Abstract
Neovascularization is a key role of renal cell carcinoma (RCC) and the status of neovascularization in RCC is closely correlated with the tumor development and patient prognosis. Endothelial progenitor cells (EPCs) are considered as important building blocks for neovascularization. However, the role of mobilized EPCs in RCC remains unknown. In this study, the orthotopic RCC model was established to investigate the distribution, frequency, and significance of mobilized EPCs. We found that circulating endothelial progenitor cell (CEPC) levels and plasma angiogenic factors (vascular endothelial growth factor (VEGF) and stromal cell-derived factor-1 (SDF-1) were higher in peripheral blood (PB) of the RCC than those in the normal group and positively correlated with each other. EPC levels in adjacent nonmalignant kidney tissue (AT) were significantly higher than those in tumor tissue (TT) and normal kidney tissue (NT), which were positively correlated with CEPC levels. VEGF, VEGF receptor-2 (Flk), and SDF-1 and its SDF-1 receptor (CXCR4) expression in AT was significantly higher than that in TT and NT. Levels of these angiogenic factors in AT were positively correlated with those in PB. Mean microvessel density (MVD) was higher in AT than in TT, and that in TT was slightly lower than that in NT. Our findings propose that mobilized EPCs play an important role in RCC neovascularization. EPCs in PB and AT can be used as a biomarker for predicting RCC progression.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Renal cell carcinoma (RCC) represents about 3 % of all cancers worldwide [1]. Due to the highly angiogenic phenotype of RCC, neovascularization is considered to have an important role in tumor progression, multiplication, metastasis, and recurrence. This is particularly the case for RCC as these are inherently vascular tumors [2, 3].
Angiogenesis, the formation of new capillaries from preexisting endothelial cells (ECs), is not the only source of tumor neovascularization. Tumor capillaries can arise from vasculogenesis, which is de novo vessel formation by endothelial progenitor cells (EPCs) [4]. Traditionally considered to occur only in the embryo, it is now thought that both angiogenesis and vasculogenesis contribute to tumor neovascularization [5]. EPCs resemble embryonic angioblasts, which characteristically migrate, proliferate, and differentiate into mature ECs [6]. EPCs in bone marrow are characterized by the expression of the three cell surface markers CD34, CD133, and Flk [7]. After migrating into the circulation and localizing in a particular organ, more mature EPCs are found that have gradually lost CD133 expression [7, 8]. Circulating endothelial progenitor cells (CEPCs) are generally defined as mononuclear cells (MNCs) that are positive for CD34 and Flk, but negative for CD133 and CD45 [7, 9, 10].
Clinical studies have reported that EPCs are recruited and homed with high specificity to solid tumors [11, 12]. Research in animal models shows that bone marrow-derived EPCs can home to tumors and become involved in tumor vasculogenesis and tumor growth, especially in the early phases [13, 14], while inhibition of EPCs mobilization can prevent tumor growth [5, 15]. In addition, studies have reported that CEPC levels were increased and associated with the clinical behavior of patients with breast cancer [5], hepatocellular carcinoma [16], and RCC [17, 18]. A previous study conducted by Yu et al. [16] suggested that more EPCs are recruited into nonmalignant liver tissue, especially into adjacent nonmalignant liver tissue. Although there are no reports providing evidence for the participation of EPCs in RCC neovascularization, our previous study in an animal model showed that EPCs participate in such process by committing to microvascular ECs in organ regeneration after partial nephrectomy [19]. These conclusions support the hypothesis that there exists a functional role for EPCs in vasculogenesis and growth of human solid tumors, suggesting that it is possible that EPCs can be either diagnostic or prognostic markers and vectors for targeting cancers [20, 21].
RCC is a highly vascularized tumor. The majority of RCC tissue exhibits a strong expression of proangiogenic factor, such as VEGF and SDF-1 [22, 23]. These angiogenic factors are important in human RCC neovascularization and growth [24, 25]. These factors are also involved in activating, mobilizing, and recruiting EPCs from the bone marrow and in promoting differentiation of EPCs into ECs in either tumor or ischemic diseases [26, 27].
Yang et al. [28] reported that the circulating level of EPCs was elevated in patients with RCC and might correlate with the aggressiveness of the tumor. Furthermore, there were positive correlations between levels of CEPCs and plasma VEGF. However, there is no report showing the exact mechanism of mobilization for EPCs in RCC.
Therefore, we hypothesize that EPCs are mobilized from the bone marrow in the RCC model by angiogenic factors (VEGF and SDF-1) and increase vasculogenesis. To test this, we examined levels of CEPCs and the distribution and numbers of EPCs in the kidney of the RCC model and analyzed the relationships between EPCs and RCC neovascularization.
Materials and methods
Establishment of orthotropic RCC model
The human ACHN RCC cell line was purchased from the American Type Culture Collection (Manassas, VA, USA). The ACHN cell line was cultured in MEM (KeyGen Biotech, Nanjing, People’s Republic of China) with 10 % fetal bovine serum (Gibco, Grand Island, NY, USA) and 1 % penicillin/streptomycin. All experimental animals were bred and housed in the animal house of the Experimental Animal Centre affiliated with Nanjing First Hospital and were maintained on a 12-h light/12-h dark cycle at 20–25 °C and received standard laboratory chow and water ad libitum. Animal experiments were approved by the Committee on the Ethics of Animal Experiments of Nanjing Medical University (Permit Number: SYXK 2009–0015).
