Abstract
Zinc (Zn) is a trace element with a variety of anti-inflammatory and antioxidant effects. Zn deficiency is related to tissue fibrosis. The present study was designed to investigate the effect of Zn on renal fibrosis. Mouse models were successfully established by feeding mice diets with different concentrations of Zn. Zn deficiency induced a decrease in Zn levels in kidney tissue. The results also revealed renal vasodilation, hyperemia, and inflammatory cell infiltration, and the levels of creatinine and urea nitrogen were increased. Furthermore, the TUNEL results showed a large degree of renal cell necrosis caused by Zn deficiency. Meanwhile, the corresponding antioxidant and anti-inflammatory regulators (MT-1, MT-2, Nrf2, and TGF-β1) were detected by RT-PCR, showing that the expression of MT-1, MT-2, and Nrf2 decreased but that TGF-β1 expression increased. The results of Sirius red staining proved that the expression of collagen was increased by Zn deficiency. The immunohistochemical experiments found that the expression of α-smooth muscle actin (α-SMA) increased. ELISA showed that the expression of Collagen I, III, and IV; fibronectin (FN); and inflammatory factors (TNF-α and IL-1β) were remarkably increased. The expression of MMP-1, MMP-2, MMP-3, MMP-7, MMP-9, MMP-12, and TIMP-1, which are extracellular matrix-regulating molecules, was detected by RT-PCR. The results showed that the expression of TIMPs was increased but that the expression of MMPs was decreased. We also obtained consistent results in vivo. All the experimental results indicated that Zn deficiency could aggravate fibrosis by increasing inflammation in the kidney.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Chronic kidney disease (CKD) is a highly prevalent disease that represents a serious hazard to both human and animal health. It is estimated that even in developed countries, more than 10% of adults suffer from some degree of CKD [1]. Related studies have shown that progressive renal disease regularly results in renal fibrosis and even renal failure [2]. Progression of CKD is usually caused by two factors: the loss of renal cells and their replacement by extracellular matrix (ECM) [3]. Thus, it is important to study the mechanism of renal fibrosis. At the same time, controlling the development of fibrosis according to its mechanism is also of great interest for controlling the development of CKD.
Some studies have shown that reactive oxygen species (ROS) play an important role in the pathogenesis of renal fibrosis [4,5,6,7]. ROS are by-products of cells undergoing aerobic respiration. Under normal body conditions, ROS maintain a dynamic balance [8]; however, studies have shown that the production of ROS in renal fibrosis is obviously excessive. Oxidative stress is viewed as an imbalance between the production of ROS and ROS elimination by protective mechanisms and can lead to inflammation [9, 10]. Therefore, the involvement of the inflammatory response in the formation of renal fibrosis also cannot be ignored [11]. As an important regulatory signal involved in the formation of renal fibrosis, TGF-β1 is secreted by inflammatory cells and activates downstream signalling pathways [12]. TGF-β1 can stimulate fibroblast proliferation, extracellular matrix synthesis (collagen types I, III, and IV and fibronectin), and epithelial-to-mesenchymal transition (EMT) [13]. When the synthesis and degradation of ECM is out of balance, this can increase the synthesis or reduce the degradation of ECM, which leads to renal fibrosis [3, 14]. In addition to TGF-β1, there is a major group of enzymes that regulate cell-matrix composition called MMPs. MMPs are Zn-dependent endopeptidases known for their ability to cleave one or more ECM constituents [15]. TIMPs are a kind of enzyme that can regulate MMP activity [16]. When the expression of MMPs is decreased and the expression of TIMPs is increased, the synthesis and degradation of ECM can become unbalanced, which can cause fibrosis.
Many studies have shown that trace elements and some other elements can inhibit or promote inflammation and oxidative stress [17,18,19]. As one of the most significant trace elements, Zn is involved in numerous biochemical pathways in cells, and it is the only metal that is a cofactor in more than 300 enzymes [20, 21]. Obviously, Zn plays an important role in animals. When the level of Zn is deficient, this can lead to disorders of body function, which can lead to several diseases. Increasing evidence suggests that Zn deficiency increases the concentrations of ROS and inflammatory factors. Zn plays a preventive role in free radical formation resulting from injury during inflammatory processes [22, 23]. Since Zn is widely distributed in various organs and maintains relatively high concentrations in the kidneys, a lack of Zn may exacerbate kidney damage [24]. Therefore, we conducted a study on the effect of Zn deficiency on renal fibrosis.
