Abstract
Renal fibrosis is the final result of the progression of chronic kidney disease (CKD) to end-stage renal disease (ESRD). Earlier studies confirmed that selenium (Se) displays a close association with kidney diseases. However, the correlation between Se and fibrosis has rarely been explored. Thus, this article mainly aimed to investigate the effect of Se deficiency on renal fibrosis and the Wnt/β-catenin signaling pathway. Twenty BALB/c mice were fed a diet containing 0.02-mg/kg Se (Se-deficient diet) or 0.18-mg/kg Se (standard diet) for 20 weeks. A human glomerular mesangial cell (HMC) cell line was transfected with lentiviral TRNAU1AP-shRNA vector to establish a stable Se deficiency model in vitro. As indicated in this study, the glutathione (GSH) content in the Se-deficient group displayed an obvious decline compared with that in the control group, whereas the content of malondialdehyde (MDA) was obviously elevated. The results of Masson staining showed fibrosis around the renal tubules, and the results of immunohistochemistry showed that the area of positive fibronectin expression increased. In the Se-deficient group, the levels of collagen I, collagen III, matrix metalloproteinase 9 (MMP9), and other fibrosis-related proteins changed significantly in vivo and in vitro. Compared with the control group, the TRNAU1AP-shRNA group showed markedly reduced cell proliferation and migration abilities. Our data indicate that Se deficiency can cause kidney damage and renal fibrosis. Furthermore, the Wnt pathway is critical for the development of tissue and organ fibrosis. The data of this study demonstrated that the expression of Wnt5a, β-catenin, and dishevelled 1 (Dvl-1) was significantly upregulated in the Se-deficient group. Therefore, the Wnt/β-catenin pathway may play an important role in renal fibrosis caused by Se deficiency.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Selenium (Se) is an essential trace element in the human body that is implicated in various biological functions, including antioxidant, antimutagenic, and cell differentiation immunomodulatory functions [1, 2]. Se deficiency is closely correlated with numerous human diseases, such as chronic kidney disease (CKD) [3], diabetes [4], and Keshan disease [5]. It has been shown that the main biological form of Se in the human body is selenoproteins that contain the amino acid selenocysteine (Sec) [6]. The synthesis of selenoproteins requires selenophosphate synthetases, including SPS1 and SPS2, as well as Secp43 [7]. Secp43, namely, selenocysteine tRNA 1 associated protein (TRNAU1AP), contains two RNA recognition motifs (RRM), as well as a Tyr-rich C-terminal with an unknown function [8, 9]. TRNAU1AP is primarily located in the nucleus and was previously identified to interact with tRNA[Ser]Sec [10]. Studies have shown that TRNAU1AP can regulate selenoprotein synthesis by its involvement in the methylation of tRNA[Ser]Sec and the intracellular distribution of soluble liver antigen (SLA) [11]. In addition, one study indicated that constitutive deletion of exons 7+8 of TRNAU1AP can cause embryonic death but is not necessary for selenoprotein synthesis in hepatocytes and neurons [9]. Glutathione peroxidase 1 (GPX1), thioredoxin reductase 1 (TrxR1), glutathione peroxidase 4 (GPX4), and selenoprotein P (SELENOP) are the four members of the mammalian selenoprotein family that are of great importance for maintaining the metabolic functions of Se [12]. Our research group previously showed that knockdown of TRNAU1AP can downregulate selenoprotein expression [13, 14]. Therefore, TRNAU1AP is crucial for the synthesis of selenoproteins.
The kidney is the major excretory and secretory organ of the human body and plays an essential role in the homeostasis of Se [15]. It has been shown that Se accumulates to the highest level in the kidney; thus, the internal Se status is expected to be closely correlated with renal function [16]. Zachara et al. reported that Se levels in tissues and serum are reduced in patients with CKD and end-stage renal disease (ESRD) [3, 17]. Guo et al. [18] found that serum Se levels in peritoneal dialysis (PD) and hemodialysis (HD) patients were significantly decreased, while the oxidative stress levels in these patients were increased. In addition, Se can protect the kidney against cadmium-induced damage [19]. In our previously published work, Se deficiency was confirmed to result in kidney damage, ultrastructural and mitochondrial functional alterations, and changes in biogenesis [14]. However, the relationship between Se deficiency and renal fibrotic lesions requires in-depth exploration.
Renal fibrosis, which is characterized by the accumulation and deposition of extracellular matrix (ECM), is the final manifestation of CKD [20, 21]. It has been reported that the synthesis and degradation of ECM are affected by numerous factors, among which matrix metalloproteinases (MMPs) are the main regulatory factors of the degradation process [22]. In addition, MMPs have been found to induce the epithelial–mesenchymal transition (EMT) process by destroying epithelial contact [23]. It is also worth noting that dysregulated activation of the Wnt/β-catenin pathway is involved in renal fibrosis [24]. Schunk et al. [25] suggested that transient Wnt/β-catenin signaling stimulates repair and regeneration after acute kidney injury (AKI), while continuous activation of the pathway promotes CKD-associated fibrogenesis. However, it remains unknown whether Se deficiency is involved in these regulatory processes correlated with renal fibrosis.
Therefore, the purpose of this study was (i) to investigate whether Se deficiency can cause kidney tissue and cell damage, (ii) to observe the effect of Se deficiency on mouse kidney histopathology, and (iii) to understand the effect of Se deficiency on fibrosis marker proteins (collagen I, collagen III, MMP2, MMP9, and E-cadherin) and the signaling molecules in the Wnt/β-catenin pathway (Wnt5a, Dvl-1, and β-catenin) that may be involved in fibrosis.
