Abstract
Human cancers are endowed with sustained vascularization capability, and their growth, invasion, and metastasis are vascularization dependent. Recently, accumulated body of evidence suggests that endothelial progenitor cells (EPCs) can support vasculogenesis and induce angiogenesis through paracrine mechanisms. In addition, numerous clinical studies have revealed the increase in the number of EPCs in the peripheral blood of cancer patients and demonstrated the correlation of circulating EPCs (CEPCs) with the clinical outcomes. This review highlights current enrichment procedures and methods for the detection of CEPCs and different biomarkers to identify CEPCs as well as the functions of EPCs in tumor vascularization. Furthermore, we systematically review available studies on the clinical relevance of CEPCs in cancer patients to explore the potential diagnostic and prognostic values of CEPCs. Although several contrasting results exist, CEPCs can conceivably serve as a promising biomarker for the early diagnosis, prognosis prediction, and treatment response indication in the future. Additionally, further well-designed clinical studies with larger sample size and unique, specific enumeration procedures are warranted to achieve further insight into the clinical implications of CEPCs.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cancer is a major public health problem which serves as the leading cause of death across the globe. An estimated 1,665,540 newly diagnosed cancer cases and 585,720 cancer-related deaths are projected to occur in the USA in 2014 [1]. Over the past few decades, numerous studies have been conducted to reveal the underlying mechanism of carcinogenesis. A rich and complex body of evidence has been generated, which suggests that sustained vascularization plays an important and fundamental role in the development and progression of human malignancies [2, 3].
Tumor neovasculature, a major hallmark of cancer, plays a crucial role in cell function and survival by supplying essential oxygen and nutrients. Tumor-related neovasculature has been conventionally recognized as the result of angiogenesis, which depends on the preexisting endothelial cells to form new capillaries [4]. Several lines of evidences have emerged indicating postnatal vasculogenesis supports as an alternative source of tumor-associated vascularization [5, 6]. Specifically, vasculogenesis is a multistep process, which includes the mobilization and homing of bone marrow (BM)-derived endothelial progenitor cells (EPCs) to neoplastic sites and subsequent proliferation and differentiation into mature endothelial cells (ECs) [7, 8]. In 1997, Asahara et al. [9] initially described EPCs as a specific subtype of stem cells that can migrate, proliferate, and differentiate into mature ECs. Since then, considerable attention has been paid on the potential roles of EPCs in the development and progression of human cancers [10–12] as well as further clinical implications [13–15]. However, inconclusive results were obtained from clinical studies. We conducted this review to present a state-of-the-art overview of the recent findings in this field, which may provide implications for the potential diagnostic and prognostic values of circulating EPCs (CEPCs) in clinical oncology.
Characteristics of EPCs
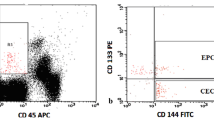
EPCs are a specific subgroup of mononuclear cells (MNCs) co-expressing vascular endothelial growth factor receptor 2 [VEGFR2, also known as kinase domain receptor (KDR) or fetal liver kinase-1 (Flk-1)] and CD34, which could proliferate and differentiate into mature ECs [9]. Intensive studies on EPCs have revealed specific membrane markers and molecular probes, such as fibroblast growth factor receptor (FGFR), Von Willebrand factor (vWF), CD38, CD31, c-kit/CD117, and vascular endothelial cadherin (VE-cadherin or CD144) [16–18]. However, given that EPCs and mature ECs share many identical surface markers, which include CD31, VEGFR2, vWF, and VE-cadherin, distinguishing EPCs and ECs is extremely difficult. Thus, an additional marker, CD133, has been suggested as a means of ensuring the identity of EPCs. CD133 is a precursor of an EC-like phenotype and has been applied in combination with CD34 and VEGFR2 to identify presumed CEPCs [19–22]. However, the CD34+CD133+VEGFR2+ cells only represent approximately 0.025 % of the peripheral blood MNCs [22], which complicates the reliable quantification of EPCs and limits the translation of prosperous findings about EPCs from bench to bedside. Furthermore, CD133 has also been suggested to be expressed in hematopoietic stem cells (HSCs) [23, 24], and cells identified by CD133 comprise one subtype of HSCs incapable of forming true endothelial phenotypes [25, 26]. Consequently, various definitions of CEPCs, such as CD34+VEGFR2+, CD34+VEGFR2+CD45−, and CD34+CD133, have been employed, which made the comparison and further integration of results across all individual studies quite challenging.
