Abstract
T helper cell 22 (Th22) is a new subset of T cells clearly separate from Th17 and other known T cell subsets with distinct gene expression and function. With the CCR6 + CCR4 + CCR10 + phenotype and aryl hydrocarbon receptor as the key transcription factor, Th22 subsets produce cytokines such as IL-22, whose function depends on the activation of signal transduction and activators of transcription 3. IL-22 was up-regulated in Rheumatoid arthritis, Crohn’s disease, Psoriasis, and atopic dermatitis patients whereas it was down-regulated in the serum of patients with sarcoidosis and systemic lupus erythematosus. Furthermore, it has been demonstrated that IL-22 may have promise as a potential therapeutic for chronic inflammatory diseases, and treatment with recombinant cytokine or gene therapy delivery of IL-22 may alleviate tissue destruction during inflammatory responses. In summary, Th22 cell plays an important and complicated role in inflammatory and autoimmune disease.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
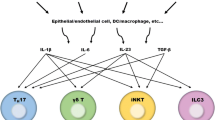
T helper cell (Th) subsets are defined according to their production of lineage-indicating cytokines and functions. They play important roles in translating antigen-specific immune responses into tissue functions or immunopathology. The identification of novel T cell subsets, such as Th17 cells, is important to define the role of the specific immune response in human disease. Across the board of different pathologies, distinct T cell subsets secrete cytokines that function not only on other immune cells, but also on target cells [1–3]. Recently, a Th22 subset of T cells was clearly separated from Th17 and other known T cell subsets with a distinct identity with respect to gene expression and function [4].
Th22
With the CCR6 + CCR4 + CCR10 + phenotype and aryl hydrocarbon receptor (AHR) as the key transcription factor, Th22 subsets produce cytokines such as IL-22, IL-26, and IL-13, of which IL-22 is the most important functional cytokine. Recent studies indicate that IL-6 and TNF-α, along with the help of plasmacytoid dendritic cells (DCs), can promote the Th22 phenotype [5].
The IL-23 and IL-6 can directly induce the production of IL-22 from both murine and human naive T cells. AHR agonists promote IL-22 production in both human and mice [6, 7]. However, the production of IL-22 from Th17 cells is differentially regulated [8]. There is a species-specific regulation of IL-17 production by AHR that may partially be attributed to the differences in the affinities of the human and mouse AHR. The affinity for the ligand of the human AHR is much lower than that of the mouse AHR. Therefore, IL-22 + IL-17 cells are only found in human [9].
IL-22 and signaling pathway
The IL-22 belongs to the IL-10 family of cytokine. Human and mouse IL-10-related-T-cell-derived inducible factor (IL-TIF) both consist of 179 amino acid long proteins and six exons spreading over approximately 6 Kb including four cysteins, and showing an overall sequence identity with IL-10 of 22% in the mouse and 25% in the human. Human IL-TIF gene is a single copy gene located on chromosome 12q15, at 90 Kb from the IFN-γ gene, and at 27 Kb from the AK155 gene, which codes for another IL-10-related cytokine. In the mouse, the IL-22 gene is located on chromosome 10, also near the IFN-γ gene. There are two copies in different mouse strains, which show 98% nucleotide identity in the coding region, named IL-TIFα and IL-TIFβ. Beside single nucleotide variations, they differ by a 658 nucleotide deletion in IL-TIFβ, including the first non-coding exon and 603 nucleotides from the promoter, suggesting that the IL-TIFβ gene is either differentially regulated, or not expressed at all [10].
Like all other members of the IL-10 family, IL-22 exerts its biological effects via members of the cytokine receptor family class 2. The cell surface IL-22 receptor complex is a heterodimeric receptor consisting of IL-22R1 and IL-10R2 chains [11]. The latter also function as an accessory receptor chain for the IL-10, IL-26, and the IL-28/IL-29 receptor complex [12]. IL-22R1 is expressed in skin, pancreas, kidney, liver, and gut but not on T cells, B cells, monocytes, or dendritic cells [11, 13–15]. In addition to the cell surface IL-22 receptor complex, there is a soluble, single-chain IL-22 receptor named IL-22 binding protein (IL-22BP), which also demonstrates the features of the extracellular domain of the class 2 cytokine receptors [15]. Indeed, IL-22BP is a kind of secreted protein that specifically binds IL-22 and seems to function as an IL-22 antagonist in vitro. In vivo, this soluble receptor might act as either a cytokine carrier molecule or a cytokine antagonist [11].
