Abstract
Boric acid is known to regulate the proliferation of cancer cells. Prostate cancer is among the types of cancer with high mortality in men. There are a few numbers of studies investigating the effects of boric acid on prostate cancer cells. The objective of the present study was to assess the effects of boric acid at concentrations higher than that can be achieved in blood by dietary intake on DU-145 human prostate cancer cells for 24 h. Firstly, we determined the cytotoxic activity of boric acid (0 to 12.5 mM) on DU-145 human prostate cancer cells by using 3-(4, 5-dimethylthiazol, 2-yl)-2, 5-diphenyl tetrazolium bromide (MTT) and defined the IC50 concentration of boric acid. Then, by employing the doses found in MTT, the levels of antioxidant-oxidant molecules and apoptotic proteins were measured and morphological changes were evaluated. We have concluded that boric acid caused oxidative stress, inhibition of cell growth, apoptosis, and morphological alterations in a concentration-dependent manner in DU-145 cells. Furthermore, treatments with increasing boric acid concentrations decreased the antioxidant levels in cells. We actually revealed that boric acid, known as an antioxidant, may prevent cell proliferation by acting as an oxidant in certain doses. Although the high IC50 concentration of boric acid is perceived to be negative, we think it provides important background for subsequent studies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Boron, a trace element for organisms, has a high affinity for oxygen and is found abundant in nature as borate forms, such as boric acid and borax [1, 2]. Boric acid, a soluble form of boron, is rapidly absorbed from the gastrointestinal tract following dietary intake, passes to the blood flow, and is involved in many physiological and biochemical processes through its antioxidant or anticancer properties [3,4,5]. The unique cytotoxic abilities of boric acid for cancer cells have been presented by some studies. Treatment of nude mice, injected with androgen-sensitive LNCaP prostate cancer cells, with boric acid led to a reduction of tumor growth and inhibition of the enzymatic activity of prostate-specific antigen [6]. In cell culture or animal studies, researchers found that boron compounds exhibit antiproliferative effects [7, 8], but its cellular effects related to cancer remain obscure. Therefore, these compounds still attract the attention of researchers due to their multifaceted effects in the treatment of cancer as well as many other diseases.
In general, although cancer types have some common features, they have some specific symptoms, too [9]. At this point, prostate cancer is the second most important cause of death in malignant tumors in men [10]. Prostate cancer along with its multifocal nature and metastatic bone invasion greatly limits the treatment strategies of this disease and reduces survival times [11]. However, previous studies showed that there is a negative correlation between the amount of dietary boron intake and prostate cancer risk [12, 13]. LNCaP, DU-145, and PC-3 human prostate cancer cell lines are used as favorable models to investigate prostate cancer risk at the molecular levels. The effects of boric acid on prostate cancer are supported by several lines of investigation. Barranco et al. (2009) demonstrated that boric acid reduced the proliferation of DU-145 human cancer prostate cells in a dose-dependent manner and inhibited cell migration and invasion [5]. It was suggested that boron compounds over a range from 1 to 1000 μM showed adverse effects on the metastatic and proliferative properties of cancer cells [7]. Previous study also displayed that boric acid caused antiproliferative effects, cell cycle inhibition, and disruption of mitochondrial functions on LNCaP, DU-145, and PC-3 human prostate cancer cell lines [14]. In addition, some in vitro studies reported that boric acid treatment affected cellular motility, morphological changes, and endoplasmic reticulum stress conditions on prostate cancer cell lines [15, 16], but the effect of boric acid on the apoptotic and oxidative processes still remains mysterious. The knowledge of cellular changes caused by boric acid is crucial for future anticancer studies, which will reveal the potential therapeutic effects of boric acid and guide other studies in this area. Therefore, the response of prostate cancer cells to boric acid needs to be elucidated at concentrations above those that can be achieved by dietary and common environmental exposures.
In the current study, we aimed at ascertaining the apoptotic and oxidative effects of different concentrations of boric acid treatment in DU-145 prostate cancer cell line in detail.
Materials and Methods
Cell Culture
DU-145 cells were obtained from the American Type Culture Collection (ATCC, Rockville, MD, USA) and were grown in high glucose DMEM supplemented with 10% (v/v) FBS and 1% (v/v) penicillin-streptomycin (100 U/mL–100 μg/mL) at 37 °C in a humidified atmosphere of 95% air and 5% CO2. The cells were cultivated in 75-cm2 cell culture flasks. Sufficiently confluent (80–90%) cells were dislodged with 0.25% trypsin-1 mM EDTA solution. Protein levels were measured by the biuret method [17].