Three experiments were conducted. Male 5-week-old BALB/c nude mice were randomly divided into three groups. All surgical procedures were done under anesthesia with 50 mg/kg body weight pentobarbital sodium and placed on a warming table in a prone position to maintain a rectal temperature of 37 °C. All efforts were done to minimize suffering. In group 1, the tumor-implanted group (RCC group), the inferior pole of the right kidney was exposed by a right transverse, 1-cm lumbotomy incision, and 1 × 106 viable ACHN cells (20 μL) were implanted into the kidney through a hole made in the renal capsule. After carefully establishing hemostasis, the abdomen was sutured. Normal saline was injected after surgery for fluid repletion, calculated to a volume of 3–5 % of the preanesthetic weight, with an addition of 0.05 mg/kg of the analgesic Temgesic in a volume of 0.1 mL. In group 2, the sham-operated group (sham group), the same operation was conducted as for group 1, except that animals were injected with 20 μL phosphate-buffered saline (PBS). In group 3, mice were normal without any treatment (normal group). After treatment, treated mice were killed at days 21, 28, 35, 42, and 49, with each group containing eight mice per time point.
Sample collection
At the end of each experimental time point, the peripheral blood, normal kidney tissue (NT), sham-operated kidney tissue (ST), tumor tissue (TT), and adjacent nonmalignant kidney tissue (AT; 0–3 mm away from the tumor edge) were harvested. NT and ST were control samples. A portion of the blood samples was processed for flow cytometry (FCM). The other blood samples were centrifuged at 3,000 rpm for 10 min and then stored at −80 °C for enzyme-linked immunosorbent assay (ELISA). A portion of each tissue sample was placed in formalin and underwent paraffin embedding. Another portion of tissue samples was saved on ice for isolation of monoplast suspensions, and the residual tissue samples were rapidly frozen in liquid N2 and stored at −80 °C.
Histological examination
Tumor tissues were stained with hematoxylin and eosin (H&E) to confirm whether the RCC implantation was successful. Tissues were embedded in paraffin and sectioned at 4 μm and stained with H&E. Sections were microscopically examined by an experienced pathologist.
Immunohistochemical staining
Mean microvessel density (MVD) was evaluated by using purified anti-mouse CD34 polyclonal antibody (1:100; Abnova, Taipei, Taiwan) and anti-mouse CD31 polyclonal antibody (1:400; Santa Cruz, CA, USA) and stained by using immunohistochemistry. Briefly, paraffin-embedded blocks were sectioned at 4 μm, dewaxed, and rehydrated. Paraffin sections were then set in citrate buffer, endogenous peroxidase was blocked with 3 % H2O2 for 15 min, and nonspecific binding sites were blocked with 4 % goat serum diluted at 1:10 in PBST (pH 7.4 PBS and 0.05 % Tween 20). Sections were then incubated overnight with anti-CD34 antibody or anti-CD31 antibody at 4 °C. On the following day, primary antibodies were detected with horseradish peroxidase-conjugated secondary antibody and developed with 3,3′-diaminobenzidine tetrahydrochloride. An immunoglobulin-negative control was used to rule out nonspecific binding. After scanning for areas with the highest levels of brown staining, the three areas of highest staining were examined under ×200 magnification, and these were taken as the MVD and expressed as the absolute number of microvessels on a microscope with an Olympus camera (Olympus Corporation, Tokyo, Japan). The number of microvessels was counted by two independent investigators. Brown-stained ECs or clusters of contiguous ECs were considered to represent a single microvessel. Counts by each investigator were averaged.
Immunofluorescence staining
The number of EPCs residing in the RCC was observed by double immunofluorescence staining. Briefly, tissue sections were deparaffinized in xylene and rehydrated in ethanol at room temperature. Endogenous peroxidase activity was blocked by 3 % H2O2 in methanol for 5 min. Sections were then washed several times in PBS containing Triton X-100 for 10 min. To block nonspecific binding of antibodies, sections were processed for 20 min with PBS containing 2 % goat serum. Blocking serum was removed and sections were incubated with 1:100 rabbit anti-CD34 antibody and 1:100 goat anti-Flk antibody (both from Abcam, Cambridge, UK) at 4 °C overnight. For controls, the primary antibodies for CD34 and Flk were replaced with normal rabbit immunoglobulin G and normal mouse immunoglobulin G, respectively (both diluted at 1:100). After washing twice with 10 mM PBS for 10 min, slides were incubated with phycoerythrin-conjugated donkey anti-goat and fluorescein isothiocyanate-conjugated chicken anti-rabbit secondary antibody (Santa Cruz) for 2 h in the dark. Finally, CD34+/Flk+ protein expression was analyzed with confocal laser scanning microscopy.
