Abstract
Wnt signaling is dysregulated in heart failure (HF) and may promote cardiac hypertrophy, fibrosis, and inflammation. Blocking the Wnt ligand Wnt5a prevents HF in animal models. However, the role of Wnt5a in human HF and its functions in cardiac cells remain unclear. Here, we investigated Wnt5a regulation in HF patients and its effects on primary mouse and human cardiac fibroblasts. Serum Wnt5a was elevated in HF patients and associated with hemodynamic, neurohormonal, and clinical measures of disease severity. In failing human hearts, Wnt5a protein correlated with interleukin (IL)-6 and tissue inhibitor of metalloproteinase (TIMP)-1. Wnt5a messenger RNA (mRNA) levels were markedly upregulated in failing myocardium and both mRNA and protein levels declined following left ventricular assist device therapy. In primary mouse and human cardiac fibroblasts, recombinant Wnt5a dose-dependently upregulated mRNA and protein release of IL-6 and TIMP-1. Wnt5a did not affect β-catenin levels, but activated extracellular signal-regulated kinase 1/2 (ERK1/2) signaling. Importantly, inhibition of ERK1/2 activation attenuated Wnt5a-induced release of IL-6 and TIMP-1. In conclusion, our results show that Wnt5a is elevated in the serum and myocardium of HF patients and is associated with measures of progressive HF. Wnt5a induces IL-6 and TIMP-1 in cardiac fibroblasts, which might promote myocardial inflammation and fibrosis, and thereby contribute to HF progression.
Key messages
• Wnt5a is elevated in serum and myocardium of HF patients and is associated with measures of progressive HF.
• In cardiac fibroblasts, Wnt5a upregulates interleukin (IL)-6 and tissue inhibitor of metalloproteinase (TIMP)-1 through the ERK pathway.
• Wnt5a-mediated effects might promote myocardial inflammation and fibrosis, and thereby contribute to HF progression.
Similar content being viewed by others

Avoid common mistakes on your manuscript.
Introduction
Heart failure (HF) is a major cause of morbidity and mortality worldwide with increasing incidence due to aging population [1]. Pathological stimuli induce a series of alterations in the heart, referred to as myocardial remodeling, where mechanically stressed cardiac cells secrete humoral factors that alter myocardial structure and function, promote cardiac hypertrophy, fibrosis, and inflammation, leading to development and progression of HF [2]. Initially, a useful compensatory response, myocardial remodeling, is harmful in the long run.
The Wnt pathway is involved in myocardial remodeling [3–5] and consists of multiple ligands and antagonists which transduce signals through a canonical, β-catenin-dependent pathway and several non-canonical ones, including the planar cell polarity, Wnt/Ca2+ [4, 6], and others, less studied, such as the extracellular-regulated kinase 1/2 (ERK1/2) cascade [7]. Outcomes of Wnt signaling depend on receptor context, cell, and tissue type [6, 8, 9]. The non-canonical ligand Wnt5a has emerged as an important player in cardiac disease, inflammatory disorders, and cancer [9, 10]. Wnt5a may induce cardiomyocyte hypertrophy while its inhibition attenuates hypertrophy [11] and HF development [12, 13]. Furthermore, Wnt5a promotes inflammation and fibrosis in multiple tissues including the lung [14, 15]. Recently, Wnt5a was implicated in fibrosis in the neonatal heart after cryoinjury [16].
The role of Wnt5a in human HF is still largely unknown. Cardiac fibroblasts (CFs) play a major role in myocardial inflammation and ECM remodeling, but the effects of Wnt5a on these cells have not been clarified. To further elucidate the role of Wnt5a in HF, we evaluated (i) the expression of Wnt5a in serum and myocardium of HF patients and associations with measures of HF severity and adverse outcome, (ii) Wnt5a-mediated effects on expression of fibrotic and inflammatory markers as well as activation of β-catenin and ERK signaling in adult mouse CFs, and (iii) confirmed the main findings in primary human CFs.
Materials and methods
Patients and study procedures
The study population consisted of 155 consecutively recruited patients with stable HF (>6 months) and reduced left ventricular (LV) ejection fraction (LVEF) of the New York Heart Association (NYHA) functional classes II–IV. The underlying cause of HF (Table 1) was based on medical history, coronary angiography, and echocardiographic examination. Patients with acute coronary syndromes within the last 6 months or with significant concomitant diseases (e.g., infection, malignancy, or autoimmune disorders) were excluded. Twenty-five age- and sex-matched healthy individuals (19 males, mean ± SD age 58.2 ± 7.8 years) served as controls (serum cohort). Echocardiography and right-sided heart catheterization was performed as described [17].
Blood sampling and biochemical analyses
Details on analysis of Wnt5a, interleukin-6 (IL-6), tissue inhibitor of metalloproteinase 1 (TIMP-1), tumor necrosis factor (TNF), chemokine (C-X-C motif) ligand 2 (CXCL2), secreted frizzled-related protein 3 (sFRP3), C-reactive protein (CRP), and N-terminal pro-brain natriuretic peptide (NT-proBNP) are given in the Supplementary Material.