The IL-22 induces signal transduction and activators of transcription (STAT) activation in several cell lines such as mesangial cells, lung, and intestinal epithelial cells, melanomas, and hepatomas [16, 17]. Cell lines such as TK-10, a renal cell carcinoma and SW480, a colon adenocarcinoma were observed to show rapid and robust STAT1, STAT3, and STAT5 activation in response to IL-22 [18]. A later study showed binding of IL-22 to its surface receptor on rat hepatoma cell line H4IIE induced the rapid activation of JAK1 and Tyk2, leading to phosphorylation of STAT1, STAT3, and STAT5. IL-22 also activated the three major MAPK pathways: the MEK-ERK-RSK, the JNK/SAPK, and the p38 kinase pathways. In addition, IL-22 induced phosphorylation of STAT3 on a serine residue. This further STAT modification was necessary for maximum transactivation and depended only marginally on the ERK pathway [19].
In addition, IL-22 has a crucial role in mucosal host defense as to induce the antimicrobial peptides human β-defensin 2 and β-defensin 3 [3].
IL-22 in inflammatory and autoimmune disease
The IL-22 is produced by special immune cell populations, including Th22, Th1, and Th17 cells, classical and non-classical (NK-22) NK cells, NKT cells, and lymphoid tissue inducer cells. The main biological role of IL-22 includes the increase of innate immunity, protection from damage, and enhancement of regeneration. Its target cells are certain tissue cells from the skin, liver and kidney, and from organs of the respiratory and gastrointestinal systems. IL-22 can play either a protective or a pathogenic role in chronic inflammatory diseases depending on the nature of the affected tissue and the local cytokine milieu. The production of IL-22 by activated immune cells is reflected in the enhanced presence of this cytokine in various chronic inflammatory diseases, especially in those associated with a dominant role in the major IL-22-producing Th cell populations (Th22, Th1, and Th17) [20].
Rheumatoid arthritis (RA)
The RA is characterized by synovial inflammation and destruction of bone and joint cartilage. It is mediated by persistent synthesis of proinflammatory cytokines and matrix metalloproteinases (MMPs) [21]. T cell activation and migration occurs as an early consequence of RA, and these cells adopt a proinflammatory phenotype. IL-22 is primarily produced by activated T cells and natural killer cells [22].
In RA, two studies point to a possible pathogenic role of IL-22, although this has not been extensively investigated thus far [23, 24]. First, IL-22 increased the proliferation and modestly promoted the production of CCL2 by RA synovial fibroblasts in vitro. A study showed up-regulation of IL-22 mRNA in RA synovial tissues and mononuclear cells both in the lining and the sublining layers of RA synovial tissues. The majority of IL-22-positive cells were synovial fibroblasts and macrophages. IL-22R1 mRNA was also expressed in RA synovial tissues. However, the majority of IL-22R1-positive cells were synovial fibroblasts only. In vitro, recombinant IL-22 increased proliferation of synovial fibroblasts derived from RA patients (RASF) and production of monocyte chemoattractant protein 1(MCP-1) by RASF above the value of medium controls and stimulated MAPK activation [23]. Second, in collagen-induced arthritis (CIA) in C57BL/6 mice, IL-22 levels were found to be higher in sera from immunized mice as compared with sera from untreated animals. IL-22-deficient (IL-22−/−) mice were less susceptible to CIA than untreated mice, as evidenced by their decreased incidence of arthritis and decreased pannus formation. In IL-22−/− mice of CIA, there were higher levels of anti-CII total IgG antibodies which could be explained by the significantly higher levels of the IgG2c isotype. Furthermore, there was reduced arthritis severity associated with a significantly lower degree of pannus formation. Lower numbers of mRNA copies of IL-1β, IL-6, TNFα, and MMP-9 were found in pooled synovium samples from immunized IL-22−/− mice. In vitro, IL-22 was found to promote osteoclastogenesis, a process that might contribute to its proinflammatory activity in CIA [24].
