Abstract
Background and aim
Solid tumors, including pediatric malignancies, depend on angiogenesis for tumor growth, invasion, and metastases. We aimed to evaluate the prognostic impact of circulating endothelial cells (CECs) and endothelial progenitor cells (EPCs) on treatment response and survival of pediatric patients with solid tumors.
Methods
A prospective study included 70 patients with different pediatric solid tumors treated with different types of chemotherapy and 20 age and sex-matched healthy children as controls. Blood samples collected at diagnosis then on day 7 and day 21 after chemotherapy. CECs and EPCs were evaluated using flow cytometry.
Results
The mean levels of CECs and EPCs of patients at diagnosis were significantly higher than controls (85.29 ± 24.78 and 26.1 ± 9.11 versus 20.08 ± 6.65; and EPCs; 2.78 ± 1.48, respectively; P < 0.001 for both). The highest levels of CECs were observed in patients with rhabdomyosarcoma (RMS). An overall increase was reported in CECs, and after the first cycle of chemotherapy, that was significantly correlated to treatment response and overall survival.
Conclusion
Pediatric patients with solid tumors have elevated levels of CECs and EPCs with more elevation after chemotherapy. The magnitude of increase of CECs occurred on day 7 after chemotherapy may be considered as an early predictor of response to therapy and outcome in pediatric patients with solid tumors.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Pediatric solid tumors have an overall better survival and outcome compared with adult cancers [1]. However, despite aggressive multimodal therapy, the prognosis remains poor for patients with metastatic or relapsed solid malignancies. To improve prognosis in these groups of patients, Oncologists need validated therapeutic approaches [2]. Among the common factors of poor prognosis is that pediatric solid malignancies display evident angiogenic potential, thus blocking tumor angiogenesis represented a new therapeutic stratification for these patients [3].
Angiogenesis is the process of new blood vessel formation from preexisting vasculature that plays a major role in the growth, development, and metastatic spread of solid malignancies [4]. Circulating endothelial cells (CECs) are mature endothelial cells that have been shed into the circulation from damaged tumor vessels [5, 6]. High levels of these cells are observed in different diseases with vascular insult [7]. The ability to quantify CECs and endothelial progenitor cells (EPCs) in peripheral blood has provided novel ways to assess vascular function in cancer [8, 9]. Some studies have demonstrated elevated CECs count in adults with malignant diseases [10]. Other studies have found that rare circulating cells with endothelial features contribute to tumor neovascularization and have shown the principle role of bone marrow-derived progenitor in metastatic dissemination [5, 11, 12]. In addition, it has been experimentally demonstrated that chemotherapy induces a rapid induction of EPCs into the peripheral circulation of mice, irrespective of the presence of tumors [13].
Thirty years ago, CECs were first described using light microscopy. Currently, they were determined by several novel assay systems, including the flow cytometry and immunomagnetic detection system using endothelial cell markers, including CD31, CD34, and CD146, and progenitor cell markers including CD133 [14]. However, the markers and method of assay differ among studies [5, 15].
Few published studies evaluated the levels of CECs and EPCs in pediatric patients with solid tumors. Here we aimed to quantify CECs and EPCs in newly diagnosed pediatric patients with solid tumors receiving different types of systemic therapy at South Egypt Cancer Institute(SECI) and correlating them to other factors related to patients (age, sex) and tumor ( type, metastases).
In addition, we investigated the relevance of changes reported in CECs and EPCs after the first cycle of chemotherapy to the type of chemotherapy used in treatment, clinical response and survival outcome.
Patients and methods
Patients
This prospective case–control study included 70 patients with de novo solid tumors presented to the Pediatric Oncology Department, South Egypt Cancer Institute (SECI), Assuit University, during the period from January 2018 to May 2019. The study was approved by the Institutional Review Board of SECI, Assuit University. Informed written consent was obtained from parents of all enrolled cases and controls.
Patients were included in the study if they have histologically confirmed newly diagnosed malignant solid tumor (either localized or metastatic), and had not yet received anticancer therapy. Age ranges from12 months to 18 years. All patients with previous chemotherapy or surgery (other than tumor biopsy) within 4 weeks were excluded.
