29-95.
Diekstra MH, Swen JJ, Boven E, Castellano D, Gelderblom H, Mathijssen RH, et al. CYP3A5 and ABCB1 polymorphisms as predictors for sunitinib outcome in metastatic renal cell carcinoma. Eur Urol. 2015;68(4):621–9. https://doi.org/10.1016/j.eururo.2015.04.018.
Qin C, Cao Q, Li P, Wang S, Wang J, Wang M, et al. The influence of genetic variants of sorafenib on clinical outcomes and toxic effects in patients with advanced renal cell carcinoma. Sci Rep. 2016;6:20089. https://doi.org/10.1038/srep20089.
Zhang J, Yang J, Chen Y, Mao Q, Li S, Xiong W, et al. Genetic variants of VEGF (rs201963 and rs3025039) and KDR (rs7667298, rs2305948, and rs1870377) are associated with Glioma Risk in a Han Chinese population: a case-control study. Mol Neurobiol. 2016;53(4):2610–8. https://doi.org/10.1007/s12035-015-9240-0.
Huez I, Creancier L, Audigier S, Gensac MC, Prats AC, Prats H. Two independent internal ribosome entry sites are involved in translation initiation of vascular endothelial growth factor mRNA. Mol Cell Biol. 1998;18(11):6178–90.
Wang Y, Zheng Y, Zhang W, Yu H, Lou K, Zhang Y, et al. Polymorphisms of KDR gene are associated with coronary heart disease. J Am Coll Cardiol. 2007;50(8):760–7. https://doi.org/10.1016/j.jacc.2007.04.074.
Yamamoto K, Shinomiya K, Ioroi T, Hirata S, Harada K, Suno M, et al. Association of single nucleotide polymorphisms in STAT3 with hand-foot skin reactions in patients with metastatic renal cell carcinoma treated with multiple tyrosine kinase inhibitors: a retrospective analysis in Japanese patients. Targ Oncol. 2016;11(1):93–9. https://doi.org/10.1007/s11523-015-0382-9.
Tsuchiya N, Narita S, Inoue T, Hasunuma N, Numakura K, Horikawa Y, et al. Risk factors for sorafenib-induced high-grade skin rash in Japanese patients with advanced renal cell carcinoma. Anticancer Drugs. 2013;24(3):310–4. https://doi.org/10.1097/CAD.0b013e32835c401c.
Franke RM, Lancaster CS, Peer CJ, Gibson AA, Kosloske AM, Orwick SJ, et al. Effect of ABCC2 (MRP2) transport function on erythromycin metabolism. Clin Pharmacol Ther. 2011;89(5):693–701. https://doi.org/10.1038/clpt.2011.25.
Liu Y, Yin Y, Sheng Q, Lu X, Wang F, Lin Z, et al. Association of ABCC2 -24C > T polymorphism with high-dose methotrexate plasma concentrations and toxicities in childhood acute lymphoblastic leukemia. PLoS ONE. 2014;9(1):e82681. https://doi.org/10.1371/journal.pone.0082681.
Hu S, Chen Z, Franke R, Orwick S, Zhao M, Rudek MA, et al. Interaction of the multikinase inhibitors sorafenib and sunitinib with solute carriers and ATP-binding cassette transporters. Clin Cancer Res Off J Am Assoc Cancer Res. 2009;15(19):6062–9. https://doi.org/10.1158/1078-0432.CCR-09-0048.
Tirona RG, Lee W, Leake BF, Lan LB, Cline CB, Lamba V, et al. The orphan nuclear receptor HNF4alpha determines PXR- and CAR-mediated xenobiotic induction of CYP3A4. Nat Med. 2003;9(2):220–4. https://doi.org/10.1038/nm815.
Bartnicka L, Kurzawski M, Drozdzik A, Plonska-Gosciniak E, Gornik W, Drozdzik M. Effect of ABCB1 (MDR1) 3435C > T and 2677G > A, T polymorphisms and P-glycoprotein inhibitors on salivary digoxin secretion in congestive heart failure patients. Pharmacol Rep PR. 2007;59(3):323–9.