The proinflammatory role of endogenous IL-22 in arthritis as promoting osteoclastogenesis and regulating antibody production is suggested. However, the uncoupling between higher antibody production and low incidence in IL-22−/− mice during CIA is unclear and needs further study.
Crohn’s disease (CD)
The CD is an inflammatory bowel disease which is characterized by a chronic, uncontrolled inflammation in the intestinal mucosa with transmural infiltration of activated immune cells such us Th1 and Th17 cells, and patchy granulomatous lesions [25, 26]. In view of the essentiality of STAT3 for IL-22’s effects, a positive role of IL-22 in inflammatory bowel diseases was suggested by the importance of STAT3 for intestinal mucosal homeostasis [27]. Furthermore, through investigation of IL-22’s effects on intestinal epithelial cells and subepithelial myofibroblasts, a role for IL-22 in inflammatory bowel disease further emerged. These cells were shown to mainly respond to IL-22 stimulation with production of a range of anti-inflammatory, regenerative, and tissue-protective proteins and enhanced migratory capacity [28–30].
Several groups tried to illuminate the meaning of IL-22 in this disorder [28, 29]. However, the exact role of this cytokine in the intestinal inflammation remains ambiguous. In contrast to these studies, Wolk’s group study focused on possible systemic effects of IL-22. They demonstrated that IL-22 was present in high quantities in the blood of CD patients in contrast to IFN-γ and IL-17. In a mouse colitis model, IL-22 mRNA expression was elevated predominantly not only in the inflamed intestine but also in the mesenteric lymph nodes. IL-22BP, the soluble receptor with a higher affinity for IL-22, demonstrated strong constitutive expression in the intestine and lymph nodes, was decreased in the inflamed intestine, but not in the mesenteric lymph nodes in the inflammatory bowel disease mode. The affinity between IL-22BP and IL-22 was also analyzed to gain an insight into the potential in vivo role of IL-22BP. These binding data strongly support an IL-22-inhibitory role of IL-22BP in vivo. To investigate the possible role of systemic IL-22 in CD, administration of IL-22 was given to healthy mice and showed an up-regulation of LPS-binding protein (LBP) blood levels reaching concentrations known to neutralize LPS. This systemic up-regulation was associated with increased hepatic but not renal or pulmonary LBP mRNA levels. In vitro, IL-22 also enhanced the secretion of LBP in human primary hepatocytes and HepG2 hepatoma cells. This increase was mainly transcriptionally regulated and synergistic with that of other LBP inducers. Finally, elevated LBP levels were detected in CD patients and the mouse colitis model. Therefore, systemic IL-22 may contribute to the prevention of systemic inflammation provoked by LPS present in the blood of CD patients through its induction of hepatic LBP [31].
Psoriasis and atopic dermatitis (AD)
The IL-22 has a regenerative and protective role on epithelial cells. These primarily non-inflammatory effects, however, might turn pathological when not tightly regulated. This could be the case in some inflammatory and autoimmune diseases [32]. Psoriasis is the first example of an organ-specific autoimmune disorder for which the role of IL-22 has been comprehensively investigated. IL-22 has been found to be a key mediator in the final phase of the psoriasis pathogenesis, the phase in which visible keratinocyte alterations are formed.
Psoriatic patients showed strongly elevated IL-22 plasma levels, which correlated with the disease severity [22]. Furthermore, IL-22 mediates IL-23-induced acanthosis and dermal inflammation through the activation of STAT3 in vivo [8]. IL-22, with keratinocytes as an important target cell, induced the expression of the antimicrobial molecules β-defensin 2, β-defensin 3, S100A7, S100A8, and S100A9, which enhance the inherent immunity of keratinocytes. High IL-22 levels in psoriatic skin were associated with strongly up-regulated cutaneous S100A7, S100A8, S100A9, and MMP1 expression [22]. Knowledge of IL-22’s role in the skin biology thus led to a revision of the current view of the final phase of psoriasis pathogenesis. In fact, aside from activation of antigen-presenting cells, IFN-γ is also a prominent inducer of keratinocyte expression of chemokines attracting Th1 cells, as well as monocytes and DCs. IL-22 promotes development of keratinocyte alterations directly by preventing their terminal differentiation, without IFN-γ or IL-17. In that way, IL-22 also provides mechanisms to prolong and amplify its own effects by inducing the production of its major signaling molecule, STAT3, and of IL-20, which has IL-22-like effects. TNF-α plays an important role in the keratinocyte effector stage as it increases the responsiveness of the keratinocytes to IL-22 via up-regulation of IL-22 receptor and signal transduction element expression [33].