Materials and Methods
Regents and Antibodies
Dulbecco’s modified Eagle’s medium (DMEM) and fetal bovine serum (FBS) were obtained from Thermo Fisher, Life Technologies (Carlsbad, CA, USA). In addition, penicillin and streptomycin were obtained from Biological Industries (Kibbutz, Beit HaEmek, Israel). Polybrene was provided by Obio Technology (Shanghai, China). Puromycin was provided by Beijing Huayueyang Bio (Beijing, China). TRIzol reagent, a PrimeScript™ 1st Strand cDNA Synthesis Kit, and a PrimeScript® RT Reagent Kit with gDNA Eraser were provided by Takara (Dalian, China). Radioimmunoprecipitation assay (RIPA) buffer was provided by Leagene Biotechnology (Beijing, China). Phosphatase inhibitors and protease inhibitors were provided by Roche (Shanghai, China). Furthermore, Masson’s trichrome staining kit was obtained from Solarbio Technology Co., Ltd. (Beijing, China). 3,3-Diaminobenzidine (DAB) was purchased from Beijing Zhongshan Golden Bridge Biotechnology Co., Ltd. (Beijing, China). Polyvinylidene difluoride (PVDF) membranes were obtained from Merck & Co Inc. (MA, USA). Primary antibodies applied in this study included an anti-SELENOP antibody purchased from Santa Cruz (CA, USA), as well as antibodies against TRNAU1AP, Wnt5a, fibronectin, E-cadherin, dishevelled 1 (Dvl-1), β-catenin, GPX1, TrxR1, and GAPDH purchased from Proteintech (Wuhan, China). In addition, antibodies against matrix metalloproteinase 2 (MMP2), matrix metalloproteinase 9 (MMP9), collagen I, and collagen III were provided by Wanleibio (Shenyang, China). Secondary antibodies (goat anti-rabbit and goat anti-mouse) were obtained from Proteintech (Wuhan, China). All primers employed were synthesized by Sangon (Shanghai, China).
Establishment of the Se Deficiency Model in Mice
This study was approved by the Institutional Animal Use and Care Committee of Harbin Medical University. All procedures conducted in studies involving animals complied with the ethical standards of the institution or practice at which the studies were conducted. Twenty male BALB/c mice weighing nearly 20 g were obtained from the Experimental Animal Center of The Second Affiliated Hospital of Harbin Medical University. In addition, BALB/c mice were randomly assigned to two groups, i.e., the Se-deficient group (n = 10) and the Se-adequate group (control group, n = 10). The environmental temperature was 23 ± 2°C, which was alternated day and night per 12 h. Twenty mice were fed a diet with all other nutritional indicators to be standard. Mice in the Se-deficient group were fed 0.02-mg/kg Se according to the AIN-93M formula (TROPHIC Animal Feed High-tech Co.) [26]. Likewise, mice in the control complied with the standard dietary formula of AIN-93M with 0.18-mg/kg Se (TROPHIC Animal Feed High-tech Co.) [26]. After 20 weeks, mice were anesthetized with ether. The left kidneys were fixed in 4% paraformaldehyde (Leagene, Beijing, China), and the right kidneys were stored at −80°C until subsequent use. After surgery, all mice were euthanized.
Cell Culture and Virus Infection
The human glomerular mesangial cell (HMC) cell line (Cobioer Biosciences Co., Ltd., China) was cultured in DMEM containing 10% FBS and 1% penicillin/streptomycin in an incubator with 5% CO2 at 37°C. Lentiviral TRNAU1AP-shRNA vector (TRNAU1AP-shRNA group) and lentiviral negative control-shRNA vector (NC-shRNA group) were provided by Obio Technology (Shanghai, China). The target sequence of human TRNAU1AP shRNA was 5′-CTGAGAAGTGTTTGCATAA-3′. In brief, HMC cells were seeded into 24-well plates at a density of 4 × 104 cells per well. Subsequently, the cell transfection of lentiviral shRNA vectors was conducted with polybrene. In addition, transfection efficiency could be improved by adding polybrene (5 μg/ml). The multiplicity of infection (MOI) used in HMC cells was 50. After 2 weeks, puromycin (2 μg/ml) was added to screen for positive cells.
Western Blot
Total protein in tissues and kidney cells was ice lyzed by RIPA buffer, phosphatase inhibitor, and protease inhibitor (100:1:1) for 15 min. After centrifugation at 4°C and 12,000 rpm for 10 min, total protein samples were collected. Protein concentrations were determined following the instructions of the bicinchoninic acid assay kit (Wuhan, China). Furthermore, protein lysates were mixed with loading buffer in a certain amount for 5 min at 95°C.
Proteins were separated by 8–12% SDS-PAGE gels for 1.5–2 h and then transferred onto PVDF membranes. Next, the membranes were blocked with 5% nonfat milk for 2 h at 25°C, washed with 1 × TBST, and then incubated with primary antibodies against GPX1 (1:1000), TrxR1 (1:1000), SELENOP (1:500), TRNAU1AP (1:2000), collagen I (1:2000), collagen III (1:2000), MMP2 (1:1000), MMP9 (1:1000), Wnt5a (1:1000), Dvl-1 (1:1000), β-catenin (1:1000), and GAPDH (1:2000) at 4°C overnight. The next day, the membranes were incubated with secondary antibodies (1:5000) for 2 h at ambient temperature. Immunoreactive bands were scanned or photographed by a BioSpectrum Imaging System (USA). Finally, the molecular mass and net optical density values of the target strips were analyzed with ImageJ software.
Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR)
Total RNA in 10 mg of kidney tissue and transfected cells was extracted with TRIzol reagent. Subsequently, the RNA samples were reverse transcribed into cDNA in accordance with the PrimeScript™ 1st Strand cDNA Synthesis Kit. qRT-PCR was performed by employing a PrimeScript® RT Reagent Kit and a LightCycler® 96 real-time PCR system (Roche, USA). The specific procedure was elucidated as follows: 95°C for 10 min, followed by 40 cycles at 95°C for 15 s and elongation at 60°C for 1 min. The primers applied here in this study are listed in Table 1. The relative expression levels of the target genes were determined with the 2−△△Ct method. All experiments were performed in triplicate.
Measurement of the Malondialdehyde (MDA) Level
The level of MDA can indicate the severity of free radical attack on cells. In the present study, kidney tissues (60 mg) were ground in cold physiological saline. The cells were lyzed with an ultrasonic breaker (JY92-2D, SCIENTZ, Ningbo, China). Subsequently, the samples were incubated in a water bath at 95°C for 40 min and were then tested by using an MDA reagent kit (Beyotime, Nanjing, China). The absorbance at 532 nm was eventually determined with a microplate scanner (Spectra Max M3, CA, USA).
Determination of the Glutathione (GSH) Content
Reduced GSH is the critical antioxidant-containing substance in cells. It is critical to antioxidants, the protection of sulfhydryl proteins, and the transport of transmembrane amino acids. Accordingly, the measurement of the content of GSH can effectively indicate the redox state of cells. In this study, the GSH content in prepared samples was determined by complying with the instructions of the GSH reagent kit (Beyotime, Nanjing, China). The absorbance at 405 nm was eventually determined with a microplate scanner.
Migration Assay
Wound scratch assay was performed to detect cell migration. HMC cells were stocked in 6-well slabs at 80–90% of confluence. After 24 h, the cells were scratched with a 200-μl pipette tip, and nonadhering cells were cleaned with sterile PBS. Subsequently, the adhering cells were incubated in a serum-free medium for 48 h. Lastly, cells were imaged with a fluorescence microscope (Olympus, Tokyo, Japan), and experimental data were analyzed with ImageJ software.
Cell Proliferation
Cell Counting Kit-8 (CCK-8) assays and colony formation assays were performed to determine the growth of cells. For the CCK-8 assay, cells were inoculated into 96-well plates at a density of 4 × 103 cells per well. Subsequently, 10 μl of CCK-8 reagent (Beyotime, Shanghai, China) was added to the respective wells and then incubated at 37°C for 2 h. Absorbance at 450 nm after 24, 48, and 72 h was determined with a microplate scanner. Specific to the colony formation assay, 750 cells were inoculated in the respective petri dishes and then cultured for 15 days. After being washed three times with PBS, formed colonies were fixed with precooled methanol for 15 min, stained with 0.1% crystal violet for 25 min, and washed again with running water. Formed colonies were photographed under a fluorescence microscope, and the number of colonies was calculated.
Masson Staining
Kidney tissues were first fixed with 4% paraformaldehyde for 24 h. Subsequently, paraffin-embedded kidney tissues were sliced into 4-μm sections, and a Masson trichrome staining was used to visualize collagen. Histomorphological variations were observed under a light microscope. In addition, Masson staining was performed to assess the amount of collagen fiber deposition in the renal stroma.
Immunohistochemistry
Paraffin sections were first dewaxed and then hydrated. Subsequently, the sections were incubated with 3% H2O2 for 30 min at ambient temperature. After washing with phosphate-buffered saline (PBS) three times (10 min each), the sections were sealed with goat serum (Zhongshan Golden Bridge, Beijing, China) at ambient temperature for 1 h prior to incubation with primary antibody (PBS diluted) overnight at 4°C. Then, the membranes were washed with PBS three times (10 min each) and incubated with secondary antibody for 30 min at ambient temperature. DAB was used as a chromogenic reagent to visualize the immune response. Finally, the sections were rehydrated with hematoxylin (Solarbio, Beijing, China) and dehydrated and sealed with resin. No antibody was applied in the negative control group.
Statistical Analysis
The GraphPad Prism was employed for all statistical analysis. Experimental data were expressed as mean ± SD. Student’s t-test was performed to compare the differences between the two groups. P < 0.05 was considered to show statistical significance.
Results
Establishment of HMC Cell Lines with Reduced TRNAU1AP Expression
Transfection efficiency was observed under a fluorescence microscope (Fig. 1a). The mRNA and protein expression levels of TRNAU1AP were detected by qRT-PCR and western blot assay, respectively. As shown in Fig. 1b, c, the protein and mRNA expression levels of TRNAU1AP in transfected cells were remarkably lower than those in the control.
Se-deficient HMC cell model was established by the transfection of lentivirus TRNAU1AP-shRNA. a The green fluorescent part indicates successful transfection. b Western blot and (c) qRT-PCR results showed that TRNAU1AP expression was decreased. The results are expressed as the means ± SD. *P < 0.05, compared with the NC-shRNA groups
Expression of Selenoprotein in Renal Tissues and Cell Lines
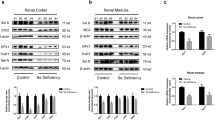
The expression levels of SELENOP, GPX1, and TrxR1 in renal tissues and cells were detected at the protein and mRNA levels. Compared with those in control tissues and cells, the protein and mRNA expression levels of SELENOP, GPX1, and TrxR1 in Se-deficient tissues and cells significantly decreased (Fig. 2), which showed that the Se-deficient mouse model and cell line model were successfully established.