MTT Cytotoxicity Assay
The cells were grown in 96 well plates at a density of 5 × 103 cells per well for MTT assay. Firstly, cells were allowed to adhere to flasks for 24 h before boric acid treatment. The stock solution of boric acid was prepared in double distilled water and filtered with 0.21-μm filters. A working solution was prepared with cell medium just before the treatment. The concentration range for boric acid was selected by preliminary experiments conducted in our laboratory. Boric acid concentrations from 0 to 12.5 mM were applied to the adhered cells in a 96-well plate for 24 h. The viability of the boric acid–untreated cells was accepted as 100% and the viability of the experimental cells was calculated accordingly. The viability percentage of DU-145 cells was calculated by following formula:
- OD:
-
optical density
IC50 and IC75 were calculated with the respective plot from MTT results. IC75 was used together with IC50 for inverted microscopy, hematoxylin-eosin, and DAPI staining. However, in immunocytochemistry, IC75 was not used because of its possibility to considerably decrease the cell number, thereby making to observe cytoplasmic immunoreactivity harder.
Cell Lysate Preparation
Cell lysates for malondialdehyde (MDA), glutathione (GSH), catalase (CAT), superoxide dismutase (SOD), total antioxidant status (TAS), total oxidant status (TOS), caspase 3 (CASP3), and cytochrome C somatic (CYCS) assays were prepared according to the following method. After boric acid treatment (6.25, 10.77, and 16.15 mM), adherent cells were washed by ice-cold phosphate-buffered saline (PBS; pH 7.0) gently, and then dislodged with trypsin, and collected by centrifugation at 1000×g for 5 min at 4 °C. The cells were washed three times in ice-cold PBS. Resuspended cells were incubated in fresh lysis buffer (10 mM Tris–HCl at pH 8.0, 20 mM EDTA, 1 mM dithiothreitol, 50 mM HEPES at pH 7.0, 1 mg/mL proteinase K) for 2 h at 4 °C. Thereafter, the cells were centrifuged at 16000×g for 10 min at 4 °C to remove cellular debris. Protein levels were measured by the biuret method [17]. The prepared cell lysates were used immediately for the assays.
Lipid Peroxidation Measurement
MDA levels of cell lysates were measured using the method reported by Ohkawa et al. [18]. MDA is accepted as an important parameter which is most commonly used in the determination of lipid peroxidation. Briefly, MDA is a pink-colored complex molecule formed by incubation with thiobarbituric acid at 95 °C for 1 h. The color density was determined spectrophotometrically at 532 nm. The results were indicated as nmol/mg protein.
Glutathione Measurement
GSH levels were determined according to the modified method of Srivastava and Beutler [19]. Briefly, it is based on the measurement of 5-thio-2-nitrobenzoic acid that occurs as a result of the reaction between GSH and 5,5′-dithiobis(2-nitrobenzoic acid) (DTNB, Ellman’s reagent). The color intensity associated with the GSH concentration was determined by measuring the absorbance values at 412 nm. Reaction medium contains 0.1 mL cell lysates, 1 mL 200 mM Tris-HCl buffer (pH = 8), and 0.1 mL Ellman’s reagent (60 mg/100 mL, 0.2 M Tris-HCl buffer pH = 6.5). GSH levels were expressed as μmol/mg protein.
Catalase Activity Measurement
CAT activity was determined by the method of Aebi [20], which is based on the decrease in absorbance of H2O2 at 240 nm. In brief, the final concentration of reaction medium adjusted to be 1 mL as follows: 50 mM sodium-potassium phosphate buffer (pH = 7), 20 mM H2O2, and 20 μL cell lysates. CAT was activated by the addition of H2O2 to the reaction medium and the absorbance changes were spectrophotometrically monitored. The results were expressed as Unit/mg protein.
Superoxide Dismutase Activity Measurement
SOD activity in cell lysates was measured by the method of Sun et al. [21]. Briefly, the determination of SOD activity is based on the reduction of the superoxide that results from the reaction of xanthine with xanthine oxidase. The reaction was started by adding 50 μL xanthine oxidase to the reaction medium. SOD activity was determined spectrophotometrically by monitoring the change in the absorbance at 560 nm for 5 min. The results were indicated as Unit/mg protein.
Total Oxidant and Total Antioxidant Status Measurements
TOS and TAS measurements were performed according to the manufacturer’s instructions (Rel Assay Diagnostics, Gaziantep, Turkey). Briefly, TAS measurement is based on the reduction of colored 3-ethylbenzothiazoline-6-sulfonate (ABTS) to colorless ABTS by antioxidants in DU-145 cell lysates. Reduction of ABTS was monitored at 660 nm. The results were expressed as mmol Trolox Equiv./L.
TOS measurement is based on the oxidation process in DU-145 cell lysates. During the oxidation of ferrous ion to ferric ion, a colored complex molecule is formed in the reaction medium. The color density, which represents the total amount of TOS in cells, was measured spectrophotometrically at 530 nm. The results were expressed as μmol H2O2 Equiv./L.
