Abstract
Prostate cancer is the main cause of cancer-related mortality in men around the world and an important health problem. DU-145 human prostate cancer cells provide an opportunity to investigate prostate cancer. Betaine has a number of anticancer effects, such as inactivation of carcinogens, inhibition of cancer cell proliferation, angiogenesis, and metastasis. However, there is no study investigating the effects of betaine on DU-145 cells. The aim of this study was to evaluate the effects of different concentrations of betaine on the oxidative stress, apoptosis, and inflammation on DU-145 cells. Firstly, we proved the cytotoxic activity of betaine (0 to 150 mg/ml) on DU-145 cells by using 3-(4, 5-dimethylthiazol, 2-yl)-2, 5-diphenyl tetrazolium bromide (MTT) and defined the optimal concentration of betaine. Then, by employing the doses found in MTT, the levels of antioxidant (GSH, SOD, CAT, and TAS) and oxidant (MDA and TOS) molecules, pro-inflammatory cytokines (TNF-a and IL-6), apoptotic proteins (CYCS and CASP3), and DNA fragmentation were measured. Morphological changes and apoptosis were evaluated using H&E technique, Bax and Bcl-2 immunohistochemistry. Results suggested that betaine caused oxidative stress, inflammation, inhibition of cell growth, apoptosis, and morphological alterations in DU-145 cells dose-dependently. Furthermore, treatments with increasing betaine concentrations decreased the antioxidant levels in cells. We actually revealed that betaine, known as an antioxidant, may prevent cell proliferation by acting as an oxidant in certain doses. In conclusion, betaine may act as a biological response modifier in prostate cancer treatment in a dose-dependent manner.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cancer is an important health problem of the world and a disease characterized by complex pathology and uncontrolled cell growth, and its incidence has been increasing every year (Clark et al. 2005). Prostate cancer (PCa) is one of the most common malignancies in the world (Ferlay et al. 2012). Although various treatment strategies such as surgery, chemotherapy, radiation, and hormone therapy are used to treat PCa, the negative side effects of current treatments are very high (Ghagane et al. 2017). Researchers developed second-generation hormone therapy drugs such as abiraterone and enzalutamide for PCa treatment (Fizazi et al. 2012; Schrader et al. 2014). However, due to the negative effects of synthetic chemotherapeutics, more natural anticancer drugs with low toxic effects are being used (Hacioglu et al. 2019). For this reason, 25% of patients with PCa use at least one alternative drug treatment method. Many agents still expect validation for PCa (Wang and Martins-Green 2014; Philippou et al. 2013).
Betaine is a constant and non-toxic natural substance with three additional methyl groups (Yu et al. 2004). Studies on several human diseases such as metabolic syndrome, Alzheimer’s disease, and cancer showed that betaine has beneficial and positive effects (Schartumhansen et al. 2013; Chen et al. 2014; Madsen et al. 2015; Ying et al. 2013). In addition, in in vitro cancer studies, betaine can inhibit the growth of cancer cells (Duong et al. 2006; Gerile et al. 2012). According to our hypothesis, betaine may be associated with PCa cancer, due to its role in modulating oxidative stress, apoptosis, and inflammation in single-carbon metabolism. Abnormal changes in DNA methylation take place in the development and progression of cancer and cause activation of certain proto-oncogenes such as c-Myc and inactivation of certain tumor suppressors such as p16. Betaine is a methyl group donor that is vital for a trans-methylation process catalyzed by betaine-homocysteine methyltransferase (BHMT) (Du et al. 2009). The presence of methyl group donors was shown to affect the methylation levels (Zeisel 2017). The methyl group donors are also implicated in the trans-sulfuration pathway that converts homocysteine to cystathionine. In these pathways, glutathione (GSH) levels are one of the important markers indicating in oxidant-antioxidant status (Mosharov et al. 2000). These metabolite level changes in trans-methylation and trans-sulfuration pathways were suggested to induce oxidative stress and apoptosis (Almashhadany et al. 2015; Baggott and Tamura 2015). Different concentrations of betaine changed methionine cycle metabolites and oxidant-antioxidant molecule levels (Pastor et al. 1996; Cavallaro et al. 2010). Administration of low-dose betaine decreased homocysteine levels and increased S-adenosyl methionine (SAM) and GSH levels (Ueland et al. 2005; Day and Kempson 2016). Nuclear factor-κB (NF-κB), an important transcription factor, controls many genes related to inflammation caused by oxidative damage. These genes include pro-inflammatory cytokines such as tumor necrosis factor-alpha (TNF-α) and interleukin 6 (IL-6) (Zhao et al. 2018). Betaine can alter these cytokine levels in various cancer diseases (Yi and Kim 2012; Kim et al. 2014). Uncontrolled cell proliferation and the inability of cells to undergo apoptotic cell death cause cancer (Salseven and Dixit 1999; Huerta et al. 2007). As a result, compounds capable of inducing cancer cell apoptosis may produce promising drugs for cancer treatment. Betaine affects caspase proteins that are mainly involved in apoptosis mechanism.
In the previous studies, betaine revealed variable results in dose-dependent manner. There are also inconsistencies in the cohort and prospective meta analyzes between betaine consumption and PCa risk (Sun et al. 2016). According to the authors’ knowledge, this is the first study investigating the effects of betaine on DU-145 human prostate cancer cells. In the current study, we aimed to evaluate the oxidative, apoptotic, inflammatory, and anti-proliferative effects of varying betaine concentrations on DU-145 prostate cancer cell line.
