Abstract
Purpose
T-helper (Th) cells abnormalities are considered to be associated with the pathogenesis of Systemic lupus erythematosus (SLE). Recently, The Th22 cells have been identified and implicated in the pathogenesis of autoimmune diseases such as Rheumatoid arthritis (RA), although therir role in Systemic lupus erythematosus (SLE) remains unclear. The present study intends to investigate their roles in SLE.
Methods
Clinical data were collected in 65 SLE patients and 30 healthy controls. The patients were divided into active and inactive groups. CD4+IFN-γ−IL-17−IL-22+Tcells (Th22 cells),CD4+ IFN-γ−IL-22−IL-17+T cells (Th17 cells),and CD4+ IFN-γ+ (Th1 cells) were assayed by flow cytometry. Serum interleukin-22 (IL-22) and IL-17 levels were measured by enzyme-linked immunosorbent assay.
Results
The main observation focused on increased Th22 cells in patients with sole lupus skin disease and decreased Th22 cells in patients with sole lupus nephritis. Likewise, concentrations of serum IL-22 were increased in patients with sole lupus skin disease, and decreased in patients with sole lupus nephritis. Additionally, there was a positive correlation between the percentage of Th22 cells and IL-22 production. The percentage of Th17 cells or concentration of serum IL-17 correlated positively with Systemic Lupus Erythematosus Disease Activity Index (SLEDAI).
Conclusion
Th22 seems to be a more significant index to predict the tissue involvement of SLE than Th17, although Th17 may play a role in the activity of SLE.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
T helper cells, also called auto-reactive effector CD4+ T cells, were first subdivided into 2 groups, Th1 and Th2 cells. Among the 2 groups, Th1 cells were believed to a main drive of autoimmune diseases [1–3]. However, this notion often faces challenges with some inconsistencies. Consequently, The identification of newer effector T-cell subsets in recent years such as Th17 and Th22 cells brings new insights to these diseases.
As distinct from classical Th1 and Th2 cells [4–9], Th17 cells, which can produce IL-17A, play a pro-inflammatory role in autoimmune diseases [10] via the up-regulation of additional proinflammatory and neutrophil-recruiting cytokines and chemokines. Furthermore, increasing evidence both in humans [11, 12] and in mouse models [13] may suggest that Th17 cells play a role in the progression of Systemic lupus erythematosus (SLE) .
Th22 cells, a new subset of CD4+ T-helper, differentiate from naïve T cells in response to TNF-α and IL-6, and is characterized by secretion of IL-22 but not IL-17 or IFN-γ [14–16]. Th22 cells also produce cytokines IL-26 and IL-13 in addition to IL-22 [16]. IL-22, being the most important functional cytokine of Th22 cells [17], acts synergistically with IL-17, TNF-a, and IFN-γ in a proinflammatory context [18] and plays an important role in autoimmune diseases. Abnormal expression of Th22 cells was seen in peripheral blood of some autoimmune diseases including psoriasis [19], systemic sclerosis (SSc) [20], and rheumatoid arthritis (RA) [21, 22]. IL-22–positive CD+ T cells were present in high quantities in psoriatic skin [15] and RA synovial tissues [23]. However, the role of Th22 cells in SLE has not been reported yet.
In present study, we measured the frequency of Th22 cells and their secretion of IL-22 in peripheral blood (PB) of patients with SLE and discussed the underlying mechanism of their roles in SLE.
Materials and Methods
Patients and Controls
A total of 65 patients meeting with the 1997 American College of Rheumatology (ACR) classification criteria for SLE [24] were recruited. This group consisted of 58 women and 7 men, with mean ± SD disease duration of 1.53 ± 1.17 years. The mean age of patients was 34 ± 11 years. Systemic lupus erythematosus disease activity index (SLEDAI) was used to determine the global disease activity [25]. Active SLE was defined by SLEDAI ≥ 5. Complicated lupus nephritis was defined according to the ACR criteria i.e., any one of the following: 1) persistent proteinuria ≥ 0.5 g/day; 2) the presence of active cellular casts; 3) biopsy evidence of lupus nephritis [26]. Complicated lupus skin disease was defined according to rash shape and/or skin histopathology. Subjects that denied any autoimmune disease and active infection without undergoing treatment with immunomodulatory drugs for any known condition were recruited as 30 healthy controls (25 females, 5 males; mean age 32 ± 10 years). Enrollment took place from March 2011 to July 2012 at the Department of Rheumatology of the Second Affiliated Hospital, Zhejiang University, Collage of Medicine. The study protocol was approved by the ethical committee of the hospital (No. 2011–107). Informed consent was obtained from each patient before being included in the study.