Huber JC, Schneeberger C, Tempfer CB. Genetic modeling of estrogen metabolism as a risk factor of hormone-dependent disorders. Maturitas. 2002;41(Suppl 1):S55–64.
Lakkireddy S, Aula S, Avn S, Kapley A, Rao Digumarti R, Jamil K. Association of the common CYP1A1*2C Variant (Ile462Val polymorphism) with chronic myeloid leukemia (CML) in patients undergoing imatinib therapy. Cell J. 2015;17(3):510–9.
Watanabe A, Yamamoto K, Ioroi T, Hirata S, Harada K, Miyake H, et al. Association of single nucleotide polymorphisms in STAT3, ABCB1, and ABCG2 with stomatitis in patients with metastatic renal cell carcinoma treated with sunitinib: a retrospective analysis in Japanese patients. Biol Pharm Bull. 2017;40(4):458–64. https://doi.org/10.1248/bpb.b16-00875.
Garcia-Donas J, Esteban E, Leandro-Garcia LJ, Castellano DE, del Alba AG, Climent MA, et al. Single nucleotide polymorphism associations with response and toxic effects in patients with advanced renal-cell carcinoma treated with first-line sunitinib: a multicentre, observational, prospective study. Lancet Oncol. 2011;12(12):1143–50. https://doi.org/10.1016/s1470-2045(11)70266-2.
Choi HY, Bae KS, Cho SH, Ghim JL, Choe S, Jung JA, et al. Impact of CYP2D6, CYP3A5, CYP2C19, CYP2A6, SLCO1B1, ABCB1, and ABCG2 gene polymorphisms on the pharmacokinetics of simvastatin and simvastatin acid. Pharmacogenet Genomics. 2015;25(12):595–608. https://doi.org/10.1097/fpc.0000000000000176.
Keskitalo JE, Pasanen MK, Neuvonen PJ, Niemi M. Different effects of the ABCG2 c.421C > A SNP on the pharmacokinetics of fluvastatin, pravastatin and simvastatin. Pharmacogenomics. 2009;10(10):1617–24. https://doi.org/10.2217/pgs.09.85.
Diekstra MH, Belaustegui A, Swen JJ, Boven E, Castellano D, Gelderblom H, et al. Sunitinib-induced hypertension in CYP3A4 rs4646437 A-allele carriers with metastatic renal cell carcinoma. Pharmacogenomics J. 2017;17(1):42–6. https://doi.org/10.1038/tpj.2015.100.
He HR, Sun JY, Ren XD, Wang TT, Zhai YJ, Chen SY, et al. Effects of CYP3A4 polymorphisms on the plasma concentration of voriconazole. Eur J Clin Microbiol Infect Dis. 2015;34(4):811–9. https://doi.org/10.1007/s10096-014-2294-5.
Choi JW, Park CS, Hwang M, Nam HY, Chang HS, Park SG, et al. A common intronic variant of CXCR3 is functionally associated with gene expression levels and the polymorphic immune cell responses to stimuli. J Allergy Clin Immunol. 2008;122(6):1119–26 e7. https://doi.org/10.1016/j.jaci.2008.09.026.
Houk BE, Bello CL, Poland B, Rosen LS, Demetri GD, Motzer RJ. Relationship between exposure to sunitinib and efficacy and tolerability endpoints in patients with cancer: results of a pharmacokinetic/pharmacodynamic meta-analysis. Cancer Chemother Pharmacol. 2010;66(2):357–71. https://doi.org/10.1007/s00280-009-1170-y.
Kim JJ, Vaziri SAJ, Rini BI, Elson P, Garcia JA, Wirka R, et al. Association of VEGF and VEGFR2 single nucleotide polymorphisms with hypertension and clinical outcome in metastatic clear cell renal cell carcinoma patients treated with sunitinib. Cancer. 2012;118(7):1946–54. https://doi.org/10.1002/cncr.26491.