Materials and methods
Cell culture
DU-145 cells were gained from the American Type Culture Collection (ATCC, Rockville, MD, USA) and cultured in Dulbecco’s Modified Eagle’s Medium (DMEM) appended with 10% (v/v) FBS and 1% (v/v) penicillin-streptomycin (100 U/ml–100 μg/ml, respectively) at 37 °C in a humidified atmosphere of 95% air and 5% CO2. The cells were harvested in 75 cm2 cell culture flasks. The cells were grown to confluence of 80–90% and detached by 0.25% trypsin 1 mM EDTA solution.
MTT cytotoxicity assay
The cells were grown in 96-well plates at a density of 5 × 103 cells per well for MTT assay. Firstly, cells were allowed to adhere to flasks for 24 h before betaine treatment. The stock solution of betaine was prepared in double-distilled water and filtered with 0.21-μm filters. A working solution was prepared with cell medium just before the treatment. The concentration range for betaine was selected by preliminary experiments conducted in our laboratory. Betaine concentrations from 0 to 150 mg/ml were applied to the adhered cells in a 96-well plate for 24 h. The viability of the betaine-untreated cells was accepted as 100% and the viability of the experimental cells was calculated accordingly. The viability percentage of DU-145 cells was calculated by following formula:
OD is the optical density.
IC25, IC50, and IC75 were calculated with the respective plot from MTT results. Betaine concentrations of 25, 40, and 50 mg/ml were used for inverted microscopy and hematoxylin-eosin staining.
Cell lysate preparation
Cell lysates for malondialdehyde (MDA), glutathione (GSH), catalase (CAT), superoxide dismutase (SOD), total antioxidant status (TAS), total oxidant status (TOS), DNA fragmentation, caspase 3 (CASP3) and cytochrome C somatic (CYCS), tumor necrosis factor-alpha (TNF-α), and interleukin-6 (IL-6) assays were prepared according to the following method. After betaine treatment (25, 40, and 50 mg/ml), adherent cells were washed by ice-cold phosphate-buffered saline (PBS; pH 7.0) gently, and then dislodged with trypsin, and collected by centrifugation at 1000×g for 5 min at 4 °C. The cells were washed three times in ice-cold PBS. Resuspended cells were incubated in fresh lysis buffer (10 mM Tris–HCl at pH 8.0, 20 mM EDTA, 1 mM dithiothreitol, 50 mM HEPES at pH 7.0, 1 mg/ml proteinase K) for 2 h at 4 °C. Thereafter, the cells were centrifuged at 16000×g for 10 min at 4 °C to remove cellular debris. Protein levels were measured by the Lowry method (Lowry et al. 1951). The prepared cell lysates were used immediately for the assays.
Biochemical measurement
TAS and TOS levels (Rel Assay Diagnostics, Gaziantep, Turkey,) in cell lysates, were measured by ELISA (PerkinElmer2030 Multilabel reader, VictorX3). The results were expressed as mmol trolox eq/l and μmol H2O2 eq/l, respectively. MDA, GSH levels, and CAT activities were measured using the method reported by his instruction (Tangjitjaroenkun et al. 2012). The MDA results were expressed as nmol/mg protein. CAT enzyme activity was measured on the absorbance values decreasing in proportion to the minute and was expressed as U/mg protein by being proportional to protein levels. SOD activities were measured with the method of Winterbourn et al. (1975). SOD activities are calculated based on the absorbance values which are changed as a result of the decrease in nitroblue tetrazolium used as substrate. Manual measurement methods were performed with Shimadzu UV-1201 spectrophotometer (Shimadzu Corporation, Kyoto, Japan). All samples were conducted independently in triplicate. All chemicals for the biochemical measurement were obtained from Sigma.
DNA fragmentation was evaluated according to the method applied by Wyllie. DNA fragmentation in cell lysates was expressed as a percentage of total DNA in the supernatant fraction (Wyllie 1980). CYCS levels and CASP3 activities were measured using a commercial kit (Cloud-Clone Corp., USA, cat no. SEA594Ra and SEA626Ra, respectively). The concentrations of CASP3 and CYCS in cell lysates were shown as ng/ml in comparison with the optical density of the standard curve.
TNF-α and IL-6 levels were measured using a commercial kit (Cloud-Clone Corp., USA, cat no. SEA133Ra and SEA079Ra). The concentrations of TNF-α and IL-6 in cell lysates were shown as pg/ml in comparison with the optical density of the standard curve.
Inverted microscopy
Cells were incubated on the coverslips in 6-well plates and treated with different betaine concentrations (25, 40, and 50 mg/ml) determined by MTT. Then, the plates were scrutinized under an inverted microscope for morphological alterations.
Hematoxylin-eosin staining
The hematoxylin-eosin staining is a widespread method used for examining cellular changes (Fischer et al. 2008). The morphological alterations of DU-145 cells were observed under a BX51 light microscope (Olympus Corporation, Tokyo, Japan) attached with a DP70 digital camera (Olympus Corporation, Tokyo, Japan). In this respect, DU-145 cells were seeded into 6-well plates until they adhered on the bottom of the flasks. After that, the cell medium was removed and the cells were rinsed with PBS. The cells were incubated with different betaine concentrations (25, 40, and 50 mg/ml) determined by MTT for 24 h. Later, the cells were fixed with 100% ice-cold methanol and rewashed with PBS. Next, hematoxylin stain was applied for 4 min and the cells were immersed into 1% ammonia solution. Subsequently, the eosin stain was applied for 5 min. Eventually, the cells were washed with distilled water, dried, and mounted with the aqueous mounting medium.
