Abstract
The trace elements and minerals in Terminalia pallida fruit ethanolic extract (TpFE) were determined by the instrument inductively coupled plasma-mass spectrometry (ICP-MS), and the cardioprotection of TpFE against isoproterenol (ISO)-administered rats was studied. Rats were pretreated with TpFE (100, 300, and 500 mg/kg bw) for 30 days, with concurrent administration of ISO (85 mg/kg bw) for two consecutive days. The levels of trace elements and minerals in TpFE were below the permitted limits of World Health Organization standards. ISO administration significantly increased the heart weight and cardiac marker enzymes in serum, xanthine oxidase, sodium, and calcium in the heart, whereas significantly decreased body weight, reduced glutathione, glutathione-S-transferase, superoxide dismutase, and potassium in the heart. Oral pretreatment of TpFE significantly prevented the ISO-induced alterations. This is the first report that revealed the determination of trace elements and mineral nutrients of TpFE by ICP-MS which plays a principal role in the herbal drug discovery for the treatment of cardiovascular diseases.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Myocardial infarction (MI) is the death of heart tissue, which occurs due to the oxygen deficiency that caused by the blood flow interruption to the myocardium. The main risk factors responsible for MI are hypercholesterolemia, hyperlipoproteinemia, diabetes mellitus, high blood pressure, and smoking [1].
Isoproterenol (ISO) acts as a sympathomimetic agonist on β-adrenergic receptor that results to injury of the heart tissue [2]. ISO generates free radicals that cause irreversible damage to the myocardium [3] and the generated oxygen-free radicals involved in the MI [4]. ISO-induced MI is the best existing experimental model, and the observed variations in animal’s myocardium are alike to that of human MI [5].
Terminalia pallida (T. pallida) popularly known as “Harad” is an endemic tree belongs to the family Combretaceae. T. pallida fruit has been appreciated as cardioprotective in the Indian ancient medicinal text Ayurveda. T. pallida fruit decoction cures diarrhea and maceration of the fruit powder has been used for diabetes treatment [6]. The ethanolic extract of T. pallida fruits (TpFE) has antibacterial and antifungal [7], antiulcer [8], antioxidant, and hepatoprotective activities [9].
Due to the increasing industrialization activity along with the pollution of environment, the determination of trace elements concentration in natural medicinal plants is crucial for their safe consumption. Furthermore, mineral elements such as macronutrients or micronutrients that are essential for human health can be harmful when they exceed their recommended levels in medicinal plants [10]. Inductively coupled plasma-mass spectroscopy (ICP-MS) is a potent instrument to determine the trace elements and minerals. ICP-MS technique is useful to analyze the sensitive range of elements from 7 to 250 (Li to U) [11]. The quantification of trace elements and minerals in medicinal plants for herbal drug quality control is essential. Hence, in this study, the trace elements such as lead (Pb), cobalt (Co), cadmium (Cd), barium (Ba), arsenic (As), and aluminum (Al) and the mineral elements such as copper (Cu), chromium (Cr), nickel (Ni), manganese (Mn), iron (Fe), potassium (K), sodium (Na), calcium (Ca), and magnesium (Mg) were analyzed by ICP-MS. In our previous study, TpFE has been standardized by LC-MS for the presence of phenolic compound gallic acid (GA), and has been reported that TpFE ameliorated myocardial injury by stabilizing the membrane bound ATPase enzymes in the myocardium [12]. The purpose of the present study is to determine the trace elements and minerals of TpFE by ICP-MS and to investigate the modulatory role of electrolytes on the membrane bound ATPases in cardioprotection.
Materials and Methods
Chemicals
Metal standard solutions were supplied from BDH laboratory, England. Nitric acid and ISO were procured from Sigma-Aldrich Co., USA. The positive control GA was obtained from SRL Pvt. Ltd., India. The rest of all reagents were of analytical high grade.
Preparation of Extract
The coarse powder of T. pallida fruits was prepared by using mechanical grinder and extracted with absolute ethanol in Soxhlet apparatus. Rotary evaporator was used to remove the solvent. The extract was orally fed to the rats by suspending in distilled water. Before the experimental study, the TpFE was analyzed for acute toxicity studies and the multiple doses such as 100, 300, and 500 mg/kg body weight (bw) were selected by following OPPTS guidelines (http://www.epa.gov/oppts/home/guideline.htm). TpFE at the dose of 80 mg/kg bw is recommended as human equivalent dose which is calculated by following US Food and Drug Administration (USFDA) guidelines [13]. Extrapolation of animal dose to humans can be done by applying the formula from Table 1.
