Abstract
Type 1 diabetes (T1D) is perceived as an autoimmune disease caused by T cell-mediated destruction of the insulin-producing pancreatic β cells. However, the number of inflammatory T cells in blood, as well as the relative importance of each cell type is unclear. Forty-two patients with T1D and 30 controls were enrolled. Circulating primary CD4+ or CD8+ T cells were quantified with 5-color flow cytometry. Serum IL-22 and IL-17 levels were examined by ELISA. Serum autoantibodies were measured by radio-binding assays, using 35S-labeled glutamic acid decarboxylase-65 (GAD65), protein tyrosine phosphatase-2 (IA-2), and zinc transporter 8 (ZnT8). Th17–Th22 and Tc1–Tc17 were significantly elevated in patients with T1D compared to control subjects, while there were no significant differences in Th1 cells. The levels of these T cells in different stages of T1D were investigated. Th22 cells showed a positive correlation with Th17 cells in T1D patients. However, we did not find any correlation between IL-17 and IL-22 in sera. Autoantibodies were not significantly different between patients with early T1D and those who have had it for a longer duration. This study indicates that Th22 may contribute to the pathogenesis of T1D. Blockade of Th22 cells might be of clinical profit in T1D patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Type 1 diabetes (T1D) occurs as a consequence of a breakdown in immune regulation, resulting in the expansion of autoreactive CD4+ and CD8+ T cells and destruction of the insulin-producing β-cells [1]. Animal models of autoimmune diabetes have indicated that IFN-γ-secreting Th1 cells are key players in the destruction of β cells as activators of cytotoxic CD8+ cells. More recently, Th17 immunity has been demonstrated in the development of autoimmune diabetes in NOD mice [2, 3] and humans [4]. Th17 cells are inflammatory CD4+ T cells that produce IL-17 [5]. IL-17 has detrimental effects on human islet cells via inflammatory responses [4]. It also mediates significant and reproducible enhancement of IL-1β/IFN-γ-induced and TNF-α/IFN-γ-induced apoptosis in human islets [6]. Circulating IL-17(+) β-cell-specific autoreactive CD4+ T cells are a feature of type 1 diabetes diagnosis [6, 7]. Th22 cells differ from Th17 and Th1 cells in their differentiation and function as they express low or undetectable Th17 and the Th1-associated transcription factors RORγt and T-bet. Notably, the Aryl hydrocarbon receptor (AHR) has been shown to be required for IL-22 production [8, 9]. This subset of IL-22-producing cells may participate in the pathology of inflammation, tumors, and autoimmune diseases [10–13]. However, there is no data about Th22 cells in patients with T1D.
Recent evidence indicates that CD8+ T cells express functions that extend beyond Ag-specific cell cytotoxicity. Type I CD8+ cytotoxic T (Tc1) cells secrete IFN-γ and kill targets by either perforin or Fas-mediated mechanisms, whereas type II CD8+ cytotoxic T cells secrete IL-4, IL-5, and IL-10, and kill targets predominantly through the perforin pathway. Notably, a subset of CD8+ T cells has recently been identified that is characterized by the expression of the transcription factor retinoic acid-related orphan receptor (ROR)γt and the production of IL-17 [14]. These cells have been termed Tc17 cells, despite being remarkably noncytotoxic with little expression of granzyme B, perforin, and Fas ligand [14, 15]. Tc17 cells have been identified during various inflammatory conditions that range from infection to cancer and autoimmunity [16–18]. In patients, CD8+ T cells producing IL-17 can be identified in chronic inflammatory lesions such as psoriatic lesions [19] and multiple sclerosis plaques [18]. At present, plasticity of the Tc17 lineage could be associated with transition to overt disease in this experimental model of T1D [20].
In this study, 5-color flow cytometry was used to identify and quantify Th1, Th17, Th22, Tc1, and Tc17 cells among circulating primary CD4+ or CD8+ T cells isolated from individuals at different stages of T1D. We also investigated their correlations in T1D. The prevalence of autoantibodies was also analyzed.