Flow cytometry
FCM was used to evaluate the degree of EPC mobilization. Blood samples (200 μL) were obtained from all mice at days 21, 28, 35, 42, and 49 after the treatment. MNCs were separated by using density-gradient centrifugation. Isolated MNCs were quickly washed with red blood cell lysis solution and gently washed once with bovine serum albumin. MNCs were incubated for 30 min at 4 °C with Alexa Fluor 488-conjugated anti-CD34 (RD, San Diego, CA, USA) and phycoerythrin-conjugated anti-Flk antibody (Miltenyi, Bergisch Gladbach, Germany). For quantitative detection of EPCs in TT and AT, FCM was applied. MNCs were isolated by a series of mechanical steps. Briefly, approximately 30 mg of fresh tissue were placed in a glass homogenizer with 2 mL PBS, homogenized, and then filtered. After washing once with bovine serum albumin, the MNC suspension was incubated with CD34 and Flk for 30 min at 4 °C. Appropriate fluorochrome-conjugated isotypes were used as controls. Immunofluorescence was detected with a BD-FACSCanto II flow cytometer after washing once, and analysis was performed with the FACSDiva software (BD, San Jose, CA, USA). The number of CD34/Flk double-positive cells within the MNCs population was used to quantify EPCs.
Enzyme-linked immunosorbent assay analysis
Measurements of plasma VEGF and SDF-1 in treated and normal groups were done by using the Quantikine mouse sandwich enzyme-linked immunosorbent assay Kit (Abcam, Cambridge, UK) according to the manufacturer’s instructions, following the collection of sera at the same time points as stated above. All assays were performed in duplicate.
Real-time polymerase chain reaction analysis
Messenger RNA (mRNA) expression of VEGF, SDF-1, Flk, and CXCR4 was assessed using quantitative PCR (qPCR). Total RNA was extracted from NT, ST, TT, and AT of three groups by using the MicroElute Total RNA Kit (Omega, Norcross, GA, USA). Total RNA concentration was measured by UV spectrophotometry at 260 nm, purity was analyzed by the 260/280 nm absorbance ratio, and then quality was confirmed by agarose gel electrophoresis. Total RNA (1 μg) was reverse transcribed by using oligo-dT primers and the Reverse Transcription System (Takara, Tokyo, Japan) according to the manufacturer’s instructions. Transcript expression was analyzed by using qPCR with SYBR Premix Ex Taq Kit (Takara, Tokyo, Japan) and a 7,500 Fast Real-Time PCR System (Applied Biosystems, San Diego, CA, USA). PCR was performed in triplicate for 40 cycles comprising an initial denaturation stage of 95 °C for 2 min, followed by 95 °C for 15 s, and finally 60 °C for 1 min. Sequences of primers, which were synthesized by Invitrogen, Co. (Carlsbad, CA, USA), were as follows:
-
Glyceraldehyde-3-phosphate dehydrogenase:
-
F: 5′-GTCTCCTGCGACTTCAACAGC-3′
-
R: 5′-GTGGTCCAGGGTTTCTTACTCC-3′
-
-
VEGF:
-
F: 5′-GCACATAGAGAGAATGAGCTTCC-3′
-
R: 5′-CTCCGCTCTGAACAAGGCT-3′
-
-
Flk:
-
F: 5′-CAAACCTCAATGTGTCTCTTTGC-3′
-
R: 5′-AGAGTAAAGCCTATCTCGCTGT-3′
-
-
SDF-1:
-
F: 5′-TGCATCAGTGACGGTAAACCA-3′
-
R: 5′-CACAGTTTGGAGTGTTGAGGAT-3′
-
-
CXCR4:
-
F: 5′-GACTGGCATAGTCGGCAATG-3′
-
R: 5′-AGAAGGGGAGTGTGATGACAAA-3′
-
A standard curve was constructed in each of the experimental repetitions by serial dilution of cDNA (1 to 1:10,000). PCR specificity was examined by dissociation curve analyses. To determine the relative concentration of the products, we used the comparative CT (2−△△CT) method, according to the instructions supplied by Applied Biosystems.
Statistical analysis
All data were expressed as mean ± standard error of mean (SEM). Statistical comparisons were done by using the t test, one-way analysis of variance (ANOVA), and Pearson’s correlation coefficients when data were normally distributed. Means of the different groups were compared by using ANOVA. Pearson’s coefficients were used to compare results of two or more subgroups. The level of significance for all comparisons was set at P < 0.05 or 0.01.
Results
Successful establishment of the orthotropic RCC model
The vast majority of xenografts (90 %) formed primary tumors. Tumor diameters were determined during necropsy and gradually increased over time (diameters at day 21, 2.16 ± 0.13 mm; day 28, 2.66 ± 0.15 mm; day 35, 3.14 ± 0.11 mm; day 42, 3.50 ± 0.19 mm; and day 49, 4.20 ± 0.14 mm; P < 0.01). Lesions were then stained with H&E and confirmed to be tumors (Fig. 1).
RCC orthotopic xenografts with primary tumors. Macroscopic appearance (b) and histopathology of the tumor in the right kidney (c) (both with black arrows), implanted with ACHN cells (a). Microscopic appearances are H&E staining sections and were examined by using light microscopy at ×200 magnification. Scale bars = 25 μm
Effects of tumor on accumulation of EPCs in RCC
Mobilized EPCs were increased in the peripheral blood (PB) of the RCC model compared to the sham and normal groups. To explore relationships between EPCs and RCC development, we first examined CEPC levels at five time points. Sham and normal groups were assessed for comparison (Fig. 2i). In the sham group, EPC levels in PB were not significantly different from those in the normal group after day 28 (P > 0.05). In the RCC group, EPC levels were significantly higher than those in the normal and sham groups (P < 0.01). These decreased to their lowest levels at day 28 and then gradually increased (P < 0.01) (Fig. 3a).