Se deficiency reduced the expression levels of selenoprotein in renal tissues and cells. a Western blot (n = 6) and b qRT-PCR (n = 4) results in Se-deficient renal tissues showed that the expressions of GPX1, TrxR1, and SELENOP were decreased. c Western blot and d qRT-PCR results in Se-deficient cells showed that the expressions of GPX1, TrxR1, and SELENOP were decreased. The results are expressed as the means ± SD. *P < 0.05, **P < 0.01, compared with the NC-shRNA/control group
Se Deficiency Reduced Antioxidant Activity
Compared with those in control tissues and cells, Se deficiency increased the MDA levels in both kidney tissues and HMC cells (Fig. 3a). However, the content of GSH was decreased in both kidney tissues and cells (Fig. 3b).
Se deficiency increased the levels of MDA in renal tissues and cells, while the levels of GSH were decreased. The levels of a MDA were increased, and the levels of b GSH were decreased in Se-deficient renal tissues (n = 10) and cells. The results are presented as the mean ± SD. *P < 0.05, **P < 0.01, compared with the control group
Se Deficiency Inhibits Cell Migration and Proliferation
As shown in Fig. 4a, the migration rate of cells transfected with TRNAU1AP lentivirus significantly decreased after 48 h, which proved that Se deficiency reduced the migration rate of cells. The CCK-8 results showed that Se-deficient cells had a significantly lower proliferation ability than control cells at 24, 48, and 72 h (Fig. 4b). In addition, a reduced proliferation ability of Se-deficient cells was observed in the colony formation assay (Fig. 4c).
Se deficiency inhibited cell migration and proliferation. The migration ability was detected by a wound scratch assay, and the proliferation ability was detected by b CCK-8 and c clone formation assays. The results are expressed as the means ± SD. *P < 0.05, **P < 0.01, compared with the NC-shRNA group
Se Deficiency Causes Renal Fibrosis
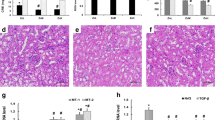
Masson staining showed blue collagen deposition in the Se-deficient group, whereas no obvious deposition was observed in the control group, which indicates fibrosis caused by Se deficiency (Fig. 5a, b). In addition, immunohistochemical staining indicated that fibronectin was highly expressed in kidney tissues of the Se-deficient group (Fig. 5c, d). Moreover, the protein and mRNA expression levels of collagen I and collagen III in kidney tissues and HMC cells were both significantly increased (Fig. 6a–d).
The effect of Se deficiency on mice kidney histology. a Representative Masson trichrome stain in mice fed with a Se-deficient diet. Bar = 100 μm. b Quantitative of Masson trichrome staining results. c Representative fibronectin immunohistochemical staining and d quantitative analysis. Bar = 50 μm. The results are presented as the mean ± SD value of three independent experiments. **P < 0.01, compared with the control group
Se deficiency increased the expressions of collagen I and collagen III in renal tissues and cells. a Western blot (n = 6) and b qRT-PCR (n = 4) results in Se-deficient renal tissues showed that the expressions of collagen I and collagen III were increased. c Western blot and d qRT-PCR results in Se-deficient cells showed that the expressions of collagen I and collagen III were increased. The results are expressed as the means ± SD. *P < 0.05, **P < 0.01, compared with the control groups
Se Deficiency leads to Changes in the Expression of MMPs and E-Cadherin
Compared with those in control tissues, the protein levels of MMP2 and E-cadherin were significantly decreased in tissues, while that of MMP9 was obviously increased (Fig. 7a), and the mRNA expression levels of these molecules were consistent with the protein levels (Fig. 7b). In HMC cells, the mRNA and protein expression levels of MMP2 and MMP9 were both significantly increased, whereas E-cadherin expression levels were significantly decreased (Fig. 7c, d).
Effects of Se deficiency on MMP2, MMP9, and E-cadherin in renal tissues and cells. a Western blot (n = 6) and b qRT-PCR (n = 4) results in Se-deficient renal tissues showed that the expression of MMP9 was increased and the expressions of MMP2 and E-cadherin were decreased. c Western blot and d qRT-PCR results in Se-deficient cells showed that the expressions of MMP2 and MMP9 were increased and the expressions of E-cadherin were decreased. The results are presented as the means ± SD. *P < 0.05, ****P < 0.0001, compared with the control/NC-shRNA groups
The Effect of Se Deficiency on Wnt/β-Catenin Pathway Components
The protein expression levels of Wnt5a, Dvl-1, and β-catenin were increased in both Se-deficient tissues and cells (Fig. 8a, c). In addition, significantly increased mRNA expression levels of downstream marker genes in the Wnt/β-catenin signaling pathway were observed (Fig. 8b, d).
Changes of Wnt/β-catenin signaling pathway components in Se-deficient models. a Western blot (n = 6) and b qRT-PCR (n = 4) results showed that the expressions of Wnt5a, Dvl-1, and β-catenin were increased in Se-deficient renal tissues. c Western blot results showed that the expression levels of Wnt5a, Dvl-1, and β-catenin were increased in Se-deficient cells. d qRT-PCR results showed that the expression levels of Dvl-1 and β-catenin were increased in Se-deficient cells. The results are expressed as the means ± SD. *P < 0.05, **P < 0.01, compared with the control groups
Discussion
In the present study, a Se-deficient mouse model and TRNAU1AP gene knockdown cell model were successfully established. The results showed that Se deficiency was capable of downregulating the expression of selenoproteins in tissues and cells, inhibiting cell migration and proliferation, and elevating the oxidative stress level. In addition, we demonstrated that Se deficiency led to the accumulation of ECM and changes in MMPs expression, thereby promoting the occurrence of fibrosis. Furthermore, upregulation of signaling molecules in the Wnt/β-catenin pathway was observed in the Se deficiency model.