Materials and methods
Patients
A total of 42 patients with T1D were enrolled in the study. This group consisted of 24 females and 18 males, with mean ± SD disease duration of 7.26 ± 6.69 years. The mean age of the patients was 23.63 ± 12.00 years. The diagnosis was based on the criteria of World Health Organization and American Diabetes Association. 30 age- and gender-matched healthy volunteers were recruited as controls. There were 14 females and 16 males, ranging from 25.43 ± 2.19 years. All of the control subjects were free of a history of T1D or autoimmune diseases. All subjects gave informed consent, and the study was approved by the relevant ethics committees.
Islet autoantibody determination
Serum autoantibodies were measured by radio-binding assays, using 35S-labeled glutamic acid decarboxylase-65 (GAD65), protein tyrosine phosphatase-2 (IA-2), and zinc transporter 8 (ZnT8). As previously described [21], antibody levels were expressed as a relative immunoprecipitation index, which is defined as (sample − negative control)/(positive control − negative control). The cut-off for positivity for GADA, IA2A, and ZnT8A was defined as a value above 0.015, 0.048, and 0.018, respectively, based on the 99th percentile of 102, 315, and 218 healthy control subjects (non-diabetic individuals without known autoimmune disease and no family history of diabetes).
Intracellular staining
Human peripheral blood mononuclear cells (PBMCs) were isolated by LymphoprepTM (Nycomed, Pharma AS, Oslo, Norway) gradients according to the manufacturer’s protocol. PBMCs were stimulated with PMA (25 ng/ml) and ionomycin (1 μg/ml) (Sigma-Aldrich) for 5 h. GolgiStop (BD Biosciences, San Jose, CA, USA) was added for at least the last 4 h of T cell activation of PBMCs, and then PBMCs were stained for surface Ags using anti-CD3 APC-eFluor® 780(eBioscience, San Diego, USA) and Anti-CD8a FITC (eBioscience, San Diego, USA), at room temperature for 30 min, respectively. After washing, cells were fixed and permeabilized using the Cytofix/Cytoperm Plus Fixation and Permeabilization Kit (BD Biosciences). Intracellular IFN-γ, IL-17, and IL-22 were stained with anti-IFN-γ-PerCP-Cy5.5 (eBioscience, San Diego, CA, USA), anti-Human IL-17A PE (eBioscience, San Diego, CA, USA), and anti-IL-22-eFluor® 660 (eBioscience, San Diego, CA, USA). Appropriate isotype controls were used to determine specific binding for each fluorescent channel: IgG1-PerCP-Cy5.5 (eBioscience, San Diego, CA, USA), IgG1-PE (eBioscience, San Diego, CA, USA), and IgG-eFluor® 660 (eBioscience, San Diego, CA, USA).
Enzyme-Linked ImmunoSorbent Assay (ELISA)
The concentrations of sera IL-17 and IL-22 from patients and healthy controls were determined using ELISA kits, according to manufacturers’ instructions (eBioscience, San Diego, USA).
Statistical Analysis
GraphPad PRISM 5.0 Software was used for statistical analysis (GraphPad Software, Inc., San Diego, CA, USA). Values were expressed as mean ± SD according to their distribution. Student’s unpaired t test was performed over all statistically significant changes between two groups. Correlations between variables were determined by Pearson’s correlation coefficient. P < 0.05 was considered to be statistically significant.