Immunocytochemistry
Cells were incubated on the coverslips in 6-well plates and treated with different betaine concentrations (40 and 50 mg/ml) determined by MTT beforehand. Twenty-four hours later, the cells were washed with PBS and fixed with 100% ice-cold methanol for 10 min. Once the cells were rinsed with PBS three times, samples were immersed in 0.2% Triton X for 5 min, and then incubated in blocking solution for 10 min. Thereafter, the anti-Bax and anti-Bcl2 antibodies were added over the fixed cells and the samples were incubated overnight at 4 °C. The moisture was maintained to keep antibodies from drying, followed the 10-min incubation with biotinylated goat anti-polyvalent reagent and in turn with streptavidin peroxidase. Next, the samples were stained with 3-amino-9-ethylcarbazole (AEC) and hematoxylin as a counterstaining, consecutively. Finally, the samples were mounted with the aqueous mounting medium.
Statistical analysis
All experiments were done in three replicates. Results were expressed as the mean ± SD (standard deviation). Statistical analyses were performed by using SPSS 21 (SPSS Inc., Chicago, IL) and Graph-Pad Prism 7 (San Diego, CA, USA) statistical software. Firstly, the Kolmogorov-Smirnov and Shapiro-Wilk normality tests were used to determine whether the data conformed to normal distribution or not. In case the data were distributed normally, the analysis of variance (ANOVA) followed by post hoc Tukey test was performed for multiple comparisons. The statistical significance was considered as p < 0.05.
Results
Inhibitory effect of betaine on DU-145 cell viability
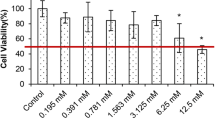
Change of DU-145 cell viabilities according to different betaine concentrations are shown in Fig. 1. The treatments with low doses of betaine concentrations (0.78, 1.56, 3.13, 6.25 mg/ml) caused a statistically insignificant increase in cell viability when compared with the control (118.4, 118.5, 103.8, and 100.2%, respectively). The betaine concentration of 50 mg/ml was the first concentration indicating a significant decrease from untreated cells, in which the cell viability was 61.7% (p < 0.001). At the betaine concentration of 75 mg/ml, the cell viability was reduced to 19.1% (p < 0.001). IC25, IC50, and IC75 concentrations of betaine in DU-145 cells for 24 h were found as 30.88, 61.77, and 92.65 mg/ml. We used the betaine concentrations (25, 40, and 50 mg/ml) lower than IC50 concentrations to show its effect on viable cells.
Inhibition of DU-145 cell proliferation treated with betaine. DU-145 cells were treated with various concentrations of betaine (0.78, 1.56, 3.13, 6.25, 12.5, 25, 50, 75, 100, and 150 mg/ml) according to the MTT assay results. Asterisk denotes significant difference of p < 0.001 when compared with the control. The results are displayed as mean ± SD of three individual 96-well plates (n = 3)
Betaine triggered oxidative stress, apoptosis, and inflammation in DU-145 cells
Change of antioxidant and oxidant molecule levels according to different betaine concentrations in DU-145 cell are shown in Fig. 2. As shown in Fig. 2a and e, the treatments with 40 and 50 mg/ml of betaine caused a significant increase in MDA and TOS levels on DU-145 cells exposed to increasing concentrations of betaine compared with the control (all p < 0.001) while 25 mg/ml betaine concentration caused insignificant increases in MDA and TOS levels compared with the control (all p > 0.05). Moreover, 50 mg/ml concentration led to the greatest increase in MDA and TOS levels (139.22 ± 3.12 nmol/mg protein and 13.74 ± 0.42 μmol H2O2 eq/l, respectively).
Antioxidant and oxidant molecule levels in DU-145 human prostate cancer cell treated with betaine. a Malondialdehyde (MDA) levels. b Glutathione (GSH) levels. c Superoxide dismutase (SOD) activity. d Catalase (CAT) activity. e Total oxidant status (TOS). f Total antioxidant status (TAS). **P < 0.0001 when compared with the control and *P < 0.05 when compared with the group between 40 and 50 mg/ml. The results are displayed as mean ± SD of three individual 96-well plates (n = 3)
On the contrary, statistically significant decreases in antioxidant defense system were observed in DU-145 cells after 40- and 50-mg/ml betaine treatments. The betaine led to a considerable reduction in SOD, CAT, GSH, and TAS levels in DU-145 cells. The activity of SOD in cells treated with increasing concentrations of betaine was shown in Fig. 2c. Unlike the 25-mg/ml concentration, 40- and 50-mg/ml betaine concentrations produced significant reductions in SOD activity (28.35 ± 0.89 and 20.42 ± 2.24 U/mg protein respectively, both p < 0.001).
It can be seen from Fig. 2b and d that GSH level and CAT activity showed significant decreases in higher concentrations of betaine (all p < 0.001). Betaine concentration at 50 mg/ml caused a 59% decrease in GSH and 33% decrease in CAT activity compared with the control. On the other hand, the lowest betaine concentration (25 mg/ml) did not cause significant alterations in GSH level and CAT activity compared with the control.
Higher betaine concentrations exerted significant decreases in the TAS levels compared with the control cells (Fig. 2f, both p < 0.001). The highest decrease (42%) in TAS level was detected in 50-mg/ml betaine concentration (1.01 ± 0.02 mmol trolox eq/l). However, 25 mg/ml betaine did not significantly affect the TAS level in cells.
We found that the 24-h betaine treatment (40 and 50 mg/ml) significantly increased the DNA fragmentation %, CYCS level, and CASP3 activity, by inducing apoptotic pathways in DU-145 cells (Fig. 3a–c, all p < 0.001). The levels of DNA fragmentation, CYCS, and CASP3 in control were 8.1%, 6.16 ± 0.04, and 3.35 ± 0.15 ng/ml, respectively. However, the levels of DNA fragmentation, CYCS, and CASP3 increased approximately twofold due to exposure to betaine concentrations compared with the control. Furthermore, the increases in DNA fragmentation, CYCS, and CASP3 were the highest in 50-mg/ml betaine–treated group.