Patients’ groups
For diagnosis of different types of solid tumors, standard histopathologic, immunohistochemical, radio-imaging, and tumor markers policies were used. Patients were categorized according to diagnosis into three groups: embryonal tumors [Neuroblastoma (NB), Wilms’ tumor (WT), Retinoblastoma (RB) and Hepatoblastoma (HB)], sarcomas [Rhabdomyosarcoma (RMS), Osteosarcoma (OS) and Ewing sarcoma (ES)] and other rare tumors.
Control group
Twenty age and sex-matched healthy children, 12 boys and 8 girls were included as a control group to determine the normal count of CECs and EPCs. They were recruited from siblings of patients attending the Pediatric Oncology Outpatient Clinic, SECI, Assiut University, Egypt.
Methods:
Blood samples were prospectively collected from patients with cancer at diagnosis before the first cycle of chemotherapy, seven days thereafter and immediately before the second cycle (day 21). The patients have received chemotherapy in a thrice-weekly schedule as neoadjuvant chemotherapy.
Routine laboratory tests as complete blood count, liver function test, and renal chemistry were done for all patients and controls.
Flow cytometric detection of CECs and EPCs
Blood samples were obtained within the first 48 h after the diagnosis of cancer and before starting chemotherapy. Blood samples were taken from freshly placed, flushed venous cannula as venipuncture associated trauma may increase the yield of endothelial cells in the blood sample.
CECs and EPCs were evaluated using a panel of monoclonal antibodies; fluorescein isothiocyanate (FITC) labeled -CD144 (Becton Dickinson (BD) Biosciences, San Jose, USA), phycoerythrin (PE) conjugated -CD133 (AC133) (Miltenyi Biotec GmbH, Bergisch Gladbach, Germany); and peridinium-chlorophyll-protein (Per-CP)-conjugated CD34 (BD Biosciences) and allophycocyanin (APC)- conjugated CD45 (BD Biosciences).
Fifty µl of the blood sample was incubated with 5 µl of CD144, CD133, CD34 and CD45 for 15 min in the dark. After incubation; RBC lysis, washing, the cells were resuspended in phosphate buffer saline (PBS).
Flow cytometric analysis was done by FACSCalibur flow cytometry with Cell Quest software (BD Biosciences). Fifty thousand events were analyzed and anti-human IgG isotype-matched negative control was used with each sample. The gating strategy to detect CECs and EPCs was based on CD45 staining to exclude hematopoietic cells. CECs were identified as cells lacking CD45 expression, positive for CD144, positive for CD34 and negative for CD133 (CD45–/CD144 + /CD34 + /CD133–) and EPCs were negative for CD45, positive for CD144, positive for CD34 and CD133 (CD45–/CD34 + CD144 + /CD133 +) (Fig. 1). The number of CECs and EPCs were expressed as absolute count per 50,000 cells. CEC/EPC levels were normalized to the baseline values and expressed as percent change to minimize variability due to a large variation in baseline CEC and EPC levels.
Flow cytometric detection of circulating endothelial cells (CECs), and circulating endothelial progenitor cells (EPCs). a The analysis gate (R1) included CD34+ CD45– cells. b The expression of CD144 and CD133 in R1 gate was detected. CECs were defined as CD45 – CD34 + CD144 + and CD133 −. EPCs were identified as CD45– CD34 + CD144 + and CD133 +
Response evaluation
Response evaluation was performed after the third cycle of chemotherapy and was defined into categories of progressive disease (PD), stable disease (SD), or partial remission (PR) according to Response Evaluation Criteria in Solid Tumors (RECIST) [16]
Statistical analysis
The follow-up of the patients was ended in May 2019. Overall survival (OS) is defined as the time from start chemotherapy to the date of patients' death or last follow-up. Event-free survival (EFS) is defined as the time that patients remain free of any events, including relapse, progression or death.
Data analysis was performed with the Statistical Package for Social Sciences (SPSS version 21). Statistical comparisons were performed using the Independent Sample T test, paired t test and Pearson correlation. To associate changes in CEC and EPC levels (separately or combined) for EFS and OS, variables were both tested as continuous variables applying univariable Cox regression proportional hazard (PH) analysis and dichotomized for Kaplan–Meier estimation. Differences were evaluated using the log-rank test, and hazard ratios were obtained. A p-value of 0.05 was considered significant in all statistical analyses.
Results
Patients’ characteristics.
Seventy patients (39 males and 31 females) were enrolled in this study. Their median age at diagnosis was 8 years ± 5.01 (ranged between 14 months and 16 years).