EPCs are mainly divided into two subtypes in terms of functional phenotypes: early and late outgrowth EPCs [27, 28]. Early outgrowth EPCs, also known as colony-forming unit-ECs (CFU-ECs) or CFU-Hill, appear after short-term (4–10 days) culture of MNCs from peripheral blood, display peak growth at 2–3 week, and live up to 4 week [9, 29]. By contrast, late outgrowth EPCs, now generally termed as endothelial colony-forming cells (ECFCs), are cobblestone-shaped cells that can be found after long-term culture (>2 week), achieve peak growth at 4–8 week, and survive up to 12 week [30–32]. Furthermore, the two types of EPCs have been proven to carry different gene expression signatures by genome-wide transcriptional profiling and protein electrophoresis methods [33]. Notably, over a decade have passed since the first description of CEPCs, but the understanding of the fundamental basic science governing the biology of EPCs is still incomplete, and no golden criteria for defining CEPCs exist.
Role of EPCs in tumor vascularization
Human cancers can sustain vascularization, and their growth, invasion, and metastasis are vascularization dependent [2, 3]. Tumor vascularization has long been considered solely through the mechanism of angiogenesis, which means sprouting from preexisting blood vessels [34, 35]. EPCs identification resulted in a paradigm shift, inducing vasculogenesis as a novel mechanism for vessel formation into the tumor setting. The first proof-of-principle of EPCs contribution to cancer-induced vasculogenesis was demonstrated by Lyden et al. in [36]. With the use of angiogenesis-defective Id-mutant mice, they reported that BM transplantation from wild-type mice rather than from Id-mutant mice could restore tumor vascularization and growth of several types of tumor cell lines [36]. In subsequent animal xenograft models, the levels of incorporated EPCs in newly formed blood vessels have been reported to be as high as 50 % [37], whereas lower but significant levels between 10 and 20 % were reported in another study [38]. However, other studies have challenged the contribution of EPCs to tumor vasculature because they hardly or even failed to observe the presence of EPCs in tumor capillaries [39–41]. In a clinical study, Peters et al. [42] analyzed tumors from six patients who developed cancers after sex-mismatched BM transplantation. Fluorescence in situ hybridization studies with sex-chromosome-specific probes have demonstrated that BM-derived EPCs contributed to tumor endothelium, but with various levels as the incorporation levels which ranged from 1 to 12.1 %.
The inconclusive or even controversial findings from the different studies suggest that EPCs may induce tumor vascularization through an alternative autocrine/paracrine mechanism [43]. This paracrine aspect of EPCs activity was supported by Gao et al., who demonstrated that blocking EPCs mobilization could cause severe angiogenesis inhibition and remarkably impair cancer progression. Furthermore, gene expression analysis of EPCs revealed the upregulation of various key proangiogenic genes, such as FGFR1, VEGF-C, and platelet-derived growth factor alpha [44]. Along this line, our own study confirmed that EPCs could induce angiogenesis by synthesizing angiogenesis factors, such as SDF-1 and VEGF [45, 46]. In summary, a growing body of evidence indicates that EPCs can directly and indirectly contribute to tumor vascularization.
Circulating levels of EPCs in cancer patients
Given the important functions of vascularization in human malignancies, both as a pathogenic mechanism and treatment target, numerous efforts have been undertaken to identify surrogate biomarkers that could accurately reflect the angiogenic/vasculogenic activity of the tumor and therapy effects on tumor vasculature. Considered as potent surrogate biomarkers, microvessel density (MVD) and VEGF expression level have been extensively investigated and proven to be of both diagnostic and prognostic values in clinical oncology [47–50]. In addition to these two biomarkers, the EPCs concentration in the peripheral blood has emerged as a promising and potent biomarker because it could reflect the tumor vascularization activity more accurately, directly, and non-invasively. Various EPCs concentration in the peripheral blood of cancer patients was first reported in 2003, during which Kim et al. examined the CEPCs levels using a culture assay of peripheral blood MNCs after recruiting 16 healthy controls and 71 newly diagnosed cases (19 breast cancer and 52 gastric cancer patients). They found that the number of CEPCs in the cancer patients was comparable to that in the healthy controls (37.6 ± 4.2 vs. 40.2 ± 10.2/mm2; P > 0.05). They also conducted subgroup analysis based on cancer types and failed to detect higher EPC concentration in the peripheral blood of both breast and gastric carcinoma patients [51]. Subsequently, Ho et al. [19] compared the circulating levels of EPCs in 80 hepatocellular carcinoma (HCC) and 14 healthy subjects by employing another culture assay described by Hill et al. [29]. They found a significantly elevated EPC level in HCC patients (P = 0001). Interestingly in the same study, fluorescence-activated cell sorting (FACS) quantification of EPCs, in which CD34, CD133, and VEGFR2 were used as markers, was also employed to validate the CFU scores.