Given its broad role in the final phase of psoriasis pathogenesis, together with the fact that TNF-α promotes the differentiation of the major IL-22-producing Th22 cells, this can explain the high therapeutic success of TNF-a blockade in psoriasis. However, targeting IL-22 would be a more promising therapeutic intervention, since such a strategy is not expected to induce major side effects, especially due to the lack of IL-22 responsiveness of immune cells.
Compared to normal skin, IL-22 was significantly up-regulated not only in psoriasis but also in AD skin lesion with a higher expression in AD than in psoriatic lesions. Interestingly, distinct IL-22 producing CD4+ and CD8+ T-cell populations were significantly increased in AD skin compared to psoriasis. While the frequency of IL-22 + CD8 + T-cell correlated with AD disease severity [34]. Actually, the expression of IL-22 in atopic skin was even slightly higher when compared to lesional psoriatic skin, which may rely on the observation that the majority of IL-22-producing cells in atopic skin are Th22 cells, whereas in psoriatic skin there is a shift to a rather equal presence of IL-22-producing Th22, Th1, and Th17 cells [15, 34]. Although the role of IL-22 in AD has not been specifically explored, it is likely to contribute to the epidermal acanthosis seen in the chronic stage.
Systemic lupus erythematosus (SLE)
The SLE is a systemic autoimmune disease, characterized by a multitude of autoantibody production, complement activation, and immune-complex deposition, which causes tissue and organ damage. Cytokines produced by abnormal Th cells have been shown to be involved in the pathogenesis of SLE [35]. Th17 cells, which selectively secrete several proinflammatory cytokines, including IL-22, have been implicated in the etiology of autoimmune disease including SLE [35, 36].
Nevertheless, the exact role of IL-22 in the pathogenesis of SLE remains to be established. The assessment of a cytokine at the serum level would certainly simplify clinical evaluation [37]. Until recently, we investigated serum IL-22 levels in SLE, in comparison with normal controls, and relations to disease activity. In the serum of patients with SLE, IL-22 levels were significantly decreased compared with normal controls. However, there was no significant difference between less active SLE and more active SLE. Correlation analysis between serum IL-22 levels and SLE Disease Activity Index (SLEDAI) showed no association. The decreased serum IL-22 level in SLE indicated that IL-22 might be protective for SLE. However, association of serum IL-22 levels with SLEDAI was not found because of the small sample size of this study [38]. Subsequently, Cheng’s group found decreased plasma IL-22 levels correlated with disease activity in SLE patients [39]. Therefore, future prospective cohort studies with large sample size will examine the role of IL-22 in chronic inflammation and the development and treatment of SLE in human. IL-22 may prove to be an important target for developing new drugs and treatments to SLE.
Others
Western blotting for IL-22 in bronchoalveolar lavage fluid demonstrated that lower levels of IL-22 were present in the patients with acute respiratory distress syndrome (ARDS) and sarcoidosis relative to control subjects [40]. IL-22 messenger RNA and protein expression are significantly elevated in T cell-mediated hepatitis induced by concanavalin A (ConA). IL-22 blockade with a neutralizing antibody reduces signal transducer and activator of STAT3 activation and worsens liver injury in T cell-mediated hepatitis, whereas injection of recombinant IL-22 attenuates such injury [41]. These data suggest the potential protective role of IL-22.
The IL-22 has only minor pro-inflammatory effects and, in some cases, is protective against autoimmune disease. This is shown in the brain, where IL-22 knockout mice develop experimental autoimmune encephalomyelitis (EAE) pathology [42].