Flow Cytometric Analysis of Th22 Cells and Th17 Cells via Intracellular Staining
PBMCs were purified from peripheral blood by centrifugation, using a Ficoll-Hypaque gradient (Amersham Pharmacia Biotech, Little Chalfont, UK), then reincubated for 4 h at 37 °C, 5 % CO2 in the presence of 25 ng/ml of phorbol myristate acetate (PMA), 1 μg/ml of ionomycin, and 1.7 μg/ml Golgiplug (monensin; all from Alexis Biochemicals, San Diego, CA, USA) to stimulate T cells but block intracellular transportation, thereby leading to an accumulation of cytokines in the cells. Then cells were stained with FITC conjugated anti-CD4 monoclonal antibodies at room temperature in the dark for 20 min, followed by stain with PE-conjugated anti-interferon (IFN)-γ monoclonal antibodies, APC-conjugated anti-IL-17 monoclonal antibodies, and PerCP-onjugated anti-IL-22 monoclonal antibodies after fixation and permeabilization. All the antibodies were from eBioscience (San Diego, CA, USA). Stained cells were analyzed by flow cytometric analysis using a FACScan cytometer equipped with Cell Quest software (BD Bioscience PharMingen).
Cytokines Assay
Serum concentrations of different cytokines were quantified by enzyme-linked immunosorbent assay (ELISA) kits for IL-22 (R&D Systems, USA) and IL-17 (eBioscience, USA). Sensitivity of the ELISA kit was 31.25 pg/ml for IL-22 and 15.6 pg/ml for IL-17.
Statistical Analysis
Data were presented as mean ± SD or median (range). Statistical significance was determined by ANOVA, and difference between two groups was measured by Newman–Keuls multiple comparison test (q test). If the data were not normally distributed, Kruskal–Wallis test (H test) and Nemenyi test were performed. Pearson or Spearman correlation test was applied for correlation analysis depending on data distribution. All tests were performed by SPSS 19.0 system. P value less than 0.05 was considered statistically significant.
Results
Clinical and Demographic Characteristics of Subjects
45 out of 65 patients with active SLE included 30 new-onset patients and 15 relapsing patients free from immunosuppressive or immunomodulatory drugs for at least 6 months when sampling. The other 20 patients with inactive SLE were on prednisolne at low dosage (5–10 mg/day) or hydroxychloroquine (200 mg/day) or azathioprine (50–100 mg/day) on a stable dose for at least 6 months prior to the study. Clinical and demographic data of SLE patients are presented in Table I.
Elevated Frequency of Th17 Cells and Unchanged Frequency of Th22 and Th1 Cells in Patients with SLE
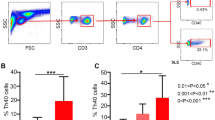
The frequency of Th22 cells (CD4+IFN-γ-IL-17−IL-22+T cells), Th17 cells (CD4+IFN-γ−IL-22−IL17+T cells) as well as Th1 cells (CD4+IFN-γ+T cells) were analyzed by their cytokine patterns after short-term activation by PMA/ionomycin. The expression of a typical dot plot of Th22, Th17 and Th1 cells in SLE patients and healthy controls is shown in Fig. 1.
The percentages of circulating Th1 cells, Th17 cells and Th22 cells in SLE patients using cytometry analysis. a Lymphocytes were gated by flow cytometry. b, d Th1(CD4+IFN-γ+) cells in SLE and healthy controls. c, e Th17 cells (CD4+IFN-γ−IL−22−IL-17+ T cells) and Th22 (CD4+IFN-γ−IL-17−IL-22+T cells) in SLE and healthy controls
Th17 cells was significantly increased in SLE patients compared to healthy controls (P = 0.003), and significant difference between active and inactive SLE patients (P = 0.000) could be seen. However, Th22 cells or Th1 cells were unchanged compared to healthy controls (Table II).