Results
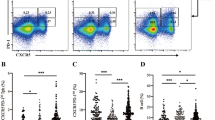
We analyzed the frequency of Th and Tc cells based on cytokine patterns by intracellular staining. Th and Tc cells were defined as CD3+CD8− T cells and CD3+CD8+ T cell, respectively. Recent study showed the role of IL-17 immunity in pathogenesis of human T1D [4]. Thus, we detected the levels of Th17 cells in our patients. Remarkably, the percentage of Th17 (IL-17+) subsets from T1D patients was significantly increased when compared to controls (5.62 ± 1.82 vs. 0.66 ± 0.32, P < 0.0001) (Fig. 1a, b). The duration of T1D in patients was divided into three groups (<5 years, 5–10 years, and >10 years). There were no differences in these 3 groups in Th17 cells (Fig. 1c). We found no significant difference in the upregulation of Th1 (IFN-γ+IL-17−) cells among patients of different T1D durations and the healthy controls (Fig. 1c). However, the total percentage of Th1 cells was slightly decreased in diabetic patients (17.38 ± 5.29 vs. 20.16 ± 6.33, P = 0.0413) (Fig. 1b).
The percentages of Th1 and Th17 cells in peripheral blood of patients with T1D. Peripheral blood mononuclear cells (PMBCs) from T1D patients (n = 42) and healthy controls (n = 30) were stained with labeled antibodies as described in Methods. a Representative dot plots of CD3+CD8−IFN-γ+IL-17− and CD3+CD8−IL-17+ cells in different groups of samples. At least about 20,000 events were analyzed for each sample. b CD3+CD8−IFN-γ+IL-17− and CD3+CD8−IL-17+ cells were compared between T1D patients and healthy controls. c The percentages of circulating Th1 and Th17 cells from T1D in different durations. Each data point represents an individual subject. The bars indicate the mean values. * P < 0.05, *** P < 0.001
In the CD8+ T cell subsets, both the Tc1 (IFN-γ+IL-17−) and Tc17(IL-17+) cells were significantly increased in T1D patients compared with healthy controls (16.49 ± 4.67 vs. 12.70 ± 5.41, P = 0.0034, 2.44 ± 0.82 vs. 1.61 ± 0.55, P < 0.0001) (Fig. 2a, b). The level of Tc1 cells did not change during the disease progress (Fig. 2c). The percentages of Tc17 cells were higher in patients who had T1D for less than 5 years. The level of Tc17 cells decreased in patients who had T1D for more than 5 years (P = 0.03). They were not significantly elevated after 10 years (Fig. 2c).
The percentages of Tc1 and Tc17 cells in peripheral blood of patients with T1D. Peripheral blood mononuclear cells (PMBCs) from T1D patients (n = 42) and healthy controls (n = 30) were stained with labeled antibodies as described in Methods. a Representative dot plots of CD3+CD8+IFN-γ+IL-17− and CD3+CD8+IL-17+ cells in different groups of samples. At least about 20,000 events were analyzed for each sample. b CD3+CD8+IFN-γ+IL-17− and CD3+CD8+IL-17+ cells were compared between T1D patients and healthy controls. c The percentages of circulating Tc1 and Tc17 cells from T1D in different durations. Each data point represents an individual subject. The bars indicate the mean values. * P < 0.05, ** P < 0.01, *** P < 0.001
The percentages of Th22 cells (IL-22+IL-17−) were increased in T1D patients compared to healthy controls (1.08 ± 0.66 vs. 0.49 ± 0.26, P < 0.0001) (Fig. 3a, b). The highest percentages of Th22 cells were found in patients who had T1D for less than 5 years. The percentages were significantly reduced for patients with T1D between 5 and 10 years (P = 0.0167). Th22 cell percentages were then sustained in patients who had T1D for more than 10 years (Fig. 3c).
The percentages of Th22 cells in peripheral blood of patients with T1D. Peripheral blood mononuclear cells (PMBCs) from T1D patients (n = 42) and healthy controls (n = 30) were stained with labeled antibodies as described in Methods. a Representative dot plots of CD3+CD8−IL-22+IL-17− cells in different groups of samples. At least about 20,000 events were analyzed for each sample. b CD3+CD8−IL-22+IL-17− cells were compared between T1D patients and healthy controls. c The percentages of circulating Th22 cells from T1D in different durations. Each data point represents an individual subject. The bars indicate the mean values. * P < 0.05, *** P < 0.001
In T1D patients, a significant positive correlation was found between Th22 cells and Th17 cells (R = 0.46, P = 0.002) (Fig. 4c). However, the percentages of Th1 cells failed to show a significant correlation with the percentage of Th17 and Th22 cells, and level of Tc1 failed to show a statistical correlation with the level of Tc17 (Fig. 4a, b, d).