Tissue sampling from human myocardium
LV myocardial biopsies were collected from (i) still-beating hearts immediately on explantation from patients with end-stage HF undergoing heart transplantation (Table 1), (ii) patients with structurally normal hearts (controls), and (iii) 18 patients with advanced HF at the time of implantation and at the time of removal (heart transplantation) of a continuous-flow LV assist device (LVAD) as detailed in the Supplementary Material.
Immunohistochemistry
Immunohistochemical staining of Wnt5a protein expression in failing human hearts obtained from HF patients after explantation was performed as detailed in the Supplementary Material.
Cardiac cell isolation and culture
Mouse cardiac cell separation, CF isolation, and culturing was performed as previously described [18, 19] and detailed in the Supplementary Material. Primary human CFs isolated from the ventricles of an adult heart were purchased from PromoCell (Heidelberg, Germany), grown and cultured as described in the Supplementary Material.
RNA extraction and reverse-transcription qPCR
Total RNA was extracted from human myocardial tissue and CF cell pellet and qPCR performed as described in the Supplementary Material.
Immunoblot analysis
Total protein was isolated from CF pellet and immunoblot analysis was performed on protein homogenates as described in the Supplementary Material.
Statistics
Statistical differences between groups were tested using Student’s t test or the Mann–Whitney U test depending on distribution, and χ 2 test for categorical variables. One-way and two-way ANOVA or Kruskal–Wallis test was used to compare greater than two groups. Association between Wnt5a tertiles and all-cause mortality or heart transplantation was evaluated by Kaplan–Meier analysis with log-rank test. Associations between Wnt5a levels and clinical parameters as well as gene expression in human myocardium were assessed with the Spearman correlation. Two-sided probability values were considered significant at p < 0.05.
Results
Serum Wnt5a is elevated and associated with disease severity in HF patients
HF patients (Table 1) had elevated Wnt5a levels compared with age- and sex-matched controls (Fig. 1a) with higher levels according to clinical disease severity (NYHA, Fig. 1b), but no relation to etiology (p = 0.64). Among hemodynamic measures (Table 2), Wnt5a positively correlated with E/A ratio as a marker of diastolic dysfunction (Fig. 1c), pulmonary artery systolic pressure (PASP), pulmonary artery resistance, and negatively correlated with cardiac output, but not LVEF (Table 2). Patients with PASP >40 mmHg, indicating pulmonary hypertension, had increased Wnt5a levels (Fig. 1d). Wnt5a correlated modestly with CRP and NT-proBNP, reflecting systemic inflammation and myocardial wall stress, respectively (Table 2).
Serum Wnt5a is elevated and associated with disease severity in heart failure patients. a Serum Wnt5a levels in HF patients (n = 155) compared to healthy controls (n = 25). b Association between Wnt5a levels and New York Heart Association (NYHA) class. c Association between serum Wnt5a levels and E/A ratio. d Serum Wnt5a in patients with pulmonary hypertension (PASP ≥40 mmHg) and without it (PASP <40 mmHg). e Kaplan–Meier curves showing adverse outcome according to Wnt5a tertiles and f tertiles of Wnt5a/sFRP3 ratio. Data are presented as median and 25th and 75th percentile. **p < 0.01, ***p < 0.001, †p < 0.05 vs controls. HF heart failure, PASP pulmonary artery systolic pressure, sFRP3 secreted frizzled-related protein 3
The ratio between Wnt5a and its antagonist sFRP3 is associated with transplant-free survival in HF patients
During a median follow-up of 5.6 (range 0.1–7.8) years, 39 (26%) patients died and an additional 14 (9%) patients underwent heart transplantation (composite outcome). The Kaplan–Meier analysis for the composite of all-cause mortality or heart transplantation based on Wnt5a tertiles was not significant, but suggested a non-linear association with outcome (Fig. 1e). Tertile 3 had a significantly higher outcome rate vs. tertile 1 and 2 combined (p = 0.025).
We have previously analyzed circulating sFRP3, an antagonist of Wnt5a, in this patient cohort [20] and using the Wnt5a/sFRP3 ratio as a surrogate for Wnt5a activity revealed a worse outcome for patients in the highest tertile (Fig. 1f). By Cox regression analysis, the hazard ratio and 95% confidence intervals, associated with a 1-SD increase in Wnt5a/sFRP3 ratio, was 2.52 (1.44–4.42), which remained significant when adjusting for age, gender, eGFR, NYHA, and NT-proBNP: 1.93 (1.07–3.51) p = 0.030.