Diekstra MH, Liu X, Swen JJ, Boven E, Castellano D, Gelderblom H, et al. Association of single nucleotide polymorphisms in IL8 and IL13 with sunitinib-induced toxicity in patients with metastatic renal cell carcinoma. Eur J Clin Pharmacol. 2015;71(12):1477–84. https://doi.org/10.1007/s00228-015-1935-7.
Hacking D, Knight JC, Rockett K, Brown H, Frampton J, Kwiatkowski DP, et al. Increased in vivo transcription of an IL-8 haplotype associated with respiratory syncytial virus disease-susceptibility. Genes Immun. 2004;5(4):274–82. https://doi.org/10.1038/sj.gene.6364067.
Amaya MP, Criado L, Blanco B, Gomez M, Torres O, Florez L, et al. Polymorphisms of pro-inflammatory cytokine genes and the risk for acute suppurative or chronic nonsuppurative apical periodontitis in a Colombian population. Int Endod J. 2013;46(1):71–8. https://doi.org/10.1111/j.1365-2591.2012.02097.x.
Xu CF, Johnson T, Garcia-Donas J, Choueiri TK, Sternberg CN, Davis ID, et al. IL8 polymorphisms and overall survival in pazopanib- or sunitinib-treated patients with renal cell carcinoma. Br J Cancer. 2015;112(7):1190–8. https://doi.org/10.1038/bjc.2015.64.
Petreaca ML, Yao M, Liu Y, Defea K, Martins-Green M. Transactivation of vascular endothelial growth factor receptor-2 by interleukin-8 (IL-8/CXCL8) is required for IL-8/CXCL8-induced endothelial permeability. Mol Biol Cell. 2007;18(12):5014–23. https://doi.org/10.1091/mbc.E07-01-0004.
Martin D, Galisteo R, Gutkind JS. CXCL8/IL8 stimulates vascular endothelial growth factor (VEGF) expression and the autocrine activation of VEGFR2 in endothelial cells by activating NFkappaB through the CBM (Carma3/Bcl10/Malt1) complex. J Biol Chem. 2009;284(10):6038–42. https://doi.org/10.1074/jbc.C800207200.
Xu CF, Reck BH, Xue Z, Huang L, Baker KL, Chen M, et al. Pazopanib-induced hyperbilirubinemia is associated with Gilbert’s syndrome UGT1A1 polymorphism. Br J Cancer. 2010;102(9):1371–7. https://doi.org/10.1038/sj.bjc.6605653.
Bosma PJ, Chowdhury JR, Bakker C, Gantla S, de Boer A, Oostra BA, et al. The genetic basis of the reduced expression of bilirubin UDP-glucuronosyltransferase 1 in Gilbert’s syndrome. New Engl J Med. 1995;333(18):1171–5. https://doi.org/10.1056/nejm199511023331802.
Zucker SD, Qin X, Rouster SD, Yu F, Green RM, Keshavan P, et al. Mechanism of indinavir-induced hyperbilirubinemia. Proc Natl Acad Sci USA. 2001;98(22):12671–6. https://doi.org/10.1073/pnas.231140698.
Danoff TM, Campbell DA, McCarthy LC, Lewis KF, Repasch MH, Saunders AM, et al. A Gilbert’s syndrome UGT1A1 variant confers susceptibility to tranilast-induced hyperbilirubinemia. Pharmacogenomics J. 2004;4(1):49–53. https://doi.org/10.1038/sj.tpj.6500221.
Singer JB, Shou Y, Giles F, Kantarjian HM, Hsu Y, Robeva AS, et al. UGT1A1 promoter polymorphism increases risk of nilotinib-induced hyperbilirubinemia. Leukemia. 2007;21(11):2311–5. https://doi.org/10.1038/sj.leu.2404827.