To determine whether this correlation reflected the cytokine levels in serum, we analyzed the level of serum IL-17 and IL-22. Although we found that IL-17 and IL-22 were significantly increased in T1D patients (27.49 ± 12.87 vs. 19.29 ± 16.39, P = 0.0239, 101.57 ± 68.32 vs. 72.43 ± 46.77, P = 0.0426) (Fig. 5a, b), there was no significant correlation between those cytokines (Fig. 5c).
ZnT8A, IA2A, and GADA were detected in 42 patients. There was no significant difference in the levels of these three antibodies between patients who had earlier stages of T1D (<5 years) and those who had T1D for over 5 years (16.67 vs. 29.17, 66.67 vs. 54.17, 16.67 vs. 33.33, P > 0.05) (Fig. 6a). At the same time, we analyzed the levels of autoantibodies in subjects who were positive for ZnT8A, IA2A, or GADA. We found that the levels of ZnT8 and IA2A were lower, while the level of GADA was higher for T1D >5 years (compared with the patients with T1D <5 years). However, no significant differences were observed between these two groups (P > 0.05) (Fig. 6b).
Discussion
T1D is perceived as an autoimmune disease caused by T cell-mediated destruction of the insulin-producing pancreatic cells. Our data are strengthened by the utilization of 5-color flow cytometry to simultaneously examine both cell surface marker expression and intracellular cytokine expression in various circulating T cell subsets. We suggest a functional disequilibrium of T cell subsets in T1D which may contribute to the inflammatory process and disease pathogenesis.
In recent years, the idea that T1D is mainly driven by the Th1 subset has been questioned. A new T cell subset, Th17 cells, the main cells producing IL-17, are increased in many models of autoimmunity and are often considered to be the principal driver of inflammation [4]. More recently, a unique IL-22-producing CD4+ T-helper subset has been identified in human blood [8, 22]. In similarity to the Th17 subset, cells with this cytokine profile have been named as the Th22 subset. They represent a distinct human T cell compared with Th1, Th2, and Th17 cells [22]. It is also difficult to generalize whether Th22 cells are anti-inflammatory or pro-inflammatory. IL-22 can play either a protective or a pathogenic role in chronic inflammatory diseases depending on the nature of the affected tissue and the local cytokine milieu. The production of IL-22 by activated immune cells is reflected in the enhanced presence of this cytokine in various chronic inflammatory diseases, especially in those associated with a dominant role in the major IL-22-producing Th cell populations (Th22 and Th17) [23]. Chen H et al. showed lower levels of IL-22 in serum of patients with T2DM in comparison to the healthy controls [24]. Previous studies suggested that Th22 cells may play important roles in certain autoimmune diseases [11, 25]. To study whether these T cell subsets were involved in the development of T1D, the level of these cells in the peripheral blood of patients with T1D and healthy controls were examined. We found that Th17 and Th22 levels were obviously increased, especially in patients with early stages of T1D (<5 years). They then retained or decreased slightly with progression of the disease. It is possible that the autoimmune attack weakness after a longer duration.
CD8+ T effector cells take center stage in the destruction of pancreatic beta cells and contribute to sustaining islet inflammation [26]. To our knowledge, this is the first study to demonstrate the presence and evaluate function of Tc1 and Tc17 cells in T1D. The role of these cells remains unclear. However, their presence was demonstrated in a mouse model of multiple sclerosis [15] and in autoimmune encephalomyelitis in humans [27]. Tc17 cells display a greatly suppressed cytotoxic function and share some key features with the Th17 subset [14, 15, 28]. We may speculate that Tc17 cooperate with Th17 and have similar functions in inflammatory conditions, including T1D and other autoimmune diseases [15, 28], infection [15, 29], and antitumor immunity [30].