Sample Digestion
The digestion of sample was performed on Topwave Analytik Jena microwave digestion system by following the recommendations of manufacturer. About 300 mg of TpFE was transferred to a digestion vessel containing 10 ml of 65% nitric acid. The operating conditions of microwave oven are given in Table 2. The TpFE sample was digested to obtain the clear solution. The digested sample was cooled to room temperature and filtered using Whatman (no. 42) filter paper. The filtrate was diluted by adding 50 ml of distilled water.
ICP-MS Analysis
The digested sample was used for trace elements and minerals analysis by using iCAP Q ICP-MS (Thermo Fisher Scientific Instrument). The instrument was optimized based on the recommendations of the manufacturer. ICP-MS operating conditions are given in Table 3. The instrument was calibrated by external calibration using blank and working standards (0.05–1 μg/ml). A multi-element standard solution with concentration 10 μg/ml was used. Each sample was analyzed in three replicates.
Animals
Male albino Wistar strain rats (100–120 g) were used by housing in polyacrylic cages. The animals were acclimatized in a well-ventilated animal house for a week and maintained in standard laboratory conditions by providing with animal diet and water ad libitum. The animal experimental study use was approved by Sri Krishnadevaraya University, Institutional Animal Ethics Committee, India (Reg. No. 470/01/a/CPCSEA).
Animal Experimental Protocol
Fifty-six rats were equally categorized into seven groups:
-
Group 1:
Control
-
Group 2:
TpFE (500 mg/kg bw)
-
Group 3:
ISO (85 mg/kg bw)
-
Group 4:
TpFE (100 mg/kg bw) + ISO
-
Group 5:
TpFE (300 mg/kg bw) + ISO
-
Group 6:
TpFE (500 mg/kg bw) + ISO
-
Group 7:
GA (15 mg/kg bw) + ISO
The positive control GA (15 mg/kg) was dissolved in saline. Intragastric tube was used for the oral treatment of TpFE and GA. The rats were administered with distilled water dissolved ISO (85 mg/kg) by subcutaneous injection. The rats were pretreated with TpFE and GA for the period of 30 days. ISO was administered on the 29th and 30th day, and the animals were sacrificed by cervical decapitation. Before 12 h of sacrifice, the rats were made fasting. Blood was collected from the heart puncture, and the serum was obtained by centrifugation at 1000 g for 10 min. The heart was separated then cleaned with ice cold saline (0.9%), and 0.1 M Tris-HCl buffer was used to prepare heart homogenate. The fresh homogenate was applied for centrifugation at 3000 rpm for 5 min, and then the collected clear supernatant was utilized for assays.
Biochemical Analysis
The serum was used to analyze marker enzymes like creatine kinase (CK), aspartate aminotransferase (AST), alanine aminotransferase (ALT), and lactate dehydrogenase (LDH) by using diagnostic kits. The electrolytes like sodium ion (Na+) and potassium ion (K+) were measured in the heart by Monozyme kits, India. The electrolyte calcium ion (Ca2+) was estimated in the heart homogenate by Span Diagnostics Reagent Kit, India. Xanthine oxidase (XOD) [14], reduced glutathione (GSH) [15], glutathione-S-transferase (GST) [16], and superoxide dismutase (SOD) [17] were analyzed in the heart homogenate. Protein level was measured by Lowry et al. method [18].
Data Statistical Study
Statistical study of data was carried out by using one-way analysis of variance (ANOVA), and the values were expressed as means ± S.D. Mean differences between the groups were determined by using Duncan’s multiple range test (DMRT). Statistically, p < 0.05 was considered as significant.
Results
The values of trace elements in TpFE are represented in Table 4. Cd and Pb concentrations in TpFE were lower than the admissible levels regulated by the World Health Organization (WHO), whereas the element As was not detected in TpFE. Cd was the lowest concentration among all the analyzed trace elements (0.011 μg/g). There are no standard WHO recommended values for the heavy metals Ba, Co, and Al.