DNA fragmentation, cytochrome C somatic (CYCS) levels, and caspase 3 (CASP3) activities in DU-145 prostate cancer cell treated with betaine. a DNA fragmentation level. b CYCS level. c CASP3 level. *P < 0.05, **P < 0.0001, and ***P < 0.01 when compared with the control. The results are displayed as mean ± SD of three individual 96-well plates (n = 3)
Changes of TNF-α and IL-6 levels according to different betaine concentrations in DU-145 cell are shown in Fig. 4. TNF-α and IL-6 levels were significantly higher in the 40 and 50-mg/ml betaine–treated groups than the control (Fig. 4a and b, all p < 0.001). However, 25 mg/ml betaine did not affect significantly the TNF-α and IL-6 levels (all p > 0.05) in DU-145 cells. The highest TNF-α (220.9 ± 0.72) and IL-6 (316.30 ± 1.30) levels were detected in the 50-mg/ml–treated group.
However, when TNF-α in the 50-mg/ml betaine–treated group significantly differs from 40-mg/ml betaine–treated group (p < 0.05), IL-6 did not differ significantly between both betaine-treated groups.
Betaine-induced morphological observations in DU-145 Cells
Inverted microscope images of DU-145 cells are shown in Fig. 5. Betaine diminished the density of DU-145 cells and caused a number of abnormalities, such as cell shrinkage and rounded cell shape in a concentration-dependent manner.
Inverted microscope images of the DU-145 cells. a Control. b 25 mg/ml betaine. c 40 mg/ml betaine. d 50-mg/ml betaine–treated groups. Note that the cell density decreases and the number of rounded and shrunken cells are increased in betaine-treated cells in a concentration-dependent manner. Objective magnification is × 10
Light microscope images of hematoxylin-eosin stained DU-145 cells are shown in Fig. 6. Untreated cells possessed typical morphology with their standard nuclei and cytoplasm (Fig. 6a and b). However, in betaine-treated cells, numerous morphological alterations were observed concentration-dependently as shrunken cells and condensed and crescent-shaped nuclei as well as decrease in cell number (Fig. 6c–f).
Hematoxylin-eosin-stained DU-145 cells. a, b Typical-shaped DU-145 cells (arrows) with their nuclei in the control group. c, d There are morphological changes in 40 mg/ml of the betaine-treated group including cellular rounding (arrow) and condensed nucleus and eosinophilic cytoplasm (lined arrow). e, f Same degenerations persist more severely in 50 mg/ml of the betaine-treated group. e Membrane blebbings (arrow), a bunch of shrunken cells (star). f Cells with condensed nuclei and eosinophilic cytoplasm (arrows). Bars in a, c, and e denote 50 μm and bars in b, d, and f indicate 20 μm
Light microscope images of Bax (apoptotic protein) and Bcl-2 (anti-apoptotic protein) immunostained DU-145 cells are shown in Fig. 7. Untreated DU-145 cells were positively immunostained with bcl-2 whereas both of betaine-treated (40 and 50 mg/ml) cells showed gradually negative staining (Fig. 7a–c). As for Bax, untreated cells were negatively immunostained with Bax whereas both of betaine-treated (40 and 50 mg/ml) cells showed gradually positive staining (Fig. 7d–f).
Bcl-2 and Bax immunostaining of control and betaine-treated DU-145 cells. a Bcl-2 staining of untreated cells. Note the positive staining. b Bcl-2 staining of 40-mg/ml betaine-treated cells. The cells are barely stained. c Bcl-2 staining of 50-mg/ml betaine–treated cells. No staining is observable. d Bax staining of untreated cells. Note that the cells stained barely. e Bax staining of 40-mg/ml betaine–treated cells. Note that the cells stained moderately. f Bax staining of 50-mg/ml betaine–treated cells. Increase in Bax staining is apparent. All bars denote 50 μm
Discussion
In recent years, there has been a growing interest in revealing the protective and therapeutic effects of natural compounds, especially for the treatment of diseases with high mortality, such as cancer. Although some scientific studies indicated that betaine has protective effects on liver and cervical carcinoma cells, its effects on prostate cancer have not been investigated sufficiently. Several in vitro and in vivo studies confirmed that betaine has a number of anticancer effects, such as inhibition of carcinogen activation, cancer cell proliferation, angiogenesis, and metastasis (Guo et al. 2015). In addition to the positive effects on metabolic diseases, there are also human studies reporting that betaine is also associated with various cancer, including breast, lung, liver, colorectal, and nasopharyngeal (Ying et al. 2013; Zeng et al. 2014; Xu et al. 2009; Zhou et al. 2017). High-dose intake of betaine reduced the risks of many cancers. In one clinical study, 100 mg of betaine and choline consumption per day reduced cancer incidence by 11% (Sun et al. 2016). However, some studies found conflicting results (Cho et al. 2007). For example, no correlation was found between colorectal cancer and betaine intake (Lee et al. 2010). In our current study, for the first time, we examined the effects of different doses of betaine on prostate cancer cell line DU-145.