Numerous studies that only used FACS technique have been conducted to reveal the dynamics of CEPCs in various cancer types such as lung cancer [22, 52–54], malignant glioma [20, 55, 56], HCC [21, 57], breast cancer [58, 59], head and neck cancer [60], ovarian cancer [61–63], cervical cancer [63], colorectal cancer [64], prostate cancer [65], renal cell carcinoma (RCC) [66, 67], osteosarcoma [68], and multiple myeloma [69] (Table 1). However, inconclusive and controversial results were obtained; although in most studies, a significantly higher EPCs concentration was observed in the peripheral blood of cancer patients. In five case–control studies, the number of CEPCs was demonstrated to be comparable to that in control subjects [51, 54, 56, 65, 68]. However, Goon et al. [59] reported a significantly lower EPCs concentration in breast cancer patients (median: 121 vs. 169 cells/ml; P < 0.05). The contrasting results may be attributed to the fact that the majority of the included patients were at early stage, and the “healthy” controls were actually patients with benign breast lesions. To validate the diagnostic value of CEPCs, the selection criteria of both cancer patients and healthy controls should be stricter because of numerous confounding factors such as background cardiovascular diseases, diabetes mellitus, and lifestyles, which include smoking status, physical exercise, among others [70, 71].
Prognostic value of CEPCs
The paradigm of CEPCs as a surrogate biomarker of vascularization has stimulated numerous researchers to explore the prognostic power of CEPCs in cancer patients. TNM classification and histological grade are the main prognostic indicators in clinical oncology. Supplementary Table S1 underscores the data from available studies [19, 22, 51–53, 55, 56, 58–62, 64, 65, 67–69, 72–74] that assessed the correlation of CEPCs with clinicopathological features such as TNM stage, pathological grade, and other specific variables, which could indirectly support the prognostic value of CEPCs in studies without long-term follow-up data. CEPCs levels have been confirmed to be significantly correlated with tumor stage [22, 61, 62, 64, 67, 69, 72, 74], tumor size [19, 58, 61], MVD [55, 73], and serum VEGF concentration [55, 67]. Further detailed information is presented in Supplementary Table S1.
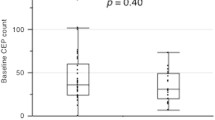
To illustrate the usefulness of the circulating levels of EPCs in predicting long-term outcomes such as progression, recurrence, or survival, the impacts of pre-treatment, post-treatment level, and/or intratreatment changes in the EPCs on disease outcome have been assessed in several studies with long-term follow-up [52–54, 61, 69, 75–77] (as summarized in Table 2). Patients with lower pre-treatment CEPCs levels have achieved longer overall survival (OS) length [52, 53, 61, 77], recurrence-free survival (RFS) [54], and response duration (RD) [69]. However, Sakamori et al. [76] failed to detect any association of EPC concentration with progression-free survival (PFS). Interestingly, Bhaskar et al. [69] analyzed the association between the number of CEPCs at baseline and RD and found that the median RD was higher (23 months) for the group with ≤19.6/μl (n = 44) than that (14 months) for the group with >19.6/μl (n = 15). Furthermore, they analyzed the prognostic power of circulating level of EPCs after therapy and confirmed that the arm with ≤6.5/μl benefitted in longer median RD (23 vs. 9 months; P = 0.001). Similarly, in another cohort of patients with multiple cancers, CEPCs level at day 7 after chemotherapy was significantly median PFS (<11.5 vs. >6.2 months; P = 0.046) [75]. The prognostic value of the changes in the number of CEPCs has also been investigated [54, 76] and will be discussed in the next section. Thus, although divergence exists, the prognostic value of CEPCs is so overwhelming, and further clinical studies are warranted to validate the conclusion.