The mineral elements such as micronutrients (Cr, Cu, Mn, and Ni) and macronutrients (Fe, Na, K, Ca, and Mg) in TpFE are represented in Table 5. The results revealed that the TpFE contained mineral elements that are necessary for human health with the concentrations ranging from 2.0 to 3805.0 μg/g. The level of Ni was not detected in TpFE. The standard international limits are not available for the mineral elements in herbal drugs.
Figures 1 and 2 represent the effect of TpFE on heart weight and body weight. ISO-administered rat group exhibited significantly (p < 0.05) increased the heart weight and significantly (p < 0.05) decreased body weight as compared to normal rat group. Oral treatment with TpFE (300 and 500 mg/kg bw) and GA (15 mg/kg bw) to ISO-induced myocardial infracted rat group decreased the heart weight significantly (p < 0.05) and increased the body weight significantly (p < 0.05) as compared to ISO-treated rat group. TpFE at 500 mg/kg bw pretreatment to ISO-administered rats group significantly (p < 0.05) increased and maintained the body weight to near normal. TpFE in 100 mg/kg bw did not show significant effect on both the heart and body weights of experimental rats.
Table 6 shows the effect of TpFE on serum marker enzymes CK, LDH, AST, and ALT. The levels of CK, LDH, AST, and ALT enzymes increased significantly (p < 0.05) in the serum of ISO administrated rats group as compared with those of control rats group. Upon pretreatment of rats with GA (15 mg/kg bw) and TpFE (100, 300, and 500 mg/kg bw) dose-dependently, the condition was reversed where the levels of cardiac marker enzymes decreased significantly (p < 0.05) in ISO-treated rat group when compared with untreated ISO rat group. TpFE at 100 mg/kg bw did not decrease significantly (p < 0.05) the activity of the enzyme CK. TpFE pretreatment at 500 mg/kg bw to ISO-administered rats group significantly (p < 0.05) increased and maintained LDH at near normal levels.
Data in Table 7 represents the effect of TpFE on the heart electrolytes such as sodium, calcium, and potassium. ISO rat group showed significant (p < 0.05) increase in the levels of sodium and calcium and significant (p < 0.05) decrease in the levels of potassium. Pretreatment with TpFE (100, 300, and 500 mg/kg bw) and GA (15 mg/kg bw) significantly (p < 0.05) restored the altered levels of sodium, potassium, and calcium in ISO-administered rats when compared to ISO alone administered rats. TpFE in 100 mg/kg bw decreased the levels of sodium and calcium, and increased the levels of potassium but not significantly (p < 0.05). There is no significant effect on the levels of electrolytes with the treatment of TpFE in 500 mg/kg bw alone.
Figure 3 depicts the effect of TpFE on the activity of XOD in normal group and ISO group rats. ISO-administered cardiotoxicity showed a significant (p < 0.05) increase in the activity of XOD when compared to control group. TpFE (100, 300, and 500 mg/kg bw) dose-dependently decreased the activity of XOD. Pretreatment with TpFE in 500 mg/kg bw and GA in 15 mg/kg bw significantly (p < 0.05) decreased the activity of XOD and maintained near to normal in ISO-administered rats when compared to the ISO alone administered rats. There is no significant change with treatment of TpFE (500 mg/kg bw) alone.
Effect of Terminalia pallida fruit ethanolic extract (TpFE) on xanthine oxidase (XOD) in the myocardium of control and isoproterenol (ISO)-administered rat groups. Results are mean ± S.D. Values with unusual lowercase letters (a, b and c) significantly differ from one another (p < 0.05, DMRT). Asterisk indicates the group that does not significantly differ from TpFE (100 mg/kg bw) + ISO-administered group. Number sign indicates the group that does not significantly differ from TpFE (300 mg/kg bw) + ISO-administered group. Euro sign indicates the group that does significantly differ from TpFE (500 mg/kg bw) pretreated group and not significantly differ from TpFE (300 mg/kg bw) + ISO-administered group
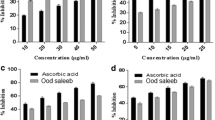
Data illustrated in Figs. 4, 5, and 6 show the effect of TpFE on antioxidants in the heart of control and experimental rats. A significant (p < 0.05) decrease is observed in the level of GSH and the activity of GSH dependent enzyme GST in the heart tissue. Further a significant (p < 0.05) decrease is observed in the activity of antiperoxidative enzyme SOD in the myocardium of ISO-treated group when compared to control group. GA (15 mg/kg bw) and TpFE (100, 300, and 500 mg/kg bw) dose-dependent pretreatments increased the antioxidants significantly (p < 0.05) when compared to ISO alone treated group. TpFE at 100 mg/kg bw did not increase the activities of GST and SOD significantly (p < 0.05) when compared to ISO-administered rats. Rats pretreated with TpFE at 500 mg/kg bw showed significant increase and maintained to near normal activities of GST and SOD compared to ISO-administered rats.