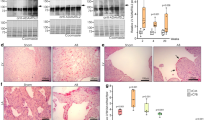
Wnt5a is increased in failing human hearts
Immunohistochemical staining demonstrated abundant Wnt5a protein expression in failing human hearts (Fig. 2a) and LV Wnt5a messenger RNA (mRNA) levels were elevated in end-stage HF compared to non-failing hearts (Fig. 2b), with no difference according to etiology (p = 0.78). Moreover, Wnt5a mRNA and protein expression decreased following LVAD treatment (Fig. 2c), suggesting that Wnt5a may be involved in myocardial remodeling. In addition, BNP mRNA levels, an established marker of wall-stress, also declined after LVAD therapy (Fig. 2d). Furthermore, when dichotomizing the change in Wnt5a mRNA during LVAD, patients with a large decrease in LV Wnt5a expression had a more pronounced decrease in BNP mRNA levels (Fig. 2e). Wnt5a LV mRNA and protein correlated negatively with LVEF and positively with NT-proBNP (Table 2). Wnt5a mRNA was positively correlated with PASP and pulmonary artery resistance (Table 2). Patients with pulmonary hypertension (PASP >40 mmHg) had increased myocardial Wnt5a mRNA levels with a similar trend for Wnt5a protein (Fig. 2f). Furthermore, LV Wnt5a protein levels correlated with IL-6 and TIMP-1 (Fig. 2g). In contrast, the canonical Wnt ligands Wnt3a and Wnt8a were barely detectable in myocardial tissue from HF patients (data not shown).
Increased Wnt5a expression in failing human myocardium. mRNA is expressed relative to GAPDH and protein is expressed ng Wnt5a/mg total protein. a Representative immunohistochemical staining of Wnt5a in failing human hearts (n = 6). b Relative mRNA expression of Wnt5a in myocardium of patients with end-stage HF (n = 82) compared to non-failing myocardium (n = 12). c Wnt5a mRNA and protein levels and d BNP mRNA levels in myocardium of HF patients before (pre) and after (post) LVAD therapy (n = 18). e The change in Wnt5a mRNA levels in relation to the change in BNP levels in patients on LVAD therapy. f Myocardial Wnt5a mRNA and protein levels in patients with pulmonary hypertension (PASP ≥40 mmHg) and without it (PASP <40 mmHg). g Correlations between myocardial Wnt5a and IL-6 and TIMP-1 levels. Ab antibody, BNP brain natriuretic peptide, HF heart failure, IL-6 interleukin-6, LV left ventricular, LVAD LV assist device, PASP pulmonary artery systolic pressure, TIMP-1 tissue inhibitor of metalloproteinase 1
Wnt5a promotes IL-6 and TIMP-1 release from mouse cardiac fibroblasts
As shown in Fig. 3a, the main cellular source of Wnt5a in mouse heart appears to be CFs, with ninefold higher expression compared to levels in cardiomyocytes. We next investigated if Wnt5a could induce production of relevant cytokines in these cells and found that Wnt5a increased protein release of IL-6 in the conditioned medium in a dose-dependent manner, while no effects were found with heat-inactivated Wnt5a (Fig. 3b). In addition to being inflammatory cells, CFs play a major role in ECM remodeling and fibrosis and as shown in Fig. 3c, a similar dose response as observed for IL-6 was found for the fibrotic marker TIMP-1.
Wnt5a promotes IL-6 and TIMP-1 release from mouse cardiac fibroblasts. mRNA is expressed relative to GAPDH. a Relative Wnt5a mRNA expression in fractionated mouse heart cells: cardiomyocytes (CM), leukocytes (CD45+), endothelial cells (CD31+), and cardiac fibroblast (CF)-rich fraction. b Protein release of IL-6 and c TIMP-1 after treatment with increasing doses of Wnt5a for 24 h. d Relative mRNA expression of IL-6 and e TIMP-1 after treatment with 30 nM Wnt5a at different time points. f Protein release of IL-6 and g TIMP-1 after treatment with 30 nM Wnt5a at different time points. Data are presented as mean ± SEM of three independent experiments. *p < 0.05, **p < 0.01, ***p < 0.001 vs control. IL-6 interleukin-6, LPS lipopolysaccharide, TIMP-1 tissue inhibitor of metalloproteinase 1
Time course experiments using 30 nM Wnt5a revealed a transient response with a maximum increase in IL-6 and TIMP-1 mRNA expression at 5 h (Fig. 3d) and 8 h (Fig. 3e), respectively, while a linear increase in protein release was observed up to 24 h (Fig. 3f, g). Other inflammatory cytokines were not affected (Online Resource 1). There were no effects of Wnt5a treatment on the mRNA expression of other fibrotic markers, α smooth muscle actin (αSMA), matrix metalloproteinase (MMP)-14 or collagen type 1, except for a minor increase in αSMA at 12 h, and modest decrease in mRNA expression was observed for MMP-2, TIMP-2, and TIMP-4 toward the end of the experiment (Online Resource 2). Finally, Wnt5a may also play a role in angiogenesis [21] but we found no effects of Wnt5a on angiogenic markers relevant for myocardial remodeling and regulation of capillary density, i.e., VEGF, SDG1, and SPRED1 (Online Resource 3).
Wnt5a does not affect β-catenin, but activates ERK1/2 signaling
Next, we investigated signaling pathways that could be involved in Wnt5a-induced IL-6 and TIMP-1 release. As shown in Fig. 4a and Online Resource 4, recombinant Wnt5a did not affect β-catenin protein levels, but reduced the expression of the canonical Wnt pathway target genes Axin2, cFos, and cJun (Fig. 4b), possibly through inhibition of canonical Wnt signaling at the level of T cell factor transcription [8].
