Abstract
The purpose of this work is to evaluate if the coffee-associated malabsorption of tablet levothyroxine (l-T4) is reduced by soft gel capsule. We recruited 8 patients with coffee-associated l-T4 malabsorption including one hypothyroid patient. For 6 months, the patients were switched to the capsule maintaining the l-T4 daily dose. Patients took the capsule with water, having coffee 1 h later (proper habit, PH) on days 1–90, or with coffee ≤5 min later (improper habit, IH) on days 91–180. After 6 months, 2 patients volunteered for an acute loading test of 600 μg l-T4 (capsule) ingested with water (PH) or with coffee (IH). In the single hypothyroid patient, the post-switch TSH ranged 0.06–0.16 mU/L (PH) versus 5.8–22.4 mU/L pre-switch (PH) and 0.025–0.29 mU/L (IH) versus 26–34 mU/L pre-switch (IH). In the other 7 patients, post-switch TSH was 0.41 ± 0.46 (PH) versus 0.28 ± 0.20 pre-switch (PH) (P = 0.61) and 0.34 ± 0.30 (IH) versus 1.23 ± 1.47 pre-switch (IH) (P < 0.001). Importantly, TSH levels in PH versus IH habit did not differ post-switch (P = 0.90), but they did pre-switch (P < 0.0001). The proportions of post-switch TSH levels <0.10 mU/L with PH (33.3 %) or with IH (33.3 %) were borderline significantly greater than the corresponding pre-switch levels with PH (10.3 %) (P = 0.088) or with IH (0 %) (P = 0.0096). In the two volunteers, the l-T4 loading test showed that coffee influenced l-T4 pharmacokinetics minimally. Soft gel capsules can be used in patients who are unable/unwilling to change their IH of taking l-T4.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Levothyroxine (l-T4) is prescribed for replacement purposes in patients with primary or central hypothyroidism, and for TSH-suppressive purposes in patients thyroidectomized for thyroid cancer or as an attempt to arrest/decrease the growth of benign thyroid nodules. l-T4 is one of the most widely dispensed medications in the world because of the high frequency of these diseases [1]. For instance, in the USA, differentiated thyroid cancer was estimated to occur in 37,200 new patients in 2009, but its incidence continues to increase [2]. The leading cause of acquired primary hypothyroidism is Hashimoto’s thyroiditis (HT). In 1996, there were 1,490,371 adults and 205,159 children in the USA affected by HT [3], which equated to an estimated prevalence of ~1 in 182 or 0.55 % or 1.5 million people [4]. The frequency of newly diagnosed overt hypothyroidism was 3 per 1,000 women and less than 1 per 1,000 men in the epidemiologic English study known as the Wickham survey, 6 per 1,000 women, and 2 per 1,000 men in an epidemiologic Danish study [5]. The incidence of HT is on the rise worldwide, and approximately one-half of HT patients have subclinical or overt hypothyroidism [6]. Congenital hypothyroidism occurs in approximately 1:2,500–3,500 newborn infants [7, 8].
l-T4 is usually taken with water in the morning before breakfast [9]. A recent trial [10] in which patients using l-T4 were switched to three different ingestion modalities (l-T4 taken in the morning prior to breakfast, during breakfast or after dinner at bedtime), showed that the pre-breakfast fasting was associated with the lowest serum levels of TSH, indicating this is the best modality to ensure the highest intestinal absorption of the hormone. Many conditions and drugs may interfere with the l-T4 malabsorption [11–15]. However, sometimes it is difficult to pinpoint the cause of l-T4 malabsorption. In patients with a cause of l-T4 malabsorption that remained unknown, the problem was addressed successfully by administering l-T4 intravenously [16], or intramuscularly [17].