Quantitative evaluation of EPCs in PB (II) and relevant tissues (II) by FACS analyses. Representative FACS data in which CD34+/Flk+ cells were identified as EPCs. PB of normal group at day 21 (A), PB of RCC group at five time points (B–F); control tissue (NT) at day 21 (G), AT at five time points (H–L), and TT at five time points (M–Q). Graphs of sham group are not shown
Percentage of CD34+/Flk+ cells within PB and relevant tissue population of MNCs. In the sham model, EPC levels in PB and NT were not significantly different from those in the normal group after day 28 (P > 0.05). Peripheral blood: in contrast, the RCC model had a marked increase in EPC number. Tissue: levels of EPCs were gradually increased in AT after day 28 (P < 0.01) and significantly higher than those in TT and NT (P < 0.01). Levels of EPCs in TT were first increased and then decreased (P < 0.01). When compared to NT, EPCs in TT were higher (P < 0.01). a Blood and b tissue
EPCs were recruited and incorporated into vessels in RCC, being especially abundant in AT. FCM and immunofluorescence analyses were performed to elucidate whether differences in morphology and function between TT and AT tissues were associated with increases in the number of EPCs (Figs. 2(II) and 4). In ST, levels of EPCs were not significantly different from those in NT after day 28 (P > 0.05). The percentage of double-positive cells were significantly elevated in AT compared to that of TT, and NT at all five time points (P < 0.01). In AT, the EPC quantity dropped to the lowest number at day 28 and then gradually increased (P < 0.01). EPC levels in TT first gradually increased then decreased but were elevated compared to those in NT (P < 0.01; Fig. 3b). An immunofluorescence assay was used to observe the precise location of EPCs in RCC. The double-positive cells were incorporated into vessel walls of different sizes, such as those in the medullopapillary region and microvessels. CD34+/Flk+ cells were mainly concentrated in vasculature of AT, but only a rare representation was observed in TT, and that was slightly higher than that in NT. In ST, however, there was a modest expression of CD34+/Flk+ cells in the vascular area (Fig. 4).
Double immunofluorescence staining to show the presence of EPCs in relevant tissues at day 35 after modeling. Cellular co-expression of CD34 (red) and Flk (green) in vasculature of tissues, indicating the presence of EPCs. In NT (a–c), there was only slight expression of EPCs. There was no significant difference in fluorescence intensity in ST (d–f) compared to that in NT. AT (g–i) showed significant increases in the fluorescence intensity of EPCs. TT (j–l) had a slightly higher fluorescence intensity than that of NT. Magnification, ×200 (a–l) and ×400 (M arrow indicated)
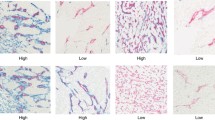
Distribution and expression of CD34 and CD31 in kidney with RCC:CD34 and CD31 rich in AT
In NT and ST, expression of CD34 was restricted to ECs mainly in the glomerular region (Fig. 5a and b). In the tumor region, however, expression of CD34 was different from that in NT (Fig. 5c). We detected the most highlighted microvessels in the AT (Fig. 5b). The image suggests that MVD-CD34 in AT was increased as compared to that in NT and TT (P < 0.01). These effects were consistent with patterns of EPC expression. The MVD-CD34 in TT was slightly lower than that in NT (P < 0.05). MVD-CD34 values (means ± SEM) were 2.78 ± 0.56 in TT, 11.65 ± 1.93 in AT, 7.37 ± 1.39 in ST, and 7.20 ± 1.18 in NT (Fig. 5e). The distribution and expression of CD31 are consistent with CD34 (Fig. 6). MVD-CD31 values (means ± SEM) were 2.18 ± 0.33 in TT, 9.65 ± 0.83 in AT, 5.37 ± 1.14 in ST, and 5.11 ± 1.36 in NT (figure not shown).
Immunohistochemical staining for CD34 at day 35 after modeling (×200 magnification). a Immunostaining for CD34 in NT. b Highlighted and diffuse positive microvessels in AT compared to those in NT and TT. c Small quantities of positive segments were found in TT but were slightly lower than those in NT. d There were no significant differences between ST and NT (P > 0.05). e Data are shown as mean ± SEM. Significant differences versus either NT or TT (*P < 0.01); significant differences versus NT (# P < 0.01)
mRNA expression of angiogenic factors and their receptors
Quantitative PCR was used to investigate the mRNA expression of several factors at the first four time points. mRNA expression of AT, VEGF, SDF-1, Flk, and CXCR4 initially decreased and then gradually increased (P < 0.01), which were consistent with the patterns of expression of EPCs. Expression of VEGF, Flk, SDF-1, and CXCR4 was significantly higher in AT compared to that in TT and NT (P < 0.01). mRNA expression of VEGF, SDF-1, Flk, and CXCR4 in TT was initially increased and then gradually decreased (P < 0.01). VEGF, Flk, SDF-1, and CXCR4 expression in TT was significantly high than that in NT, except at day 21 (P < 0.01) (Fig. 7).