Wnt5a activates ERK1/2 in mouse cardiac fibroblasts. mRNA is expressed relative to GAPDH. a Representative immunoblot and quantification of β-catenin and β-tubulin (loading control) in CFs treated with 30 nM Wnt5a for 3 h. b Relative mRNA expression of β-catenin target genes in CFs treated with 30 nM Wnt5a. c Representative immunoblot and quantification of p-ERK1/2, total ERK1/2, and β-tubulin (loading control) in CFs treated with 30 nM Wnt5a. d Relative mRNA expression and protein release of IL-6 and e TIMP-1 from CFs treated with recombinant Wnt5a, MEK inhibitor PD98059, or a combination of both for 8 h. Data are shown as mean ± SEM of three independent experiments. *p < 0.05, **p < 0.01, ***p < 0.001 vs control; #p < 0.05, ###p < 0.001 vs Wnt5a. CFs cardiac fibroblasts, ERK1/2 extracellular signal-regulated kinase 1/2, IL-6 interleukin-6, MEK mitogen-activated protein kinase kinase, TIMP-1 tissue inhibitor of metalloproteinase 1
The ERK1/2 pathway has previously been demonstrated to be involved in IL-6 and TIMP-1 upregulation [22, 23] and Wnt5a markedly increased ERK1/2 phosphorylation (Fig. 4c and Online Resource 4). Furthermore, blocking ERK1/2 activation with the MEK inhibitor PD98059 suppressed Wnt5a-induced mRNA expression and protein release of IL-6 and TIMP-1 (Fig. 4d, e), indicating that Wnt5a-induced IL-6 and TIMP-1 release is dependent on ERK1/2.
Wnt5a increases sFRP3 release from mouse cardiac fibroblasts
The Wnt antagonist sFRP3 may bind and antagonize the effects of Wnt5a [24] and is abundantly expressed in cardiac cells and in particular CFs [20]. Recombinant Wnt5a increased sFRP3 protein release in a dose- and time-dependent manner (Fig. 5a, b), but did not alter sFRP3 mRNA expression (Fig. 5c). Moreover, the MEK inhibitor had no effect on sFRP3 protein release (Fig. 5d), suggesting that ERK1/2 is not required for Wnt5a-induced sFRP3 release.
Wnt5a increases sFRP3 release from mouse cardiac fibroblasts. mRNA is expressed relative to GAPDH. a sFRP3 release from CFs after treatment with different doses of Wnt5a or LPS. b sFRP3 release and c mRNA expression in CFs after treatment with 30 nM Wnt5a for different time points. d sFRP3 release from CFs treated with recombinant Wnt5a, MEK inhibitor PD98059, or a combination of both. Data are mean ± SEM of three independent experiments. ***p < 0.001. CFs cardiac fibroblasts, sFRP3 secreted frizzled-related protein 3, LPS lipopolysaccharide, MEK mitogen-activated protein kinase kinase
Wnt5a promotes IL-6 and TIMP-1 release through the ERK1/2 signaling in human cardiac fibroblasts
We next aimed to confirm our main findings from primary mouse CFs in primary human CFs. We found a similar pattern of Wnt5a responses in primary human CFs with an increase in mRNA expression and release of IL-6 and TIMP-1 in a dose- and time-dependent manner (Fig. 6a–f). Moreover, inhibition of ERK1/2 activation suppressed Wnt5a-induced IL-6 and TIMP-1 mRNA levels and protein release, although the effect on IL-6 protein levels did not reach statistical significance (Fig. 6g, h).
Wnt5a promotes IL-6 and TIMP-1 release from human cardiac fibroblasts. mRNA is expressed relative to GAPDH. a Protein release of IL-6 and b TIMP-1 after treatment with increasing doses of Wnt5a for 24 h. c Relative mRNA expression of IL-6 and d TIMP-1 after treatment with 30 nM Wnt5a at different time points. e Protein release of IL-6 and f TIMP-1 after treatment with 30 nM Wnt5a at different time points. g Relative mRNA expression and protein release of IL-6 and h TIMP-1 from CFs treated with recombinant Wnt5a, MEK inhibitor PD98059, or a combination of both for 8 h. Data are mean ± SEM of a representative experiment from three independent experiments. *p < 0.05, **p < 0.01, ***p < 0.001 vs control; #p < 0.05, ##p < 0.01, ###p < 0.001 vs Wnt5a. IL-6 interleukin-6, LPS lipopolysaccharide, TIMP-1 tissue inhibitor of metalloproteinase 1
Discussion
Several lines of evidence support a critical role for dysregulated Wnt signaling in the pathogenesis of cardiac disease [3–5]. Herein, we provide clinical support by demonstrating elevated systemic and myocardial Wnt5a in HF patients, associated with hemodynamic, neurohormonal, and clinical measures of disease severity. We show that myocardial Wnt5a protein correlated with IL-6 and TIMP-1, markers of inflammation and fibrosis. Furthermore, Wnt5a induced IL-6 and TIMP-1 through ERK1/2 activation in vitro. Our findings suggest that Wnt5a-induced non-canonical Wnt signaling may contribute to HF progression.