Embryonal tumors were the most predominant solid tumors amongst patients in this study reported in nearly half of patients, followed by sarcomas in 36%. Forty-seven percent of patients had metastatic disease at presentation. The patients were treated with various types of chemotherapy. We categorized them according to treatment received into two groups: patients treated with platinum-based chemotherapy (58.5%) and those treated with other chemotherapeutic agents. Table 1 shows the baseline clinical characteristics of patients with pediatric solid tumors enrolled in the study and their correlation to CECs and EPCs mean levels at initial diagnosis.
Among the 70 patients enrolled in the study, the mean CECs and EPCs levels during their initial diagnosis were (85.29 ± 24.78 and 26.1 ± 9.11, respectively). That was significantly high compared to the levels reported in the control group (CECs; 20.08 ± 6.65; P < 0.001 and EPCs; 2.78 ± 1.48; P < 0.001). The mean levels of CECs and EPC sin pediatric patients with solid tumors versus the control group were shown in Table 1.
Correlation of CECs and EPCs at initial diagnosis with characteristics of patients with pediatric solid tumors
Baseline CECs levels were not correlated with the gender (P = 0.63) of the study group. However, a significant correlation was observed between CEC levels and the types of tumors (P = 0.049), the highest levels reported among patients with sarcomas (94.26 ± 20.03). No statistically significant difference was reported in the mean levels of CECs between patients with localized or metastatic disease (P < 0.292) at diagnosis. Regarding EPCs, their levels were not correlated to any clinical or pathological characteristics of patients during their initial diagnosis Table 2.
In this study, we also monitored our patients for changes that occurred in the levels of both CECs and EPCs during the first cycle of chemotherapy. Table 1 shows the reported changes in CECs and EPCs on day 7 and day 21. Only 65 patients out of the whole study group were evaluable for the analysis of these changes. The remaining 5 patients did not finish their blood sampling. Then, we studied the association between these changes and the type of treatment used, the response to treatment, and the survival outcomes of the patients.
An overall increase was reported in CECs and EPCs levels after the first cycle of chemotherapy. At day 7 after chemotherapy, CECs were significantly increased (mean, 100.27%; 95% confidence interval [CI], 88%–112%; P < 0 0.01) compared to baseline. On day 21, further significant increase in these cells were reported (mean; 228%; 95% confidence interval [CI], 206%–250%; P < 0 0.01) compared to day seven.
Similarly, a significant increase in EPCs levels were observed at day 7 (mean, 62%, 95% CI, 53%–71%; P = 0.02), and further increase at day 21 (mean, 85.8% (95% CI, 72%–99%; P < 0.01) were reported.
Among the studied factors, the response to treatment was the only significant factor that inversely correlated to the reported increased CECs levels one week after chemotherapy (mean, 122.4 ± 50.9, 144.72 ± 45.8, and 173.46 ± 44.76 for PR, SD and PD, respectively, P = 0.045). The reported increase in EPCs level both in days 7 and 21 did not show significant difference considering the type of tumor, presence of metastasis at initial diagnosis or type of treatment received.
As regard EPCs, the changes reported in their levels at day 7 found to be significantly related only to the tumor type, patients with sarcoma showed the highest values of increase in EPCs levels (mean, 81% (95% CI, 32%–130%; P < 0.002) compared to other types of tumors. At day 21, the reported increase in EPCs levels was comparable for all types of tumors at day 21.
Changes in CEC and EPC Levels and relation to outcome
At the time of survival analysis, 47 patients were alive, with a median follow-up of 22 ± 9.56 months (range 3–34 months), while 18 patients died within 6–30 months after diagnosis. Estimated OS and EFS of the all patients with malignant solid tumors were 72.3 and 67.6%, respectively.
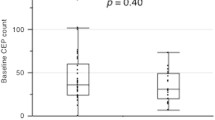
The baseline levels of CECs and EPCs did not show any significant effect on the outcome of the study group (OS and EFS), as shown in Table 3. However, a significant correlation was found between changes reported in the CECs level after chemotherapy and the OS of the patients. At day 7, patients with low CECs changes had a higher survival rate compared to patients with high changes in the level of CECs (71.4% versus 56.5%, respectively; P = 0.011) (Fig. 2). Similarly, on day 21; OS was 76.7% versus 57.1% for the patients with low and high changes in the CECs levels, respectively; P = 0.002 (Fig. 3).