Materials and Methods
Establishment and Design of the Experimental Animal Model
For the experiment, all mice were provided by the Huazhong Agricultural University Laboratory Animal Research Center (Wuhan, China), which were fed and used in accordance with animal management guidelines. Eighty adult-weaned male BALB/c mice (3–4 weeks old, weighing 20–25 g) were divided into three equal groups. One group of mice was fed a diet with normal Zn levels (34 mg/kg). One group was fed a diet with a high Zn level (100 mg/kg), and the remaining group was fed a diet with a deficient Zn level (2 mg/kg). The rat food was provided by the Nantong Trophi Feed Company. All mice were kept and fed in a sunny, temperature-controlled room for 6 weeks. After the mice were killed, their kidney tissues were divided into three groups as follows: the Zn-deficient group (ZnL, 2 mg Zn/kg), Zn-normal group (ZnM, 34 mg Zn/kg), and Zn-high group (ZnH, 100 mg Zn/kg). The kidney tissue was placed in liquid nitrogen and stored at − 80 °C.
Histopathological Analysis
Appropriate amounts of kidney tissues were obtained and fixed in 10% formalin for 1 day. Paraffin-embedded samples were obtained, deparaffinized with xylene, and rehydrated with a graded alcohol series prior to staining and analysis. The sections were stained with hematoxylin and eosin.
Sirius Red Staining
Sirius red was used to stain the paraffin sections with a thickness of 4 μm, which were prepared by gradient dehydration, transparentizing, and embedding. Paraffin was removed with toluene-anhydrous alcohol. Then, the tissues were rehydrated by placing them in anhydrous alcohol, 70% alcohol, 50% alcohol, and finally water. The tissue sections were stained with a mixture of 0.1% Sirius red F3BA and 0.25% Fast Green FCF at room temperature for 45 min. Then, they were dehydrated, transparentized, and sealed. The deposition of the fibrous component Collagens (dyed red) in kidney tissues was observed under an optical microscope, and semiquantitative analysis was conducted using a digital image collection and analysis system.
Immunohistochemistry
The kidney tissue block was routinely dewaxed and hydrated, and nonspecific protein binding was blocked after antigen retrieval. The primary antibody, which can specifically bind to α-SMA, was incubated with the tissue at 37 °C for 1 h or overnight at 4 °C. Then, the tissue was rewarmed at 37 °C for 45 min. The secondary antibody with a detectable label was incubated with the tissue at 37 °C for 30 min to 1 h. Then, we stained the tissue with DAB and added ddH2O. The samples were stained with hematoxylin, decolorized, and transparentized. After drying, the neutral resin sealant was observed under a light microscope.
ROS Detection
Fresh tissue samples were washed with PBS, after that weigh 100 mg of tissue samples accurately, and add 1 ml buffer A homogenizing thoroughly with glass homogenizer. Centrifugation was performed at the condition of 4 °C, 1000 g for 10 min. After abandoning the precipitate, we took the supernatant. Add 200 μl supernatant of homogenate and 2 μl 013 probe to 96-well plate, and blow them with pipette to make them fully mixed. Incubate it at 37 °C in the dark for 30 min. The 96-well plate was placed in a fluoroenzyme labeling instrument, and the excitation wavelength was 488, and the emission wavelength was 610 nm to detect the fluorescence intensity. Another 50 μl supernatant homogenate was taken and diluted with PBS for about 30 times, 100 μl of which was used for protein quantification. DHE ROS kit was used for testing. The DHE solution was diluted to the appropriate concentration to replace the cell culture solution. The HEK293T cells were incubated at 37 °C for 10–90 min in dark and then washed with fresh solution. Fluorescence microscopy was used for detection.
Measurement of Creatinine and BUN
Creatinine and BUN were the blood evaluation index of renal function [25]. Blood samples from the mice were taken by eye enucleation. After all the mice were fixed, surgical clippings were used to remove their whiskers to prevent blood from causing hemolysis. Use tweezers to remove the eyeballs quickly, and make blood flow from the orbit into the EP tubes. Put them in ice bath for 30 min, and then use the centrifugal to separate the supernatant. Blood routine examination of creatinine and urea nitrogen was performed to compare normal values.