Relative expression of VEGF (a), Flk (b), SDF-1 (c), and CXCR4 (d) mRNA. For above mRNA expression, there were no significant differences between NT and ST after day 28 (P > 0.05). Significant differences versus either NT or TT (*P < 0.05); significant differences versus NT (# P < 0.05); significant differences in either AT or TT versus neighboring time group (**P < 0.05)
Plasma VEGF and SDF-1 as indicators of mobilization and recruitment of EPCs to RCC
The mean level of plasma VEGF and SDF-1 in the RCC group was higher than that in the normal and sham groups (P < 0.01; Fig. 8). In the PB of the RCC group, there was a reasonable correlation between CEPC levels and plasma VEGF (r = 0.584; Fig. 9a). For its part, there was a correlation between CEPCs and SDF-1 (r = 0.479; Fig. 9b). Furthermore, there was a correlation in AT between plasma factors and tissue mRNA for VEGF (r = 0.618; Fig. 9c) and SDF-1 (r = 0.421; Fig. 9d). There was no correlation between PB and TT for VEGF or SDF-1 (data not shown). For the analysis of EPCs, we also found a similar situation. There was a significant correlation between EPC levels in PB and those in AT (r = 0.857; Fig. 9e), which were consistent with MVD and tumor diameters. However, there was no correlation between EPC levels in PB and those in TT (data not shown).
Plasma VEGF and SDF-1 in the RCC group versus the normal and sham group. Plasma VEGF and SDF-1 levels in the RCC group were significantly higher compared to those in the other group. Significant differences versus either sham or normal group (*P < 0.01); significant differences in RCC model versus neighboring time group (**P < 0.05)
Correlation analyses. a, b Pearson’s correlation analysis revealed that levels of CEPCs were correlated with those plasma factors (VEGF and SDF-1). c, d Plasma factors (VEGF and SDF-1) levels were correlated with those of related genes in AT. e Levels of CEPCs were correlated with those of EPCs in AT
Discussion
Neovascularization is a crucial factor for RCC growth and metastasis. The role of EPCs in tumor neovascularization is still debated [29, 30]. Although the mobilization of EPCs has been demonstrated in patients with RCC [28], the precise mechanism for mobilization and the role of EPCs in vascularization are not well understood. An understanding of the mobilization mechanisms and role of EPCs in RCC neovascularization may provide novel insight and a basis for changes in current therapeutic and diagnostic strategies for patients with RCC.
The clinical significance of CEPCs has been considered for lung cancer [12], hepatic carcinomas [31], breast cancer [32], and RCC [17, 18]. Although studies have reported a higher level of EPCs and angiogenic factors in peripheral blood of patients with RCC or hepatocellular carcinoma (HCC) compared to healthy controls [28, 31], mechanisms for EPC mobilization have not been investigated in RCC. Richter-Ehrenstein et al. [33] reported that CEPCs were strongly correlated with angiogenic factors released by tumor cells, and the tumor, as a source of angiogenic chemokines, is most important for recruiting EPCs [34]. Involvement of VEGF in EPCs growth has been questioned when the cells are mobiled in RCC patients [35]. However, studies have demonstrated that VEGF could stimulate EPCs migration through the VEGF/Flk pathway [25]. Agents that promote EPCs mobilization may act through the matrix metalloproteinases (MMP-9) [36, 37]. VEGF can activate MMP-9, which may make EPCs from stationary into proliferation [38]. SDF-1 was found to be highly expressed in bone marrow, and a high-level of CXCR4 expression was also found in EPCs. SDF-1 induces EPCs migration, protects it from apoptosis, and the SDF-1/CXCR4 axis has been confirmed to play a key role in EPCs mobilization in response to either hypoxia or ischemia [39, 40]. In the current investigation, we found a clear correlation between plasma factors and AT factors (VEGF and SDF-1). Levels of CEPCs in the RCC model gradually increased after day 28 and were significantly higher than those in the normal model. These correlated with plasma factors (VEGF and SDF-1) and were consistent with MVD and tumor diameters. We reasonably inferred that with RCC proliferation, the EPCs were mobilized into the circulation by mobilization factors released from RCC and subsequently entered into kidney via the blood stream. Mice with larger RCC had higher levels of EPCs. These results imply that EPCs might independently contribute to tumor growth. Our results support the significance of monitoring CEPC levels during tumor progression [41, 42].