The Deviation of the Frequency of Th22 Cells in SLE Patient with Sole Lupus Skin Disease or Sole Lupus Nephritis
To understand the mechanism of Th22 in different tissue, we stratified the patients into different groups. The first group contained the patients with sole skin involvement, the number of which is 17 out of a total of 25 patients with skin impairments. The other group contained 19 out of 23 patients with sole kidney involvement. The stratum analysis revealed an opposite change of Th22 cells in the above mentioned groups of patients. Th22 cells were significantly elevated in patients with sole lupus skin disease, while they decreased in patients with sole lupus nephritis [(2.03 ± 1.02 %) and (0.60 ± 0.19 %), respectively versus (1.28 ± 0.40 %); (P = 0.000, 0.020respectively)] (Fig. 2a).
The percentages of circulating Th1 cells, Th17 cells and Th22 cells in both SLE patients with sole lupus skin disease and with sole lupus nephritis. a The percentage of Th22 cells significantly increased in SLE patients with sole lupus skin disease and decreased in patients with sole lupus nephritis. Compared to healthy controls, *p < 0.05. b The percentage of Th17 cells in both SLE patients with sole lupus skin disease and sole lupus nephritis increased significantly. Compared to healthy controls, *p < 0.05. c There was no significant difference of the percentage of Th1 between SLE patients either with sole lupus skin disease or with sole lupus nephritis and healthy controls
Th17 cells was significantly increased in both patients with sole lupus skin disease and patients with sole lupus nephritis compared with healthy controls [(2.02 ± 0. 82 %) and (1.98 ± 0.62 %) respectively, versus (1.13 ± 0.65 %); (P = 0.000, 0.000 respectively)] (Fig. 2b) . For Th1 cells, there was no obvious difference between two groups of patients and healthy controls (Fig. 2c).
The Deviation of Serum Level of IL-22 in SLE Patients with Sole Lupus Skin Disease or Sole Lupus Nephritis
Serum IL-22 was unchanged in SLE [median, 196.26 pg/ml (range 36.80–531.70)], compared with healthy controls [median, 187.61 pg/ml (range 56.00–429.00); P = 0.725], and no significant difference in serum IL-22 between active [median, 202.41 pg/ml (range, 36.80–531.70)] and inactive SLE patients [median 182.42 pg/ml (range, 78.00–427.00); P = 0.529] could be seen. However, similar to the change of Th22 cells results, the serum level of IL-22 was significantly increased in patients with sole lupus skin disease [median, 289.34 pg/ml (range 89.70–531.70); P = 0.001], while it decreased in patients only with sole lupus nephritis [median, 82.44 pg/ml (range, 36.80–178.50); P = 0.024] (Fig. 3a).
Concentration of IL-22 and IL-17 in serum from both SLE patients with sole lupus skin disease and with sole lupus nephritis. a The level of IL-22 increased significantly in patients with sole lupus skin disease and decreased in SLE patients with sole lupus nephritis. Compared with healthy controls, *p < 0.05. b The level of IL-17 increased significantly in both patients with sole lupus skin disease and with sole lupus nephritis compared with healthy controls respectively *p < 0.05
An increased level of IL-17 was seen in SLE [median, 73.08 pg/ml (range, 17.00.–176.00)], compared to healthy controls [median, 46.76 pg/ml (range, 16.05–173.20); P = 0.001]. Moreover, the level of IL-17 in active SLE patients [median, 82.44 pg/ml (range, 53.05–176.00)] was significantly higher than that in inactive SLE patients [median, 41.02 pg/ml (range, 17.00–156.70); P = 0.002], and compared to healthy controls, the level of IL-17 was increased in both SLE patients with sole lupus skin disease [median, 77.78 pg/ml (range, 35.00–172.00); P = 0.004] and SLE patients with sole lupus nephritis [median, 85.20 pg/ml (range, 23.10–160.40); P = 0.000] (Fig. 3b).