IL-22 as a potential therapeutic target for chronic inflammatory diseases
As discussed above, IL-22 may have promise as a potential therapeutic target for chronic inflammatory diseases, and treatment with recombinant cytokine or gene therapy delivery of IL-22 may alleviate tissue destruction during inflammatory responses [43]. Therapeutic control of IL-22 could therefore be beneficial in chronic remodeling diseases with high tissue turnover, but would probably require long-term medication. Furthermore, IL-22 acts synergistically with IL-17, TNF-a, and IFN-γ in a pro-inflammatory context and thus has to be regarded as an ambivalent cytokine.
Experimental delivery of IL-22 has been efficacious in treating autoimmune disorders such as experimental autoimmune myocarditis in rats [44, 45]. Suppressing the immune system via anti-inflammatory treatments such as TNF-α inhibitors can lead to unwanted dampening of the immune response, weakening its ability to respond to infection. On the other hand, IL-22 is an ideal therapeutic candidate since it will specifically affect tissue responses and not have direct effects on the immune response [43].
Conclusion
Th22 cells represent a T cell subset which is not only defined by a stable and distinct expression profile, but also characterized by what we believe to be a novel functional profile. The identification of Th22 cells provides a cellular target for therapeutic intervention and may shed light on thus far unknown pathways in the control of inflammatory diseases. However, several points should be realized: first, IL-22 is expressed by not only Th22 but also Th17 and NK cells, so that its function could not absolutely identify Th22’s function; second, it is also difficult to generalize whether Th22 cells is anti-inflammatory or pro-inflammatory; third, the differentiation, regulation, downstream pathways of Th22 and the relationship between Th22 and Th17 are not clear. Therefore, further studies are required, especially in human systems, to comprehensively explore the therapeutic potential of Th22 in inflammatory and autoimmune disease.
References
Burgler S, Ouaked N, Bassin C, Basinski TM, Mantel PY, Siegmund K, Meyer N, Akdis CA, Schmidt-Weber CB (2009) Differentiation and functional analysis of human T(H)17 cells. J Allergy Clin Immunol 123(3):588–595. doi:10.1016/j.jaci.2008.12.017 595 e581–587
Eyerich K, Pennino D, Scarponi C, Foerster S, Nasorri F, Behrendt H, Ring J, Traidl-Hoffmann C, Albanesi C, Cavani A (2009) IL-17 in atopic eczema: linking allergen-specific adaptive and microbial-triggered innate immune response. J Allergy Clin Immunol 123(1):59–66. doi:10.1016/j.jaci.2008.10.031 e54
Aujla SJ, Chan YR, Zheng M, Fei M, Askew DJ, Pociask DA, Reinhart TA, McAllister F, Edeal J, Gaus K, Husain S, Kreindler JL, Dubin PJ, Pilewski JM, Myerburg MM, Mason CA, Iwakura Y, Kolls JK (2008) IL-22 mediates mucosal host defense against Gram-negative bacterial pneumonia. Nat Med 14(3):275–281. doi:10.1038/nm1710
Eyerich S, Eyerich K, Pennino D, Carbone T, Nasorri F, Pallotta S, Cianfarani F, Odorisio T, Traidl-Hoffmann C, Behrendt H, Durham SR, Schmidt-Weber CB, Cavani A (2009) Th22 cells represent a distinct human T cell subset involved in epidermal immunity and remodeling. J Clin Invest 119(12):3573–3585. doi:10.1172/JCI40202