It is generally accepted that tumor vascularization arises exclusively from endothelial sprouting. In this regard, Shirakawa et al. [13] used tumor models to show that EPCs are also involved in tumor vasculogenesis, especially in the tissue surrounding the tumor. Until recently, some clinical studies have indicated that tumor-adjacent tissues are rich in EPCs when compared to tumor tissue, and a higher microvessel density was detected in tissues adjacent to tumors [16]. In this study, the EPC levels in TT initially increased and then decreased and were significantly lower than those in AT. This change may be induced by the AT because, after EPCs were initially recruited to the transplanted tumor, rapid tumor proliferation occurred, resulting in augmented blood supply. The EPCs were then mobilized to AT for neovascularization, which is necessary for tumor invasion. Moreover, the number of EPCs in AT was gradually increased after day 28. High levels of EPCs before day 21 are partly due to recovery of the lesion after surgical injury. The increasing numbers of cells revealed that AT enhances mobilization of EPCs and vascularization. Although the exact distribution of mobilized EPCs in the target organ is still unclear [13, 14], it is clear that recruitment into target tissues is a prerequisite for EPCs effectiveness. However, further tumor growth is accompanied by the formation of tumor vessels as the RCC needs more EPCs, in accordance with RCC neovasculature development. Image data substantiated this hypothesis. EPCs were mainly concentrated in AT rather than TT. Our previous studies confirmed that the MVD in tumor-adjacent tissue was higher than that in normal kidney [25], but MVD in TT was lower compared to that in NT. It may be that in the early stage of tumor development, vessels mainly derive from the surrounding normal tissue, and a larger number of capillaries appear late. Increasing EPC numbers in AT was consistent with the increasing tumor diameter and MVD. Therefore, mobilized EPCs participate in tumor vasculogenesis of RCC. EPCs in AT could promote RCC invasion.
Some studies have reported that proangiogenic factors, such as VEGF [43] and SDF-1 [22], have a higher expression in the surrounding tissue than in tumors, which is consistent with our results. Plasma VEGF and SDF-1 were positively correlated with values in AT. These findings are consistent with the EPC levels between PB and AT. Suggested reasons for differences in levels of EPCs have been discussed above. EPCs were mobilized into the circulation and then finally deposited on RCC tissues specifically due to the induction of a high local content of angiogenic factors (i.e., VEGF and SDF-1). It is established that expression of VEGF and SDF-1 in tumor cells is upregulated by hypoxia. In addition, EPCs can synthesize VEGF and SDF-1 [25, 44, 45]. Furthermore, tumor stroma provides pathways for neovessels and serve as a reservoir for growth factors and other macromolecules [46]. CXCR4 and Flk showed a positive feedback action. SDF-1/CXCR4 and VEGF/Flk signaling pathways perhaps play a major role in the vascularization process.
There has been a debate on whether EPCs and plasma angiogenic factors are true reflections of tumor activity in cancer patients, and whether they derive from peripheral blood. Previous reports have indicated the clinical significance of CEPCs in lung cancer, hepatic carcinomas, breast cancer, and RCC. Zhu et al. [47] found that EPCs mobilized into the circulation and then finally became arrested on HCC. In this study, there was a considerable correlation between EPC levels and angiogenic factors (VEGF and SDF-1) in PB and AT. However, precise mechanism for mobilization of EPCs requires further investigation. EPCs in AT and PB may be used as a biomarker to predict RCC progression.
In conclusion, despite remaining uncertainties, our findings suggest that mobilized EPCs participate in RCC neovascularization and may serve as biomarkers for predicting RCC progression. As EPCs are endowed with the capacity to home to the tumor vasculature, they might be used to deliver drugs. Therefore, the identification of chemokines and cytokines that are involved in the recruitment of EPCs in RCC might provide new targets for RCC treatment.
References
Ljungberg B, Hanbury DC, Kuczyk MA, Merseburger AS, Mulders PF, Patard JJ, et al. Renal cell carcinoma guideline. Eur Urol. 2007;51(6):1502–10.
MacLennan GT, Bostwick DG. Microvessel density in renal cell carcinoma: lack of prognostic significance. Urology. 1995;46(1):27–30.
Yoshino S, Kato M, Okada K. Evaluation of the prognostic significance of microvessel count and tumor size in renal cell carcinoma. Int J Urol. 1998;5(2):119–23.
Asahara T, Murohara T, Sullivan A, Silver M, van der Zee R, Li T, et al. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997;275(5302):964–7.
Davidoff AM, Ng CY, Brown P, Leary MA, Spurbeck WW, Zhou J, et al. Bone marrow-derived cells contribute to tumor neovasculature and, when modified to express an angiogenesis inhibitor, can restrict tumor growth in mice. Clin Cancer Res. 2001;7(9):2870–9.
Caprioli A, Minko K, Drevon C, Eichmann A, Dieterlen-Lievre F, Jaffredo T. Hemangioblast commitment in the avian allantois: cellular and molecular aspects. Dev Biol. 2001;238(1):64–78.
Hristov M, Erl W, Weber PC. Endothelial progenitor cells: mobilization, differentiation, and homing. Arterioscler Thromb Vasc Biol. 2003;23(7):1185–9.
Peichev M, Naiyer AJ, Pereira D, Zhu Z, Lane WJ, Williams M, et al. Expression of VEGFR-2 and AC133 by circulating human CD34(+) cells identifies a population of functional endothelial precursors. Blood. 2000;95(3):952–8.
Timmermans F, Van Hauwermeiren F, De Smedt M, Raedt R, Plasschaert F, De Buyzere ML, et al. Endothelial outgrowth cells are not derived from CD133+ cells or CD45+ hematopoietic precursors. Arterioscler Thromb Vasc Biol. 2007;27(7):1572–9.
Rafii S. Circulating endothelial precursors: mystery, reality, and promise. J Clin Invest. 2000;105(1):17–9.