Positive Correlation Both Between Th22 Cells and Serum Level of IL-22 and Between Th17 Cells and Serum Level of IL-17 in Patients with SLE
In patients with SLE, a positive correlation between Th22 cells and serum level of IL-22 (r = 0.855, p = 0.000) are noted (Fig. 4a). In contrast, Th1 or Th17 cells failed to show a statistical correlation with serum level of IL-22 (P = 0.407, or P = 0.282). There were correlations between the frequency of Th17 and serum level of IL-17 (r = 0.771, p = 0.000) (Fig. 4b).
Correlation between different Th cells and related cytokines. a Correlation between the percentage of Th22 cells and the serumIL-22 concentrations in SLE patients. A positive correlation was discovered between Th22 cells and serum level of IL-22. b Correlation between the percentage of Th17 cells and the serumIL-17 concentrations. A positive correlation was discovered between Th17 cells and serum level of IL-17
Th17 Cells and Serum Level of IL-17 Positively Correlated with the Disease Activity in SLE Patients
There were no correlations between the frequency of Th22 or Th1 cells and SLEDAI (p = 0.070, p = 0.120, respectively) in SLE patients. But Th17 cells showed a positive correlation with SLEDAI (r = 0.279, p = 0.012) (Fig. 5a).
Correlation between the Th17 cells/serum IL-17 and SLEDAI. a Correlation between the percentage of Th17 cells and SLEDAI. A positive correlation was found between Th17 cells and SLEDAI. b Correlation between the serum IL-17 and SLEDAI. A positive correlation was found between the serum level of IL-17 and SLEDAI
The serum level of IL-22 was not correlated with SLEDAI (P = 0.282) in SLE patients, but a positive correlation was found between serum level of IL-17and SLEDAI (r = 0.211, p = 0.046) (Fig. 5b).
Discussion
Recent research have identified that Th22 cells contribute to some autoimmune diseases. However, there are no studies on Th22 cells in SLE patients. Therefore, we have measured the frequency of Th22 cells and the serum level of IL-22 in peripheral blood of SLE patients within the entire study, and the subsequent overall results revealed that there was no difference of the frequency of Th22 cells between SLE patients and health controls. Nevertheless, further analysis found that a difference of frequency also occurred with patients possessing a different subtype of SLE. Our studies revealed elevated Th22 cells in patients with sole lupus skin disease and decreased Th22 cells in patients with sole lupus nephritis. The serum level of IL-22 also indicated similar changes to those of Th22 cells and a positive correlation with the frequency of Th22 cells.
As for the mechanism which is underlying the phenomenon of the elevation of Th22 cells in patients with sole lupus skin disease,the following studies might shed some light on the subject. Studies showed that Th22 cells express the chemokine receptors CCR6, CCR4 and CCR10 [14, 16]. Besides IL-22, Th22 cells also produce IL-13 [16] or other factors which may result in tissue remodeling, such as several fibroblast growth factor isoforms [15]that are consistent with the role of Th22 cells in the crosstalk between the immune system and the non-hematopoietic stromal cells. The expression of two skin-homing receptors CCR4 and CCR10 on the surface of Th22 cells implies that Th22 cells will inevitably migrate to the skin. Therefore, we speculate that increased Th22 cells of peripheral blood in SLE patients with lupus skin disease may indeed reach the skin and could be a significant factor in the development of lupus skin diseases.