Our group demonstrated that there are patients in whom failure of TSH to be normalized or suppressed by conventional l-T4 tablets was associated solely with the habit of swallowing the tablet with coffee or with water, or followed by taking coffee within the very first few minutes later, a sequence that we designated as improper habit (IH) [18]. In contrast, instructing these patients to postpone taking coffee by 60 min (proper habit, PH) resulted in normalization or suppression of serum TSH. Studying volunteers under an acute oral loading of l-T4 with either water or coffee and in vitro studies confirmed the interference of coffee [18]. Over the following 12 months, we observed another 6 cases [19]. As clearly described previously, a number of patients found difficult to comply with the PH of postponing coffee by 1 h, therefore increasing the frequency of relapse. To the best of our knowledge, data on the interference exerted on l-T4 intestinal absorption by American coffee are unavailable in the scientific literature but are present in patients’ blogs on the web. An illustrative case is a lady who, upon exchange of thyroid patients’ opinions that followed the publication of our paper [18], admits that her high TSH levels coincided with her habit of consuming coffee (“I have been told to take the meds with 8 oz of water. I take mine with coffee every morning … at the exact time every morning, … I can give up a lot of things but coffee is not one of them” [20]).
A new formulation of l-T4 in a capsule as opposed to the classic tablet (Tirosint® capsules, manufactured by IBSA Institut Biochimique SA, Lugano, Switzerland) is available [21]. This pearl-shaped capsule formulation contains T4 dissolved in glycerine and has soft gelatin as a protecting shell. We reasoned that, by rendering l-T4 less available for quick mixing with fluids/solids capable of sequestering l-T4 in the gastrointestinal tract, this formulation could benefit patients with coffee-associated l-T4 malabsorption. The crossover study on eight such patients described here shows that this was indeed the case.
Subjects and Methods
Upon informed consent and Internal Review Board approval, we selected eight adult outpatients, in whom the usual habit of ingesting l-T4 with plain black coffee or with water but followed by coffee within the next 5 min caused serum TSH not to be normalized (n = 1) or not to be suppressed (n = 7). In contrast, switch to the PH of ingesting the same dose of l-T4 caused serum TSH to be normalized or suppressed. These 8 patients are different from the patients described in references [18] and [19]. Coffee was consumed as such (that is, with no milk, cream, chocolate, or liquors added), brewed either by the classical Italian coffeemaker “caffettiera” or by a household espresso machine. Also, the same patient used to drink more than one brand of ground coffee.
The patient under replacement dose of l-T4 was a 44-year-old woman with HT-related hypothyroidism when first observed. Of the remaining 7 patients under l-T4 suppressive doses of l-T4 for treatment of benign nodular goiter or recurrence of nodules after thyroidectomy, 6 were women (41–64-year-old when first observed) and 1 was a man (64-year-old when first observed). With the IH or the PH, the l-T4 tablet taken by any given patient was the same. Careful history taking excluded use of drugs known to interfere with l-T4 absorption or metabolism. No patient reported intolerance to lactose, and all patients tested negative at the biochemical screening for celiac disease based on assay analysis of serum antibodies to transglutaminase, gliadin, and endomysium [13, 14].
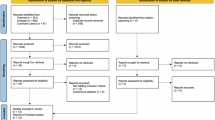
The study design is illustrated in Fig. 1. On day 0 (time zero, T0), the patients performed the blood sampling to measure TSH (and FT3 and FT4 as well) after ~2 months of taking the l-T4 tablet in the “compromise habit”. Under this habit, patients took the l-T4 tablet with water but had plain black coffee 20 min later. The purpose was to gather further evidence of the insufficient time lag between l-T4 ingestion and coffee drinking, as compared with the 60 min time lag of the PH. On day 1 and for the subsequent 89 days, patients took l-T4 at the same dose as in the compromise habit but with two changes: the formulation was switched to the soft gel capsule and coffee was consumed 60 min after l-T4 ingestion (PH). From days 91 through 180, the switch was to the IH. The name of this brand formulation of soft gel capsule is Tirosint® except in Italy, where the brand name is Syntroxine®.