Regarding EFS, it did not correlate to the changes reported in CECs level at day 7 after chemotherapy; EFS for those with low CECs changes was 66.7% versus 52.2% for patients with high change; P = 0.28. However, the correlation approached significance between EFS and changes reported on day 21; EFS was 73.1% versus 51.4% for the patients with low and high changes in the CECs levels, respectively; P = 0.051. Neither changes reported in EPC at day 7 nor day 21 after chemotherapy found to be correlated either to OS (P = 0.29 and 0.43, respectively) or EFS (P = 0.4 at day 7 and P = 0.39 at day 21).
Discussion
The progress in the treatment of childhood malignancies is a true success story, and survival rates are currently approaching 80% in developed countries. Advances reported in both diagnostic and management approaches have contributed equally to this success [17].
In spite of that, the prognosis remains inferior for some groups of patients. This diversity in clinical behavior of pediatric malignancies mandates searching for new prognostic factors that may shed new light on the behavior of pediatric malignancies and give rise to novel therapeutic approaches appropriately adjusted to improve the prognosis in this pediatric population [2].
Pediatric solid tumors possess a strong angiogenic activity. In the present study, we investigated the role of two angiogenic markers (CECs and EPCs) in pediatric patients with newly diagnosed various solid tumors.
The increased levels of CECs and EPCs in the study group with pediatric solid tumors may indicate the role of angiogenesis in the pathogenesis of pediatric solid tumors in association with widespread vascular damage and disruption that occurred in the endothelium of these tumors. Angiogenesis plays a key role in the growth, development, and metastatic dissemination of solid malignancies [4]. Interestingly, we reported a significant association between baseline levels of CECs and type of tumor with the highest levels reported in patients with sarcomas. Our finding is supported by several reports [10, 18,19,20]. In contrast, DuBois et al., 2012 [19] found no difference in CEC levels between patients with osteosarcoma in their study and the controls.
Here, we noticed that differences in the levels of CECs were independent of gender and the used chemotherapeutic agents. Our results came in agreement with Taylor et al., 2009 [18] and DuBois et al., 2012 [19]who reported similar results amongst their patients.
Despite the increased level of CECs and EPCs in our patients, we did not find any relation between CECs and EPCs elevations and metastatic disease at presentation or metastatic progression. This was reported by El-Housieni et al., 2004 [21]. However, others reported that EPCs play a role in the tumor progression by promoting the progression of avascular micrometastasis to vascularized macrometastases [22, 23]. Other studies have demonstrated that CECs elevations were associated with tumor stage and prognosis [8, 11, 24]. This discrepancy between our results and other studies could be explained by the heterogeneity and the small number of enrolled patients.
The changes that occurred in the levels of CECs and EPCs after chemotherapy could be due to bone marrow depression and recovery that results with chemotherapy. This might be of importance when considering these cells as a potential goal for therapy [25]. We reported a significant increase in CECs and EPCs in the blood of patients after the induction treatment, which was not limited to specific types of chemotherapeutic agents. This is comparable to other similar studies including adult population with different types of cancer [13, 25] CECs and EPCs may release after chemotherapy as a part of reactive host response independent of tumor type and chemotherapy regimen (Roodhart et al., [25]. CECs and EPCs have been found to stimulate tumor growth, metastasis formation, and limit chemotherapeutic efficacy by prevention of necrosis [13, 22] In our study, we found increased levels of CECs and EPCs in our patients with cancer treated with different chemotherapeutic regimen with no significant difference between types of chemotherapeutic agents. However, significant changes in the mean levels of CECs were reported among different tumor types, again the sarcoma reported the highest levels of change in EPCs after chemotherapy
We also found a correlation between CECs levels after one week of chemotherapy and the response to treatment (P = 0.045). This finding could be of interest because this is the earliest time point that could be used as a predictor for treatment response. Subsequently, a significant correlation was reported between changes in CECs on day 7 and day 21 after receiving chemotherapy, and the outcome of our patients with a more increase of CEC levels at 7 days showed a significant association toward poor OS (P = 0.01). Equally, on day 21, a large change in CEC levels was correlated with the poor OS as well (P = 0.002). This is supported by several studies including adult patients [25,26,27] In addition, Roodhart et al.[25]in a study on adults’ population with cancer reported that “The magnitude of the increase in CEC levels after chemotherapy correlates with response and survival”. In contrast, DuBois et al. [19] reported that there is no effect of angiogenic markers on the outcome of patients with osteosarcoma.