Duhen T, Geiger R, Jarrossay D, Lanzavecchia A, Sallusto F (2009) Production of interleukin 22 but not interleukin 17 by a subset of human skin-homing memory T cells. Nat Immunol 10(8):857–863. doi:10.1038/ni.1767
Veldhoen M, Hirota K, Westendorf AM, Buer J, Dumoutier L, Renauld JC, Stockinger B (2008) The aryl hydrocarbon receptor links TH17-cell-mediated autoimmunity to environmental toxins. Nature 453(7191):106–109. doi:10.1038/nature06881
Quintana FJ, Basso AS, Iglesias AH, Korn T, Farez MF, Bettelli E, Caccamo M, Oukka M, Weiner HL (2008) Control of T(reg) and T(H)17 cell differentiation by the aryl hydrocarbon receptor. Nature 453(7191):65–71. doi:10.1038/nature06880
Zheng Y, Danilenko DM, Valdez P, Kasman I, Eastham-Anderson J, Wu J, Ouyang W (2007) Interleukin-22, a T(H)17 cytokine, mediates IL-23-induced dermal inflammation and acanthosis. Nature 445(7128):648–651. doi:10.1038/nature05505
Okey AB, Franc MA, Moffat ID, Tijet N, Boutros PC, Korkalainen M, Tuomisto J, Pohjanvirta R (2005) Toxicological implications of polymorphisms in receptors for xenobiotic chemicals: the case of the aryl hydrocarbon receptor. Toxicol Appl Pharmacol 207(2 Suppl):43–51. doi:10.1016/j.taap.2004.12.028
Dumoutier L, Van Roost E, Ameye G, Michaux L, Renauld JC (2000) IL-TIF/IL-22: genomic organization and mapping of the human and mouse genes. Genes Immun 1(8):488–494. doi:10.1038/sj.gene.6363716
Renauld JC (2003) Class II cytokine receptors and their ligands: key antiviral and inflammatory modulators. Nat Rev Immunol 3(8):667–676
Langer JA, Cutrone EC, Kotenko S (2004) The Class II cytokine receptor (CRF2) family: overview and patterns of receptor-ligand interactions. Cytokine Growth Factor Rev 15(1):33–48
Wolk K, Sabat R (2006) Interleukin-22: a novel T- and NK-cell derived cytokine that regulates the biology of tissue cells. Cytokine Growth Factor Rev 17(5):367–380. doi:10.1016/j.cytogfr.2006.09.001
Wolk K, Kunz S, Asadullah K, Sabat R (2002) Cutting edge: immune cells as sources and targets of the IL-10 family members? J Immunol 168(11):5397–5402
Wolk K, Kunz S, Witte E, Friedrich M, Asadullah K, Sabat R (2004) IL-22 increases the innate immunity of tissues. Immunity 21(2):241–254. doi:10.1016/j.immuni.2004.07.007
Dumoutier L, Louahed J, Renauld JC (2000) Cloning and characterization of IL-10-related T cell-derived inducible factor (IL-TIF), a novel cytokine structurally related to IL-10 and inducible by IL-9. J Immunol 164(4):1814–1819
Dumoutier L, Van Roost E, Colau D, Renauld JC (2000) Human interleukin-10-related T cell-derived inducible factor: molecular cloning and functional characterization as an hepatocyte-stimulating factor. Proc Natl Acad Sci USA 97(18):10144–10149. doi:10.1073/pnas.170291697
Xie MH, Aggarwal S, Ho WH, Foster J, Zhang Z, Stinson J, Wood WI, Goddard AD, Gurney AL (2000) Interleukin (IL)-22, a novel human cytokine that signals through the interferon receptor-related proteins CRF2-4 and IL-22R. J Biol Chem 275(40):31335–31339. doi:10.1074/jbc.M005304200
Lejeune D, Dumoutier L, Constantinescu S, Kruijer W, Schuringa JJ, Renauld JC (2002) Interleukin-22 (IL-22) activates the JAK/STAT, ERK, JNK, and p38 MAP kinase pathways in a rat hepatoma cell line. Pathways that are shared with and distinct from IL-10. J Biol Chem 277(37):33676–33682. doi:10.1074/jbc.M204204200
Witte E, Witte K, Warszawska K, Sabat R, Wolk K (2010) Interleukin-22: a cytokine produced by T, NK and NKT cell subsets, with importance in the innate immune defense and tissue protection. Cytokine Growth Factor Rev 21(5):365–379. doi:10.1016/j.cytogfr.2010.08.002