In this study, there was a positive correlation between Th22 cells and Th17 cells in patients with T1D, suggesting that differentiation of Th22 and Th17 cells may be driven in an isotropic manner in T1D. IL-17 and IL-22 have overlaps regarding their cellular sources, biochemical structure, signaling cascades in target cells, and function [31]. IL-23 is essential for human Th17 differentiation [32] and IL-23 treatment can induce IL-22 production [33]. Recent study has demonstrated that IL-23 gene expression is upregulated in T1D [34]. In addition, IL-6 is not only required for IL-17 induction from naïve T cells [35, 36] but also can promote the expression of IL-22 [33]. These might contribute to the positive correlation between Th22 cells and Th17 cells in our study. However, we did not find any significant correlation between IL-17 and IL-22 in the sera. The potential correlations between Th22 cells and Th17 cells need further research.
T1D is characterized by the presence of a series of autoantibodies targeting islet molecules, including insulin, GAD65, IA-2, and ZnT8 [37]. New-onset patients treated with insulin develop high levels of insulin antibodies. So IAAs were not detected in our study. Islet autoantibodies have a number of distinguishing characteristics. IAAs often are (but not always) the first autoantibody to appear in children followed from birth [38]; and at onset of diabetes, their levels are inversely related to age of diabetes onset [39, 40]. GADAs change the least with age of onset. ZnT8As are rapidly lost after diabetes onset [41], and IA2As are very specific, identifying a particularly high diabetes risk of prospectively followed nondiabetic children [42]. We did not observe significant differences between patients who have had T1D for less than 5 years and those who have had T1D for more than 5 years within the small number of patients analyzed. However, given these unique properties for each of the islet autoantibodies, it is perhaps not surprising that patients had a differential effect upon the measured antibodies.
In conclusion, we observed the change of circulating Th17, Th22, Th1, Tc1, and Tc17 cells from individuals in different stages of T1D. Our data demonstrated that the elevated Th22 cells correlated with Th17 cells in T1D patients. Th22 may contribute to the pathogenesis of T1D. Blockade of Th22 cells might be of clinical profit in T1D patients.
References
J.A. Bluestone, K. Herold, G. Eisenbarth, Genetics, pathogenesis and clinical interventions in type 1 diabetes. Nature 464(7293), 1293–1300 (2010)
J.A. Emamaullee, J. Davis, S. Merani, C. Toso, J.F. Elliott, A. Thiesen, A.M. Shapiro, Inhibition of Th17 cells regulates autoimmune diabetes in NOD mice. Diabetes 58(6), 1302–1311 (2009)
R. Jain, D.M. Tartar, R.K. Gregg, R.D. Divekar, J.J. Bell, H.H. Lee, P. Yu, J.S. Ellis, C.M. Hoeman, C.L. Franklin, H. Zaghouani, Innocuous IFNgamma induced by adjuvant-free antigen restores normoglycemia in NOD mice through inhibition of IL-17 production. J. Exp. Med. 205(1), 207–218 (2008)