CEPCs as an indicator of treatment response
The efficacy of antitumor treatments is typically assessed by measuring the direct effects on tumor burden and/or survival and is mostly confined to imaging techniques such as X-ray, computed tomography, magnetic resonance imaging, and ultrasound [78]. However, the assessment of these parameters cannot reflect the efficacy immediately and directly, thus necessitating a reliable surrogate biomarker. The potential of CEPCs in the prediction and response monitoring to a therapeutic intervention is determined by distinguishing the impact of various interventions (e.g., surgery, chemotherapy, and radiation) on CEPCs in different cancers, including breast cancer [59, 72], head and neck cancer [60], lung cancer [22, 54, 76], ovarian cancer [61, 63], RCC [66, 67], colorectal cancer [79], multiple myeloma [69], glioma [56], gastric cancer [74], and mixed types [75] (Table 3). In all studies, the CEPCs levels were quantified by FACS technique, which are not presented in Table 3.
With respect to chemotherapy (including anti-angiogenic target therapy), a significantly decreased CEPCs concentration was demonstrated in three studies [69, 72, 76], whereas others reported no significant changes [22, 79], and another presented a remarkably higher EPCs level post-treatment [75]. Roodhart et al. analyzed the changes in CEPCs in a cohort of patients receiving chemotherapy and found that the increase in EPCs level started a few hours after the initiation of chemotherapy, exceeded to 114 % (95 % CI 78–151 %; NS) after 7 days and continued to increase to 304 % (95 % CI 176–1,431 %; P < 0.01) on day 21, and was not limited to the regimens applied in the chemotherapy [75]. However, they included heterogeneous population of chemotherapy and cancer types as well as applied the definition of EPCs as CD31+CD133+CD45− cells, which could partially explain the contradictory results. Notably, timing is a relevant issue because the circulating level of EPCs changes time-dependently [76].
The postoperative EPCs concentration in cancer patients has been compared with the preoperative level in several studies, but inconclusive results were obtained [54, 56, 59, 63, 66, 67, 74]. Yang and his colleagues quantified the CEPCs levels in 38 RCC patients who underwent surgery and found that the CEPCs level 3 months after surgery was significantly lower than the preoperative level (P < 0.001; Table 3) [67]. However, similar results have not been replicated in other studies. Such contradictory findings could be explained by confounding factors such as surgery types, BM-EPCs mobilization, and consumption caused by recruitment to surgical injury sites and to stress reaction to surgical insult [80]. Additionally, the time to assess the post-surgery CEPCs varies greatly, ranging from 2 days to 6 months. Furthermore, EPCs concentration has been examined in a cohort of patients with head and neck cancer who had been receiving radiation therapy, but no significant change was found [60].
The prognostic value of varying CEPCs post-treatment has been investigated in two clinical studies [54, 76] (Table 2). After classifying 38 patients according to the changes in the number of CEPCs relative to day 1 level before the second cycle of chemotherapy, Sakamori et al. [76] demonstrated that patients with high percentage changes in the number of CEPCs did not achieve a significantly longer median PFS (139 vs. 120 days; P = 0.295). Subsequently, Pirro et al. reported that a total of 15 non-small cell lung cancer patients experienced a 48-h postoperative increase in the number of CEPCs, whereas the remaining 19 patients had stable or decreased EPCs level compared with preoperative levels. Thus, patients with stable or decreased EPC level achieved a significantly longer RFS (P = 0.012) [54]. Therefore, CEPCs can act as potent indicator of clinical response to various interventions after validation by further large-scale clinical studies.
Future directions and conclusions
Further, basic studies are required with the following objectives: To clarify the origin, function, and molecular pathways of EPCs; to refine the identification, isolation, and molecular characterization of CEPCs; and to understand the process of tumor vascularization. After the unique specific markers have been determined, higher-quality clinical studies with larger sample size to allow adjusted analysis focusing on clinically important outcomes will aid in clarifying the potent diagnostic and prognostic values of CEPCs. Despite several diverging results, our review of the available literature apparently supports the diagnostic and prognostic power of CEPCs in clinical oncology.
References
Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2014;64(1):9–29.
Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100(1):57–70.
Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–74.
Carmeliet P, Jain RK. Molecular mechanisms and clinical applications of angiogenesis. Nature. 2011;473(7347):298–307.
Liu J, Huang J, Yao WY, Ben QW, Chen DF, He XY, et al. The origins of vacularization in tumors. Front Biosci (Landmark Ed). 2012;17:2559–65.
Hardee ME, Zagzag D. Mechanisms of glioma-associated neovascularization. Am J Pathol. 2012;181(4):1126–41.
Asahara T, Kawamoto A. Endothelial progenitor cells for postnatal vasculogenesis. Am J Physiol Cell Physiol. 2004;287(3):C572–9.