HEK 293T Cell Culture
The HEK293T cell line was cultured in the presence of 10% fetal bovine serum. The cells were maintained in Dulbecco’s modified Eagle medium (DMEM) supplemented with 10% fetal bovine serum, 100 U/ml penicillin, and 100 mg/ml Streptomycin, at 37 °C and 5% CO2.After the cells are grown in the cell flask, they are inoculated in a six-well plate. Three groups of serum-free culture medium with successively reduced zinc concentration were prepared (0, 10, 25 μM/L).
Quantitative Real-Time PCR Analysis
Total RNA was extracted from kidney tissue and HEK293T cells, and the concentration and purity of RNA were determined by ultraviolet spectrophotometry (260/280 ratio). Equal amounts of RNA were then reverse-transcribed to synthesize complementary DNA (cDNA of the sample). Then, we selected and designed primers and used glyceraldehyde 3-phosphate dehydrogenase (GAPDH) as an internal control gene; reactions were carried out using the SYBR Green I system. Finally, a Roche Light Cycler 96 instrument that was developed in Switzerland was used for the determination of the target gene expression levels (TIMP-1; MMP-1, 3, 7, 9, 12; MT-1, 2; TGF-β1; IL-1β; TNF-α). Each sample was repeated three times. GAPDH was used as an internal reference gene. The relative abundance of mRNA for each gene was calculated according to the 2−ΔΔCt method as the previous study [26].
ELISA
Kidney tissues were weighed, homogenized with phosphate-buffered saline on ice, and centrifuged at 2000 rpm for 40 min at 4 °C and then the supernatants were collected. All the supernatants were stored at − 20 °C prior to measurements. HEK293T cells were grown and then incubated in serum-free RPMI. The supernatant was then harvested for ELISA. The inflammatory cytokines (TNF-α, IL-1β) and the proteins associated with fibrosis (Collagen I, III, IV, and FN) were detected with mouse ELISA kits in accordance with the instructions of the microplate reader to get the OD value under the conditions of 450 nm within 15 mins. Meanwhile, the results are expressed as the mean ± standard deviation of all the independent experiments.
Data analysis
All statistical analyses were performed using the SPSS version 17.0 software (SPSS Inc., Chicago, IL, USA). T tests were conducted, and results were represented in the formant of mean ± standard deviation (M ± SD). Samples with different superscript letters indicate that there is significant difference among ZnL, ZnM, and ZnH. (p < 0.05).
Results
In vivo
Effects of Zn Deficiency on Injury and Correlation of Regulators
Blood from mice was collected by enucleation of the eyeball. Meanwhile, renal function examination was performed on three groups of samples, and the results showed that (Fig. 1a) the creatinine and urea nitrogen levels in ZnL were higher than those in ZnM and ZnH. Furthermore, ROS kits were used for detection. The ROS results are shown in Fig. 1b. Compared with that in ZnM, the ROS content in ZnL increased significantly, but the increase in ZnH was not obvious, with no significant change. Inflammatory factors were detected by ELISA. It was obvious that compared with those in ZnM, the inflammatory factors in ZnL increased significantly, while those in ZnH did not.
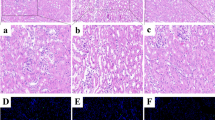
Analysis of injury and the correlation of regulators in the kidney. Protein expression was detected with ELISA kits. RT-qPCR was used to verify mRNA expression. GAPDH was used as a control. a Results showing CRE and BUN content. b The results showing reactive oxygen species (ROS) levels. c TNF-α and IL-1β protein content. d Results of H&E staining of kidney tissue, ZnL. e Results of H&E staining of kidney tissue, ZnM. f Results of H&E staining of kidney tissue, ZnH. g The mRNA levels of MT-1 and MT-2. h The mRNA levels of Nrf2 and TGF-β1. ZnL (Zn-low, 2 mg Zn/kg). ZnM (Zn-normal, 34 mg Zn/kg). ZnH (Zn-high, 100 mg Zn/kg). Asterisk represents a comparison with ZnM. Number sign represents a comparison with ZnL. The values are presented as the mean ± SD of three independent experiments. P < 0.05, significantly different from ZnM
The kidney sections subjected to H&E staining are shown in Fig. 1d–f. Compared with ZnM, ZnL showed obvious vascular congestion, renal corneal injury with loss of filtration space, and infiltration of many inflammatory cells. There was no significant difference between ZnH and the other groups.