Conclusions
Pediatric patients with solid tumors have elevated levels of CECs and EPCs with more elevation after chemotherapy. The magnitude of increase of CECs occurred on day 7 after chemotherapy may be considered as an early predictor of response to therapy and outcome in pediatric patients with solid tumors.
Data availability
Data available on request due to privacy/ethical restrictions.
Abbreviations
- CECs:
-
Circulating endothelial cells
- EPCs:
-
Endothelial progenitor cells
- ES:
-
Ewing sarcoma
- HB:
-
Hepatoblastoma
- NB:
-
Neuroblastoma
- RB:
-
Retinoblastoma
- RMS:
-
Rhabdomyosarcoma
- OS:
-
Osteosarcoma
- WT:
-
Wilms’ tumor
References
McGregor LM, Metzger ML, Sanders R, Santana VM (2007) Pediatric Cancers in the New Millennium: Dramatic Progress, New Challenges: Page 6 of 6. Oncology 21:7
Vassal G (2005) Has chemotherapy reached its limits in pediatric cancers? Eur J Cancer 41:564–575
Bender JLG, Adamson PC, Reid JM, Xu L, Baruchel S, Shaked Y, Kerbel RS, Cooney-Qualter EM, Stempak D, Chen HX (2008) Phase I trial and pharmacokinetic study of bevacizumab in pediatric patients with refractory solid tumors: a Children's Oncology Group Study. J Clin Oncol 26:399–405
Carmeliet P, Jain RK (2000) Angiogenesis in cancer and other diseases. Nature 407:249
Bertolini F, Shaked Y, Mancuso P, Kerbel RS (2006) The multifaceted circulating endothelial cell in cancer: towards marker and target identification. Nat Rev Cancer 6:835
Strijbos M, Gratama J-W, Kraan J, Lamers C, Den Bakker M, Sleijfer S (2008) Circulating endothelial cells in oncology: pitfalls and promises. Br J Cancer 98:1731
Blann AD, Woywodt A, Bertolini F, Bull TM, Buyon JP, Clancy RM, Haubitz M, Hebbel RP, Lip GY, Mancuso P (2005) Circulating endothelial cells. Thromb Haemost 94:228–235
Mancuso P, Bertolini F (2010) Circulating endothelial cells as biomarkers in clinical oncology. Microvasc Res 79:224–228
Zahran AM, Abdel-Rahim MH, Refaat A, Sayed M, Othman MM, Khalak LMR, Hetta HF (2020) Circulating hematopoietic stem cells, endothelial progenitor cells and cancer stem cells in hepatocellular carcinoma patients: contribution to diagnosis and prognosis. Acta Oncol 59:33–39
Kawaishi M, Fujiwara Y, Fukui T, Kato T, Yamada K, Ohe Y, Kunitoh H, Sekine I, Yamamoto N, Nokihara H (2009) Circulating endothelial cells in non-small cell lung cancer patients treated with carboplatin and paclitaxel. J Thorac Oncol 4:208–213
Ahn G-O, Brown JM (2009) Role of endothelial progenitors and other bone marrow-derived cells in the development of the tumor vasculature. Angiogenesis 12:159–164
Nolan DJ, Ciarrocchi A, Mellick AS, Jaggi JS, Bambino K, Gupta S, Heikamp E, McDevitt MR, Scheinberg DA, Benezra R (2007) Bone marrow-derived endothelial progenitor cells are a major determinant of nascent tumor neovascularization. Genes Dev 21:1546–1558
Shaked Y, Henke E, Roodhart JM, Mancuso P, Langenberg MH, Colleoni M, Daenen LG, Man S, Xu P, Emmenegger U (2008) Rapid chemotherapy-induced acute endothelial progenitor cell mobilization: implications for antiangiogenic drugs as chemosensitizing agents. Cancer Cell 14:263–273
Strijbos MH, Kraan J, den Bakker MA, Lambrecht BN, Sleijfer S, Gratama JW (2007) Cells meeting our immunophenotypic criteria of endothelial cells are large platelets. Cytometry Part B: Clinical Cytometry: The Journal of the International Society for Analytical Cytology 72:86–93
Yoder MC, Ingram DA (2009) Endothelial progenitor cell: ongoing controversy for defining these cells and their role in neoangiogenesis in the murine system. Curr Opin Hematol 16:269–273
Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S, Mooney M, Rubinstein L, Shankar L, Dodd L, Kaplan R, Lacombe D, Verweij J (2009) New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 45:228–247