Arend WP, Dayer JM (1995) Inhibition of the production and effects of interleukin-1 and tumor necrosis factor alpha in rheumatoid arthritis. Arthritis Rheum 38(2):151–160
Wolk K, Witte E, Wallace E, Docke WD, Kunz S, Asadullah K, Volk HD, Sterry W, Sabat R (2006) IL-22 regulates the expression of genes responsible for antimicrobial defense, cellular differentiation, and mobility in keratinocytes: a potential role in psoriasis. Eur J Immunol 36(5):1309–1323. doi:10.1002/eji.200535503
Ikeuchi H, Kuroiwa T, Hiramatsu N, Kaneko Y, Hiromura K, Ueki K, Nojima Y (2005) Expression of interleukin-22 in rheumatoid arthritis: potential role as a proinflammatory cytokine. Arthritis Rheum 52(4):1037–1046. doi:10.1002/art.20965
Geboes L, Dumoutier L, Kelchtermans H, Schurgers E, Mitera T, Renauld JC, Matthys P (2009) Proinflammatory role of the Th17 cytokine interleukin-22 in collagen-induced arthritis in C57BL/6 mice. Arthritis Rheum 60(2):390–395. doi:10.1002/art.24220
Cobrin GM, Abreu MT (2005) Defects in mucosal immunity leading to Crohn’s disease. Immunol Rev 206:277–295. doi:10.1111/j.0105-2896.2005.00293.x
Fuss IJ, Becker C, Yang Z, Groden C, Hornung RL, Heller F, Neurath MF, Strober W, Mannon PJ (2006) Both IL-12p70 and IL-23 are synthesized during active Crohn’s disease and are down-regulated by treatment with anti-IL-12 p40 monoclonal antibody. Inflamm Bowel Dis 12(1):9–15
Pickert G, Neufert C, Leppkes M, Zheng Y, Wittkopf N, Warntjen M, Lehr HA, Hirth S, Weigmann B, Wirtz S, Ouyang W, Neurath MF, Becker C (2009) STAT3 links IL-22 signaling in intestinal epithelial cells to mucosal wound healing. J Exp Med 206(7):1465–1472. doi:10.1084/jem.20082683
Andoh A, Zhang Z, Inatomi O, Fujino S, Deguchi Y, Araki Y, Tsujikawa T, Kitoh K, Kim-Mitsuyama S, Takayanagi A, Shimizu N, Fujiyama Y (2005) Interleukin-22, a member of the IL-10 subfamily, induces inflammatory responses in colonic subepithelial myofibroblasts. Gastroenterology 129(3):969–984. doi:10.1053/j.gastro.2005.06.071
Brand S, Beigel F, Olszak T, Zitzmann K, Eichhorst ST, Otte JM, Diepolder H, Marquardt A, Jagla W, Popp A, Leclair S, Herrmann K, Seiderer J, Ochsenkuhn T, Goke B, Auernhammer CJ, Dambacher J (2006) IL-22 is increased in active Crohn’s disease and promotes proinflammatory gene expression and intestinal epithelial cell migration. Am J Physiol Gastrointest Liver Physiol 290(4):G827–G838. doi:10.1152/ajpgi.00513.2005
Sekikawa A, Fukui H, Suzuki K, Karibe T, Fujii S, Ichikawa K, Tomita S, Imura J, Shiratori K, Chiba T, Fujimori T (2010) Involvement of the IL-22/REG Ialpha axis in ulcerative colitis. Lab Invest 90(3):496–505. doi:10.1038/labinvest.2009.147
Wolk K, Witte E, Hoffmann U, Doecke WD, Endesfelder S, Asadullah K, Sterry W, Volk HD, Wittig BM, Sabat R (2007) IL-22 induces lipopolysaccharide-binding protein in hepatocytes: a potential systemic role of IL-22 in Crohn’s disease. J Immunol 178(9):5973–5981
Eyerich S, Eyerich K, Cavani A, Schmidt-Weber C (2010) IL-17 and IL-22: siblings, not twins. Trends Immunol 31(9):354–361. doi:10.1016/j.it.2010.06.004
Wolk K, Haugen HS, Xu W, Witte E, Waggie K, Anderson M, Vom Baur E, Witte K, Warszawska K, Philipp S, Johnson-Leger C, Volk HD, Sterry W, Sabat R (2009) IL-22 and IL-20 are key mediators of the epidermal alterations in psoriasis while IL-17 and IFN-gamma are not. J Mol Med 87(5):523–536. doi:10.1007/s00109-009-0457-0