Effect of Terminalia pallida fruit ethanolic extract (TpFE) on the level of antioxidant reduced glutathione (GSH) in the myocardium of control and isoproterenol (ISO)-administered rat groups. Results are mean ± S.D. Values with unusual lowercase letters (a, b, c, d, and e) significantly differ from one another (p < 0.05, DMRT)
Effect of Terminalia pallida fruit ethanolic extract (TpFE) on the activity of antioxidant enzyme glutathione-S-transferase (GST) in the myocardium of control and isoproterenol (ISO)-administered rat groups. Results are mean ± S.D. Values with unusual lowercase letters (a, b, and c) significantly differ from one another (p < 0.05, DMRT)
Effect of Terminalia pallida fruit ethanolic extract (TpFE) on the activity of antioxidant enzyme superoxide dismutase (SOD) in the myocardium of control and isoproterenol (ISO)-administered rat groups. Results are mean ± S.D. Values with unusual lowercase letters (a, b, and c) significantly differ from one another (p < 0.05, DMRT). Asterisk indicates the group that does not significantly differ from TpFE (300 mg/kg bw) + ISO-administered group
Discussion
The herbal medicine development involved various processing steps like cultivation, collection, and harvesting which may contaminate with heavy toxic metals. The heavy toxic element accumulation in medicinal plants depends on plant species, composition of soil, climate factors, and environmental pollution [19]. Heavy metal toxicity is the major hindrance in the global acceptance of herbal medicine. ICP-MS is an appropriate choice to determine the heavy toxic metals and mineral elements in medicinal plants. The ICP-MS analyzed heavy toxic metals such as Cd and Pd in TpFE were low which met WHO standards. The levels of Cd, Pb, Ba, and Co are consistent with previous studies [20, 21]. Although the levels of Al are high in TpFE, our reported results are less than the values reported for Al in medicinal plants like Juniper, Thyme, Mentha, and Curcuma [20]. Minerals are the essential nutrients for human health. The levels of Fe in TpFE are under the physiological limits of plants. The highest concentration of calcium usually found in plants [22]. In our study also, we observed higher calcium concentrations. The abundance of minerals such as Cr, Cu, Mn, Fe, Na, K, Ca, and Mg in TpFE was in good agreement with the previous reports [20, 23, 24].
Cardiac necrosis caused by the injection of ISO at the dose of 85 mg/kg has been used as a well-established standardized experimental model of MI in rats, which is useful to discover numerous cardioprotective compounds [25] and antioxidants. In this study, an increased heart weight has been observed which may be due to the accumulation of fluid content in edematous fluid compartments of intramuscular spaces and necrosis of myocardial fibers, and decreased body weight has been observed which may be due to reduction activity of food consumption by the ISO-administered rats [26]. The decreased heart weight and increased body weight by TpFE may relate the amelioration of cardiac necrosis. The results are in line with previous report [27].
ISO treatment causes cardiac damage resulting in enhanced cell membrane permeability by which cardiac enzymes were released into the circulation of blood. In the present study, the marker enzymes of serum CK, LDH, AST, and ALT were extremely increased in ISO-treated rats. TpFE treatment significantly encountered the toxic effects of ISO by decreasing the level of all these enzymes. The reduced myocardial damage and decreased levels of cardiac enzymes in serum may be due to the cardioprotection of TpFE. This result is in concord with the earlier report [28].