Experimental studies implicate canonical and non-canonical Wnt pathway activation in processes that lead to HF development, such as cardiac hypertrophy, fibrosis, myocardial remodeling, and vascular calcification [3–5]. In contrast, the canonical Wnt3a and Wnt8a ligands were scarcely expressed in our myocardial tissue from end-stage HF patients. However, our findings, showing upregulated systemic and myocardial levels of Wnt5a that were associated with measures of disease severity, support a role for the non-canonical Wnt activation in clinical HF.
Several reports have identified a critical role of Wnt signaling in pulmonary hypertension [25, 26], pulmonary remodeling, and fibrosis [14, 15]. Long-standing pulmonary arterial pressure overload may eventually lead to RV failure, characterized by increased filling pressures, diastolic dysfunction [27], and extensive remodeling in the pulmonary vasculature [28]. Our finding that systemic and myocardial Wnt5a were elevated in pulmonary hypertension may suggest a role for Wnt5a in RV failure and the progression to end-stage HF. Moreover, circulating Wnt5a was associated with elevated E/A ratio, a measure of diastolic dysfunction, which is often caused by heart stiffness and fibrosis [29]. Wnt5a is implicated in fibrosis of multiple organs [14, 15, 30], and the association between the E/A ratio and serum Wnt5a could reflect increased ECM turnover and release of Wnt5a into the circulation. Furthermore, myocardial Wnt5a protein levels correlated with IL-6 and TIMP-1, which have an established pathophysiological role in HF. Both are elevated in HF patients, IL-6 increases collagen synthesis and causes myocardial fibrosis, concentric hypertrophy and diastolic dysfunction [31, 32], while TIMP-1 promotes cardiac fibrosis [33–35].
CFs are among the most abundant cell types in the heart and have major roles in inflammation and ECM regulation by producing structural proteins (e.g., collagen), enzymes (i.e., MMPs), and inhibitors (i.e., TIMPs). A balanced regulation of these proteins is necessary for normal ECM homeostasis and alterations in their expression and activity can lead to pathological remodeling and fibrosis [36]. Herein, we show that Wnt5a was most abundantly expressed in mouse CFs and enhanced the mRNA expression and release of TIMP-1 through ERK1/2 signaling in primary mouse and human CFs. ERK1/2 is known to contribute to cardiac fibrosis by stimulating collagen synthesis and TIMP-1 expression [22, 37], while ERK1/2 inhibition ameliorates fibrosis and cardiac dysfunction [37]. Thus, our findings suggest that Wnt5a could be involved in HF progression by promoting cardiac fibrosis through TIMP-1 and ERK1/2. There is limited data on Wnt5a effects on ECM markers in CFs. No effects of Wnt5a overexpression on TIMP-1 were observed in C2C12 myoblasts [38], while Wnt5a increased ECM production by elevating fibronectin and α5-integrin levels in lung fibroblasts [15] and MMP-3 activity in rat-dental fibroblast-like cells [39]; Wnt5a may activate ERK1/2 leading to the induction of IL-6 in bone marrow stromal cells [23] and CCL2 in neutrophils [40]. Similar to TIMP-1, Wnt5a dose dependently enhanced IL-6 expression and release from mouse and human CFs, suggesting that Wnt5a may modulate inflammatory responses in cardiac cells in an ERK-dependent manner. Wnt5a has been shown to promote HF development by stimulating hypertrophic responses in cardiomyocytes [11]. Our study suggests that Wnt5a could also promote HF progression by inducing inflammatory and pro-fibrotic responses in CFs, which could contribute to systolic and diastolic dysfunction. However, experimental models are needed to evaluate the precise role of Wnt5a and its therapeutic potential in HF.
Little is known about the regulation of Wnt5a in CFs. In lung fibroblasts, Wnt5a is induced by transforming growth factor β1, a key modulator of ECM remodeling and fibrosis [14]. The transcription factors NF-kappaB and MAP kinases may also have distinct roles in determining the activity of Wnt5a promoters [41]. Increasing evidence suggests that microRNAs also may regulate Wnt5a [42–44], e.g., miR-223 upregulates Wnt5a expression [42]. Interestingly, DNA damage decreases miR223 [45] and oxidative stress and DNA damage have been linked to HF [46]. Thus, a decrease in miR-223 due to DNA damage could potentially cause Wnt5a upregulation in HF patients. In addition, miR126 and inflammation have been linked to decreased angiogenesis in HF in particular in the context of RV remodeling and pulmonary hypertension [47, 48], but we found no effects of Wnt5a on relevant angiogenic markers, although this should be more thoroughly investigated in forthcoming studies.