Betaine plays a crucial role in the betaine-homocysteine and DNA methylation mechanisms. The disruption of DNA methylation mechanism, such as excessive methylation of tumor suppressor genes, low methylation of oncogene, and pro-metastatic genes, is considered to be associated with almost all types of cancers (Shao et al. 2011). The betaine is well-known to present a carbon unit in single-carbon metabolism. Thus, a breakdown in single-carbon metabolism can affect the level of gene expression, leading to disruption of the DNA repair process and formation of carcinogenesis. There is such a mechanism in cancer prevention studies (Iorio et al. 2010; Katz-Brull et al. 2002). Since DNA fragmentation is one of the important parameters pointing to this process, we aimed to investigate the effect of different doses of betaine on DNA degradation in DU-145 cells. There are studies showing that betaine intake from outside produces significant effects on some sulfur-containing amino acids (Craig 2004). For example, such a reinforcement effectively increases methionine and its metabolites (Cholewa et al. 2014). Betaine may alter GSH metabolism. Betaine could significantly increase the SAM:SAH ratio, GSH levels, and the activity of methionine adenosyl transferases (MAT), which are implicated in single-carbon metabolism (Kwon et al. 2009). All these studies encouraged us to measure GSH levels.
In the current study, according to our results, high-dose betaine decreased cell viability and caused morphological changes in DU-145 cells. Application of different concentrations of betaine (1–4% w/v) to Hepa 1-6 and C34 liver cancer cell lines for 2 and 4 days resulted in no statistical difference in total cell viability (Oliva et al. 2012). Betaine at concentrations ˂ 10 mM did not cause any detectable cytotoxicity in N9 microglial cell. However, higher doses of betaine (10–100 mM) decreased cell viability (Shi et al. 2019). In in vitro studies, exogenous betaine administration increased cell growth and decreased necrosis in HeLa cervical carcinoma cells (Guo et al. 2015) and primary lymphocyte cells (Ji et al. 2009) at low doses. The former study also showed that high concentration of betaine (> 5.0 mg/ml) inhibited the proliferation and increased necrosis in the HeLa cells. As for our study, while we did not detect any statistically significant change in cell viability up to betaine concentrations of 25 mg/ml, we observed a significant reduction in DU-145 cell viability at high-dose betaine concentrations when compared with the control.
Epidemiology and clinical studies demonstrated that reactive oxygen species (ROS) play a crucial role in carcinogenesis (Franco et al. 2008; Abrahim et al. 2012). The imbalance of oxidant-antioxidant system and increased oxidative stress are the features focused on mostly in cancer research. A study related to betaine consumption found that betaine decreased lipid peroxidation products and suppressed oxidative stress by increasing the amount of the main antioxidant enzymes, including GPx and SOD in rat liver (Alirezaei et al. 2014). SOD activities diminish in many cancers depending on excess production of ROS. In a study, betaine-treated HeLa cells showed a dose-dependent decrease in Cu/Zn-SOD activities (Guo et al. 2015). In a different cell line, the study of bovine mammary epithelial cells (mammary alveolar cells, MAC-T), betaine (25 mM) pretreatment reversed the heat-induced reduction in total antioxidant status by restoring SOD and CAT activities and reducing MDA content (Li et al. 2019). Our results show that betaine (40 and 50 mg/ml) lowered GSH levels, SOD and CAT activities, and elevated MDA levels in DU-145 cells. In total, we demonstrated the change of these measurements with TAS and TOS levels.
The ratio between Bax and Bcl protein expression is often used as apoptotic index (Tang et al. 2017). The genes encoding Bcl-2 and Bax regulate cell apoptosis. That is, the Bcl-2 protein encoded by BCL-2 gene resists cell apoptosis and Bax shows the opposite effect. Low-dose betaine therapy increased cellular Bcl-2/Bax ratio in HeLa cell, thereby inhibiting cell apoptosis and promoting cell proliferation (Guo et al. 2015). However, high concentration of betaine inhibited the growth of cervical cancer cells and activated caspase 3 apoptosis signaling pathway, thereby triggering apoptosis in the same study. Similarly, we observed that high-dose betaine concentrations promoted cell apoptosis and inhibited cell proliferation in DU-145 cells. Betaine diminished the ratio of Bax/Bcl-2 and caspase-3 activity in MAC-T cell (Li et al. 2019). Betaine added to hepatocytes showed an increase in caspase 3 activity (Kharbanda et al. 2005). DNA fragmentation is an indicator of cellular apoptosis (Kerr and Winterfold 1994). Some plant extracts and their phenolic acids were applied to DU-145 cells and no DNA fragmentation was detected (Yumrutaş et al. 2018). In contrast, high-dose betaine, as a natural compound, induced DNA fragmentation in our study.
Although NF-kB is generally considered an anti-apoptotic and pro-inflammatory mediator, it has a complex role together with TNF-α in cell signaling (Lin et al. 1999). Four percent of betaine effectively reduced A549 cell viability by augmenting TNF-α levels (Bingula et al. 2016). In our study, 40- and 50-mg/ml betaine doses applied to DU 145 cells increased pro-inflamatory cytokines, TNF-α, and IL-6.
As for the limitation of our study, we could not examine SAM:SAH ratio and homocysteine and methionine levels. In our future studies, we plan to search the levels of these metabolites and gene expression in the pathway of single-carbon metabolism.
Conclusions
In the present study, betaine inhibited the proliferation and caused the deformities and apoptosis in DU-145 cancer cells concentration-dependently. Furthermore, high-dose betaine treatment triggered oxidative stress, apoptosis, and inflammation by increasing TOS levels decreasing GSH and TAS in DU-145 prostate cancer cell line. Our results suggest that dose-dependent betaine supplementation may be a new alternative source for traditional procedures in the treatment of prostate cancer.