In accordance with increasing number of Th22 cells, elevated serum IL-22 concentration was observed in patients with sole lupus skin diseases. IL-22 is a member of the IL-10 family of cytokines and stands for a crucial effector molecule of activated Th22 cells [14]. In addition, IL-22R1 is only expressed on stromal cells, which include keratinocytes, myofibroblasts and epithelial cells, but not on hematological cells. [27]. IL-22 also provides unidirectional signaling from the immune system to the stroma by downstream STAT3 activation in stromal cells resulting in the secretion of a variety of factors that include antimicrobial peptides and chemokines [28, 29]. Consequently, this stromal IL-22/STAT3 pathway can be exemplified by induction of a psoriasis-like autoimmune disease [30, 31]. A further implication of our studies show that Th22 cells might mediate dermal inflammation through the activation of IL-22/STAT3 pathway in lupus skin disease. Furthermore, another main cytokine, TNF-α, secreted by Th22 cells, is also an important pathogenic cytokine in lupus skin disease. It could enhance the responsiveness of the keratinocytes to IL-22 via up-regulation of IL-22 receptor and signal transduction element expression [32], as well as continuing to amplify and/or prolong these IL-22 effects. In addition, TNF-α also induce ICAM-1 expression, DCs migration and lymphocyte infiltration [33, 34], any of which can contribute to skin lesion. Th22 cells, therefore, may be involved in the pathogenesis of lupus skin disease by secretion of proinflammatory cytokines such as IL-22 and TNF-α.
On the other hand, the number of Th22 cells and the level of IL-22 in patients of lupus nephritis had a large reduction. The differentiation of Th22 cells was induced by DCs in a dependant IL-6 and TNF-αway, and the subsequent removal of TNF-α minimized the development of Th22 cells [14]. Decreased levels of TNF-α in the peripheral blood were already found in lupus nephritis [35, 36], which, according to the results of our study, might account for the decreased number of Th22 cells in peripheral blood of patients with lupus nephritis. In humans, the kidney expresses high level of IL-22R1 [27]. The decreased serum IL-22 level in SLE patient with lupus nephritis indicated that IL-22 might be a protection against lupus nephritis.
The results of a majority of previous research suggests that Th17 cells have been linked to the development of SLE [11, 12]. It has been detected that IL-17, secreted by Th17 cells, not only exerts a synergisticinfluence on the inflammatory reaction to induce an increased production of a panel of proinflammatory cytokines and the generation of auto-antibodies in SLE [12, 37], but also make contribution to the pathogenesis of lupus nephritis through both the induction of IgG, anti-dsDNA overproduction and IL-6 over-expression of PBMC in patients with lupus nephritis[38]. This investigation discovered the positive correlation between both Th17 cells and serum level of IL-17, and between their level and disease activity. Overall, our results validate the reliability of previous scientific findings [39, 40] and profoundly support the view that Th17 cells obviously traced back to the pathogenesis of SLE with an unprecedented significance.
Though the development of SLE has already bonded with Th1 cells, the intervention of Th1 in peripheral blood is more controversial for both decreased and unchanged Th1 profile than has been previously reported in SLE patients [41, 42]. In our results, we discovered no alteration in the number of Th1 cells in patients with SLE either, lupus skin disease, or lupus nephritis.
In conclusion, the results of our present investigation suggest that Th22 cells might be a good index to define the different tissue involvement in SLE patients. The limitation of this investigation is its small sample size. Therefore, future prospective cohort studies with large sample size are necessary to examine the role of IL-22 in SLE.
References
Mosmann TR, Coffman RL. TH1 and TH2 cells: different patterns of lymphokine secretion lead to different functional properties. Annu Rev Immunol. 1989;7:145–73.
Dolhain RJ, van der Heiden AN, ter Haar NT, Breedveld FC, Miltenburg AM. Shift toward T lymphocytes with a T helper 1 cytokine-secretion profile in the joints of patients with rheumatoid arthritis. Arthritis Rheum. 1996;39:1961–9.
Wedderburn LR, Robinson N, Patel A, Varsani H, Woo P. Selective recruitment of polarized T cells expressing CCR5 and CXCR3 to the inflamed joints of children with juvenile idiopathic arthritis. Arthritis Rheum. 2000;43:765–74.
Weaver CT, Harrington LE, Mangan PR, Gavrieli M, Murphy KM. Th17: an effector CD4 T cell lineage with regulatory T cell ties. Immunity. 2006;24:677–88.
Mangan PR, Harrington LE, O’Quinn DB, Helms WS, Bullard DC, Elson CO, et al. Transforming growth factor-β induces development of the TH17 lineage. Nature. 2006;441:231–4.
Harrington LE, Hatton RD, Mangan PR, Turner H, Murphy TL, Murphy KM, et al. Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat Immunol. 2005;6:1123–32.