Patenaude A, Parker J, Karsan A. Involvement of endothelial progenitor cells in tumor vascularization. Microvasc Res. 2010;79(3):217–23.
Asahara T, Murohara T, Sullivan A, Silver M, van der Zee R, Li T, et al. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997;275(5302):964–7.
Ding YT, Kumar S, Yu DC. The role of endothelial progenitor cells in tumour vasculogenesis. Pathobiology. 2008;75(5):265–73.
Ahn JB, Rha SY, Shin SJ, Jeung HC, Kim TS, Zhang X, et al. Circulating endothelial progenitor cells (EPC) for tumor vasculogenesis in gastric cancer patients. Cancer Lett. 2010;288(1):124–32.
Moschetta M, Mishima Y, Sahin I, Manier S, Glavey S, Vacca A, et al. Role of endothelial progenitor cells in cancer progression. Biochim Biophys Acta. 2014;1846(1):26–39.
Roncalli JG, Tongers J, Renault MA, Losordo DW. Endothelial progenitor cells in regenerative medicine and cancer: a decade of research. Trends Biotechnol. 2008;26(5):276–83.
Dome B, Timar J, Ladanyi A, Paku S, Renyi-Vamos F, Klepetko W, et al. Circulating endothelial cells, bone marrow-derived endothelial progenitor cells and proangiogenic hematopoietic cells in cancer: from biology to therapy. Crit Rev Oncol Hematol. 2009;69(2):108–24.
Le Bourhis X, Romon R, Hondermarck H. Role of endothelial progenitor cells in breast cancer angiogenesis: from fundamental research to clinical ramifications. Breast Cancer Res Treat. 2010;120(1):17–24.
Burger PE, Coetzee S, McKeehan WL, Kan M, Cook P, Fan Y, et al. Fibroblast growth factor receptor-1 is expressed by endothelial progenitor cells. Blood. 2002;100(10):3527–35.
Heissig B, Werb Z, Rafii S, Hattori K. Role of c-kit/Kit ligand signaling in regulating vasculogenesis. Thromb Haemost. 2003;90(4):570–6.
Fadini GP, Losordo D, Dimmeler S. Critical reevaluation of endothelial progenitor cell phenotypes for therapeutic and diagnostic use. Circ Res. 2012;110(4):624–37.
Ho JW, Pang RW, Lau C, Sun CK, Yu WC, Fan ST, et al. Significance of circulating endothelial progenitor cells in hepatocellular carcinoma. Hepatology. 2006;44(4):836–43.
Zheng PP, Hop WC, Luider TM, Sillevis Smitt PA, Kros JM. Increased levels of circulating endothelial progenitor cells and circulating endothelial nitric oxide synthase in patients with gliomas. Ann Neurol. 2007;62(1):40–8.
Sieghart W, Fellner S, Reiberger T, Ulbrich G, Ferlitsch A, Wacheck V, et al. Differential role of circulating endothelial progenitor cells in cirrhotic patients with or without hepatocellular carcinoma. Dig Liver Dis. 2009;41(12):902–6.
Nowak K, Rafat N, Belle S, Weiss C, Hanusch C, Hohenberger P, et al. Circulating endothelial progenitor cells are increased in human lung cancer and correlate with stage of disease. Eur J Cardiothorac Surg. 2010;37(4):758–63.
Wognum AW, Eaves AC, Thomas TE. Identification and isolation of hematopoietic stem cells. Arch Med Res. 2003;34(6):461–75.
Mizrak D, Brittan M, Alison M. CD133: molecule of the moment. J Pathol. 2008;214(1):3–9.
Timmermans F, Van Hauwermeiren F, De Smedt M, Raedt R, Plasschaert F, De Buyzere ML, et al. Endothelial outgrowth cells are not derived from CD133+ cells or CD45+ hematopoietic precursors. Arterioscler Thromb Vasc Biol. 2007;27(7):1572–9.
Case J, Mead LE, Bessler WK, Prater D, White HA, Saadatzadeh MR, et al. Human CD34+AC133+VEGFR-2+ cells are not endothelial progenitor cells but distinct, primitive hematopoietic progenitors. Exp Hematol. 2007;35(7):1109–18.
Prater DN, Case J, Ingram DA, Yoder MC. Working hypothesis to redefine endothelial progenitor cells. Leukemia. 2007;21(6):1141–9.