Figure 1 g and h shows that four genes were found with changes in the kidneys of mice. Among the four genes, one was upregulated, and the other three were downregulated. To further confirm the effects of Zn on these genes, RT-qPCR was carried out. The results showed that when the level of Zn decreased, the mRNA levels of TGF-β1 were significantly increased (P < 0.05) and those of MT-1, MT-2, and Nrf2 were significantly decreased (P < 0.05). In addition, when the Zn content increased, the mRNA levels of the MTs increased significantly, but the changes in Nrf2 and TGF-β1 were not obvious.
Correlation Between Zn Deficiency and Renal Fibrosis
Sirius red staining was performed on to determine the kidney tissue fibrosis index based on collagen to observe renal fibrosis levels by using a digital image acquisition system. The results confirmed that the levels of collagen glomerular deposition in ZnL shown in Fig. 2a were significantly increased compared with those in the other groups (Fig. 2b–c).
Fibrosis detection in kidney tissues. The expression of collagen I, III, IV and FN was detected with ELISA kits. a The results of Sirius red staining, ZnL. b The results of Sirius red staining, ZnM. c The results of Sirius red staining, ZnH. Red represents the collagen fibers. d The α-SMA immunohistochemistry results, ZnL. e The α-SMA immunohistochemistry results, ZnM. f The α-SMA immunohistochemistry results, ZnH. Brownish-yellow particles represent α-SMA, and blue represents the nucleus. g The expression of collagen I. h The expression of collagen III and IV. i The expression of FN. ZnL (Zn-low, 2 mg Zn/kg). ZnM (Zn-normal, 34 mg Zn/kg). ZnH (Zn-high, 100 mg Zn/kg). Asterisk represents a comparison with ZnM. Number sign represents a comparison with ZnL. The values are presented as the mean ± SD of three independent experiments. P < 0.05, significantly different from ZnM.
Immunohistochemistry showed that α-SMA was expressed in proliferative fibrous tissue in mouse kidney. The results of immunohistochemistry showed that the more severe the Zn deficiency, the more brown-yellow particles that existed, which meant that α-SMA was more highly expressed.
The expression of collagen I, III, and IV and FN were detected by ELISA. The results are shown in Fig. 2g–i. The expression of collagen and fibronectin in ZnL were evidently increased, while the increases in ZnH were not obvious compared with those in ZnM.
Effects of Zn Deficiency on TIMPs and MMPs
The formation of renal fibrosis is related to the regulation of TIMPs and MMPs. The results of the experiments were measured by RT-qPCR. As shown in Fig. 3, compared with that in ZnM, the expression of TIMP-1 in ZnL increased significantly (P < 0.05), while expression of MMP-1, MMP-3, MMP-7, MMP-9, and MMP-12 decreased significantly (P < 0.05). Moreover, the results showed that TIMP-1 expression decreased with increasing Zn content, while the expression of MMP-1, MMP-3, MMP-7, MMP-9, and MMP-12 showed the opposite trend. This also indicated that Zn content can affect the expression of TIMPs and MMPs to some extent.
Expression of TIMPs/MMPs in kidney tissues. RT-qPCR was used to verify the expression of mRNA. GAPDH was used as a control. a The mRNA level of TIMP-1. b The mRNA level of MMP-1. c The mRNA level of MMP-3. d The mRNA level of MMP-7. e The mRNA level of MMP-9. f The mRNA level of MMP-12. ZnL (Zn-low, 2 mg Zn/kg). ZnM (Zn-normal, 34 mg Zn/kg). ZnH (Zn-high, 100 mg Zn/kg). Asterisk represents a comparison with ZnM. Number sign represents a comparison with ZnL. The values are presented as the mean ± SD of three independent experiments. P < 0.05, significantly different from ZnM
In Vitro
Effects of Zn Deficiency on Correlation of Regulators, ROS, and Inflammatory Factors
Figure 4 a and b show that expression of four genes were associated with Zn in HEK 293T cells. Among the four genes, one was upregulated, and the other three were downregulated. To further confirm the effects, RT-qPCR was carried out. The results showed that when the level of Zn decreased, the mRNA levels of TGF-β1 were significantly increased (P < 0.05) and those of MT-1, MT-2 and Nrf2 were significantly decreased (P < 0.05).