Hilbe W, Dirnhofer S, Oberwasserlechner F, Schmid T, Gunsilius E, Hilbe G, et al. CD133 positive endothelial progenitor cells contribute to the tumour vasculature in non-small cell lung cancer. J Clin Pathol. 2004;57(9):965–9.
Dome B, Timar J, Dobos J, Meszaros L, Raso E, Paku S, et al. Identification and clinical significance of circulating endothelial progenitor cells in human non-small cell lung cancer. Cancer Res. 2006;66(14):7341–7.
Shirakawa K, Furuhata S, Watanabe I, Hayase H, Shimizu A, Ikarashi Y, et al. Induction of vasculogenesis in breast cancer models. Br J Cancer. 2002;87(12):1454–61.
Arbab AS, Pandit SD, Anderson SA, Yocum GT, Bur M, Frenkel V, et al. Magnetic resonance imaging and confocal microscopy studies of magnetically labeled endothelial progenitor cells trafficking to sites of tumor angiogenesis. Stem Cells. 2006;24(3):671–8.
Gao D, Nolan DJ, Mellick AS, Bambino K, McDonnell K, Mittal V. Endothelial progenitor cells control the angiogenic switch in mouse lung metastasis. Science. 2008;319(5860):195–8.
Yu D, Sun X, Qiu Y, Zhou J, Wu Y, Zhuang L, et al. Identification and clinical significance of mobilized endothelial progenitor cells in tumor vasculogenesis of hepatocellular carcinoma. Clin Cancer Res. 2007;13(13):3814–24.
Bhatt RS, Zurita AJ, O’Neill A, Norden-Zfoni A, Zhang L, Wu HK, et al. Increased mobilisation of circulating endothelial progenitors in von Hippel-Lindau disease and renal cell carcinoma. Br J Cancer. 2011;105(1):112–7.
Farace F, Gross-Goupil M, Tournay E, Taylor M, Vimond N, Jacques N, et al. Levels of circulating CD45(dim)CD34(+)VEGFR2(+) progenitor cells correlate with outcome in metastatic renal cell carcinoma patients treated with tyrosine kinase inhibitors. Br J Cancer. 2011;104(7):1144–50.
Liu H, Wu R, Jia RP, Zhong B, Zhu JG, Yu P, et al. Ischemic preconditioning increases endothelial progenitor cell number to attenuate partial nephrectomy-induced ischemia/reperfusion injury. PLoS One. 2013;8(1):e55389.
De Palma M, Venneri MA, Roca C, Naldini L. Targeting exogenous genes to tumor angiogenesis by transplantation of genetically modified hematopoietic stem cells. Nat Med. 2003;9(6):789–95.
Beaudry P, Force J, Naumov GN, Wang A, Baker CH, Ryan A, et al. Differential effects of vascular endothelial growth factor receptor-2 inhibitor ZD6474 on circulating endothelial progenitors and mature circulating endothelial cells: implications for use as a surrogate marker of antiangiogenic activity. Clin Cancer Res. 2005;11(9):3514–22.
Wang L, Chen W, Gao L, Yang Q, Liu B, Wu Z, et al. High expression of CXCR4, CXCR7 and SDF-1 predicts poor survival in renal cell carcinoma. World J Surg. 2012;10:212.
Patard JJ, Rioux-Leclercq N, Masson D, Zerrouki S, Jouan F, Collet N, et al. Absence of VHL gene alteration and high VEGF expression are associated with tumour aggressiveness and poor survival of renal-cell carcinoma. Br J Cancer. 2009;101(8):1417–24.
Zhao FL, Guo W. Expression of stromal derived factor-1 (SDF-1) and chemokine receptor (CXCR4) in bone metastasis of renal carcinoma. Mol Biol Rep. 2011;38(2):1039–45.
Zhao Y, Yu P, Wu R, Ge YZ, Wu JP, Zhu JG, et al. Renal cell carcinoma-adjacent tissues enhance mobilization and recruitment of endothelial progenitor cells to promote the invasion of the neoplasm. Biomed Pharmacother. 2013;67(7):643–9.
Hattori K, Dias S, Heissig B, Hackett NR, Lyden D, Tateno M, et al. Vascular endothelial growth factor and angiopoietin-1 stimulate postnatal hematopoiesis by recruitment of vasculogenic and hematopoietic stem cells. J Exp Med. 2001;193(9):1005–14.
Petit I, Jin D, Rafii S. The SDF-1-CXCR4 signaling pathway: a molecular hub modulating neo-angiogenesis. Trends Immunol. 2007;28(7):299–307.
Yang B, Gu W, Peng B, Xu Y, Liu M, Che J, et al. High level of circulating endothelial progenitor cells positively correlates with serum vascular endothelial growth factor in patients with renal cell carcinoma. J Urol. 2012;188(6):2055–61.
Hagensen MK, Raarup MK, Mortensen MB, Thim T, Nyengaard JR, Falk E, et al. Circulating endothelial progenitor cells do not contribute to regeneration of endothelium after murine arterial injury. Cardiovasc Res. 2012;93(2):223–31.
Wickersheim A, Kerber M, de Miguel LS, Plate KH, Machein MR, et al. Endothelial progenitor cells do not contribute to tumor endothelium in primary and metastatic tumors. Int J Cancer. 2009;125(8):1771–7.