References
Abrahim NN, Kanthimathi MS, Abdul-Aziz A (2012) Piper betle shows antioxidant activities, inhibits MCF-7 cell proliferation and increases activities of catalase and superoxide dismutase. BMC Complement Altern Med 12:220. https://doi.org/10.1186/1472-6882-12-220
Alirezaei M, Jelodar G, Ghayemi Z, Mehr MK (2014) Antioxidant and methyl donor effects of betaine versus ethanol-induced oxidative stress in the rat liver. Comp Clin Pathol 23(1):161–168. https://doi.org/10.1007/s00580-012-1589-0
Almashhadany A, Shackebaei D, Van der Touw T, Jones GL, Suleiman MS, King N (2015) Homocysteine exposure impairs myocardial resistance to ischaemia reperfusion and oxidative stress. Cell Physiol Biochem 37(6):2265–2274. https://doi.org/10.1159/000438582
Baggott JE, Tamura T (2015) Homocysteine, iron and cardiovascular disease: a hypothesis. Nutrients 7(2):1108–1118. https://doi.org/10.3390/nu7021108
Bingula R, Dupuis C, Pichon C, Berthon JY, Filaire M, Pigeon L, Filaire E (2016) Study of the effects of betaine and/or C-phycocyanin on the growth of lung cancer A549 cells in vitro and in vivo. J Oncol 2016:1–11. https://doi.org/10.1155/2016/8162952
Cavallaro RA, Fuso A, Nicolia V, Scarpa S (2010) S-adenosylmethionine prevents oxidative stress and modulates glutathione metabolism in TgCRND8 mice fed a B-vitamin deficient diet. J Alzheimers Dis 20(4):997–1002. https://doi.org/10.3233/JAD-2010-091666
Chen YM, Liu Y, Liu YH, Wang X, Guan K (2014) Zhu HL (2014) Higher serum concentrations of betaine rather than choline is associated with better profiles of DXA-derived body fat and fat distribution in Chinese adults. Int J Obes 39(3):465–471. https://doi.org/10.1038/ijo.2014.158
Cho E, Holmes M, Hankinson SE, Willett WC (2007) Nutrients involved in one-carbon metabolism and risk of breast cancer among premenopausal women. Cancer Epidemiol Biomark Prev 16(12):2787–2790. https://doi.org/10.1158/1055-9965.EPI-07-0683
Cholewa JM, Guimarães-Ferreira L, Zanchi NE (2014) Effects of betaine on performance and body composition: a review of recent findings and potential mechanisms. Amino Acids 46(8):1785–1793. https://doi.org/10.1007/s00726-014-1748-5
Clark HP, Carson WF, Kavanagh PV, Ho CPH, Shen P, Zagoria RJ (2005) Staging and current treatment of hepatocellular carcinoma. Radiographics 25:3–23. https://doi.org/10.1148/rg.25si055507
Craig SA (2004) Betaine in human nutrition. Am J Clin Nutr 80(3):539–549. https://doi.org/10.1093/ajcn/80.3.539
Day CR, Kempson SA (2016) Betaine chemistry, roles, and potential use in liver disease. Biochim Biophys Acta 1860:1098–1106. https://doi.org/10.1016/j.bbagen.2016.02.001
Du YP, Peng JS, Sun A, Tang ZH, Ling WH, Zhu HL (2009) Assessment of the effect of betaine on p16 and c-myc DNA methylation and mRNA expression in a chemical induced rat liver cancer model. BMC Cancer 9(1):261. https://doi.org/10.1186/1471-2407-9-261
Duong FH, Christen V, Filipowicz M, Heim MH (2006) S-Adenosylmethionine and betaine correct hepatitis C virus induced inhibition of interferon signaling in vitro. Hepatology 43:796–806. https://doi.org/. https://doi.org/10.1002/hep.21116
Ferlay J, Soerjomataram I, Ervik M, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F (2012) GLOBOCAN 2012 v1.0, Cancer incidence and mortality worldwide: IARC CancerBase No. 11 [Internet], International Agency for Research on Cancer, Anonymous, Lyon, France, 2013, p 2015
Fischer AH, Jacobson KA, Rose J, Zeller R (2008) Hematoxylin and eosin staining of tissue and cell sections. Cold Spring Harbor Protocols (5) pdb-prot4986
Fizazi K, Scher HI, Molina A, Logothetis CJ, Chi KN, Jones RJ, Staffurth JN, North S, Vogelzang NJ (2012) Saad F (2012) Abiraterone acetate for treatment of metastatic castration-resistant prostate cancer: final overall survival analysis of the COU-AA-301 randomised, double-blind, placebo-controlled phase 3 study. Lancet Oncol 13:983–992. https://doi.org/10.1016/S1470-2045(12)70379-0
Franco R, Schoneveld O, Georgakilas AG, Panayiotidis MI (2008) Oxidative stress, DNA methylation and carcinogenesis. Cancer Lett 266:6–11. https://doi.org/10.1016/j.canlet.2008.02.026
Gerile SC, Ohyama K, Masuko T, Kusama T, Morita K, Sogawa N, Kitayama S (2012) Inhibitory action of antidepressants on mouse betaine/GABA transporter (BGT1) heterologously expressed in cell cultures. Int J Mol Sci 13(3):2578–2589. https://doi.org/10.3390/ijms13032578
Ghagane SC, Puranik SI, Nerli RB, Hiremath MB (2017) Evaluation of in vitro antioxidant and anticancer activity of Allophylus cobbe leaf extracts on DU-145 and PC-3 human prostate cancer cell lines. Cytotechnology 69(1):167–177