Se plays an important biological role in the body mainly through selenoproteins [27]. At least 25 kinds of selenoproteins that are beneficial to the human body have been identified to date [1]. Selenoprotein has been confirmed to play an important role in kidney disease [28]. GPX1 is a main member of the GPX family that has antioxidant functions [29]. Previous reports indicated that GPX1 can remove peroxides and peroxynitrite that can cause renal damage [30]. SELENOP is the main component of the serum Se content, which is synthesized and then transported to the kidney [31, 32]. The SELENOP content is negatively correlated with renal function in patients with chronic kidney disease who do not require hemodialysis [33]. TrxR1 exists in the cytoplasm and is ubiquitous in humans [34]. It has been shown that TrxR can reduce the excessive production of ROS to protect cells from oxidative stress and maintain the ascorbic acid cycle [35]. In addition, TrxR can reduce renal ischemia-reperfusion injury [36]. Recent studies have reported that TrxR activity was decreased in the liver and kidney of mice fed low Se [37]. As expected, the expression levels of GPX1, SELENOP, and TrxR1 were significantly decreased in our Se-deficient cell model and Se-deficient mouse model.
The kidney is an important oxygen-consuming organ. Chronic renal hypoxia can induce oxidative stress, which may lead to renal damage and even renal fibrosis, such as tubulointerstitial fibrosis and glomerulosclerosis, through a variety of mechanisms [38,39,40]. Previous studies indicated that Se can inhibit the increased oxidative stress induced by cyclophosphamide and protect the kidney from damage [41]. Xu et al. [42] found that Se deficiency induced the accumulation of oxygen free radicals in and weakened the antioxidant capacity of mouse macrophages. In addition, it was reported that Se supplementation can prevent and treat aluminum-induced glomerulonephritis [43]. In this study, a decreased level of GSH was observed in the Se-deficient group, while the level of MDA was increased, which suggested that Se deficiency can elevate the level of oxidative stress and reduce the antioxidant capacity in the kidney.
The glomeruli and tubules are necessary to maintain the basic structure of the kidney. Glomerular mesangial cells are astrocytes that have contractile abilities and can synthesize cytokines, and phenotypic transformation will take place during injury [44]. Glomerular mesangial cell damage is characterized by apoptosis, dissolution and migration, and excessive production of matrix and reactive oxygen species, which can cause fibrosis and even glomerulosclerosis [45]. Accordingly, HMC cells were selected and then subjected to knockdown to establish a cell line with Se deficiency. The results showed that under the influence of Se deficiency, the migration rate of HMC cells was reduced, and the proliferation decreased. It has been indicated that the number of glomerular capillary rings is decreased with a decrease in the number of glomerular mesangial cells and that failure of mesangial cells to migrate to the developing glomerulus causes congenital diffuse mesangial sclerosis [45].
Renal fibrosis is a pathophysiological change and is a gradual process of renal function decline from health to injury to loss of function. The occurrence of fibrosis involves various factors, including inflammation, oxidative stress, the role and signal cascade of various cytokines, apoptosis, proliferation and activation of fibroblasts, and the transformation of epithelial cells to fibroblasts [46]. Selenium deficiency can cause kidney damage, and whether kidney injury could lead to fibrosis is an interesting issue worth further investigation. In the present study, Masson staining results showed that collagen deposition was distributed around the renal tubules of Se-deficient mice. It was reported that excessive deposition of ECM components, such as collagen I, collagen III, and fibronectin, can cause renal fibrosis and destroy the normal structure of the kidney, further leading to the loss of renal function [47, 48]. Wright et al. [49] found that the increase in ECM and the contents of MMP2 and MMP9 in lupus nephritis can promote renal fibrosis. In our experimental results, the expression levels of collagen I and collagen III were noticeably increased. Similarly, the immunohistochemical results showed that fibronectin was highly expressed in Se-deficient mice. These results confirmed that Se deficiency could lead to an increase in ECM, thereby promoting the occurrence of fibrosis. In addition, we found that the levels of MMPs varied. MMPs are a family of zinc-containing endopeptidases that were initially considered to protect the kidney by antagonizing the accumulation of ECM [50]. With the deepening of research, it was found that the role of MMPs is not limited to matrix digestion but is related to many kidney diseases [51]. For example, MMP2 and MMP9 can degrade type IV collagen of the glomerular basement membrane (GBM) [52]. Previous studies showed that the contents of MMP-9 and MMP-2 were increased with extended ligation times in the tissue homogenate of unilateral ureteral obstruction (UUO) mice [53]. Han et al. [54] demonstrated that MMP1 and MMP3 were upregulated in the renal tissue of rats fed with low Se. Our findings suggested that the protein and mRNA expression of MMP9 significantly increased both in vitro and in vivo; however, MMP2 was significantly upregulated in vitro and downregulated in vivo. The difference in MMP2 expression in vivo and in vitro may be attributed to the biological heterogeneity between animal models and cell models. EMT plays an important role in the development of fibrotic diseases, and the downregulation of E-cadherin, a glycoprotein mediating cell adhesion, is a hallmark of EMT [55]. Our results showed that Se deficiency contributed to the decrease in E-cadherin expression. In summary, Se deficiency may lead to renal fibrosis through the abnormal accumulation of ECM, the aberrant secretion of MMPs, and changes in EMT processes.