This crossover study was necessarily open-label because of the peculiar pearl-shaped form of the soft gel capsule as well as the nature of the liquids (water or coffee) used to ingest l-T4. In addition to having maintained the same brand l-T4 and not having taken (and continued not taking during the 180 days of the study) drugs known to interfere with l-T4 absorption or metabolism, another criterion for having selected the said 8 outpatients was that serum TSH had been assayed monthly (on days 30, 60, 90, 120, 150), between 7 and 9 a.m. prior to the ingestion of the daily dose of l-T4, at the same laboratory with the same kit. Because of the very large geographic area covered by our University Hospital, we spared the patients’ long and expensive monthly trips to our medical center in order to have serum TSH measured in the laboratory. Serum TSH was measured in the laboratory of our University Hospital in serum samples from the two patients who volunteered for the acute loading test (see below). TSH and T4 in all serum samples of the loading test were assayed in a single run with an electrochemiluminescence immunoassay (Roche Diagnostics GmbH, Mannheim, Germany). Serum T4 levels were corrected for baseline T4. At the end of the study, and again upon informed consent, two acute oral loading tests with the soft gel capsule (six 100 μg capsules for a total of 600 μg) were performed. In one test, capsules were swallowed with natural water and in the other with coffee.
The order of water or coffee was random. The coffee used in the test was taken from a vending machine located in the same floor as our division, as reported previously [18].
Statistics
Data are presented as mean ± SD. Comparisons between means of serum TSH levels was by ANOVA, which was preceded by log-10 transformation because of their non-Gaussian distribution. The level of statistical significance was set at P < 0.05, with P value between 0.05 and 0.10 considered borderline significant.
Results
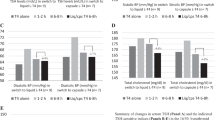
We refer to the serum TSH levels obtained when taking the tablets or the capsules as the pre-switch or post-switch levels. The first are summarized in Table 1 and changes observed after switching are summarized in Fig. 2, with one illustrative case presented in Fig. 3.
Replacement therapy
In the hypothyroid patient (case 1), serum TSH ranged between 26 and 34 mU/L over the interval of 2–36 months preceding the beginning of our study; during this interval, the patient adhered to the IH. Serum TSH fell insufficiently to 22.4 mU/L when taking coffee was postponed by 20 min (compromise habit), but it fell more markedly to 5.8 mU/L when coffee was postponed by 60 min (PH). The improvement of absorption with the same PH and the same daily dose, but in the form of the soft gel capsule was such that TSH became very low (0.06 mU/L) and clinical thyrotoxicosis appeared as confirmed by increased FT4 (22 pmol/l, n.v. 9–20). Thus, at time T2 the daily dose of l-T4 had to be decreased restoring euthyroidism and progressive return to unsuppressed TSH levels in the second part of the study (Fig. 2, top).
TSH-suppressive therapy
In the other 7 patients (cases 2–8), the purpose of l-T4 was TSH-suppressive. However, in no patients a TSH value below 0.10 mU/L could be achieved with the IH (Table 1); only in 1/7 patient’s serum TSH fell below the lower normal limit. In contrast, with the PH, 3/7 patients’ had serum TSH levels below 0.10 mU/L, and another 3 below the lower normal limit (Table 1).
After the switch to the soft gel capsule, while following the PH, 4/7 patients (57.1 %) exhibited TSH values <0.10 mU/L while taking the capsule compared to 3/7 (42.9 %) while taking the tablet (P = 0.50). Of the total 21 TSH values at days 1–90, 7 (33.3 %) were <0.10 mU/L, in contrast with only 3/29 (10.3 %) such values under the PH with the tablets (P = 0.088). The 21 TSH levels averaged 0.41 ± 0.46 (median 0.15 mU/L), which is not different from the average of 0.28 ± 0.20 (median 0.20 mU/L) of the 29 pre-switching values (P = 0.61) (Fig. 2, bottom).
With the IH, because of the said decrease in the daily dose of l-T4, 3/7 patients (42.8 %) had serum TSH <0.10 mU/L as compared to 0/7 pre-switch (P = 0.19). Of the 21 TSH measurements at days 90–180, there were 7 values <0.1 mU/L (33.3 %) as compared with 0/18 pre-switching values (P = 0.0096). These 21 TSH levels averaged 0.34 ± 0.30 (median 0.34), which is significantly lower than 1.23 ± 1.47 (median 0.84) of the 18 pre-switching values (P < 0.001) (Fig. 2, bottom).