Literature survey revealed that very little research work has been executed to study the effect of ISO-induced myocardial necrosis on electrolytes. Hence, our study designed to investigate the role of ISO on electrolytes in myocardial infarcted rats. Electrolyte imbalance may cause severe and even life threatening metabolic disorders of the heart, the liver, the lungs, and the kidneys [29]. The electrolytes Na+ and K+ play a vital role in cardiovascular activities [30]. The heart tissue of ISO-administered rat group exhibited a significant increase in the concentration of sodium and calcium ions and a significant decrease in the concentration of potassium ions when compared to control rats. The result is in concurrent with an earlier report [31]. The resulted changes in the concentrations of Na+, K+, and Ca2+ ions in the heart tissue of ISO-administered rats could be due to altered activity of ATPase enzymes in the cell membrane as a result of lipid peroxidation produced by the action of ISO [32]. In our earlier study, we reported the variation of ATPase enzymes during ISO-induced MI in rats [12]. The elevated levels of free fatty acids in myocardium noncompetitively that inhibit the enzyme Na+-K+ ATPase has been reported [33]. The sodium channel inhibition may be the cause for the accumulated and increased levels of intracellular sodium ions. Potassium channels are sensitive to ATP and closed when the level of ATP is high. Reduction of ATP concentration during ISO-induced ischemia leads to the potassium channel opening that further promoting the decreased concentration of potassium ions in the myocardium [34]. Elevated concentration of intracellular sodium ions also resulted in depressed effects of calcium ions and augment Ca2+ influx [35]. ISO showed the increase formation of cyclic adenosine monophosphate (cAMP) with the enhanced activity of adenylate cyclase enzyme. The cAMP molecules involved in the phosphorylation of various sites on C-terminal chains and enhance the opening of calcium channel [36]. This may be the cause for increased levels of calcium ions in myocardial tissue. TpFE pretreatment in ISO-administered rats can ameliorate the altered levels of sodium, potassium, and calcium ions. This protective effect of TpFE could be due to the preventive activity of oxidative damage of “SH” group on ATPases across the blockage of lipid peroxidation of membrane lipids. This indicates the membrane stabilization property of TpFE.
XOD is a predominant free radical generating system [37]. During ischemia, xanthine dehydrogenase is transformed to XOD by the degradation of adenosine nucleotide into hypoxanthine and xanthine. XOD acts on xanthine and hypoxanthine to generate oxygen-free radicals [38]. The reports revealed that free radicals derived from XOD can alter myocardial structure and depress myocardial function [39]. In this study, TpFE pretreatment reduced the activity of XOD in ISO-administered rats. This might be due to the inhibition of superoxide-free radical generation by TpFE. The result is in accordance with previous report [40].
GSH is an antioxidant that plays a vital role in the removal of reactive oxygen species such as superoxide anions, alkoxy radicals, and hydrogen peroxide, and acts as a substrate for GST and GPx [41]. Among the free radical scavenging enzymes, SOD is the most important antioxidant enzyme. SOD protects against oxidative damage by decomposing O2 and H2O2 prior to their participation in the formation of hydroxyl radicals [42]. ISO metabolic products increase oxidative stress and generate redundant free radicals [4]. Many reports reveal that oxidative damage accompanied by free radicals progresses the pathogenesis of cardiac damage [43]. ISO-administered cardiac injury occurs due to free radicals generated oxidative stress which leads to necrosis of the heart. In this investigation, a significant (p < 0.05) decrease in GSH content, GST, and SOD activity has been reported in ISO group when compared to normal group. It could be due to the elevation of reactive oxygen species such as superoxide and H2O2, which involves to the antioxidants inhibition. The decreased concentration of GSH in the heart of ISO-treated rats may be due to the increased utilization of GSH to augment the activity of GST and in preserving SH group containing proteins from lipid peroxides. GST activity reduced when glutathione availability reduced on ISO administration [44]. TpFE pretreatment exhibited improvement in both nonenzymatic and enzymatic antioxidants such as GSH, GST and SOD. The result is in consistent with earlier report [45]. The antioxidant activity of TpFE was due to its natural constituents like phenolic acids, saponins, flavonoids, triterpenes, and alkaloids [ 46, 47]. The compound GA which was standardized in TpFE has proved as strong antioxidant [48], and it might be responsible for the antioxidant activity of TpFE.