Ho JW, Pang RW, Lau C, Sun CK, Yu WC, Fan ST, et al. Significance of circulating endothelial progenitor cells in hepatocellular carcinoma. Hepatology. 2006;44(4):836–43.
Fürstenberger G, von Moos R, Lucas R, Thurlimann B, Senn HJ, Hamacher J, et al. Circulating endothelial cells and angiogenic serum factors during neoadjuvant chemotherapy of primary breast cancer. Br J Cancer. 2006;94(4):524–31.
Richter-Ehrenstein C, Rentzsch J, Runkel S, Schneider A, Schonfelder G. Endothelial progenitor cells in breast cancer patients. Breast Cancer Res Treat. 2007;106(3):343–9.
Poon RT, Lau CP, Cheung ST, Yu WC, Fan ST. Quantitative correlation of serum levels and tumor expression of vascular endothelial growth factor in patients with hepatocellular carcinoma. Cancer Res. 2003;63(12):3121–6.
Moccia F, Dragoni S, Poletto V, Rosti V, Tanzi F, Ganini C, et al. Orai1 and transient receptor potential channels as novel molecular targets to impair tumor neovascularization in renal cell carcinoma and other malignancies. Anti Cancer Agents Med Chem. 2014;14(2):296–312.
Kollet O, Shivtiel S, Chen YQ, Suriawinata J, Thung SN, Dabeva MD, et al. HGF, SDF-1, and MMP-9 are involved in stress-induced human CD34+ stem cell recruitment to the liver. J Clin Invest. 2003;112:160–9.
Heissig B, Hattori K, Dias S, Friedrich M, Ferris B, Hackett NR, et al. Recruitment of stem and progenitor cells from the bone marrow niche requires MMP-9 mediated release of kit-ligand. Cell. 2002;109:625–37.
Khakoo AY, Finkel T. Endothelial progenitor cells. Annu Rev Med. 2005;56:79–101.
Yamaguchi J, Kusano KF, Masuo O, Kawamoto A, Silver M, Murasawa S, et al. Stromal cell-derived factor-1 effects on ex vivo expanded endothelial progenitor cell recruitment for ischemic neovascularization. Circulation. 2003;107(9):1322–8.
Ceradini DJ, Kulkarni AR, Callaghan MJ, Tepper OM, Bastidas N, Kleinman ME, et al. Progenitor cell trafficking is regulated by hypoxic gradients through HIF-1 induction of SDF-1. Nat Med. 2004;10(8):858–64.
Naik RP, Jin D, Chuang E, Gold EG, Tousimis EA, Moore AL, et al. Circulating endothelial progenitor cells correlate to stage in patients with invasive breast cancer. Breast Cancer Res Treat. 2008;107(1):133–8.
Taylor M, Rössler J, Geoerger B, Laplanche A, Hartmann O, Vassal G, et al. High levels of circulating VEGFR2+ bone marrow-derived progenitor cells correlate with metastatic disease in patients with pediatric solid malignancies. Clin Cancer Res. 2009;15(14):4561–71.
Deli G, Jin CH, Mu R, Yang S, Liang Y, Chen D, et al. Immunohistochemical assessment of angiogenesis in hepatocellular carcinoma and surrounding cirrhotic liver tissues. World J Gastroenterol. 2005;11(7):960–3.
Urbich C, Aicher A, Heeschen C, Dernbach E, Hofmann WK, Zeiher AM, et al. Soluble factors released by endothelial progenitor cells promote migration of endothelial cells and cardiac resident progenitor cells. J Mol Cell Cardiol. 2005;39(5):733–42.
Abe-Yoshio Y, Abe K, Miyazaki M, Furusu A, Nishino T, Harada T, et al. Involvement of bone marrow-derived endothelial progenitor cells in glomerular capillary repair in habu snake venom-induced glomerulonephritis. Virchows Arch. 2008;453(1):97–106.
Yoshiji H, Harris SR, Thorgeirsson UP. Vascular endothelial growth factor is essential for initial but not continued in vivo growth of human breast carcinoma cells. Cancer Res. 1997;57(18):3924–8.
Zhu H, Shao Q, Sun X, Deng Z, Yuan X, Yu D, et al. The mobilization, recruitment and contribution of bone marrow-derived endothelial progenitor cells to the tumor neovascularization occur at an early stage and throughout the entire process of hepatocellular carcinoma growth. Oncol Rep. 2012;28(4):24.
Acknowledgments
This work was supported by grants from the National Natural Science Foundation of China (No. 81070597 and 81370853), Science and Education Development Program of the Jiangsu Province Health Board (No. LJ201107), and Six Talent Peaks of the Jiangsu Province Health Bureau (No. 2011-WS-093).
Conflicts of interest
None
Author information
Authors and Affiliations
Corresponding author
Additional information
Peng Yu and Yu-Zheng Ge contributed equally to this work.
Rights and permissions
About this article
Cite this article
Yu, P., Ge, YZ., Zhao, Y. et al. Identification and significance of mobilized endothelial progenitor cells in tumor neovascularization of renal cell carcinoma. Tumor Biol. 35, 9331–9341 (2014). https://doi.org/10.1007/s13277-014-2205-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13277-014-2205-5