After the switch to the soft gel capsule, the average of 0.34 ± 0.30 mU/L while following the IH did not differ from the average of 0.41 ± 0.46 mU/L while following the PH (P = 0.90). This is in contrast with the significant gap when tablets were taken between the IH and the PH (1.23 ± 1.47 vs. 0.28 ± 0.20 mU/L, P < 0.001).
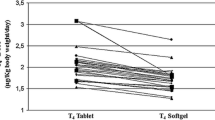
Figure 3 illustrates the reciprocal changes in serum TSH and FT4 in one example patient.
Acute oral loading test
Data for the two patients who gave consent are summarized in Fig. 4, where they are contrasted with the equivalent data from the previous study but performed with tablet l-T4 on other patients [18]. It is evident that the effect of coffee on the soft gel capsule formulation is negligible, and particularly so on the parameter C max (peak), compared with the greater magnitude effect of coffee on the tablet formulation. Strikingly enough, at the late time (24 and 48 h) the negative sign in the most comprehensive parameter (AUC) switched often to a positive sign (+2.2 % and −1.9 %, case 2; +5.2 % and +4.3 %, case 8). These two times were not tested in the previous study [18]. However, the delay in reaching the peak (T max) is comparable between the two formulations.
Indices of T4 absorption in the acute oral loading test of the same dose of LT4 contained in the same formulation (soft gel capsule) that was swallowed, by two patients (cases 2 and 8), with two different liquids (one cup of natural water or one cup of espresso coffee) in two separate occasions. The data (water vs. coffee) are contrasted with the results from a previous study (11) conducted with the similar two-liquid challenge in different patients who ingested LT4 contained in tablet (Eutirox®). In the previous study, the test lasted 4 h
Discussion
Tea and coffee are among the most consumed beverages worldwide. With almost 50 %of the world population using to take plain coffee [22], it is estimated that 4 billion of coffee cups are consumed daily in the world, 400 million of which in the U.S. [22, 23]. In Italy, 41 million people consume 43 billion cups yearly and 70 million cups daily, 57 % of which mainly in the morning [23, 24].
It is the experience of co-author S.B. (unpublished data), based on his weekly outpatient clinics at this university hospital, that the rate of newly discovered patients with coffee interference of l-T4 absorption during 11 consecutive working months approximates 3 %. However, within the group of patients with serum TSH inappropriately high or unsuppressed in the face of an adequate daily dosage of l-T4, T4 malabsorption by coffee accounts for one-fifth of the cases (S.B. unpublished data). Assuming that (i) for every 2,000,000 adult inhabitants, there are 1,000,000 adults drinking coffee in the morning; (ii) of these 1,000,000 adults, one-tenth is taking l-T4 (=100,000 adults); (iii) of these 100,000 adults, 1 % has l-T4 malabsoprtion (=1,000); (iv) coffee accounts for one-fifth of the causes of malabsorption in these 1,000 adults who each year would come at observation at the ambulatory service of a tertiary university hospital referral, then in each year there would be ~200 cases of l-T4 malabsorption caused by coffee/2,000,000 adult inhabitants. With approximately 50 million adult inhabitants in Italy, this would amount to 5,000 adult cases per year who malabsorb l-T4 tablets due to coffee. Extrapolating to the U.S. and rounding off to 300 million as the adult population, there would be an estimated total of 30,000 adult cases of l-T4 malabsorption due to coffee each year.
Generally, the issue of l-T4 malabsorption is approached by increasing the daily dose in order to reach target TSH levels, but this approach exposes the patient to the risk of overtreatment, and related consequences such as those concerning the heart [25] and the bone [26].