As a complex and critical developmental pathway, the evolutionarily conserved Wnt/β-catenin signaling pathway plays an important role in maintaining tissue homeostasis and organ repair after injury [56]. The Wnt/β-catenin signaling pathway is inactive in normal kidneys [57]. However, Wnt signaling is activated in the UUO rat model [58]. In addition, studies have suggested that the continuous activation of Wnt/β-catenin signaling plays a crucial role in driving the progression of AKI to CKD [59]. To explore the changes in the Wnt/β-catenin signaling pathway after selenium deficiency, we selected three key signaling molecules: Wnt5a, Dvl-1, and β-catenin. Wnt5a is a member of the Wnt family and is a secretory protein [60]. Previous studies have shown that Wnt5a is highly expressed in various fibrotic diseases [61, 62], and Wnt5a binds to FZD4 and then activates Dvl-1, which can cause free β-catenin to increase as well [63, 64]. Our study showed that in the Se-deficient model, Se deficiency was accompanied by high expression of Wnt5a and that the levels of free β-catenin and Dvl-1 were also increased. Thus, we hypothesized that the Wnt/β-catenin signaling pathway may be involved in the renal fibrosis induced by Se deficiency, although the underlying mechanisms require further study.
Conclusion
In conclusion, our results suggest that Se deficiency aggravates oxidative stress, ECM deposition, and abnormal MMPs secretion in tissues and cells, which causes renal fibrosis. In addition, Wnt/β-catenin may be involved in the process of renal fibrosis caused by Se deficiency. Further studies are warranted to enhance our understanding of Se deficiency in renal fibrosis.
References
Schwarz K, Foltz CM (1999) Selenium as an integral part of factor 3 against dietary necrotic liver degeneration. Nutrition 15:255
Lu J, Holmgren A (2009) Selenoproteins. J Biol Chem 284:723–727
Zachara BA (2015) Selenium and selenium-dependent antioxidants in chronic kidney disease. Adv Clin Chem 68:131–151
Kilinc M, Guven MA, Ezer M, Ertas IE, Coskun A (2008) Evaluation of serum selenium levels in Turkish women with gestational diabetes mellitus, glucose intolerants, and normal controls. Biol Trace Elem Res 123:35–40
Zhou H, Wang T, Li Q, Li D (2018) Prevention of Keshan disease by selenium supplementation: a systematic review and meta-analysis. Biol Trace Elem Res 186:98–105
Xu XM, Carlson BA, Zhang Y, Mix H, Kryukov GV, Glass RS, Berry MJ, Gladyshev VN, Hatfield DL (2007) New developments in selenium biochemistry: selenocysteine biosynthesis in eukaryotes and archaea. Biol Trace Elem Res 119:234–241
Seale LA, Gilman CL, Moorman BP, Berry MJ, Grau EG, Seale AP (2014) Effects of acclimation salinity on the expression of selenoproteins in the tilapia, Oreochromis mossambicus. J Trace Elem Med Biol 28:284–292
Cassago A, Rodrigues EM, Prieto EL, Gaston KW, Alfonzo JD, Iribar MP, Berry MJ, Cruz AK, Thiemann OH (2006) Identification of Leishmania selenoproteins and SECIS element. Mol Biochem Parasitol 149:128–134
Mahdi Y, Xu XM, Carlson BA, Fradejas N, Günter P, Braun D, Southon E, Tessarollo L, Hatfield DL, Schweizer U (2015) Expression of selenoproteins is maintained in mice carrying mutations in SECp43, the tRNA selenocysteine 1 associated protein (Trnau1ap). PLoS One 10:e0127349
Xu XM, Mix H, Carlson BA, Grabowski PJ, Gladyshev VN, Berry MJ, Hatfield DL (2005) Evidence for direct roles of two additional factors, SECp43 and soluble liver antigen, in the selenoprotein synthesis machinery. J Biol Chem 280:41568–41575
Small-Howard A, Morozova N, Stoytcheva Z, Forry EP, Mansell JB, Harney JW, Carlson BA, Xu XM, Hatfield DL, Berry MJ (2006) Supramolecular complexes mediate selenocysteine incorporation in vivo. Mol Cell Biol 26:2337–2346
Chen LL, Huang JQ, Xiao Y, Wu YY, Ren FZ, Lei XG (2020) Knockout of selenoprotein V affects regulation of selenoprotein expression by dietary selenium and fat intakes in mice. J Nutr 150:483–491
Hu X, Luo J, Lai H, Li M, Zheng X, Nie T, Li F, Li H (2018) Knockdown of TRNAU1AP inhibits the proliferation and migration of NIH3T3, JEG-3 and Bewo cells via the PI3K/Akt signaling pathway. Biochem Biophys Res Commun 503:521–527
Lai H, Nie T, Zhang Y, Chen Y, Tao J, Lin T, Ge T, Li F, Li H (2021) Selenium deficiency-induced damage and altered expression of mitochondrial biogenesis markers in the kidneys of mice. Biol Trace Elem Res 199:185–196
Thomson CD, Packer MA, Butler JA, Duffield AJ, O'Donaghue KL, Whanger PD (2001) Urinary selenium and iodine during pregnancy and lactation. J Trace Elem Med Biol 14:210–217
Zachara BA, Salak A, Koterska D, Manitius J, Wasowicz W (2004) Selenium and glutathione peroxidases in blood of patients with different stages of chronic renal failure. J Trace Elem Med Biol 17:291–299