Caspase 3 and Cytochrome C Somatic Assays
CASP3 and CYCS levels in DU-145 cell lysates were measured using a colorimetric commercial kit that has been pre-coated with an antibody specific to CASP3 and CYCS, respectively (Cloud-Clone Corp., USA). Briefly, cells were lysed according to the manufacturer’s protocol. Cells were washed by cold phosphate buffer (pH = 7) and then gathered by centrifugation at 1500×g for 10 min. Resuspended cells in fresh lysis buffer were subjected to homogenization for 1 min and centrifuged at 1500×g for 5 min at 2–8 °C to remove cellular debris. Cell lysates were stored at − 20 °C until the measurement day. The enzyme-substrate reactions for CASP3 and CYCS were performed according to the manufacturer’s instructions and then color changes were determined spectrophotometrically at 450 nm. The concentrations of CASP3 and CYCS in cell lysates were indicated as ng/mL by comparing with the optical density of the standard curve.
Inverted Microscopy
Cells were incubated on the coverslips in six-well plates and treated with different boric acid concentrations (6.25, 10.77, and 16.15 mM) determined by MTT. Then, the plates were scrutinized under an inverted microscope with terms of morphological alterations.
Hematoxylin-eosin Staining
The hematoxylin-eosin staining is a widespread method used for examining cellular changes [22]. The morphological alterations of DU-145 cells were observed under a BX51 light microscope (Olympus Corporation, Tokyo, Japan) attached with a DP70 digital camera (Olympus Corporation, Tokyo, Japan). In this respect, DU-145 cells were seeded into six-well plates until they adhered on the bottom of the flasks. After that, the cell medium was removed and the cells were rinsed with PBS. The cells were incubated with different boric acid concentrations (10.77 and 16.15 mM) determined by MTT for 24 h. Later, the cells were fixed with 100% ice-cold methanol and rewashed with PBS. Next, hematoxylin stain was applied for 4 min and the cells were immersed into 1% ammonia solution. Subsequently, the eosin stain was applied for 5 min. Eventually, the cells were washed with distilled water, dried, and mounted with the aqueous mounting medium.
Immunocytochemistry
Cells were incubated on the coverslips in six-well plates and treated with different boric acid concentrations (6.25 and 10.77 mM) determined by MTT. After 24 h, the cells were washed with PBS and fixed with 100% ice-cold methanol for 10 min. Once the cells were rinsed with PBS three times, samples were immersed in 0.2% Triton X for 5 min, and then incubated in blocking solution for 10 min. Thereafter, the anti-Bax antibody was added over the fixed cells and the samples were incubated overnight at 4 °C. The moisture was maintained to keep antibodies from drying, followed by the 10-min incubation with biotinylated goat anti-polyvalent reagent and in turn with streptavidin peroxidase. Next, the samples were stained with AEC and hematoxylin as a counterstaining, consecutively. Finally, the samples were mounted with the aqueous mounting medium.
DAPI Staining
After 24-h boric acid treatment (10.77 and 16.15 mM), DU-145 cells were spread onto the slides and fixed with 100% ice-cold methanol for 10 min. Blocking was performed with the goat serum (species from which secondary antibody was gained), respectively. Then the samples were incubated with DAPI for 30 min in a dark place. Finally, samples were mounted with an aqueous medium and observed under a fluorescence microscope.
Statistical Analysis
For MTT analysis, three independent experiments were done at different times in separate 96-well plates. For each biochemical parameter, three individual 25-cm2 flasks for each group were used. Three replications of measurement were performed in all analyses. Results were expressed as the mean ± SD (standard deviation). For statistical analyses, we used SPSS 21 software. Firstly, the Kolmogorov-Smirnov and Shapiro-Wilk normality tests were used to determine whether the data conformed to normal distribution. In case the data distributed normally, the analysis of variance (ANOVA) and posthoc Tukey tests were performed. Kruskal-Wallis ANOVA posthoc Dunn’s multiple comparison test was used for the data that did not fit normal distribution. The statistical significance was considered as P < 0.05.
Results
Boric Acid Has an Inhibitory Effect on DU-145 Cell Viability
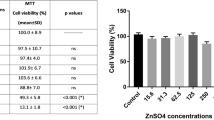
It was detected that the treatments with 3.13, 1.56, 0.78, 0.39, and 0.19 mM of boric acid caused a statistically insignificant decrease in cell viability when compared with the control (84.3, 78.5, 84.4, 89.0, and 87.8%, respectively) as indicated in Fig. 1.
The boric acid concentration of 6.25 mM was the first concentration indicating a significant decrease from untreated cells, in which the cell viability was 61% (P < 0.001). At the boric acid concentration of 12.5 mM, the cell viability was reduced to 45.8% (P < 0.001). IC50 and IC75 concentrations of boric acid in DU-145 cells for 24 h were found as 10.77 and 16.15 mM (Fig. 1 and Table 1).