Timmermans F, Plum J, Yoder MC, Ingram DA, Vandekerckhove B, Case J. Endothelial progenitor cells: identity defined? J Cell Mol Med. 2009;13(1):87–102.
Hill JM, Zalos G, Halcox JP, Schenke WH, Waclawiw MA, Quyyumi AA, et al. Circulating endothelial progenitor cells, vascular function, and cardiovascular risk. N Engl J Med. 2003;348(7):593–600.
Hur J, Yoon CH, Kim HS, Choi JH, Kang HJ, Hwang KK, et al. Characterization of two types of endothelial progenitor cells and their different contributions to neovasculogenesis. Arterioscler Thromb Vasc Biol. 2004;24(2):288–93.
Ingram DA, Mead LE, Tanaka H, Meade V, Fenoglio A, Mortell K, et al. Identification of a novel hierarchy of endothelial progenitor cells using human peripheral and umbilical cord blood. Blood. 2004;104(9):2752–60.
Yoder MC, Mead LE, Prater D, Krier TR, Mroueh KN, Li F, et al. Redefining endothelial progenitor cells via clonal analysis and hematopoietic stem/progenitor cell principals. Blood. 2007;109(5):1801–9.
Medina RJ, O’Neill CL, Sweeney M, Guduric-Fuchs J, Gardiner TA, Simpson DA, et al. Molecular analysis of endothelial progenitor cell (EPC) subtypes reveals two distinct cell populations with different identities. BMC Med Genomics. 2010;3:18.
Folkman J, Shing Y. Angiogenesis. J Biol Chem. 1992;267(16):10931–4.
Folkman J. The role of angiogenesis in tumor growth. Semin Cancer Biol. 1992;3(2):65–71.
Lyden D, Hattori K, Dias S, Costa C, Blaikie P, Butros L, et al. Impaired recruitment of bone-marrow-derived endothelial and hematopoietic precursor cells blocks tumor angiogenesis and growth. Nat Med. 2001;7(11):1194–201.
Garcia-Barros M, Paris F, Cordon-Cardo C, Lyden D, Rafii S, Haimovitz-Friedman A, et al. Tumor response to radiotherapy regulated by endothelial cell apoptosis. Science. 2003;300(5622):1155–9.
Goon PK, Lip GY, Boos CJ, Stonelake PS, Blann AD. Circulating endothelial cells, endothelial progenitor cells, and endothelial microparticles in cancer. Neoplasia. 2006;8(2):79–88.
Machein MR, Renninger S, de Lima-Hahn E, Plate KH. Minor contribution of bone marrow-derived endothelial progenitors to the vascularization of murine gliomas. Brain Pathol. 2003;13(4):582–97.
De Palma M, Venneri MA, Roca C, Naldini L. Targeting exogenous genes to tumor angiogenesis by transplantation of genetically modified hematopoietic stem cells. Nat Med. 2003;9(6):789–95.
Gothert JR, Gustin SE, van Eekelen JA, Schmidt U, Hall MA, Jane SM, et al. Genetically tagging endothelial cells in vivo: bone marrow-derived cells do not contribute to tumor endothelium. Blood. 2004;104(6):1769–77.
Peters BA, Diaz LA, Polyak K, Meszler L, Romans K, Guinan EC, et al. Contribution of bone marrow-derived endothelial cells to human tumor vasculature. Nat Med. 2005;11(3):261–2.
Asahara T, Kawamoto A, Masuda H. Concise review: circulating endothelial progenitor cells for vascular medicine. Stem Cells. 2011;29(11):1650–5.
Gao D, Nolan DJ, Mellick AS, Bambino K, McDonnell K, Mittal V. Endothelial progenitor cells control the angiogenic switch in mouse lung metastasis. Science. 2008;319(5860):195–8.
Zhao Y, Yu P, Wu R, Ge Y, Wu J, Zhu J, et al. Renal cell carcinoma-adjacent tissues enhance mobilization and recruitment of endothelial progenitor cells to promote the invasion of the neoplasm. Biomed Pharmacother. 2013;67(7):643–9.
Yu P, Ge YZ, Zhao Y, Wu JP, Wu R, Zhou LH, et al. Identification and significance of mobilized endothelial progenitor cells in tumor neovascularization of renal cell carcinoma. Tumour Biol. 2014;35(9):9331–41.
Uzzan B, Nicolas P, Cucherat M, Perret GY. Microvessel density as a prognostic factor in women with breast cancer: a systematic review of the literature and meta-analysis. Cancer Res. 2004;64(9):2941–55.