We have recently demonstrated increased circulating and myocardial expression of Wnt antagonist sFRP3 in HF, with high levels in CFs [20, 49]. Our finding that sFRP3 is released from mouse CFs upon Wnt5a treatment may suggest that enhanced sFRP3 in HF could reflect active Wnt signaling and represent a negative feedback loop to limit Wnt pathway activity. A more prominent effect of a high Wnt5a/sFRP3 ratio on adverse outcome, however, could indicate that sFRP3 is unable to adequately antagonize Wnt5a in HF patients with end-stage disease.
Limitations in our study include that samples were obtained from HF patients with reduced ejection fraction and our results may therefore not apply to patients with preserved systolic function. Also, we did not have healthy myocardial tissue for protein analysis and comparison with failing myocardium.
Conclusions
Our study demonstrates that HF patients are characterized by increased systemic and myocardial Wnt5a levels that correlate with indices of disease progression as well as myocardial IL-6 and TIMP-1 protein levels. In primary mouse and human CFs, Wnt5a induces IL-6 and TIMP-1 production through the non-canonical ERK1/2 pathway. These Wnt5a-mediated effects could promote myocardial inflammation and fibrosis, and thereby contribute to HF progression. Further mechanistic studies on Wnt5a signaling in HF are needed to elucidate possible therapeutic potential of this novel pathway.
References
Liu L, Eisen HJ (2014) Epidemiology of heart failure and scope of the problem. Cardiol Clin 32:1–8 vii
Distefano G, Sciacca P (2012) Molecular pathogenesis of myocardial remodeling and new potential therapeutic targets in chronic heart failure. Ital J Pediatr 38:41
ter Horst P, Smits JF, Blankesteijn WM (2012) The Wnt/frizzled pathway as a therapeutic target for cardiac hypertrophy: where do we stand? Acta Physiol (Oxf) 204:110–117
Dawson K, Aflaki M, Nattel S (2013) Role of the Wnt-frizzled system in cardiac pathophysiology: a rapidly developing, poorly understood area with enormous potential. J Physiol 591:1409–1432
Tao H, Yang J-J, Shi K-H, Li J (2016) Wnt signaling pathway in cardiac fibrosis: new insights and directions. Metabolism 65:30–40
Lerner UH, Ohlsson C (2015) The WNT system: background and its role in bone. J Intern Med 277:630–649
Bikkavilli RK, Malbon CC (2009) Mitogen-activated protein kinases and Wnt/beta-catenin signaling: molecular conversations among signaling pathways. Commun Integr Biol 2:46–49
Mikels AJ, Nusse R (2006) Purified Wnt5a protein activates or inhibits beta-catenin-TCF signaling depending on receptor context. PLoS Biol 4:e115
Kikuchi A, Yamamoto H, Sato A, Matsumoto S (2012) Wnt5a: its signalling, functions and implication in diseases. Acta Physiol (Oxf) 204:17–33
Bhatt PM, Malgor R (2014) Wnt5a: a player in the pathogenesis of atherosclerosis and other inflammatory disorders. Atherosclerosis 237:155–162
Hagenmueller M, Riffel JH, Bernhold E, Fan J, Katus HA, Hardt SE (2014) Dapper-1 is essential for Wnt5a induced cardiomyocyte hypertrophy by regulating the Wnt/PCP pathway. FEBS Lett 588:2230–2237
Laeremans H, Hackeng TM, van Zandvoort MA, Thijssen VL, Janssen BJ, Ottenheijm HC, Smits JF, Blankesteijn WM (2011) Blocking of frizzled signaling with a homologous peptide fragment of wnt3a/wnt5a reduces infarct expansion and prevents the development of heart failure after myocardial infarction. Circulation 124:1626–1635
Hermans K, Uitterdijk A, de Wijs-Meijler D, Daskalopoulos E, Verzijl A, Sneep S, Blonden L, Reiss I, Duncker D, Blankesteijn WM et al (2015) UM206, a Peptide Fragment of Wnt5a, Attenuates Adverse Remodeling after Myocardial Infarction in Swine. The FASEB Journal 29
Newman DR, Sills WS, Hanrahan K, Ziegler A, Tidd KM, Cook E, Sannes PL (2016) Expression of WNT5A in idiopathic pulmonary fibrosis and its control by TGF-beta and WNT7B in human lung fibroblasts. J Histochem Cytochem 64:99–111
Vuga LJ, Ben-Yehudah A, Kovkarova-Naumovski E, Oriss T, Gibson KF, Feghali-Bostwick C, Kaminski N (2009) WNT5A is a regulator of fibroblast proliferation and resistance to apoptosis. Am J Respir Cell Mol Biol 41:583–589
Mizutani M, Wu JC, Nusse R (2016) Fibrosis of the neonatal mouse heart after cryoinjury is accompanied by Wnt signaling activation and Epicardial-to-mesenchymal transition. J Am Heart Assoc 4:e002457