Boric Acid Triggered Apoptosis and Oxidative Stress in DU-145 Cells
As shown in Fig. 2b and f, the treatments with 6.25, 10.77, and 16.15 mM of boric acid caused a significant increase in MDA and TOS levels on DU-145 cells exposed to increasing concentrations of boric acid compared with control. The boric acid concentration of 10.77 mM resulted in an about threefold increase in MDA and TOS levels compared with the control (P < 0.001). Moreover, this concentration led to the greatest increase in MDA and TOS levels (275.49 ± 11.92 nmol/mg protein and 14.71 ± 1.25 μmol H2O2 Equiv./L, respectively).
Antioxidant and oxidant molecule levels in DU-145 prostate cancer cell treated with boric acid. a Glutathione (GSH) levels. b Malondialdehyde (MDA) levels. c Superoxide dismutase (SOD) activity. d Catalase (CAT) activity. e Total antioxidant status (TAS). f Total oxidant status (TOS). *P < 0.05, **P < 0.01, and ***P < 0.001 when compared with the control. The results are displayed as mean ± SD of three separate experiments conducted in 25-cm2 flasks (n = 3)
On the contrary, a statistically significant decrease in antioxidant defense system was observed in DU-145 cells after boric acid treatment. The boric acid led to a considerable reduction in SOD, CAT, GSH, and TAS levels in DU-145 cells. The activity of SOD in cells treated with increasing concentrations of boric acid was shown in Fig. 2c. Unlike the others, 10.77 mM boric acid concentration produced a statistically significant reduction in SOD activity (38.83 ± 4.1 U/mg protein, P < 0.01).
It can be seen from Fig. 2a and d that CAT activity and GSH level showed a decrease in a concentration-dependent manner. The boric acid concentration of 16.15 mM caused a decrease of 49% in CAT activity and a decrease of 93% in GSH compared with the control. On the other hand, the lowest boric acid concentration (6.25 mM) did not cause a statistically significant alteration in CAT level compared with the control.
Similar to CAT and GSH results, boric acid exerted a concentration-dependent decrease in the TAS levels (Fig. 2e). The boric acid concentration of 10.77 mM led to the highest decrease in TAS level (13.72 ± 3.63 mmol Trolox Equiv./L) which demonstrated inhibition of 58% in TAS level compared with the control. However, 16.15 mM boric acid did not affect the TAS level in cells.
We found that 24-h boric acid treatment (6.25, 10.77, and 16.15 mM) significantly increased the CYCS and CASP3 levels by inducing apoptotic pathways in DU-145 cells (Fig. 3a and b). The levels of CYCS and CASP3 in the control were 0.64 ± 0.11 ng/mL and 1.16 ± 0.5 ng/mL, respectively. However, the levels of CYCS and CASP3 increased approximately tenfold and sevenfold, respectively, due to exposure to boric acid concentrations compared with the control. Furthermore, the increases in CYCS and CASP3 levels were the highest in the 10.77-mM boric acid–treated group (~ 900% and ~ 500% of the control, respectively).
Cytochrome C somatic (CYCS) and caspase 3 (CASP3) levels in DU-145 prostate cancer cell treated with boric acid. a CYCS level. b CASP3 level. *P < 0.05 and **P < 0.01 when compared with the control. The results are displayed as mean ± SD of three separate experiments conducted in 25-cm2 flasks (n = 3)
Boric Acid–Induced Morphological Changes in DU-145 Cells
In inverted microscope images (Fig. 4), boric acid decreased the number of DU-145 cells and led to several degenerations including cellular shrinkage and rounded cells in a concentration-dependent manner.
In hematoxylin-eosin staining (Fig. 5), DU-145 cells in the control group had normal morphology with its typical nucleus and cytoplasm. No morphological abnormalities were observed. In contrast, in boric acid–treated cells, numerous morphological alterations were observed concentration-dependently as shrunken cells, condensed, and crescent-shaped nuclei as well as decrease in cell number.
Hematoxylin-eosin staining of boric acid–treated DU-145 cells. a, d Regular-shaped DU-145 cells with their nuclei in the control group. b, e There are morphological abnormalities in 10.77 mM of the boric acid–treated group including condensed nucleus and shrunken cells. c, f Similar abnormalities persist more severely in 16.15 mM of boric acid–treated group as well as kidney-shaped nucleus. Note of the lower density of cells in the high concentration boric acid group. Bars in a, b, and c indicate 100 μm and bars in d, e, and f indicate 20 μm
DAPI-stained sections in Fig. 6a–c
The untreated cells had normal nuclear morphology. However, boric acid–treated cells had nuclear abnormalities including nuclear fragmentation and condensation. In addition, in Fig. 6d–f, the untreated cells were hardly stained with Bax antibody. On the other hand, boric acid treatment increased Bax staining concentration dependently.