J. Honkanen, J.K. Nieminen, R. Gao, K. Luopajarvi, H.M. Salo, J. Ilonen, M. Knip, T. Otonkoski, O. Vaarala, IL-17 immunity in human type 1 diabetes. J Immunol 185(3), 1959–1967 (2010)
P. Miossec, T. Korn, V.K. Kuchroo, Interleukin-17 and type 17 helper T cells. N. Engl. J. Med. 361(9), 888–898 (2009)
S. Arif, F. Moore, K. Marks, T. Bouckenooghe, C.M. Dayan, R. Planas, M. Vives-Pi, J. Powrie, T. Tree, P. Marchetti, G.C. Huang, E.N. Gurzov, R. Pujol-Borrell, D.L. Eizirik, M. Peakman, Peripheral and islet interleukin-17 pathway activation characterizes human autoimmune diabetes and promotes cytokine-mediated beta-cell death. Diabetes 60(8), 2112–2119 (2011)
M. Ryba-Stanislawowska, M. Skrzypkowska, M. Mysliwiec, J. Mysliwska, Loss of the balance between CD4(+)Foxp3(+) regulatory T cells and CD4(+)IL17A(+) Th17 cells in patients with type 1 diabetes. Hum. Immunol. 74(6), 701–707 (2013)
S. Trifari, C.D. Kaplan, E.H. Tran, N.K. Crellin, H. Spits, Identification of a human helper T cell population that has abundant production of interleukin 22 and is distinct from T(H)-17, T(H)1 and T(H)2 cells. Nat. Immunol. 10(8), 864–871 (2009)
M. Veldhoen, K. Hirota, A.M. Westendorf, J. Buer, L. Dumoutier, J.C. Renauld, B. Stockinger, The aryl hydrocarbon receptor links TH17-cell-mediated autoimmunity to environmental toxins. Nature 453(7191), 106–109 (2008)
L. Zhang, Y.G. Li, Y.H. Li, L. Qi, X.G. Liu, C.Z. Yuan, N.W. Hu, D.X. Ma, Z.F. Li, Q. Yang, W. Li, J.M. Li, Increased frequencies of Th22 cells as well as Th17 cells in the peripheral blood of patients with ankylosing spondylitis and rheumatoid arthritis. PLoS One 7(4), e31000 (2012)
S. Kagami, H.L. Rizzo, J.J. Lee, Y. Koguchi, A. Blauvelt, Circulating Th17, Th22, and Th1 cells are increased in psoriasis. J. Invest. Dermatol. 130(5), 1373–1383 (2010)
T. Liu, L. Peng, P. Yu, Y. Zhao, Y. Shi, X. Mao, W. Chen, P. Cheng, T. Wang, N. Chen, J. Zhang, X. Liu, N. Li, G. Guo, W. Tong, Y. Zhuang, Q. Zou, Increased circulating Th22 and Th17 cells are associated with tumor progression and patient survival in human gastric cancer. J. Clin. Immunol. 32(6), 1332–1339 (2012)
M.E. Truchetet, N.C. Brembilla, E. Montanari, Y. Allanore, C. Chizzolini, Increased frequency of circulating Th22 in addition to Th17 and Th2 lymphocytes in systemic sclerosis: association with interstitial lung disease. Arthritis Res. Ther. 13(5), R166 (2011)
T. Kondo, H. Takata, F. Matsuki, M. Takiguchi, Cutting edge: Phenotypic characterization and differentiation of human CD8+ T cells producing IL-17. J. Immunol. 182(4), 1794–1798 (2009)
M. Huber, S. Heink, H. Grothe, A. Guralnik, K. Reinhard, K. Elflein, T. Hunig, H.W. Mittrucker, A. Brustle, T. Kamradt, M. Lohoff, A Th17-like developmental process leads to CD8(+) Tc17 cells with reduced cytotoxic activity. Eur. J. Immunol. 39(7), 1716–1725 (2009)
A.M. Intlekofer, A. Banerjee, N. Takemoto, S.M. Gordon, C.S. Dejong, H. Shin, C.A. Hunter, E.J. Wherry, T. Lindsten, S.L. Reiner, Anomalous type 17 response to viral infection by CD8+ T cells lacking T-bet and eomesodermin. Science 321(5887), 408–411 (2008)