Trivella M, Pezzella F, Pastorino U, Harris AL, Altman DG. Microvessel density as a prognostic factor in non-small-cell lung carcinoma: a meta-analysis of individual patient data. Lancet Oncol. 2007;8(6):488–99.
Zhao SF, Yang XD, Lu MX, Sun GW, Wang YX, Zhang YK, et al. Prognostic significance of VEGF immunohistochemical expression in oral cancer: a meta-analysis of the literature. Tumour Biol. 2013;34(5):3165–71.
Peng L, Zhan P, Zhou Y, Fang W, Zhao P, Zheng Y, et al. Prognostic significance of vascular endothelial growth factor immunohistochemical expression in gastric cancer: a meta-analysis. Mol Biol Rep. 2012;39(10):9473–84.
Kim HK, Song KS, Kim HO, Chung JH, Lee KR, Lee YJ, et al. Circulating numbers of endothelial progenitor cells in patients with gastric and breast cancer. Cancer Lett. 2003;198(1):83–8.
Dome B, Timar J, Dobos J, Meszaros L, Raso E, Paku S, et al. Identification and clinical significance of circulating endothelial progenitor cells in human non-small cell lung cancer. Cancer Res. 2006;66(14):7341–7.
Bogos K, Renyi-Vamos F, Dobos J, Kenessey I, Tovari J, Timar J, et al. High VEGFR-3-positive circulating lymphatic/vascular endothelial progenitor cell level is associated with poor prognosis in human small cell lung cancer. Clin Cancer Res. 2009;15(5):1741–6.
Pirro M, Cagini L, Paciullo F, Pecoriello R, Mannarino MR, Bagaglia F, et al. Baseline and post-surgery endothelial progenitor cell levels in patients with early-stage non-small-cell lung carcinoma: impact on cancer recurrence and survival. Eur J Cardiothorac Surg. 2013;44(4):e245–52.
Rafat N, Beck G, Schulte J, Tuettenberg J, Vajkoczy P. Circulating endothelial progenitor cells in malignant gliomas. J Neurosurg. 2010;112(1):43–9.
Corsini E, Ciusani E, Gaviani P, Silvani A, Canazza A, Bernardi G, et al. Decrease in circulating endothelial progenitor cells in treated glioma patients. J Neurooncol. 2012;108(1):123–9.
Yu D, Sun X, Qiu Y, Zhou J, Wu Y, Zhuang L, et al. Identification and clinical significance of mobilized endothelial progenitor cells in tumor vasculogenesis of hepatocellular carcinoma. Clin Cancer Res. 2007;13(13):3814–24.
Richter-Ehrenstein C, Rentzsch J, Runkel S, Schneider A, Schonfelder G. Endothelial progenitor cells in breast cancer patients. Breast Cancer Res Treat. 2007;106(3):343–9.
Goon PK, Lip GY, Stonelake PS, Blann AD. Circulating endothelial cells and circulating progenitor cells in breast cancer: relationship to endothelial damage/dysfunction/apoptosis, clinicopathologic factors, and the Nottingham Prognostic Index. Neoplasia. 2009;11(8):771–9.
Brunner M, Thurnher D, Heiduschka G, Grasl M, Brostjan C, Erovic BM. Elevated levels of circulating endothelial progenitor cells in head and neck cancer patients. J Surg Oncol. 2008;98(7):545–50.
Su Y, Zheng L, Wang Q, Li W, Cai Z, Xiong S, et al. Quantity and clinical relevance of circulating endothelial progenitor cells in human ovarian cancer. J Exp Clin Cancer Res. 2010;29:27.
Qiu H, Cao L, Wang D, Xu H, Liang Z. High levels of circulating CD34+/VEGFR3+ lymphatic/vascular endothelial progenitor cells is correlated with lymph node metastasis in patients with epithelial ovarian cancer. J Obstet Gynaecol Res. 2013;39(7):1268–75.
Kim YB, Chung YW, Bae HS, Lee JK, Lee NW, Lee KW, et al. Circulating endothelial progenitor cells in gynaecological cancer. J Int Med Res. 2013;41(2):293–9.
Ramcharan SK, Lip GY, Stonelake PS, Blann AD. Angiogenin outperforms VEGF, EPCs and CECs in predicting Dukes’ and AJCC stage in colorectal cancer. Eur J Clin Invest. 2013;43(8):801–8.