Low SK, Fukunaga K, Takahashi A, Matsuda K, Hongo F, Nakanishi H, et al. Association study of a functional variant on ABCG2 gene with sunitinib-induced severe adverse drug reaction. PLoS ONE. 2016;11(2):e0148177. https://doi.org/10.1371/journal.pone.0148177.
Xu CF, Reck BH, Goodman VL, Xue Z, Huang L, Barnes MR, et al. Association of the hemochromatosis gene with pazopanib-induced transaminase elevation in renal cell carcinoma. J Hepatol. 2011;54(6):1237–43. https://doi.org/10.1016/j.jhep.2010.09.028.
Olynyk JK, Knuiman MW, Divitini ML, Bartholomew HC, Cullen DJ, Powell LW. Effects of HFE gene mutations and alcohol on iron status, liver biochemistry and morbidity. J Gastroenterol Hepatol. 2005;20(9):1435–41. https://doi.org/10.1111/j.1440-1746.2005.03967.x.
Nelson JE, Bhattacharya R, Lindor KD, Chalasani N, Raaka S, Heathcote EJ, et al. HFE C282Y mutations are associated with advanced hepatic fibrosis in Caucasians with nonalcoholic steatohepatitis. Hepatology. 2007;46(3):723–9. https://doi.org/10.1002/hep.21742.
Forooghian F, Das B. Anti-angiogenic effects of ribonucleic acid interference targeting vascular endothelial growth factor and hypoxia-inducible factor-1alpha. Am J Ophthalmol. 2007;144(5):761–8. https://doi.org/10.1016/j.ajo.2007.07.022.
Xu CF, Johnson T, Wang X, Carpenter C, Graves AP, Warren L, et al. HLA-B*57:01 confers susceptibility to pazopanib-associated liver injury in patients with cancer. Clin Cancer Res. 2016;22(6):1371–7. https://doi.org/10.1158/1078-0432.CCR-15-2044.
Sharma SK, Balamurugan A, Saha PK, Pandey RM, Mehra NK. Evaluation of clinical and immunogenetic risk factors for the development of hepatotoxicity during antituberculosis treatment. Am J Respir Crit Care Med. 2002;166(7):916–9. https://doi.org/10.1164/rccm.2108091.
Hirata K, Takagi H, Yamamoto M, Matsumoto T, Nishiya T, Mori K, et al. Ticlopidine-induced hepatotoxicity is associated with specific human leukocyte antigen genomic subtypes in Japanese patients: a preliminary case-control study. Pharmacogenomics J. 2008;8(1):29–33. https://doi.org/10.1038/sj.tpj.6500442.
O’Donohue J, Oien KA, Donaldson P, Underhill J, Clare M, MacSween RN, et al. Co-amoxiclav jaundice: clinical and histological features and HLA class II association. Gut. 2000;47(5):717–20.
Kindmark A, Jawaid A, Harbron CG, Barratt BJ, Bengtsson OF, Andersson TB, et al. Genome-wide pharmacogenetic investigation of a hepatic adverse event without clinical signs of immunopathology suggests an underlying immune pathogenesis. Pharmacogenomics J. 2008;8(3):186–95. https://doi.org/10.1038/sj.tpj.6500458.
Daly AK, Donaldson PT, Bhatnagar P, Shen Y, Pe’er I, Floratos A, et al. HLA-B*5701 genotype is a major determinant of drug-induced liver injury due to flucloxacillin. Nat Genet. 2009;41(7):816–9. https://doi.org/10.1038/ng.379.
Zimmerman EI, Hu S, Roberts JL, Gibson AA, Orwick SJ, Li L, et al. Contribution of OATP1B1 and OATP1B3 to the disposition of sorafenib and sorafenib-glucuronide. Clin Cancer Res. 2013;19(6):1458–66. https://doi.org/10.1158/1078-0432.CCR-12-3306.
Hu S, Mathijssen RH, de Bruijn P, Baker SD, Sparreboom A. Inhibition of OATP1B1 by tyrosine kinase inhibitors: in vitro-in vivo correlations. Br J Cancer. 2014;110(4):894–8. https://doi.org/10.1038/bjc.2013.811.