Analysis of the correlation of regulators, ROS, and inflammatory factors in HEK 239T cells. The qPCR was used to verify the expression of mRNA. GAPDH was used as a control. Protein expression was detected with ELISA kits. a The mRNA levels of MT-1 and MT-2. b The mRNA levels of Nrf2 and TGF-β1. c The results for ROS levels. d The mRNA levels of TNF-α and IL-1β. e TNF-α and IL-1β protein content. ZnL (Zn-low, 2 mg Zn/kg). ZnM (Zn-normal, 34 mg Zn/kg). ZnH (Zn-high, 100 mg Zn/kg). Asterisk represents a comparison with ZnM. Number sign represents a comparison with ZnL. The values are presented as the mean ± SD of three independent experiments. P < 0.05, significantly different from ZnM
The results of ROS detection in HEK 293T cells are shown as follows (Fig. 3d). Compared with that in ZnH and ZnM, the ROS content in ZnL was significantly increased. To further confirm the effects of Zn deficiency on ROS, the fluorescence intensity in each group was measured. The higher the fluorescence intensity, the higher the content of ROS. The intensity in ZnL was much higher than that in the other two groups. Zn deficiency has been shown to cause an imbalance between ROS production and elimination.
The detection of inflammatory factors in HEK 293T cells was carried out at the gene and protein levels. Figure 4 d illustrates that a total of two inflammatory-related genes were found in HEK 293T cells. Both genes were upregulated. To further confirm the effects of Zn on these genes, RT-qPCR was carried out. The results showed that the mRNA levels of IL-1β and TNF-α were significantly increased (P < 0.05) in ZnL. Meanwhile, inflammatory proteins were also detected by ELISA. It was obvious that compared with those in ZnM, the levels of inflammatory proteins in ZnL increased significantly, while those in ZnH did not.
Effects of Zn Deficiency on MMPs and TIMP Expression
Figure 5 shows that six genes were found in HEK 293T cells. Among the six genes, one was upregulated, and the other five were downregulated. To further confirm the effects of Zn on these genes, RT-qPCR was carried out. The results showed that the mRNA levels of TIMP-1 were significantly increased (P < 0.05) and those of MMP-1, MMP-3, MMP-7, MMP-9, and MMP-12 were significantly decreased (P < 0.05) in ZnL HEK 293T cells.
Expression of TIMPs/MMPs in HEK 239T cells. RT-qPCR was used to verify mRNA expression. GAPDH was used as a control. a The mRNA level of TIMP-1. b The mRNA level of MMP-1. c The mRNA level of MMP-3. d The mRNA level of MMP-7. e The mRNA level of MMP-9. f The mRNA level of MMP-12. ZnL (Zn-low, 2 mg Zn/kg). ZnM (Zn-normal, 34 mg Zn/kg). ZnH (Zn-high, 100 mg Zn/kg). Asterisk represents a comparison with ZnM. Number sign represents a comparison with ZnL. The values are presented as the mean ± SD of three independent experiments. P < 0.05, significantly different from ZnM
The Relationship Between Zn Deficiency and Fibrosis
Cellular supernatants were used in cell experiments. The expression of Collagen I, III, and IV and FN was detected by ELISA. The results are shown in Fig. 6. The expression of Collagen I, III, and IV and FN in ZnL were increased. Compared with those in ZnM, the increases in ZnH were not obvious. All the results of the in vitro experiments were consistent with those of the in vivo experiments.
Analysis of protein content related to fibrosis in HEK 293T cells. The expression of collagen I, III, and IV and FN was detected with ELISA kits. a The expression of collagen I. b The expression of collagen III and IV. c The expression of FN. ZnL (Zn-low, 2 mg Zn/kg). ZnM (Zn-normal, 34 mg Zn/kg). ZnH (Zn-high, 100 mg Zn/kg). Asterisk represents a comparison with ZnM. Number sign represents a comparison with ZnL. The values are presented as the mean ± SD of three independent experiments. P < 0.05, significantly different from ZnM
Discussion
Zn is crucial for the functions of numerous proteins that regulate the functions of various other proteins, including metalloproteins. Zn is an essential micronutrient in many cellular processes, so it has important biological significance. In this experiment, a Zn-deficient mouse kidney model was successfully established through a Zn-deficient diet. Studies have shown that when the body is Zn-deficient, it can cause damage to the kidneys [27]. Kidney function was evaluated by tracking the serum levels of urea and creatinine. These two parameters are usually altered in the presence of substantial damage to kidney nephrons [28]. ZnL showed a significant rise in these two kidney function markers as a consequence of impaired kidney function.