DAPI and Bax staining of boric acid–treated DU-145 cells. a The nuclei of regular-shaped DU-145 cells in the control group. b Nuclear fragmentations are apparent in 10.77 mM boric acid–treated group. c Nuclear condensations and abnormalities are quite observable in 16.15 mM boric acid–treated group. d The untreated cells did not stain with Bax. e The Bax proteins of some cells were stained positively in 10.77 mM boric acid–treated group. f The Bax proteins of most of the cells were stained positively in 16.15 mM boric acid–treated group. Arrows indicate the nuclear abnormalities in DU-145 cells treated with boric acid. All bars indicate 20 μm
Discussion
Boron compounds and their salts are utilized as an antiseptic agent to kill the bacteria and fungi in the industrial areas. Boric acid was reported to be used for preserving some food products due to its ability of inhibiting microorganism growth [23]. Boric acid is a soft organic Lewis acid with competitively inhibiting features [24]. This characteristic of boric acid renders it a promising anticancer agent, as it alters specific signaling mechanisms related to metastatic prostate cancer cells. [25]. In recent years, boron compounds have been used in cancer-associated studies. In addition, boron-derived compounds such as phenylboronic acid, guanidine biboric acid, dihydroxy boron hydrochloride monohydrate, boric acid, and sodium tetraborate were documented to have cytotoxic effects on B16F10 murine melanoma, HL-60, and U-937 human leukemia cells [26, 27]. High boron intake was suggested to be protective against lung cancer [28]. Al-Ali and Gonzalez-Sarmiento (2017) reported that 5 mM boric acid concentration inhibited the growth of H1299 and COR-L23p lung cancer cells less than 25%, while 25 mM boric acid concentration treatment led to a more than 75% decrease in cell viability after a 7-day experiment [29]. It was also indicated that the expression of proliferation-inducing proteins in prostate cancer cells did not alter following 1 and 2-day treatments of different boric acid doses (0 to 1000 μM) [7]. In the present study, we selected boric acid as a cytotoxic agent because of its aforementioned antiproliferative and apoptotic effects on different cancer cell lines. To date, most studies have examined the long-term (for 7–8 days) effects of boric acid on cell proliferation and cytotoxicity. Therefore, in this study, we proposed to show the responses of DU-145 cells to 24-h boric acid treatment. We organized our results by dividing into three aspects as oxidative stress, apoptosis, and morphological changes.
Oxidative stress accelerates when the levels of enzymatic and non-enzymatic antioxidant molecules involved in the cellular defense mechanism diminish. Cancer cells are characterized by a higher rate of reactive oxygen species (ROS) production than normal cells due to their redox balance deterioration. For example, ROS production in lymphocytes of leukemia patients has been found to be increased compared with normal cells [30]. The imbalance between the antioxidant and oxidant molecules in cancer cells paves the way for its utilization in the cancer therapy that aspires to increase ROS formation and to reduce the antioxidant defense, thereby increasing the oxidant burden of cancer cells and ultimately activating cell death pathways, especially apoptosis [31]. To exemplify, the generation of ROS was reported to lead to apoptosis in human colon cancer HCT116 cells [32]. Furthermore, the induction of oxidative stress and the depletion of GSH were found to result in the activation of apoptosis and necrosis in HeLa cells [33]. SOD and CAT enzymes take over a pivotal role in oxidative damage. SOD catalyzes superoxide to H2O2, and then, H2O2 is converted to H2O by CAT. Previous studies demonstrated that boron compounds (50 and 500 mg/L) caused a dose-dependent decrease in antioxidant enzyme activities of erythrocytes. In this study, not only SOD but also CAT showed a statistically significant decrease in boric acid–treated cells [34]. Consistent with this study, the present study obviously demonstrated in DU145 cells that boric acid concentrations of 6.25, 10.77, and 16.15 mM induced oxidative stress by decreasing the enzymatic and non-enzymatic antioxidants, that is, SOD and CAT. To the best of our knowledge, this is the first data indicating that boric acid inhibited SOD and CAT activities in DU-145 cells. We also found that the boric acid treatment caused a marked increase in MDA levels, an important product of lipid peroxidation via oxidative stress [35]. DU-145 cell membranes might not be protected against ROS-induced lipid peroxidation which stems from the decrease in SOD and CAT activities. GSH is a tripeptide structure and an important antioxidant in cytosol. The decrease in GSH levels contributes to induction of oxidative stress [36]. Therefore, intracellular GSH levels are critical for cell death mechanisms [37]. In our study, we determined an inverse proportion between boric acid concentrations and GSH levels in DU-145 cells. It is noteworthy in the present study that the ascending boric acid concentrations indicated a positive correlation up to 10.77 mM with oxidative stress in prostate cancer cells, which was also supported by the increase in TOS levels and the decrease in TAS capacities in treated cells.