Norum HM, Gullestad L, Abraityte A, Broch K, Aakhus S, Aukrust P, Ueland T (2016) Increased serum levels of the notch ligand DLL1 are associated with diastolic dysfunction, reduced exercise capacity, and adverse outcome in chronic heart failure. J Card Fail 22:218–223
Zhou Y-Y, Wang S-Q, Zhu W-Z, Chruscinski A, Kobilka BK, Ziman B, Wang S, Lakatta EG, Cheng H, Xiao R-P (2000) Culture and adenoviral infection of adult mouse cardiac myocytes: methods for cellular genetic physiology. Am J Physiol Heart Circ Physiol 279:H429–H436
Ohm IK, Alfsnes K, Belland Olsen M, Ranheim T, Sandanger O, Dahl TB, Aukrust P, Finsen AV, Yndestad A, Vinge LE (2014) Toll-like receptor 9 mediated responses in cardiac fibroblasts. PLoS One 9:e104398
Askevold ET, Aukrust P, Nymo SH, Lunde IG, Kaasboll OJ, Aakhus S, Florholmen G, Ohm IK, Strand ME, Attramadal H et al (2014) The cardiokine secreted frizzled-related protein 3, a modulator of Wnt signalling, in clinical and experimental heart failure. J Intern Med 275:621–630
Korn C, Scholz B, Hu J, Srivastava K, Wojtarowicz J, Arnsperger T, Adams RH, Boutros M, Augustin HG, Augustin I (2014) Endothelial cell-derived non-canonical Wnt ligands control vascular pruning in angiogenesis. Development 141:1757–1766
Tong L, Smyth D, Kerr C, Catterall J, Richards CD (2004) Mitogen-activated protein kinases Erk1/2 and p38 are required for maximal regulation of TIMP-1 by oncostatin M in murine fibroblasts. Cell Signal 16:1123–1132
Rauner M, Stein N, Winzer M, Goettsch C, Zwerina J, Schett G, Distler JH, Albers J, Schulze J, Schinke T et al (2012) WNT5A is induced by inflammatory mediators in bone marrow stromal cells and regulates cytokine and chemokine production. J Bone Miner Res 27:575–585
Wawrzak D, Metioui M, Willems E, Hendrickx M, de Genst E, Leyns L (2007) Wnt3a binds to several sFRPs in the nanomolar range. Biochem Biophys Res Commun 357:1119–1123
Wu D, Talbot CC, Liu Q, Jing Z-C, Damico RL, Tuder R, Barnes KC, Hassoun PM, Gao L (2016) Identifying microRNAs targeting Wnt/β-catenin pathway in end-stage idiopathic pulmonary arterial hypertension. J Mol Med 94:875–885
Boucherat O, Bonnet S (2016) MicroRNA signature of end-stage idiopathic pulmonary arterial hypertension: clinical correlations and regulation of WNT signaling. J Mol Med (Berl) 94:849–851
Voelkel NF, Quaife RA, Leinwand LA, Barst RJ, McGoon MD, Meldrum DR, Dupuis J, Long CS, Rubin LJ, Smart FW et al (2006) Right ventricular function and failure: report of a National Heart, Lung, and Blood Institute working group on cellular and molecular mechanisms of right heart failure. Circulation 114:1883–1891
Shimoda LA, Laurie SS (2013) Vascular remodeling in pulmonary hypertension. J Mol Med (Berl) 91:297–309
Burlew BS, Weber KT (2002) Cardiac fibrosis as a cause of diastolic dysfunction. Herz 27:92–98
Li X, Yamagata K, Nishita M, Endo M, Arfian N, Rikitake Y, Emoto N, Hirata K, Tanaka Y, Minami Y (2013) Activation of Wnt5a-Ror2 signaling associated with epithelial-to-mesenchymal transition of tubular epithelial cells during renal fibrosis. Genes Cells 18:608–619
Hartford M, Wiklund O, Mattsson Hulten L, Persson A, Karlsson T, Herlitz J, Caidahl K (2007) C-reactive protein, interleukin-6, secretory phospholipase A2 group IIA and intercellular adhesion molecule-1 in the prediction of late outcome events after acute coronary syndromes. J Intern Med 262:526–536
Fontes JA, Rose NR, Cihakova D (2015) The varying faces of IL-6: from cardiac protection to cardiac failure. Cytokine 74:62–68
Lindsay MM, Maxwell P, Dunn FG (2002) TIMP-1: a marker of left ventricular diastolic dysfunction and fibrosis in hypertension. Hypertension 40:136–141
Heymans S, Schroen B, Vermeersch P, Milting H, Gao F, Kassner A, Gillijns H, Herijgers P, Flameng W, Carmeliet P et al (2005) Increased cardiac expression of tissue inhibitor of metalloproteinase-1 and tissue inhibitor of metalloproteinase-2 is related to cardiac fibrosis and dysfunction in the chronic pressure-overloaded human heart. Circulation 112:1136–1144
Jordan A, Roldan V, Garcia M, Monmeneu J, de Burgos FG, Lip GY, Marin F (2007) Matrix metalloproteinase-1 and its inhibitor, TIMP-1, in systolic heart failure: relation to functional data and prognosis. J Intern Med 262:385–392
Fan D, Takawale A, Lee J, Kassiri Z (2012) Cardiac fibroblasts, fibrosis and extracellular matrix remodeling in heart disease. Fibrogenesis Tissue Repair 5:15