Roman M, Jitaru P, Barbante C (2014) Selenium biochemistry and its role for human health. Metallomics 6:25–54
Guo CH, Wang CL, Chen PC, Yang TC (2011) Linkage of some trace elements, peripheral blood lymphocytes, inflammation, and oxidative stress in patients undergoing either hemodialysis or peritoneal dialysis. Perit Dial Int 31:583–591
Chen J, He W, Zhu X, Yang S, Yu T, Ma W (2020) Epidemiological study of kidney health in an area with high levels of soil cadmium and selenium: does selenium protect against cadmium-induced kidney injury? Sci Total Environ 698:134106
Yang L (2019) How acute kidney injury contributes to renal fibrosis. Adv Exp Med Biol 1165:117–142
Shrestha A, Che RC, Zhang AH (2019) Role of aldosterone in renal fibrosis. Adv Exp Med Biol 1165:325–346
Cui N, Hu M, Khalil RA (2017) Biochemical and biological attributes of matrix metalloproteinases. Prog Mol Biol Transl Sci 147:1–73
Zheng G, Lyons JG, Tan TK, Wang Y, Hsu TT, Min D, Succar L, Rangan GK, Hu M, Henderson BR, Alexander SI, Harris DCH (2009) Disruption of E-cadherin by matrix metalloproteinase directly mediates epithelial-mesenchymal transition downstream of transforming growth factor-beta1 in renal tubular epithelial cells. Am J Pathol 175:580–591
Zuo Y, Liu Y (2018) New insights into the role and mechanism of Wnt/beta-catenin signalling in kidney fibrosis. Nephrology (Carlton) 23:38–43
Schunk SJ, Floege J, Fliser D, Speer T (2021) WNT-β-catenin signalling — a versatile player in kidney injury and repair. Nat Rev Nephrol 17:172–184
Reeves PG (1997) Components of the AIN-93 diets as improvements in the AIN-76A diet. J Nutr 127:838S–841S
Moghadaszadeh B, Beggs AH (2006) Selenoproteins and their impact on human health through diverse physiological pathways. Physiology (Bethesda) 21:307–315
Iglesias P, Selgas R, Romero S, Díez JJ (2013) Selenium and kidney disease. J Nephrol 26:266–272
Brigelius-Flohé R, Maiorino M (2013) Glutathione peroxidases. Biochim Biophys Acta 1830:3289–3303
de Haan JB, Stefanovic N, Nikolic-Paterson D, Scurr LL, Croft KD, Mori TA, Hertzog P, Kola I, Atkins RC, Tesch GH (2005) Kidney expression of glutathione peroxidase-1 is not protective against streptozotocin-induced diabetic nephropathy. Am J Physiol Ren Physiol 289:F544–F551
Hill KE, Zhou J, McMahan WJ et al (2003) Deletion of selenoprotein P alters distribution of selenium in the mouse. J Biol Chem 278:13640–13646
Schweizer U, Streckfuss F, Pelt P et al (2005) Hepatically derived selenoprotein P is a key factor for kidney but not for brain selenium supply. Biochem J 386:221–226
Reinhardt W, Dolff S, Benson S, Broecker-Preuß M, Behrendt S, Hög A, Führer D, Schomburg L, Köhrle J (2015) Chronic kidney disease distinctly affects relationship between selenoprotein P status and serum thyroid hormone parameters. Thyroid 25:1091–1096
Yuan D, Zhan XA, Wang YX (2012) Effect of selenium sources on the expression of cellular glutathione peroxidase and cytoplasmic thioredoxin reductase in the liver and kidney of broiler breeders and their offspring. Poult Sci 91:936–942
Mustacich D, Powis G (2000) Thioredoxin reductase. Biochem J 346(Pt 1):1–8
Godoy JR, Oesteritz S, Hanschmann EM, Ockenga W, Ackermann W, Lillig CH (2011) Segment-specific overexpression of redoxins after renal ischemia and reperfusion: protective roles of glutaredoxin 2, peroxiredoxin 3, and peroxiredoxin 6. Free Radic Biol Med 51:552–561
Hill KE, McCollum GW, Boeglin ME, Burk RF (1997) Thioredoxin reductase activity is decreased by selenium deficiency. Biochem Biophys Res Commun 234:293–295
Jha JC, Gray SP, Barit D, Okabe J, el-Osta A, Namikoshi T, Thallas-Bonke V, Wingler K, Szyndralewiez C, Heitz F, Touyz RM, Cooper ME, Schmidt HHHW, Jandeleit-Dahm KA (2014) Genetic targeting or pharmacologic inhibition of NADPH oxidase nox4 provides renoprotection in long-term diabetic nephropathy. J Am Soc Nephrol 25:1237–1254
Cho S, Yu SL, Kang J, Jeong BY, Lee HY, Park CG, Yu YB, Jin DC, Hwang WM, Yun SR, Song HS, Park MH, Yoon SH (2019) NADPH oxidase 4 mediates TGF-beta1/Smad signaling pathway induced acute kidney injury in hypoxia. PLoS One 14:e0219483
Su H, Wan C, Song A et al (2019) Oxidative stress and renal fibrosis: mechanisms and therapies. Adv Exp Med Biol 1165:585–604
Gunes S, Sahinturk V, Uslu S, Ayhanci A, Kacar S, Uyar R (2018) Protective effects of selenium on cyclophosphamide-induced oxidative stress and kidney injury. Biol Trace Elem Res 185:116–123
Xu J, Gong Y, Sun Y, Cai J, Liu Q, Bao J, Yang J, Zhang Z (2020) Impact of selenium deficiency on inflammation, oxidative stress, and phagocytosis in mouse macrophages. Biol Trace Elem Res 194:237–243