Nograles KE, Zaba LC, Shemer A, Fuentes-Duculan J, Cardinale I, Kikuchi T, Ramon M, Bergman R, Krueger JG, Guttman-Yassky E (2009) IL-22-producing “T22” T cells account for upregulated IL-22 in atopic dermatitis despite reduced IL-17-producing TH17 T cells. J Allergy Clin Immunol 123(6):1244–1252. doi:10.1016/j.jaci.2009.03.041 e1242
Schmidt-Weber CB, Akdis M, Akdis CA (2007) TH17 cells in the big picture of immunology. J Allergy Clin Immunol 120(2):247–254. doi:10.1016/j.jaci.2007.06.039
Pan HF, Ye DQ, Li XP (2008) Type 17 T-helper cells might be a promising therapeutic target for systemic lupus erythematosus. Nat Clin Pract Rheumatol 4(7):352–353. doi:10.1038/ncprheum0815
Ciprandi G, Fenoglio D, De Amici M, Quaglini S, Negrini S, Filaci G (2008) Serum IL-17 levels in patients with allergic rhinitis. J Allergy Clin Immunol 122(3):650–651. doi:10.1016/j.jaci.2008.06.005 e652
Pan HF, Zhao XF, Yuan H, Zhang WH, Li XP, Wang GH, Wu GC, Tang XW, Li WX, Li LH, Feng JB, Hu CS, Ye DQ (2009) Decreased serum IL-22 levels in patients with systemic lupus erythematosus. Clin Chim Acta 401(1–2):179–180. doi:10.1016/j.cca.2008.11.009
Cheng F, Guo Z, Xu H, Yan D, Li Q (2009) Decreased plasma IL22 levels, but not increased IL17 and IL23 levels, correlate with disease activity in patients with systemic lupus erythematosus. Ann Rheum Dis 68(4):604–606. doi:10.1136/ard.2008.097089
Whittington HA, Armstrong L, Uppington KM, Millar AB (2004) Interleukin-22: a potential immunomodulatory molecule in the lung. Am J Respir Cell Mol Biol 31(2):220–226. doi:10.1165/rcmb.2003-0285OC
Radaeva S, Sun R, Pan HN, Hong F, Gao B (2004) Interleukin 22 (IL-22) plays a protective role in T cell-mediated murine hepatitis: IL-22 is a survival factor for hepatocytes via STAT3 activation. Hepatology 39(5):1332–1342. doi:10.1002/hep.20184
Kreymborg K, Etzensperger R, Dumoutier L, Haak S, Rebollo A, Buch T, Heppner FL, Renauld JC, Becher B (2007) IL-22 is expressed by Th17 cells in an IL-23-dependent fashion, but not required for the development of autoimmune encephalomyelitis. J Immunol 179(12):8098–8104
Zenewicz LA, Yancopoulos GD, Valenzuela DM, Murphy AJ, Karow M, Flavell RA (2007) Interleukin-22 but not interleukin-17 provides protection to hepatocytes during acute liver inflammation. Immunity 27(4):647–659. doi:10.1016/j.immuni.2007.07.023
Chang H, Hanawa H, Liu H, Yoshida T, Hayashi M, Watanabe R, Abe S, Toba K, Yoshida K, Elnaggar R, Minagawa S, Okura Y, Kato K, Kodama M, Maruyama H, Miyazaki J, Aizawa Y (2006) Hydrodynamic-based delivery of an interleukin-22-Ig fusion gene ameliorates experimental autoimmune myocarditis in rats. J Immunol 177(6):3635–3643
Pan H, Hong F, Radaeva S, Gao B (2004) Hydrodynamic gene delivery of interleukin-22 protects the mouse liver from concanavalin A-, carbon tetrachloride-, and Fas ligand-induced injury via activation of STAT3. Cell Mol Immunol 1(1):43–49
Acknowledgments
The study was supported by grants from the key program of National Natural Science Foundation of China (30830089).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhang, N., Pan, HF. & Ye, DQ. Th22 in inflammatory and autoimmune disease: prospects for therapeutic intervention. Mol Cell Biochem 353, 41–46 (2011). https://doi.org/10.1007/s11010-011-0772-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-011-0772-y