Apoptosis is a form of programmed cell death that regulates cell proliferation. The basic principle of widely used anticancer agents, like temozolomide, tamoxifen, and casodex, is to inhibit proliferation and to provoke apoptosis in cancer cells [38]. The apoptotic pathways are frequently activated by ROS, which increases the mitochondrial membrane permeability, causing the release of pro-apoptotic molecules, such as CYCS, an intermembrane space protein responsible for mitochondrial electron transport, to the cytosol [39]. Mitochondrial release of CYCS initiates the caspase activation [40]. CASP3, an aspartate-specific cysteine protease, is an important executioner caspase and is involved in apoptotic pathways [41]. Barranco et al. (2004) showed that boric acid (100–1000 μM) treatment did not induce an increase in CASP3 activity in DU-145 cells compared with untreated cells [42]. However, our results indicated that boric acid concentrations up to 10.77 mM led to a concentration-dependent rise in CASP3 and CYCS levels in DU-145 cells. Bax as a pro-apoptotic protein plays a crucial role in controlling apoptosis [43]. ROS production was documented to enhance Bax expression and cause apoptosis in H1299, A549, H226, and H322 lung cancer cells [44]. Similarly, we also displayed an increase in Bax staining following the boric acid treatment.
Moreover, we used the inverted, fluorescence, and light microscope images to assess the morphological changes in DU-145 cells. Boric acid–treated cells adhered to the bottom of the flasks weakly in our study. It is suggested in DU-145 cells that boron compounds selectively affect actin distribution and force generation required for cell adhesion, spreading, and migration [25]. Boric acid–induced morphological changes in DU145 cells were observed in the study of Barranco and Eckhert (2006) [15]. Consistently, we also observed similar morphological abnormalities in our study. DAPI staining was performed to detect nuclear morphological changes. Canturk et al. (2016) detected nuclear fragmentations in boron compound–treated leukemia cells [27]. Likewise, the DAPI images of boric acid–treated cells showed a concentration-dependent increase in nuclear abnormalities in our study.
Kobylewski et al. (2017) denounced that lower boric acid concentration (10 μM) inhibited cell viability without causing apoptosis in prostate cancer cells [16]. Bradke et al. (2008) found that boron compounds did not inhibit cell migration and viability after 24-h treatment, but caused abnormalities in cell morphology and a significant decrease in cell viability after 8-day treatment [7]. High doses of boron compounds showed inhibiting effects on the activities of antioxidant enzymes and the glutathione levels in peripheral human erythrocytes [34]. Altogether, boric acid exerts major cytotoxic influences by reducing antioxidant levels and hence activating apoptosis.
Conclusions
To summarize, in the present study, boric acid inhibited the proliferation and caused the deformities and apoptosis in DU-145 cancer cells concentration dependently. In addition, boric acid treatment in DU-145 prostate cancer cell line triggered oxidative stress by decreasing TAS and increasing TOS levels. The most critical limitation of this study was the use of boric acid at high concentrations. Therefore, in our future studies, we propose to investigate the effects of boron nanoparticles on prostate cancer cells, by doing so, we think to reduce the effective boric acid concentration. Further investigations associated with the effects of boric acid on cancer cells are also required.
References
Devirian TA, Volpe SL (2003) The physiological effects of dietary boron. Crit Rev Food Sci Nutr 43:219–231. https://doi.org/10.1080/10408690390826491
Moore JA (1997) An assessment of boric acid and borax using the IEHR evaluative process for assessing human developmental and reproductive toxicity of agents. Expert Scientific Committee Reprod Toxicol 11:123–160. https://doi.org/10.1016/S0890-6238(96)00204-3
Nielsen FH (1994) Biochemical and physiologic consequences of boron deprivation in humans. Environ Health Perspect 102:59–63. https://doi.org/10.1289/ehp.94102s759