Park H, Li Z, Yang XO, Chang SH, Nurieva R, Wang YH, et al. A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nat Immunol. 2005;6:1133–41.
Bettelli E, Korn T, Oukka M, Kuchroo VK. Induction and effector functions of TH17 cells. Nature. 2008;453:1051–7.
Miossec P, Korn T, Kuchroo VK. Interleukin-17 and type 17 helper T cells. New Engl J Med. 2009;361:888–98.
Dardalhon V, Korn T, Kuchroo VK, Anderson AC. Role of Th1 and Th17 cells in organ-specific autoimmunity. J Autoimmun. 2008;31:252–6.
Sullivan KE, Piliero LM, Dharia T, Goldman D, Petri MA. 3- polymorphisms of ETS1 are associated with different clinical phenotypes in SLE. Hum Mutat. 2000;16:49–53.
Wong CK, Ho CY, Li EK, Lam CW. Elevation of proinflammatory Cytokine (IL-18, IL-17, IL-12) and Th2 cytokine (IL-4) concentrations in patients with systemic lupus erythematosus. Lupus. 2000;9:589–93.
Hsu HC, Yang P, Wang J, Wu Q, Myers R, Chen J, et al. Interleukin 17-producing T helper cells and interleukin 17 orchestrate autoreactive germinal center development in autoimmune BXD2 mice. Nat Immunol. 2008;9:166–75.
Duhen T, Geiger R, Jarrossay D, Lanzavecchia A, Sallusto F. Production of interleukin 22 but not interleukin17 by a subset of human skin-homing memory T cells. Nat Immunol. 2009;10:857–63.
Eyerich S, Eyerich K, Pennino D, Carbone T, Nasorri F, Pallotta S, et al. Th22 cells represent a distinct human T cell subsetinvolved in epidermal immunity and remodeling. J Clin Invest. 2009;119:3573–85.
Trifari S, Kaplan C, Tran E, Crellin N, Spits H. Identification of a human helper T cell population that has abundant production of interleukin 22 and is distinct from T(H)-17, T(H)1 and T(H)2 cells. Nat Immunol. 2009;10:864–71.
Dumoutier L, Louahed J, Renauld JC. Cloning and characterization of IL-10-related Tcell-derived inducible factor (IL-TIF), a novel cytokine structurally related to IL-10 and inducible by IL-9. J Immunol. 2000;164:1814–9.
Zhang N, Pan H-F, Ye D-Q. Th22 in inflammatory and autoimmune disease: prospects for therapeutic intervention. Mol Cell Biochem. 2011;353:41–6.
Kagami S, Rizzo HL, Lee JJ, Koguchi Y, Blauvelt A. Circulating Th17, Th22, and Th1 cells are increased in psoriasis. J Invest Dermatol. 2010;130:1373–83.
Truchetet ME, Brembillal NC, Montanari E, et al. Increased frequency of circulating Th22 in addition to Th17 and Th2 lymphocytes in systemic sclerosis: association with interstitial lung disease. Arthritis Res Ther. 2011;13:R166.
Shen H, Goodall JC, Hill Gaston JS. Frequency and phenotype of peripheral blood Th17 cells in ankylosing spondylitis and rheumatoid arthritis. Arthritis Rheum. 2009;60:1647–56.
Zhang L, Li JM, Liu XG, et al. Elevated Th22 Cells Correlated with Th17 Cells in Patients with Rheumatoid Arthritis. J Clin Immunol. 2011;31:606–14.
Ikeuchi H, Kuroiwa T, Hiramatsu N, et al. Expression of interleukin-22 in rheumatoid arthritis: potential role as a proinflammatory cytokine. Arthritis Rheum. 2005;52(4):1037–46.
Hochberg MC. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1997;40:1725.
Gladman DD, Ibanez D, Urowitz MB. Systemic Lupus Erythematosus Disease Activity Index 2000. J Rheumatol. 2002;29:288–91.
Pan HF, Fnag XH, Wu GC, et al. Anti-neutrophil cytoplasmic antibodies in new-onset Systemic Lupus Erythematosus and lupus nephritis. Inflammation. 2008;31:260–5.