Blann AD, Balakrishnan B, Shantsila E, Ryan P, Lip GY. Endothelial progenitor cells and circulating endothelial cells in early prostate cancer: a comparison with plasma vascular markers. Prostate. 2011;71(10):1047–53.
Bhatt RS, Zurita AJ, O’Neill A, Norden-Zfoni A, Zhang L, Wu HK, et al. Increased mobilisation of circulating endothelial progenitors in von Hippel-Lindau disease and renal cell carcinoma. Br J Cancer. 2011;105(1):112–7.
Yang B, Gu W, Peng B, Xu Y, Liu M, Che J, et al. High level of circulating endothelial progenitor cells positively correlates with serum vascular endothelial growth factor in patients with renal cell carcinoma. J Urol. 2012;188(6):2055–61.
DuBois SG, Stempak D, Wu B, Mokhtari RB, Nayar R, Janeway KA, et al. Circulating endothelial cells and circulating endothelial precursor cells in patients with osteosarcoma. Pediatr Blood Cancer. 2012;58(2):181–4.
Bhaskar A, Gupta R, Kumar L, Sharma A, Sharma MC, Kalaivani M, et al. Circulating endothelial progenitor cells as potential prognostic biomarker in multiple myeloma. Leuk Lymphoma. 2012;53(4):635–40.
Umemura T, Higashi Y. Endothelial progenitor cells: therapeutic target for cardiovascular diseases. J Pharmacol Sci. 2008;108(1):1–6.
Mayr M, Niederseer D, Niebauer J. From bench to bedside: what physicians need to know about endothelial progenitor cells. Am J Med. 2011;124(6):489–97.
Naik RP, Jin D, Chuang E, Gold EG, Tousimis EA, Moore AL, et al. Circulating endothelial progenitor cells correlate to stage in patients with invasive breast cancer. Breast Cancer Res Treat. 2008;107(1):133–8.
Maeda R, Ishii G, Ito M, Hishida T, Yoshida J, Nishimura M, et al. Number of circulating endothelial progenitor cells and intratumoral microvessel density in non-small cell lung cancer patients: differences in angiogenic status between adenocarcinoma histologic subtypes. J Thorac Oncol. 2012;7(3):503–11.
Ha X, Zhao M, Zhao H, Peng J, Deng Z, Dong J, et al. Identification and clinical significance of circulating endothelial progenitor cells in gastric cancer. Biomarkers. 2013;18(6):487–92.
Roodhart JM, Langenberg MH, Vermaat JS, Lolkema MP, Baars A, Giles RH, et al. Late release of circulating endothelial cells and endothelial progenitor cells after chemotherapy predicts response and survival in cancer patients. Neoplasia. 2010;12(1):87–94.
Sakamori Y, Masago K, Ohmori K, Togashi Y, Nagai H, Okuda C, et al. Increase in circulating endothelial progenitor cells predicts response in patients with advanced non-small-cell lung cancer. Cancer Sci. 2012;103(6):1065–70.
Massard C, Borget I, Le Deley MC, Taylor M, Gomez-Roca C, Soria JC, et al. Prognostic value of circulating VEGFR2+ bone marrow-derived progenitor cells in patients with advanced cancer. Eur J Cancer. 2012;48(9):1354–62.
Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45(2):228–47.
Pohl M, Werner N, Munding J, Tannapfel A, Graeven U, Nickenig G, et al. Biomarkers of anti-angiogenic therapy in metastatic colorectal cancer (mCRC): original data and review of the literature. Z Gastroenterol. 2011;49(10):1398–406.
Condon ET, Wang JH, Redmond HP. Surgical injury induces the mobilization of endothelial progenitor cells. Surgery. 2004;135(6):657–61.
Acknowledgments
We apologize that we could not cite many important original papers because of space limitations. This project was supported by Grants from the National Natural Science Foundation of China (81070597 and 81370853), Science and Education Development Program of the Jiangsu Province Health Board (LJ201107), Six Talent Peaks of the Jiangsu Province Health Bureau (2011-WS-093), and Research and Innovation Program for Graduates of Jiangsu Province (CXZZ13_0583).
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Additional information
Yu-Zheng Ge and Ran Wu have contributed equally to this paper.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ge, YZ., Wu, R., Lu, TZ. et al. Circulating endothelial progenitor cell: a promising biomarker in clinical oncology. Med Oncol 32, 332 (2015). https://doi.org/10.1007/s12032-014-0332-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12032-014-0332-x