It is likely that the new formulation of the soft gel capsule would work also for the l-T4 malabsorption caused by American coffee. We are confident about this conclusion based on an acute oral loading test study in 16 volunteers [27]. In this study, in which 8 volunteers swallowed 600 μg l-T4 as a tablet with 240 mL American coffee and another 8 volunteers swallowed 600 μg l-T4 as a soft gel capsule, two important pharmacokinetic indices (AUC and C max) were greater in the second group of volunteers over a 12-h post-ingestion time course.
In brief, Tirosint® capsules can be used in patients who are unable/unwilling to change their IH of taking l-T4 with coffee or shortly prior to coffee. Chances are that patients affected by conditions that impair intestinal l-T4 absorption (e.g. bariatric surgery, celiac disease), could benefit from this new formulation.
References
http://www.drugs.com/top200_units.html (2012). Accessed 11 Jan 2012
D.S. Cooper, G.M. Doherty, B.R. Haugen, R.T. Kloos, S.L. Lee, S.J. Mandel, E.L. Mazzaferri, B. McIver, F. Pacini, M. Schlumberger, S.I. Sherman, D.L. Steward, R.M. Tuttle, Revised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid 19, 1167–1214 (2009)
N.R. Rose, I.R. Mackay, The Autoimmune Diseases, 3rd edn (1998). http://www.wrongdiagnosis.com/h/hypothyroidism/prevalence-types.htm. Accessed 11 Jan 2012
http://www.wrongdiagnosis.com/h/hypothyroidism/prevalence-types.htm. Accessed 11 Jan 2012
M.J. Vanderpump, The epidemiology of thyroid diseases, in Werner and Ingbar’s The Thyroid. A fundamental and Clinical Text, vol. 9, ed. by L.E. Braverman, R.D. Utiger (Lippincott Williams & Wilkins, Philadelphia, 2005), pp. 398–406
S. Benvenga, F. Trimarchi, Changed presentation of Hashimoto’s thyroiditis in North-Eastern Sicily and Calabria (Southern Italy) based on a 31-year experience. Thyroid 18, 429–441 (2008)
L. Santarpia, M. Valenzise, G. Di Pasquale, T. Arrigo, G. San Martino, M.P. Cicciò, F. Trimarchi, F. De Luca, S. Benvenga, TTF2/FOXE1 gene polymorphisms in Sicilian patients with permanent primary congenital hypothyroidism. J. Endocrinol. Investig. 30, 13–19 (2007)
G. Van Vliet, Hypothyroidsm in infants and children: congenital hypothyroidism, in Werner and Ingbar’s The Thyroid. A Fundamental and Clinical Text, vol. 9, ed. by L.E. Braverman, R.D. Utiger (Lippincott Williams & Wilkins, Philadelphia, 2005), pp. 1033–1041
M.J. Lamson, C.L. Pamplin, R.L. Rolleri, I. Klein, Quantitation of a substantial reduction in levothyroxine (T4) absorption by food. Thyroid 14, 876 (2004)
T.G. Bach-Huynh, B. Nayak, J. Loh, S. Soldin, J. Jonklaas, Timing of levothyroxine administration affects serum thyrotropin concentration. J. Clin. Endocrinol. Metab. 94, 3905–3912 (2009)
S. Benvenga, L. Bartolone, S. Squadrito, F. Lo Giudice, F. Trimarchi, Delayed intestinal absorption of levothyroxine. Thyroid 5, 249–253 (1995)
L. Liwanpo, J.M. Hershman, Conditions and drugs interfering with thyroxine absorption. Best Pract. Res. Clin. Endocrinol. Metab. 23, 781–792 (2009)
D. Collins, R. Wilcox, M. Nathan, R. Zubarik, Celiac disease and hypothyroidism. Am. J. Med. 125, 278–282 (2012)
C. Virili, G. Bassotti, M.G. Santaguida, R. Iuorio, S.C. Del Duca, V. Mercuri, A. Picarelli, P. Gargiulo, L. Gargano, M. Centanni, Atypical celiac disease as cause of increased need for thyroxine: a systematic study. J. Clin. Endocrinol. Metab. 97, E419–E422 (2012)