Wolk K, Kunz S, Witte E, Friedrich M, Asadullah K, Sabat R. IL-22 increases the innate immunity of tissues. Immunity. 2004;21:241–54.
Wolk K, Witte E, Witte K, Warszawska K, Sabat R. Biology of interleukin-22. Semin Immunopathol. 2010;32:17–31.
Zenewicz LA, Yancopoulos GD, Valenzuela DM, Murphy AJ, Stevens S, Flavell RA. Innate and adaptive interleukin-22 protects mice from inflammatory bowel disease. Immunity. 2008;29:947–57.
Zheng Y, Danilenko DM, Valdez P, Kasman I, Eastham-Anderson J, Wu J, et al. Interleukin-22, a T(H)17 cytokine, mediates IL-23-induced dermal inflammation and acanthosis. Nature. 2007;445:648–51.
Ma HL, Liang S, Li J, Napierata L, Brown T, Benoit S, et al. IL-22 is required for Th17 cell-mediated pathology in a mouse model of psoriasis-like skin inflammation. J Clin Invest. 2008;118:597–607.
Wolk K, Haugen HS, Xu W, Witte E, Waggie K, Anderson M, et al. IL-22 and IL-20 are key mediators of the epidermal alterations in psoriasis while IL-17 and IFN-gamma are not. J Mol Med. 2009;87(5):523–36.
Kolb H, Kolb-Bachofen V. Nitric oxide: a pathogenetic factor in autoimmunity. Immunol Today. 1992;13:157–1601.
Jonuleit H, Dnop J, End AH. Cytokines and their effects on maturation, differentiation and migration of dendratic cells. Arch Dermatol Res. 1996;289:1.
Fairhurst AM, Mathian A, Connolly JE, et al. Systemic TNF-αdrives kidney nephritis in B6.Sle123 mice. Eur J Immunol. 2008;38(7):1948–60.
Jacob CO, Fronek Z, Lewis GD, et al. Heritable major histocompatibility complex class II-associated difference in production of tumor necrosis factor alpha: relevance to genetic predisposition to systemic lupus erythematosus. Proc Nacl Acad Sci U S A. 1990;87:1233–7.
Doreau A, Belot A, Bastid J, Riche B, Trescol-Biemont MC, Ranchin B, et al. Interleukin 17 acts in synergy with B cell-activating factor to influence B cell biology and the pathophysiology of systemic lupus erythematosus. Nat Immunol. 2009;10:778–85.
Dong G, Ye R, Shi W, et al. IL-17 induces autoantibody overproduction and peripheral blood mononuclear cell overexpression of IL-6 in lupus nephritis patients. Chin Med J (Engl). 2003;116:543–8.
Ma J, Yu J, Tao X, et al. The imbalance between regulatory and IL-17-secreting CD4+ T cells in lupus patients. Clin Rheumatol. 2010;29:1251–8.
Henriques A, Ines L, Couto M, et al. Frequency and functional activity of Th17, Tcl7 and other T-cell subsets in Systemic Lupus Erythematosus. Cell Immunol. 2010;264:97–103.
Funauclli MS, Ikoma H, Enomoto, et al. Decreased Thl-like and increased Th2-like cells in systemic lupus erythematosus. Scand J Rheumatol. 1998;27(3):p219–24.
Chang DM, Su WL, Chu SJ. The expression and significance of intracellular T helper cytokines in systemic 1upus erythematosus. Immunol Invest. 2002;31(1):1–12.
Acknowledgments
This study was partially supported by grant from Science Foundation of Science and Technology Department of Zhejiang Province (No.2007C3305) and (No.2009C3012-4).
Disclosures
The authors have no financial conflict of interest.
The authors have nothing to disclose.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yang, Xy., Wang, Hy., Zhao, Xy. et al. Th22, but not Th17 Might be a Good Index to Predict the Tissue Involvement of Systemic Lupus Erythematosus. J Clin Immunol 33, 767–774 (2013). https://doi.org/10.1007/s10875-013-9878-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10875-013-9878-1