Cao C, Luo J, Li X, Zhang M, Zhang H, Zhang J, Wang K (2018) Selenium-rich yeast protects against aluminum-induced renal inflammation and ionic disturbances. Biol Trace Elem Res 186:467–473
Migliorini A, Ebid R, Scherbaum CR, Anders HJ (2013) The danger control concept in kidney disease: mesangial cells. J Nephrol 26:437–449
Schlöndorff D, Banas B (2009) The mesangial cell revisited: no cell is an island. J Am Soc Nephrol 20:1179–1187
Humphreys BD (2018) Mechanisms of renal fibrosis. Annu Rev Physiol 80:309–326
Hijmans RS, Rasmussen DG, Yazdani S et al (2017) Urinary collagen degradation products as early markers of progressive renal fibrosis. J Transl Med 15:63
Bülow RD, Boor P (2019) Extracellular matrix in kidney fibrosis: more than just a scaffold. J Histochem Cytochem 67:643–661
Wright RD, Dimou P, Northey SJ, Beresford MW (2019) Mesangial cells are key contributors to the fibrotic damage seen in the lupus nephritis glomerulus. J Inflamm (Lond) 16:22
Catania JM, Chen G, Parrish AR (2007) Role of matrix metalloproteinases in renal pathophysiologies. Am J Physiol Ren Physiol 292:905–911
Tan RJ, Liu Y (2012) Matrix metalloproteinases in kidney homeostasis and diseases. Am J Physiol Ren Physiol 302:1351–1361
Phillips TM, Fadia M, Lea-Henry TN, Smiles J, Walters GD, Jiang SH (2017) MMP2 and MMP9 associate with crescentic glomerulonephritis. Clin Kidney J 10:215–220
Yu XA, Hu Y, Zhang Y, Zhang R, Bai X, Gu L, Gao H, Li R, Tian J, Yu BY (2020) Integrating the polydopamine nanosphere/aptamers nanoplatform with a DNase-I-assisted recycling amplification strategy for simultaneous detection of MMP-9 and MMP-2 during renal interstitial fibrosis. ACS Sens 5:1119–1125
Han J, Liang H, Yi J, Tan W, He S, Wu X, Shi X, Ma J, Guo X (2016) Selenium deficiency induced damages and altered expressions of metalloproteinases and their inhibitors (MMP1/3, TIMP1/3) in the kidneys of growing rats. J Trace Elem Med Biol 34:1–9
Aban CE, Lombardi A, Neiman G, Biani MC, la Greca A, Waisman A, Moro LN, Sevlever G, Miriuka S, Luzzani C (2021) Downregulation of E-cadherin in pluripotent stem cells triggers partial EMT. Sci Rep 11:2048
Tan RJ, Zhou D, Zhou L, Liu Y (2014) Wnt/β-catenin signaling and kidney fibrosis. Kidney Int Suppl (2011) 4:84–90
He X (2008) Cilia put a brake on Wnt signalling. Nat Cell Biol 10:11–13
Zhang ZH, He JQ, Zhao YY, Chen HC, Tan NH (2020) Asiatic acid prevents renal fibrosis in UUO rats via promoting the production of 15d-PGJ2, an endogenous ligand of PPAR-γ. Acta Pharmacol Sin 41:373–382
Xiao L, Zhou D, Tan RJ, Fu H, Zhou L, Hou FF, Liu Y (2016) Sustained activation of Wnt/β-catenin signaling drives AKI to CKD progression. J Am Soc Nephrol 27:1727–1740
Wang Y, Zhou CJ, Liu Y (2018) Wnt signaling in kidney development and disease. Prog Mol Biol Transl Sci 153:181–207
Abraityte A, Vinge LE, Askevold ET, Lekva T, Michelsen AE, Ranheim T, Alfsnes K, Fiane A, Aakhus S, Lunde IG, Dahl CP, Aukrust P, Christensen G, Gullestad L, Yndestad A, Ueland T (2017) Wnt5a is elevated in heart failure and affects cardiac fibroblast function. J Mol Med (Berl) 95:767–777
Rashid ST, Humphries JD, Byron A, Dhar A, Askari JA, Selley JN, Knight D, Goldin RD, Thursz M, Humphries MJ (2012) Proteomic analysis of extracellular matrix from the hepatic stellate cell line LX-2 identifies CYR61 and Wnt-5a as novel constituents of fibrotic liver. J Proteome Res 11:4052–4064
Chien AJ, Conrad WH, Moon RT (2009) A Wnt survival guide: from flies to human disease. J Invest Dermatol 129:1614–1627
Igota S, Tosa M, Murakami M, Egawa S, Shimizu H, Hyakusoku H, Ghazizadeh M (2013) Identification and characterization of Wnt signaling pathway in keloid pathogenesis. Int J Med Sci 10:344–354
Acknowledgements
We sincerely thank the writing assistance provided by Dr. Chuang Nie (Department of Epidemiology, College of Public Health, Harbin Medical University) and additional editorial support from American Journal Experts.
Availability of data and materials
The data during the current study are available from the corresponding author on reasonable request.
Code availability
Not applicable.
Funding
This work was supported financially by the Natural Science Foundation of China (No. 81472929).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical Approval
All procedures involving animals were in compliance with the Animal Ethics Committee of Harbin Medical University.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Lin, T., Tao, J., Chen, Y. et al. Selenium Deficiency Leads to Changes in Renal Fibrosis Marker Proteins and Wnt/β-Catenin Signaling Pathway Components. Biol Trace Elem Res 200, 1127–1139 (2022). https://doi.org/10.1007/s12011-021-02730-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-021-02730-1