Guo Y, Xu LS, Zhang D, Liao YP, Wang HP, Lan ZH, Guan WJ, Liu CQ (2015) Betaine effects on morphology, proliferation, and p53 induced apoptosis of hela cervical carcinoma cells in vitro. Asian Pac J Cancer Prev 16(8):3195–3201. https://doi.org/10.7314/APJCP.2015.16.8.3195
Hacioglu C, Kar F, Kacar S, Sahinturk V, Kanbak G (2019) High concentrations of boric acid trigger concentration-dependent oxidative stress, apoptotic pathways and morphological alterations in DU-145 human prostate cancer cell line. Biol Trace Elem Res:1–10
Huerta S, Gaulet EJ, Haerta-Yepez S, Livingston EH (2007) Screening and detection of apoptosis. J Surg Res 1:143–156. https://doi.org/10.1016/j.jss.2006.07.034
Iorio E, Ricci A, Bagnoli M, Pisanu ME, Castellano G, Di Vito M, Venturini E, Glunde K, Bhujwalla ZM, Mezzanzanica D, Canevari S, Podo F (2010) Activation of phosphatidylcholine cycle enzymes in human epithelial ovarian cancer cells. Cancer Res 70:2126–2135. https://doi.org/10.1158/0008-5472.CAN-09-3833
Ji Y, Gao S, Feng X, He L (2009) Calcium channel mechanism by which betaine promotes proliferation of lymphocytes in mice. China J Chin Materia Medica 34(15):1959–1963
Katz-Brull R, Seger D, Rivenson-Segal D, Rushkin E, Degani H (2002) Metabolic markers of breast cancer: enhanced choline metabolism and reduced choline-ether-phospholipid synthesis. Cancer Res 62:1966–1970
Kerr JFR, Winterfold CM (1994) Apoptosis its signifcance in cancer and cancer therapy. Cancer 73:2013–2026
Kharbanda KK, Rogers DD II, Mailliard ME, Siford GL, Barak AJ, Beckenhauer HC, Sorrell MF, Tuma DJ (2005) Role of elevated S-adenosylhomocysteine in rat hepatocyte apoptosis: protection by betaine. Biochem Pharmacol 70(12):1883–1890. https://doi.org/10.1016/j.bcp.2005.09.021
Kim DH, Sung B, Kang YJ, Jang JY, Hwang SY, Lee Y, Kim M, Im E, Yoon J, Kim C, Chung H, Kim N (2014) Anti-inflammatory effects of betaine on AOM/DSS-induced colon tumorigenesis in ICR male mice. Int J Oncol 45(3):1250–1256. https://doi.org/10.3892/ijo.2014.2515
Kwon DY, Jung YS, Kim SJ, Park HK, Park JH, Kim YC (2009) Impaired sulfur-amino acid metabolism and oxidative stress in nonalcoholic fatty liver are alleviated by betaine supplementation in rats. J Nutr 139(1):63–68. https://doi.org/10.3945/jn.108.094771
Lee JE, Giovannucci E, Fuchs CS, Willett WC, Zeisel SH, Cho E (2010) Choline and betaine intake and the risk of colorectal cancer in men. Cancer Epidemiol Biomarkers Prev 19(3):884–887. https://doi.org/10.1158/1055-9965.EPI-09-1295
Li C, Wang Y, Li L, Han Z, Mao S, Wang G (2019) Betaine protects against heat exposure–induced oxidative stress and apoptosis in bovine mammary epithelial cells via regulation of ROS production. Cell Stress Chaperones 24(2):453–460. https://doi.org/10.1007/s12192-019-00982-4
Lin B, Williams-Skipp C, Tao Y, Schleicher MS, Cano LL, Duke RC, Scheinman RI (1999) NF-휅B functions as both a proapoptotic and antiapoptotic regulatory factor within a single cell type. Cell Death Differ 6(6):570–582
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275
Madsen SK, Rajagopalan P, Joshi SH, Toga AW, Thompson PM (2015) Higher homocysteine associated with thinner cortical gray matter in 803 participants from the Alzheimer’s Disease Neuroimaging Initiative. Neurobiol Aging 36:S203–S210. https://doi.org/10.1016/j.neurobiolaging.2014.01.154
Mosharov E, Cranford MR, Banerjee R (2000) The quantitatively important relationship between homocysteine metabolism and glutathione synthesis by the transsulfuration pathway and its regulation by redox changes. Biochemistry 39(42):13005–13011. https://doi.org/10.1021/bi001088w
Oliva J, Zhong J, Buslon VS, French SW (2012) The effect of SAMe and betaine on Hepa 1–6, C34 and E47 liver cell survival in vitro. Exp Mol Pathol 92(1):126–130. https://doi.org/10.1016/j.yexmp.2011.10.001
Pastor A, Collado PS, Almar M, Gonzálezgallego J (1996) Microsomal function in biliary obstructed rats: effects of S-adenosylmethionine. J Hepatol 24(3):353–359. https://doi.org/10.1016/S0168-8278(96)80016-X
Philippou V, Hadjipavlou M, Khan S, Rane A (2013) Complementary and alternative medicine (CAM) in prostate and bladder cancer. BJU Int 112:1073–1079. https://doi.org/10.1111/bju.12062
Salseven GS, Dixit VM (1999) Caspase activation: the induced proximity model. Proc Natl Acad Sci U S A 96:10964–10967. https://doi.org/10.1073/pnas.96.20.10964
Schartumhansen H, Ueland PM, Pedersen ER, Meyer K, Ebbing M, Bleie Ø, Svingen FT, Seifert R, Vikse BE, Nygård O (2013) Assessment of urinary betaine as a marker of diabetes mellitus in cardiovascular patients. PLoS One 8:e69454. https://doi.org/10.1371/journal.pone.0069454