Pawa S, Ali S (2006) Boron ameliorates fulminant hepatic failure by counteracting the changes associated with the oxidative stress. Chem Biol Interact 160:89–98. https://doi.org/10.1016/j.cbi.2005.12.002
Barranco WT, Kim DH, Stella SL Jr, Eckhert CD (2008) Boric acid inhibits stored Ca(2+) release in DU-145 prostate cancer cells. Cell Biol Toxicol 25:309–320. https://doi.org/10.1007/s10565-008-9085-7
Gallardo-Williams MT, Chapin RE, King PE, Moser GJ, Goldsworthy TL, Morrison JP, Maronpot RR (2004) Boron supplementation inhibits the growth and local expression of IGF-1 in human prostate adenocarcinoma (LNCaP) tumors in nude mice. Toxicol Pathol 32(1):73–78. https://doi.org/10.1080/01926230490260899
Bradke TM, Hall C, Carper SW, Plopper GE (2008) Phenylboronic acid selectively inhibits human prostate and breast cancer cell migration and decreases viability. Cell Adhes Migr 2:153–160. https://doi.org/10.4161/cam.2.3.6484
Zafar H, Ali S (2013) Boron inhibits the proliferating cell nuclear antigen index, molybdenum containing proteins and ameliorates oxidative stress in hepatocellular carcinoma. Arch Biochem Biophys 529:66–74. https://doi.org/10.1016/j.abb.2012.11.008
Kreeger PK, Lauffenburger DA (2010) Cancer systems biology: a network modeling perspective. Carcinogenesis 31:2–8. https://doi.org/10.1093/carcin/bgp261
Karantanos T, Corn PG, Thompson TC (2013) Prostate cancer progression after androgen deprivation therapy: mechanisms of castrate resistance and novel therapeutic approaches. Oncogene 32:5501–5511. https://doi.org/10.1038/onc.2013.206
Algaba F, Montironi R (2010) Impact of prostate cancer multifocality on its biology and treatment. J Endourol 24:799–804. https://doi.org/10.1089/end.2009.0462
Cui Y, Winton MI, Zhang ZF, Rainey C, Marshall J, De Kernion JB, Eckhert CD (2004) Dietary boron intake and prostate cancer risk. Oncol Rep 11:887–892. https://doi.org/10.3892/or.11.4.887
Barranco WT, Hudak PF, Eckhert CD (2007) Evaluation of ecological and in vitro effects of boron on prostate cancer risk. Cancer Causes Control 18:71–77. https://doi.org/10.1007/s10552-006-0077-8
Barranco W, Eckhert C (2004) Boric acid inhibits prostate cancer cell proliferation. Cancer Lett 216:21–29. https://doi.org/10.1016/j.canlet.2004.06.001
Barranco W, Eckhert CD (2006) Cellular changes in boric acid treated DU145 prostate cancer cells. Br J Cancer 94:884–890. https://doi.org/10.1038/sj.bjc.6603009
Kobylewski SE, Henderson KA, Yamada KE, Eckhert CD (2017) Activation of the EIF2α/ATF4 and ATF6 pathways in DU-145 cells by boric acid at the concentration reported in men at the US mean boron intake. Biol Trace Elem Res 176:278–293. https://doi.org/10.1007/s12011-016-0824-y
Gornall AG, Bardawill CJ, David MM (1949) Determination of serum proteins by means of the biuret reaction. J Biol Chem 177:751–766
Ohkawa H, Ohishi N, Yagi K (1979) Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem 95:351–358. https://doi.org/10.1016/0003-2697(79)90738-3
Srivastava SK, Beutler E (1968) Accurate measurement of oxidized glutathione content of human, rabbit, and rat red blood cells and tissues. Anal Biochem 25:70–76. https://doi.org/10.1016/0003-2697(68)90082-1
Aebi H (1984) [13] Catalase in vitro. Methods Enzymol 105:121–126. https://doi.org/10.1016/S0076-6879(84)05016-3
Sun Y, Oberley LW, Li Y (1988) A simple method for clinical assay of superoxide dismutase. Clin Chem 34:497–500
Fischer AH, Jacobson KA, Rose J, Zeller R (2008) Hematoxylin and eosin staining of tissue and cell sections. Cold Spring Harb Protoc 2008:pdb.prot4986. https://doi.org/10.1101/pdb.prot4986
Yiu PH, See J, Rajan A, Bong CFJ (2008) Boric acid levels in fresh noodles and fish ball. Am J Agric Biol Sci 3:476–481. https://doi.org/10.3844/ajabssp.2008.476.481
Yang W, Gao X, Wang B (2003) Boronic acid compounds as potential pharmaceutical agents. Med Res Rev 23:346–368. https://doi.org/10.1002/med.10043
McAuley EM, Bradke TA, Plopper GE (2011) Phenylboronic acid is a more potent inhibitor than boric acid of key signaling networks involved in cancer cell migration. Cell Adhes Migr 5(5):382–386. https://doi.org/10.4161/cam.5.5.18162