Zn has anti-inflammatory and antioxidant effects [29]; when the body is in a Zn-deficient state, the capacity of anti-inflammatory and antioxidant activities is frequently reduced, which increases the risk of the development of disease. MTs are a class of Zn-binding proteins whose stimulation and expression can significantly reduce ROS levels and regulate inflammation [30, 31]. The transcription factor Nrf2 is a cellular antitoxin and antioxidant [32, 33]. After activation, Nrf2 can upregulate its downstream antioxidant factors through nuclear translocation and exert its effects on antioxidant damage. Zn is also a regulator of Nrf2 [34]. Thus, in this experiment, ROS content was measured in renal tissues and HEK 293T cells by using ROS assay kits. At the same time, the expression levels of MT-1, MT-2, Nrf2, IL-1β, and TNF-α were detected by RT-qPCR. The experimental results showed that ROS content and IL-1β and TNF-α levels increased significantly in both the ZnL tissue and cell groups. Based on the H&E staining of tissues, it could be easily seen that there was increased inflammatory cell infiltration in the tissue. All of the above results proved that Zn deficiency could cause oxidative damage and inflammation in the kidney. The expression of MT-1, MT-2, and Nrf2 were decreased in ZnL, indicating that Zn deficiency could reduce the antioxidant capacity of the body.
TGF-β1 is a major driver of fibrosis via its activation of its downstream effector small mother against decapentaplegic (Smad) signalling [35]. TGF-β1 exerts its fibrotic activity through stimulation of fibroblast proliferation and extracellular matrix synthesis (collagen I, III, IV and fibronectin) [12]. α-Smooth muscle actin (αSMA) is the most characteristic marker of pathological fibroblasts [36]; therefore, it was detected by immunohistochemistry in this experiment. The expression of TGF-β1 was obviously increased, which demonstrated the initiation of renal fibrosis. ELISA kits were used to measure collagen (collagen types I, III, IV and fibronectin) expression in tissues and HEK 293T cells. The results in both tissues and cells showed that the expression of collagen in the ZnL group was increased compared with that in the other groups. Immunohistochemistry showed that there were more brown-yellow particles in the ZD group than in the other groups, which indicated that the expression of α-SMA was notably increased. The results of Sirius red staining showed that a large number of collagen fibers were dyed red in ZnL. All of the above results confirm that zinc deficiency indeed caused renal fibrosis.
Renal fibrosis is caused by excessive deposition and modification of extracellular matrix (ECM) in the renal parenchyma in response to injury and inflammation [37]. Matrix metalloproteinases (MMPs) are proteolytic enzymes involved in physiological processes such as tissue repair [38] that can remodel the ECM. TIMPs are inhibitors of MMPs, and TIMPs and MMPs interact with each other to balance ECM synthesis and degradation [39]. Under normal physiological conditions, their expression and activity are strictly controlled, while under pathological conditions, their expression is often problematic [40]. In this study, the expression of TIMP-1, MMP-1, MMP-3, MMP-7, MMP-9, and MMP-12 in renal tissues and cells was detected by RT-qPCR. The results showed that the expression of MMP-1, MMP-3, MMP-7, MMP-9, and MMP-12 in ZnL were decreased compared with those in other groups, while the expression of TIMP-1 in ZnL was increased. Therefore, ECM production and degradation were out of balance, causing renal fibrosis.
Experiments have shown that Zn deficiency in mice can lead to ROS accumulation and an inflammatory response, resulting in renal fibrosis. When chronic kidney disease occurs, the loss of Zn increases, which can accelerate the development of kidney disease in the body. Therefore, appropriate Zn supplementation for chronic kidney disease may be a new strategy for delaying the development of kidney disease.