R. Padwal, D. Brocks, A.M. Sharma, A systematic review of drug absorption following bariatric surgery and its theoretical implications. Obes. Rev. 11, 41–50 (2010)
A. Tönjes, S. Karger, C.A. Koch, R. Paschke, A. Tannapfel, M. Stumvoll, D. Fuhrer, Impaired enteral levothyroxine absorption in hypothyroidism refractory to oral therapy after thyroid ablation for papillary thyroid cancer: case report and kinetic studies. Thyroid 16, 1047–1051 (2006)
L. Anderson, F. Joseph, N. Goenka, V. Patel, Isolated thyroxine malabsorption treated with intramuscular thyroxine injections. Am. J. Med. Sci. 337, 150–152 (2009)
S. Benvenga, L. Bartolone, M.A. Pappalardo, A. Russo, D. Lapa, G. Giorgianni, G. Saraceno, F. Trimarchi, Altered intestinal absorption of l-thyroxine caused by coffee. Thyroid 18, 293–301 (2008)
A. Sindoni, R. Vita, S. Fusco, G. Saraceno, M.A. Pappalardo, O.R. Cotta, S. Grasso, F. Trimarchi, S. Benvenga, Case report. Coffee impairs intestinal absorption of levothyroxine: report of additional cases. Hot Thyroidol. HT05/09, April 2009 (http://www.hotthyroidology.com)
http://thyroid.about.com/b/2008/09/03/coffee-thyroid-drugs.htm. Accessed 11 Jan 2012
P. Colucci, P. D’Angelo, G. Mautone, C. Scarsi, M.P. Ducharme, Pharmacokinetic equivalence of a levothyroxine sodium soft capsule manufactured using the new food and drug administration potency guidelines in healthy volunteers under fasting conditions. Ther. Drug Monit. 33, 355–361 (2011)
http://www.torrefazionebugella.it/numeri.html. Accessed 11 Jan 2012
http://caffeamodomio.com/il-caffe-e-la-sua-storia/diffusione-in-italia-e-nel-mondo/il-mercato-del-caffe-e-il-suo-ruolo-nel-mondo/. Accessed 11 Jan 2012
http://www.italyecipes.com/ricette/rubriche/dettaglio_rubrica_ita.asp?index=15&titolo=NERO%20E%20BOLLENTE:%20IL%20CAFFE%27. Accessed 11 Jan 2012
T.H. Collet, J. Gussekloo, D.C. Bauer, W.P. den Elzen, A.R. Cappola, P. Balmer, G. Iervasi, B.O. Asvold, J.A. Sgarbi, H. Völzke, B. Gencer, R.M. Maciel, S. Molinaro, A. Bremner, R.N. Luben, P. Maisonneuve, J. Cornuz, A.B. Newman, K.T. Khaw, R.G. Westendorp, J.A. Franklyn, E. Vittinghoff, J.P. Walsh, N. Rodondi, The thyroid studies collaboration. Subclinical hyperthyroidism and the risk of coronary heart disease and mortality. Arch. Intern. Med. 172, 799–809 (2012)
G. Mazziotti, T. Porcelli, I. Patelli, P.P. Vescovi, A. Giustina, Serum TSH values and risk of vertebral fractures in euthyroid post-menopausal women with low bone mineral density. Bone 46, 747–751 (2010)
M.P. Ducharme, S. Benvenga, Comparison of the intestinal absorption of levothyroxine (LT4): tablet versus soft gel capsule formulation. Endocr. Rev. 32, P3–P625 (2011)
Conflict of interest
IBSA Institut Biochimique SA (Lugano, Switzerland) furnished the principal investigator (S.B.) with the capsules for the entire duration of the study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Vita, R., Saraceno, G., Trimarchi, F. et al. A novel formulation of l-thyroxine (l-T4) reduces the problem of l-T4 malabsorption by coffee observed with traditional tablet formulations. Endocrine 43, 154–160 (2013). https://doi.org/10.1007/s12020-012-9772-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12020-012-9772-2