Deshayes S, Cabral H, Ishii T, Miura Y, Kobayashi S, Yamashita T, Matsumoto A, Miyahara Y, Nishiyama N, Kataoka K (2013) Phenylboronic acid-installed polymeric micelles for targeting sialylated epitopes in solid tumors. J Am Chem Soc 135(41):15501–15507. https://doi.org/10.1021/ja406406h
Canturk Z, Tunali Y, Korkmaz S, Gulbas Z (2016) Cytotoxic and apoptotic effects of boron compounds on leukemia cell line. Cytotechnology 68(1):87–93. https://doi.org/10.1007/s10616-014-9755-7
Mahabir S, Spitz MR, Barrera SL, Dong YQ, Eastham C, Forman MR (2008) Dietary boron and hormone replacement therapy as risk factors for lung cancer in women. Am J Epidemiol 167(9):1070–1080. https://doi.org/10.1093/aje/kwn021
Al-Ali R, Gonzalez-Sarmiento R (2017) High concentrations of boric acid induce autophagy in cancer cell lines. bioRxiv 192441:1–9. https://doi.org/10.1101/193441
Kamiguti AS, Serrander L, Lin K, Harris RJ, Cawley JC, Allsup DJ, Slupsky JR, Krause KH, Zuzel M (2005) Expression and activity of NOX5 in the circulating malignant B cells of hairy cell leukemia. J Immunol 175:8424–8430. https://doi.org/10.4049/jimmunol.175.12.8424
Bai X, Ma Y, Zhang G (2015) Butein suppresses cervical cancer growth through the PI3K/AKT/mTOR pathway. Oncol Rep 33:3085–3092. https://doi.org/10.3892/or.2015.3922
Kim DH, Park KW, Chae IG, Kundu J, Kim EH, Kundu JK, Chun KS (2016) Carnosic acid inhibits STAT3 signaling and induces apoptosis through generation of ROS in human colon cancer HCT116 cells. Mol Carcinog 55:1096–1110. https://doi.org/10.1002/mc.22353
You BR, Shin HR, Han BR, Kim SH, Park WH (2015) Auranofin induces apoptosis and necrosis in HeLa cells via oxidative stress and glutathione depletion. Mol Med Rep 11:1428–1434. https://doi.org/10.3892/mmr.2014.2830
Türkez H, Geyikoğlu F, Tatar A, Keleş S, Ozkan A (2007) Effects of some boron compounds on peripheral human blood. Z Naturforsch C 62:889–896. https://doi.org/10.1515/znc-2007-11-1218
Kim H, Oh E, Im H, Mun J, Yang M, Khim JY, Lee E, Lim SH, Kong MH, Lee M, Sul D (2006) Oxidative damages in the DNA, lipids, and proteins of rats exposed to isofluranes and alcohols. Toxicology 220:169–178. https://doi.org/10.1016/j.tox.2005.12.010
Meister A (1983) Selective modification of glutathione metabolism. Science 220:472–477. https://doi.org/10.1126/science.6836290
Ortega AL, Mena S, Estrela JM (2011) Glutathione in cancer cell death. Cancers (Basel) 3(1):1285–1310. https://doi.org/10.3390/cancers3011285
He W, Liu R, Yang SH, Yuan F (2015) Chemotherapeutic effect of tamoxifen on temozolomide-resistant gliomas. Anti-Cancer Drugs 26:293–300. https://doi.org/10.1097/CAD.0000000000000197
Matsuura K, Canfield K, Feng W, Kurokawa M (2016) Metabolic regulation of apoptosis in cancer. Int Rev Cell Mol Biol 327:43–87. https://doi.org/10.1016/bs.ircmb.2016.06.006
Gogvadze V, Orrenius S, Zhivotovsky B (2006) Multiple pathways of cytochrome c release from mitochondria in apoptosis. Biochim Biophys Acta 1757(5-6):639–647. https://doi.org/10.1016/j.bbabio.2006.03.016
Seervi M, Xue D (2015) Mitochondrial cell death pathways in Caenorhabiditis elegans. Curr Top Dev Biol 114:43–65. https://doi.org/10.1016/bs.ctdb.2015.07.019
Barranco WT, Eckhert CD (2004) Boric acid inhibits human prostate cancer cell proliferation. Cancer Lett 216:21–29. https://doi.org/10.1016/j.canlet.2004.06.001
Wu M, Zhang H, Hu J, Weng Z, Li C, Li H, Zhao Y, Mei X, Ren F, Li L (2013) Isoalantolactone inhibits UM-SCC-10A cell growth via cell cycle arrest and apoptosis induction. PLoS One 8:76000. https://doi.org/10.1371/journal.pone.0076000
Honda T, Coppola S, Ghibelli L, Cho SH, Kagawa S, Spurgers KB, Brisbay SM, Roth JA, Meyn RE, Fang B, McDonnell TJ (2004) GSH depletion enhances adenoviral bax-induced apoptosis in lung cancer cells. Cancer Gene Ther 11(4):249–255. https://doi.org/10.1038/sj.cgt.7700684
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Hacioglu, C., Kar, F., Kacar, S. et al. High Concentrations of Boric Acid Trigger Concentration-Dependent Oxidative Stress, Apoptotic Pathways and Morphological Alterations in DU-145 Human Prostate Cancer Cell Line. Biol Trace Elem Res 193, 400–409 (2020). https://doi.org/10.1007/s12011-019-01739-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-019-01739-x