Schrader AJ, Boegemann M, Ohlmann CH, Schnoeller TJ, Krabbe LM, Hajili T, Jentzmik F, Stoeckle M, Schrader M, Herrmann E, Cronauer MV (2014) Enzalutamide in castration-resistant prostate cancer patients progressing after docetaxel and abiraterone. Eur Urol 65:30–36. https://doi.org/10.1016/j.eururo.2013.06.042
Shao C, Sun W, Tan M, Glazer C, Bhan S, Zhong X, Fakhry C, Sharma R, Westra WH, Hoque MO, Moskaluk CA, Sidransky D, Califano JA, Ha PK (2011) Integrated, genome-wide screening for hypomethylated oncogenes in salivary gland adenoid cystic carcinoma. Clin Cancer Res 17:4320–4330. https://doi.org/10.1158/1078-0432.CCR-10-2992
Shi H, Wang XL, Quan HF, Yan L, Pei XY, Wang R, Peng XD (2019) Effects of betaine on LPS-stimulated activation of microglial M1/M2 phenotypes by suppressing TLR4/NF-κB Pathways in N9 Cells. Molecules 24(2):367. https://doi.org/10.3390/molecules24020367
Sun S, Li X, Ren A, Du M, Du H, Shu Y, Wang W (2016) Choline and betaine consumption lowers cancer risk: a meta-analysis of epidemiologic studies. Sci Rep 6:35547. https://doi.org/10.1038/srep35547
Tang Y, Yu F, Zhang G, Yang Z, Huang F, Ding G (2017) A purified serine protease from Nereis virens and its impaction of apoptosis on human lung cancer cells. Molecules 22(7):1123. https://doi.org/10.3390/molecules22071123
Tangjitjaroenkun J, Supabphol R, Chavasiri W (2012) Antioxidant effect of zanthoxylum limonella Alston. J Med Plant Res 6:1407–1414. https://doi.org/10.5897/JMPR10.846
Ueland PM, Holm PI, Hustad S (2005) Betaine: a key modulator of one-carbon metabolism and homocysteine status. Clin Chem Lab Med 43:1069–1075. https://doi.org/10.1515/CCLM.2005.187
Wang L, Martins-Green M (2014) Pomegranate and its components as alternative treatment for prostate cancer. Int J Mol Sci 15:14949–14966. https://doi.org/10.3390/ijms150914949
Winterbourn CC, Hawkins RE, Brian M, Carrell RW (1975) The estimation of red cell superoxide dismutase activity. J Lab Clin Med 85:337–341
Wyllie AH (1980) Glucocorticoid-induced thymocyte apoptosis is associated with endogenous endonuclease activation. Nature 284(5756):555–556
Xu X, Gammon MD, Zeisel SH, Bradshaw PT, Wetmur JG, Teitelbaum SL, Neugut AI, Santella RM, Chen J (2009) High intakes of choline and betaine reduce breast cancer mortality in a population-based study. FASEB J 23(11):4022–4028. https://doi.org/10.1096/fj.09-136507
Yi EY, Kim YJ (2012) Betaine inhibits in vitro and in vivo angiogenesis through suppression of the NF-kappaB and Akt signaling pathways. Int J Oncol 41(5):1879–1885. https://doi.org/10.3892/ijo.2012.1616
Ying J, Rahbar MH, Hallman DM, Hernandez LM, Spitz MR, Forman MR, Gorlova OR (2013) Associations between dietary intake of choline and betaine and lung cancer risk. PLoS One 8(2):e54561. https://doi.org/10.1371/journal.pone.0054561
Yu DY, Xu ZR, Li WF (2004) Effects of betaine on growth performance and carcass characteristics in growing pigs. Asian Australas J Anim Sci 17(12):490–493. https://doi.org/10.5713/ajas.2004.1700
Yumrutaş Ö, Pehlivan M, Güven C, Bozgeyik I, Bozgeyik E, Yumrutaş P, Üçkardeş F (2018) Investigation of cytotoxic effect of salvia pilifera extracts and synthetic chlorogenic and caffeic acids on DU145 prostate cancer cells line. Kahramanmaraş Sütçü İmam Üniversitesi Tarım ve Doğa Dergisi 21(2):141–147. https://doi.org/10.18016/ksudobil.302249
Zeisel S (2017) Choline, other methyl-donors and epigenetics. Nutrients 9(5):445. https://doi.org/10.3390/nu9050445
Zeng F, Xu C, Liu Y, Fan Y, Lin X, Lu Y, Zhang C, Chen Y (2014) Choline and betaine intakes are associated with reduced risk of nasopharyngeal carcinoma in adults: a case–control study. Br J Cancer 110(3):808–816. https://doi.org/10.1038/bjc.2013.686
Zhao G, He F, Wu C, Li P, Li N, Deng J, Peng Y (2018) Betaine in inflammation: mechanistic aspects and applications. Front Immunol 9. https://doi.org/10.3389/fimmu.2018.01070
Zhou RF, Chen XL, Zhou ZG, Zhang YJ, Lan QY, Liao GC, Chen YM, Zhu HL (2017) Higher dietary intakes of choline and betaine are associated with a lower risk of primary liver cancer: a case-control study. Sci Rep 7(1):679. https://doi.org/10.1038/s41598-017-00773-w
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kar, F., Hacioglu, C., Kacar, S. et al. Betaine suppresses cell proliferation by increasing oxidative stress–mediated apoptosis and inflammation in DU-145 human prostate cancer cell line. Cell Stress and Chaperones 24, 871–881 (2019). https://doi.org/10.1007/s12192-019-01022-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12192-019-01022-x