References
Lopez-Novoa JM, Martinez-Salgado C, Rodriguez-Pena AB, Lopez-Hernandez FJ (2010) Common pathophysiological mechanisms of chronic kidney disease: therapeutic perspectives. Pharmacol Ther 128:61–81
Nogueira A, Pires MJ, Oliveira PA (2017) Pathophysiological mechanisms of renal fibrosis: a review of animal models and therapeutic strategies. In vivo 31:1–22
Fogo AB (2001) Progression and potential regression of glomerulosclerosis. Kidney Int 59:804–819
Manickam N, Patel M, Griendling KK, Gorin Y, Barnes JL (2014) RhoA/Rho kinase mediates TGF-beta1-induced kidney myofibroblast activation through Poldip2/Nox4-derived reactive oxygen species. Am J Physiol Ren Physiol 307:F159–F171
Bondi CD, Manickam N, Lee DY, Block K, Gorin Y, Abboud HE, Barnes JL (2010) NAD(P)H oxidase mediates TGF-beta1-induced activation of kidney myofibroblasts. J Am Soc Nephrol : JASN 21:93–102
Cheng TH, Cheng PY, Shih NL, Chen IB, Wang DL, Chen JJ (2003) Involvement of reactive oxygen species in angiotensin II-induced endothelin-1 gene expression in rat cardiac fibroblasts. J Am Coll Cardiol 42:1845–1854
Park SA, Kim MJ, Park SY, Kim JS, Lee SJ, Woo HA, Kim DK, Nam JS, Sheen YY (2015) EW-7197 inhibits hepatic, renal, and pulmonary fibrosis by blocking TGF-beta/Smad and ROS signaling. Cell Mol Life Sci : CMLS 72:2023–2039
Kerr ME, Bender CM, Monti EJ (1996) An introduction to oxygen free radicals. Heart Lung : the journal of critical care 25:200–209 quiz 210-1
Wang S, Chi Q, Hu X, Cong Y, Li S (2019) Hydrogen sulfide-induced oxidative stress leads to excessive mitochondrial fission to activate apoptosis in broiler myocardia. Ecotoxicol Environ Saf 183:109578
Xin C, Guangliang S, Qing Z, Qingqing L, Hang Y, Yiming Z, Shu L (2020) Astilbin protects chicken peripheral blood lymphocytes from cadmium-induced necroptosis via oxidative stress and the PI3K/Akt pathway. Ecotoxicol Environ Saf 190:110064
Meng XM, Nikolic-Paterson DJ, Lan HY (2014) Inflammatory processes in renal fibrosis. Nat Rev Nephrol 10:493–503
Lan HY (2011) Diverse roles of TGF-beta/Smads in renal fibrosis and inflammation. Int J Biol Sci 7:1056–1067
Loboda A, Sobczak M, Jozkowicz A, Dulak J (2016) TGF-beta1/Smads and miR-21 in renal fibrosis and inflammation. Mediat Inflamm 2016:8319283
Liu WH, Tang NN, Zhang QD (2009) Could mycophenolate mofetil combined with benazapril delay tubulointerstitial fibrosis in 5/6 nephrectomized rats? Chin Med J 122:199–204
Kapoor C, Vaidya S, Wadhwan V, Hitesh KG, Pathak A (2016) Seesaw of matrix metalloproteinases (MMPs). J Cancer Res Ther 12:28–35
Arpino V, Brock M, Gill SE (2015) The role of TIMPs in regulation of extracellular matrix proteolysis. Matrix biology : journal of the International Society for Matrix Biology 44-46:247–254
Wang L, Shi X, Zheng S, Xu S (2020) Selenium deficiency exacerbates LPS-induced necroptosis by regulating miR-16-5p targeting PI3K in chicken tracheal tissue. Metallomics. 12:562–571. https://doi.org/10.1039/c9mt00302a
Zhang Z, Liu Q, Yang J, Yao H, Fan R, Cao C, Liu C, Zhang S, Lei X, Xu S (2020) The proteomic profiling of multiple tissue damage in chickens for a selenium deficiency biomarker discovery. Food Funct 11(2):1312–1321
Liu J, Wang S, Zhang Q, Li X, Xu S (2020) Selenomethionine alleviates LPS-induced chicken myocardial inflammation by regulating the miR-128-3p-p38 MAPK axis and oxidative stress. Metallomics. 12(1):54–64
Rink L, Gabriel P (2000) Zinc and the immune system. Proc Nutr Soc 59:541–552
Chasapis CT, Loutsidou AC, Spiliopoulou CA, Stefanidou ME (2012) Zinc and human health: an update. Arch Toxicol 86:521–534