Yanagisawa T, Bartels U, Bouffet E (2008) Role of prognostic factors in the management of pediatric solid tumors. Ann N Y Acad Sci 1138:32–42
Taylor M, Rössler J, Geoerger B, Laplanche A, Hartmann O, Vassal G, Farace F (2009) High levels of circulating VEGFR2+ bone marrow–derived progenitor cells correlate with metastatic disease in patients with pediatric solid malignancies. Clin Cancer Res 15:4561–4571
DuBois SG, Stempak D, Wu B, Mokhtari RB, Nayar R, Janeway KA, Goldsby R, Grier HE, Baruchel S (2012) Circulating endothelial cells and circulating endothelial precursor cells in patients with osteosarcoma. Pediatr Blood Cancer 58:181–184
Greenfield JP, Jin DK, Young LM, Christos PJ, Abrey L, Rafii S, Gutin PH (2009) Surrogate markers predict angiogenic potential and survival in patients with glioblastoma multiforme. Neurosurgery 64:819–827
El-Houseini ME, Abdel-Azim SA, El-Desouky GI, Abdel-Hady S, El-Hamad MF, Kamel AM (2004) Clinical significance of vascular endothelial growth factor (VEGF) in sera of patients with pediatric malignancies. J Egypt Natl Cancer Inst 16:57–61
Gao D, Nolan DJ, Mellick AS, Bambino K, McDonnell K, Mittal V (2008) Endothelial progenitor cells control the angiogenic switch in mouse lung metastasis. Science 319:195–198
Dina A, Zahava V, Iness M (2005) The role of vascular endothelial growth factor in Langerhans cell histiocytosis. J Pediatr Hematol Oncol 27:62–66
Beerepoot L, Mehra N, Vermaat J, Zonnenberg B, Gebbink M, Voest E (2004) Increased levels of viable circulating endothelial cells are an indicator of progressive disease in cancer patients. Ann Oncol 15:139–145
Roodhart JM, Langenberg MH, Vermaat JS, Lolkema MP, Baars A, Giles RH, Witteveen EO, Voest EE (2010) Late release of circulating endothelial cells and endothelial progenitor cells after chemotherapy predicts response and survival in cancer patients. Neoplasia 12:87–94
Fürstenberger G, Von Moos R, Lucas R, Thürlimann B, Senn H, Hamacher J, Boneberg E (2006) Circulating endothelial cells and angiogenic serum factors during neoadjuvant chemotherapy of primary breast cancer. Br J Cancer 94:524
Dome B, Timar J, Dobos J, Meszaros L, Raso E, Paku S, Kenessey I, Ostoros G, Magyar M, Ladanyi A (2006) Identification and clinical significance of circulating endothelial progenitor cells in human non–small cell lung cancer. Can Res 66:7341–7347
Acknowledgment
The authors acknowledge the research unit, South Egypt Cancer Institute for supporting this research
Author information
Authors and Affiliations
Contributions
HAS, HFH, EAS, AMZ conceived and designed the experiments. HAS recruited patients, carried out the clinical investigations and collected patients’ clinical data. HFH, EAS, AMZ performed the experiments. AMZ, HFH shared in the analysis of the flow cytometry data. HFH, EAS, AMZ performed the statistical analysis. HAS, HFH, EAS, AMZ accomplished the interpretation of results and wrote the initial draft. All authors participated in critical review and revision of the final manuscript. HFH is the corresponding author and managed the submission of the manuscript.
Corresponding author
Ethics declarations
Conflict of interests
The authors declare that they have no conflict of interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sayed, H.A.R., Hetta, H.F., Shafik, E.A. et al. Flow cytometric analysis of circulating endothelial cells and endothelial progenitor cells in pediatric solid tumors: prognostic impact on treatment response and survival. Cancer Immunol Immunother 70, 755–761 (2021). https://doi.org/10.1007/s00262-020-02719-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00262-020-02719-0