Cheng M, Wu G, Song Y, Wang L, Tu L, Zhang L, Zhang C (2016) Celastrol-induced suppression of the MiR-21/ERK Signalling pathway attenuates cardiac fibrosis and dysfunction. Cell Physiol Biochem 38:1928–1938
Nakashima A, Tamura M (2006) Regulation of matrix metalloproteinase-13 and tissue inhibitor of matrix metalloproteinase-1 gene expression by WNT3A and bone morphogenetic protein-2 in osteoblastic differentiation. Front Biosci 11:1667–1678
Ozeki N, Yamaguchi H, Hase N, Hiyama T, Kawai R, Kondo A, Nakata K, Mogi M (2015) Polyphosphate-induced matrix metalloproteinase-3-mediated proliferation in rat dental pulp fibroblast-like cells is mediated by a Wnt5 signaling cascade. Biosci Trends 9:160–168
Jung YS, Lee HY, Kim SD, Park JS, Kim JK, Suh PG, Bae YS (2013) Wnt5a stimulates chemotactic migration and chemokine production in human neutrophils. Exp Mol Med 45:e27
Katula KS, Joyner-Powell NB, Hsu CC, Kuk A (2012) Differential regulation of the mouse and human Wnt5a alternative promoters A and B. DNA Cell Biol 31:1585–1597
Bai C, Li X, Gao Y, Lu T, Wang K, Li Q, Xiong H, Chen J, Zhang P, Wang W et al (2014) MicroRNAs regulate the Wnt/Ca2+ signaling pathway to promote the secretion of insulin in pancreatic nestin-positive progenitor cells. bioRxiv. doi:10.1101/003913
Chen QY, Jiao DM, Zhu Y, Hu H, Wang J, Tang X, Chen J, Yan L (2016) Identification of carcinogenic potential-associated molecular mechanisms in CD133(+) A549 cells based on microRNA profiles. Tumour Biol 37:521–530
Zhang Y, Liu Z, Zhou M, Liu C (2016) MicroRNA-129-5p inhibits vascular smooth muscle cell proliferation by targeting Wnt5a. Exp Ther Med 12:2651–2656
Meloche J, Le Guen M, Potus F, Vinck J, Ranchoux B, Johnson I, Antigny F, Tremblay E, Breuils-Bonnet S, Perros F et al (2015) miR-223 reverses experimental pulmonary arterial hypertension. Am J Physiol Cell Physiol 309:C363–C372
Tsutsui H, Kinugawa S, Matsushima S (2008) Oxidative stress and mitochondrial DNA damage in heart failure. Circ J 72 Suppl A: A31-37
Potus F, Ruffenach G, Dahou A, Thebault C, Breuils-Bonnet S, Tremblay E, Nadeau V, Paradis R, Graydon C, Wong R et al (2015) Downregulation of MicroRNA-126 contributes to the failing right ventricle in pulmonary arterial hypertension. Circulation 132:932–943
Sutendra G, Dromparis P, Paulin R, Zervopoulos S, Haromy A, Nagendran J, Michelakis ED (2013) A metabolic remodeling in right ventricular hypertrophy is associated with decreased angiogenesis and a transition from a compensated to a decompensated state in pulmonary hypertension. J Mol Med (Berl) 91:1315–1327
Askevold ET, Gullestad L, Nymo S, Kjekshus J, Yndestad A, Latini R, Cleland JG, McMurray JJ, Aukrust P, Ueland T (2015) Secreted frizzled related protein 3 in chronic heart failure: analysis from the Controlled Rosuvastatin Multinational Trial in Heart Failure (CORONA). PLoS One 10:e0133970
Acknowledgements
We are grateful to the patients and the animal facility staff at Oslo University Hospital, Oslo, Norway, for contributing to our research.
This work was supported by the South-Eastern Norway Regional Health Authority [grant number 2013041], the Research Council of Norway, Anders Jahre’s Fund for the Promotion of Science, Norway, and the Simon Fougner Hartmanns Family Fund, Denmark.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Human studies conformed to the Declaration of Helsinki and were approved by the South Eastern Regional Committee for Medical and Health Research Ethics. Written informed consent was obtained from all individuals. Animal experiments were approved by the animal research committee and were carried out in accordance with institutional guidelines and conformed to the Guide for the Care and Use of Laboratory Animals published by the U.S. National Institutes of Health (NIH Publication No. 85-23, revised 2011).
Conflict of interest
The authors declare that they have no competing interests.
Electronic supplementary material
ESM 1
(PDF 439 kb)
Rights and permissions
About this article
Cite this article
Abraityte, A., Vinge, L.E., Askevold, E.T. et al. Wnt5a is elevated in heart failure and affects cardiac fibroblast function. J Mol Med 95, 767–777 (2017). https://doi.org/10.1007/s00109-017-1529-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00109-017-1529-1