Zinc and health: current status and future directions (2000) Proceedings of a workshop. Bethesda, Maryland, USA. November 4-5, 1998. J Nutr 130:1341S–1519S
Stefanidou M, Maravelias C, Dona A, Spiliopoulou C (2006) Zinc: a multipurpose trace element. Arch Toxicol 80:1–9
Jarosz M, Olbert M, Wyszogrodzka G, Mlyniec K, Librowski T (2017) Antioxidant and anti-inflammatory effects of zinc Zinc-dependent NF-kappaB signaling. Inflammopharmacology 25:11–24
Sun M, Zhuo W, Guo S, Liao S, Shi D, Liu J, Cheng Z, Liu Y, Niu X, Wang S, Yang D (2012) Serological survey of canine dirofilariosis in Chongqing, Kunming, Nanchang, Fuzhou, Guangzhou, Shenzhen, and Nanning in Southern China. Vet Parasitol 185(2-4):225–228
Liu J, Cheng Z, Zhou D, Zhang L, Yan Z, Wang Z, Yang D, Liu Y, Chai T (2011) Synthesis, cloning, and expression of Mycoplasma suis inorganic pyrophosphatase gene using PCR-based accurate synthesis and overlap-extension PCR, and its immunogenicity analysis. Res Vet Sci 91(3):e100–e102
Li MS, Adesina SE, Ellis CL, Gooch JL, Hoover RS, Williams CR (2017) NADPH oxidase-2 mediates zinc deficiency-induced oxidative stress and kidney damage. Am J Physiol Cell Physiol 312:C47–C55
Nalobin DS, Krasnov MS, Alipkina SI, Syrchina MS, Yamskova VP, Yamskov IA (2016) Effect of bioregulators isolated from rat liver and blood serum on the state of murine liver in roller organotypic culture after CCl4-induced fibrosis. Bull Exp Biol Med 161:604–609
Prasad AS (2014) Zinc: an antioxidant and anti-inflammatory agent: role of zinc in degenerative disorders of aging. J Trace Elem Med Biol : organ of the Society for Minerals and Trace Elements 28:364–371
Baltaci AK, Yuce K, Mogulkoc R (2018) Zinc metabolism and metallothioneins. Biol Trace Elem Res 183:22–31
Duerr GD, Dewald D, Schmitz EJ, Verfuerth L, Keppel K, Peigney C et al (2016) Metallothioneins 1 and 2 modulate inflammation and support remodeling in ischemic cardiomyopathy in mice. Mediat Inflamm 2016:7174127
Kobayashi M, Yamamoto M (2006) Nrf2-Keap1 regulation of cellular defense mechanisms against electrophiles and reactive oxygen species. Adv Enzym Regul 46:113–140
Ma Q (2013) Role of nrf2 in oxidative stress and toxicity. Annu Rev Pharmacol Toxicol 53:401–426
Rezaei KA, Chen Y, Cai J, Sternberg P (2008) Modulation of Nrf2-dependent antioxidant functions in the RPE by Zip2, a zinc transporter protein. Invest Ophthalmol Vis Sci 49:1665–1670
Hu HH, Chen DQ, Wang YN, Feng YL, Cao G, Vaziri ND, Zhao YY (2018) New insights into TGF-beta/Smad signaling in tissue fibrosis. Chem Biol Interact 292:76–83
Sun KH, Chang Y, Reed NI, Sheppard D (2016) α-Smooth muscle actin is an inconsistent marker of fibroblasts responsible for force-dependent TGFbeta activation or collagen production across multiple models of organ fibrosis. Am J Physiol Lung Cell Mol Physiol 310:L824–L836
Zhang X, Ritter JK, Li N (2018) Sphingosine-1-phosphate pathway in renal fibrosis. Am J Physiol Ren Physiol 315:F752–F756
Sternlicht MD, Werb Z (2001) How matrix metalloproteinases regulate cell behavior. Annu Rev Cell Dev Biol 17:463–516
Malemud CJ (2006) Matrix metalloproteinases (MMPs) in health and disease: an overview. Front Biosci : a journal and virtual library 11:1696–1701
Parks WC, Wilson CL, Lopez-Boado YS (2004) Matrix metalloproteinases as modulators of inflammation and innate immunity. Nat Rev Immunol 4:617–629
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Xu, R., Chen, My., Liang, W. et al. Zinc Deficiency Aggravation of ROS and Inflammatory Injury Leading to Renal Fibrosis in Mice. Biol Trace Elem Res 199, 622–632 (2021). https://doi.org/10.1007/s12011-020-02184-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-020-02184-x