D.M. Kuang, C. Peng, Q. Zhao, Y. Wu, L.Y. Zhu, J. Wang, X.Y. Yin, L. Li, L. Zheng, Tumor-activated monocytes promote expansion of IL-17-producing CD8 + T cells in hepatocellular carcinoma patients. J. Immunol. 185(3), 1544–1549 (2010)
J.S. Tzartos, M.A. Friese, M.J. Craner, J. Palace, J. Newcombe, M.M. Esiri, L. Fugger, Interleukin-17 production in central nervous system-infiltrating T cells and glial cells is associated with active disease in multiple sclerosis. Am. J. Pathol. 172(1), 146–155 (2008)
P.C. Res, G. Piskin, O.J. de Boer, C.M. van der Loos, P. Teeling, J.D. Bos, M.B. Teunissen, Overrepresentation of IL-17A and IL-22 producing CD8 T cells in lesional skin suggests their involvement in the pathogenesis of psoriasis. PLoS One 5(11), e14108 (2010)
A. Saxena, S. Desbois, N. Carrie, M. Lawand, L.T. Mars, R.S. Liblau, Tc17 CD8+ T cells potentiate Th1-mediated autoimmune diabetes in a mouse model. J. Immunol. 189(6), 3140–3149 (2012)
L. Yu, K. Herold, H. Krause-Steinrauf, P.L. McGee, B. Bundy, A. Pugliese, J. Krischer, G.S. Eisenbarth, Rituximab selectively suppresses specific islet antibodies. Diabetes 60(10), 2560–2565 (2011)
T. Duhen, R. Geiger, D. Jarrossay, A. Lanzavecchia, F. Sallusto, Production of interleukin 22 but not interleukin 17 by a subset of human skin-homing memory T cells. Nat. Immunol. 10(8), 857–863 (2009)
N. Zhang, H.F. Pan, D.Q. Ye, Th22 in inflammatory and autoimmune disease: prospects for therapeutic intervention. Mol. Cell. Biochem. 353(1–2), 41–46 (2011)
H. Chen, F. Wen, X. Zhang, S.B. Su, Expression of T-helper-associated cytokines in patients with type 2 diabetes mellitus with retinopathy. Molecular vision 18, 219–226 (2012)
X.Y. Yang, H.Y. Wang, X.Y. Zhao, L.J. Wang, Q.H. Lv, Q.Q. Wang, Th22, but not Th17 Might be a Good Index to Predict the Tissue Involvement of Systemic Lupus Erythematosus. J. Clin. Immunol. 33(4), 767–774 (2013)
S. Tsai, A. Shameli, P. Santamaria, CD8 + T cells in type 1 diabetes. Adv. Immunol. 100, 79–124 (2008)
M. Huber, S. Heink, A. Pagenstecher, K. Reinhard, J. Ritter, A. Visekruna, A. Guralnik, N. Bollig, K. Jeltsch, C. Heinemann, E. Wittmann, T. Buch, O. Prazeres da Costa, A. Brustle, D. Brenner, T.W. Mak, H.W. Mittrucker, B. Tackenberg, T. Kamradt, M. Lohoff, IL-17A secretion by CD8+ T cells supports Th17-mediated autoimmune encephalomyelitis. J. Clin. Investig. 123(1), 247–260 (2013)
C. Ortega, A.S. Fernandez, J.M. Carrillo, P. Romero, I.J. Molina, J.C. Moreno, M. Santamaria, IL-17-producing CD8+ T lymphocytes from psoriasis skin plaques are cytotoxic effector cells that secrete Th17-related cytokines. J. Leukoc. Biol. 86(2), 435–443 (2009)
H. Hamada, L. Garcia-Hernandez Mde, J.B. Reome, S.K. Misra, T.M. Strutt, K.K. McKinstry, A.M. Cooper, S.L. Swain, R.W. Dutton, Tc17, a unique subset of CD8 T cells that can protect against lethal influenza challenge. J. Immunol. 182(6), 3469–3481 (2009)
C.S. Hinrichs, A. Kaiser, C.M. Paulos, L. Cassard, L. Sanchez-Perez, B. Heemskerk, C. Wrzesinski, Z.A. Borman, P. Muranski, N.P. Restifo, Type 17 CD8 + T cells display enhanced antitumor immunity. Blood 114(3), 596–599 (2009)
S. Eyerich, K. Eyerich, A. Cavani, C. Schmidt-Weber, IL-17 and IL-22: siblings, not twins. Trends Immunol. 31(9), 354–361 (2010)