Niemi M, Pasanen MK, Neuvonen PJ. Organic anion transporting polypeptide 1B1: a genetically polymorphic transporter of major importance for hepatic drug uptake. Pharmacol Rev. 2011;63(1):157–81. https://doi.org/10.1124/pr.110.002857.
Maayah ZH, Ansari MA, El Gendy MA, Al-Arifi MN, Korashy HM. Development of cardiac hypertrophy by sunitinib in vivo and in vitro rat cardiomyocytes is influenced by the aryl hydrocarbon receptor signaling pathway. Arch Toxicol. 2014;88(3):725–38. https://doi.org/10.1007/s00204-013-1159-5.
Maayah ZH, El Gendy MA, El-Kadi AO, Korashy HM. Sunitinib, a tyrosine kinase inhibitor, induces cytochrome P450 1A1 gene in human breast cancer MCF7 cells through ligand-independent aryl hydrocarbon receptor activation. Arch Toxicol. 2013;87(5):847–56. https://doi.org/10.1007/s00204-012-0996-y.
Lu J, Zhao Q, Zhai YJ, He HR, Yang LH, Gao F, et al. Genetic polymorphisms of CYP1A1 and risk of leukemia: a meta-analysis. OncoTargets Ther. 2015;8:2883–902. https://doi.org/10.2147/OTT.S92259.
Han F, Tan Y, Cui W, Dong L, Li W. Novel insights into etiologies of leukemia: a HuGE review and meta-analysis of CYP1A1 polymorphisms and leukemia risk. Am J Epidemiol. 2013;178(4):493–507. https://doi.org/10.1093/aje/kwt016.
Crofts F, Taioli E, Trachman J, Cosma GN, Currie D, Toniolo P, et al. Functional significance of different human CYP1A1 genotypes. Carcinogenesis. 1994;15(12):2961–3.
van der Veldt AA, Eechoute K, Gelderblom H, Gietema J, Guchelaar HJ, van Erp NP, et al. Genetic polymorphisms associated with a prolonged progression-free survival in patients with metastatic renal cell cancer treated with sunitinib. Clin Cancer Res. 2011;17(3):620–9. https://doi.org/10.1158/1078-0432.ccr-10-1828.
Carow CE, Levenstein M, Kaufmann SH, Chen J, Amin S, Rockwell P, et al. Expression of the hematopoietic growth factor receptor FLT3 (STK-1/Flk2) in human leukemias. Blood. 1996;87(3):1089–96.
Nakao M, Yokota S, Iwai T, Kaneko H, Horiike S, Kashima K, et al. Internal tandem duplication of the flt3 gene found in acute myeloid leukemia. Leukemia. 1996;10(12):1911–8.
Pascual T, Apellaniz-Ruiz M, Pernaut C, Cueto-Felgueroso C, Villalba P, Alvarez C, et al. Polymorphisms associated with everolimus pharmacokinetics, toxicity and survival in metastatic breast cancer. PLoS ONE. 2017;12(7):e0180192. https://doi.org/10.1371/journal.pone.0180192.
de Velasco G, Gray KP, Hamieh L, Urun Y, Carol HA, Fay AP, et al. Pharmacogenomic markers of targeted therapy toxicity in patients with metastatic renal cell carcinoma. Eur Urol Focus. 2016;2(6):633–9. https://doi.org/10.1016/j.euf.2016.03.017.
Acknowledgements
The authors would like to thank Enago (www.enago.jp) for the English language review.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declared no conflicts of interest.
Human and animal rights
This article does not contain any studies with human participants or animals performed by the authors.
Rights and permissions
About this article
Cite this article
Yamamoto, K., Yano, I. Genetic polymorphisms associated with adverse reactions of molecular-targeted therapies in renal cell carcinoma. Med Oncol 35, 16 (2018). https://doi.org/10.1007/s12032-017-1077-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12032-017-1077-0