E. Volpe, N. Servant, R. Zollinger, S.I. Bogiatzi, P. Hupe, E. Barillot, V. Soumelis, A critical function for transforming growth factor-beta, interleukin 23 and proinflammatory cytokines in driving and modulating human T(H)-17 responses. Nat. Immunol. 9(6), 650–657 (2008)
Y. Zheng, D.M. Danilenko, P. Valdez, I. Kasman, J. Eastham-Anderson, J. Wu, W. Ouyang, Interleukin-22, a T(H)17 cytokine, mediates IL-23-induced dermal inflammation and acanthosis. Nature 445(7128), 648–651 (2007)
F. Abbasi, P. Amiri, F.A. Sayahpour, S. Pirmoradi, M. Abolhalaj, B. Larijani, J.T. Bazzaz, M.M. Amoli, TGF-beta and IL-23 gene expression in unstimulated PBMCs of patients with diabetes. Endocrine 41(3), 430–434 (2012)
P.R. Mangan, L.E. Harrington, D.B. O’Quinn, W.S. Helms, D.C. Bullard, C.O. Elson, R.D. Hatton, S.M. Wahl, T.R. Schoeb, C.T. Weaver, Transforming growth factor-beta induces development of the T(H)17 lineage. Nature 441(7090), 231–234 (2006)
M. Ryba-Stanislawowska, M. Skrzypkowska, J. Mysliwska, M. Mysliwiec, The serum IL-6 profile and Treg/Th17 peripheral cell populations in patients with type 1 diabetes. Mediators Inflamm. 2013, 205284 (2013)
P.J. Bingley, Clinical applications of diabetes antibody testing. J. Clin. Endocrinol. Metab. 95(1), 25–33 (2010)
P. Achenbach, K. Koczwara, A. Knopff, H. Naserke, A.G. Ziegler, E. Bonifacio, Mature high-affinity immune responses to (pro) insulin anticipate the autoimmune cascade that leads to type 1 diabetes. J. Clin. Invest. 114(4), 589–597 (2004)
P. Vardi, A.G. Ziegler, J.H. Mathews, S. Dib, R.J. Keller, A.T. Ricker, J.I. Wolfsdorf, R.D. Herskowitz, A. Rabizadeh, G.S. Eisenbarth et al., Concentration of insulin autoantibodies at onset of type I diabetes. Inverse log-linear correlation with age. Diabetes Care 11(9), 736–739 (1988)
S.A. Arslanian, D.J. Becker, B. Rabin, R. Atchison, M. Eberhardt, D. Cavender, J. Dorman, A.L. Drash, Correlates of insulin antibodies in newly diagnosed children with insulin-dependent diabetes before insulin therapy. Diabetes 34(9), 926–930 (1985)
J.M. Wenzlau, M. Walter, T.J. Gardner, L.M. Frisch, L. Yu, G.S. Eisenbarth, A.G. Ziegler, H.W. Davidson, J.C. Hutton, Kinetics of the post-onset decline in zinc transporter 8 autoantibodies in type 1 diabetic human subjects. J. Clin. Endocrinol. Metab. 95(10), 4712–4719 (2010)
P. Achenbach, E. Bonifacio, A.J. Williams, A.G. Ziegler, E.A. Gale, P.J. Bingley, Autoantibodies to IA-2beta improve diabetes risk assessment in high-risk relatives. Diabetologia 51(3), 488–492 (2008)
Acknowledgments
The study was supported by Grants from the National Natural Science Foundation of China (number 30971405, 81270897) and a Project Funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD).
Conflict of interest
The authors report no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Xu, X., Zheng, S., Yang, F. et al. Increased Th22 cells are independently associated with Th17 cells in type 1 diabetes. Endocrine 46, 90–98 (2014). https://doi.org/10.1007/s12020-013-0030-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12020-013-0030-z