Conclusion
The results of present study concluded that trace elements analyzed by ICP-MS in TpFE were within the acceptable limits; thus, the extract is safe and the TpFE could serve as a good source of macro- and micronutrients. The supplementation of TpFE dose-dependently exerts notable protection on myocardium by virtue of its strong antioxidant activity. TpFE at the dose of 500 mg/kg bw effectively ameliorated the heart weight, the body weight, and the activities of cardiac marker enzymes, electrolytes, XOD, and antioxidants in ISO-induced myocardial infarcted rats. T. pallida fruit could be used as a medicinal food for the treatment of cardiovascular ailments. Further T. pallida fruit could be used in herbal drug development for the treatment of cardiovascular ailments.
References:
Khader YS, Rice J, John L, Abueita O (2003) Oral contraceptives use and the risk of myocardial infarction: a meta-analysis. Contraception 68:11–17
Sushamakumari S, Jayadeep A, Kumar JS, Menon VP (1989) Effect of carnitine on malondialdehyde, taurine and glutathione levels in heart of rats subjected to myocardial stress by isoproterenol. Ind J Exp Biol 27:134–137
Chatelain P, Gremel M, Brotelle R (1987) Prevention by amiodarone of phospholipid depletion in isoproterenol-induced ischemia in rats. Eur J Pharmacol 144:83–90
Singal PK, Kapur N, Dhillon KS, Beamish RE, Dhalla NS (1982) Role of free radicals in catecholamine-induced cardiomyopathy. Can J Physiol Pharmacol 60:1390–1397
Nirmala C, Puvanakrishnan R (1996) Protective role of curcumin against isoproterenol induced myocardial infarction in rats. Mol Cell Biochem 159:85–93
Thammanna Rao KN, Nagaraju N (1990) Medicinal plants of tirumala. TT Devasthanams, Tirupati
Jeevan Ram A, Bhakshu LM, Venkata Raju RR (2004) In vitro antimicrobial activity of certain medicinal plants from Eastern Ghats, India, used for skin diseases. J Ethnopharmacol 90:353–357
Gupta M, Mazumder UK, Manikandan L, Bhattacharya S, Senthilkumar GP, Suresh R (2005) Antiulcer activity of ethanol extract of Terminalia pallida Brandis. in Swiss albino rats. J Ethnopharmacol 97:405–408
Palani S, Raja S, Venkadesan D, Karthi S, Sakthivel K, Senthil Kumar B (2009) Antioxidant activity and hepatoprotective potential of Terminalia pallida. Arch Appl Sci Res 1:18–28
Mustafa S, Mustafa T, Ibrahim N, Hayati S (2004) Comparison of microwave, dry and wet digestion procedures for the determination of trace metals contents in spice samples produced in Turkey. J Food Drug Anal 12:254–258
Sun S, Li J (2015) Determination of Zr, Nb, Mo, Sn, Hf, Ta and W in seawater by N-benzoyl-N-phenylhydroxylamine extraction chromatographic resin and inductively coupled plasma-mass spectrometry. Microchem J 119:102–107
Shaik AH, Rasool SN, Vikram Kumar Reddy A, Abdul Kareem M, Saayi Krushna G, Lakshmi Devi K (2012) Cardioprotective effect of HPLC standardized ethanolic extract of Terminalia pallida fruits against isoproterenol-induced myocardial infarction in albino rats. J Ethnopharmacol 141:33–40
US Food and Drug Administration (USFDA) (2005). Guidance for Industry: estimating the maximum safe starting dose in adult healthy volunteer. US Food and Drug Administration, Rockville
Bergmeyer HU, Gawehn K, Grassl M (1974) Xanthine oxidase. In: Bergmeyer HU (ed) Methods of enzymatic analysis. New York, p 521–522
Ellman GL (1959) Tissue sulfhydryl groups. Arch Biochem Biophys 82:70–77
Habig WH, Pabst MJ, Jakoby WB (1974) Glutathione-S-transferase, the first enzymatic step in mercapturic acid formation. J Biol Chem 249:7130–7139
Marklund S, Marklund G (1974) Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur J Biochem 47:469–474
Lowry OH, Rose Brough NJ, Randal RJ (1951) Protein measurements with the Folin phenol reagent. J Biol Chem 193:265–275
Lasisi AA, Yusuff AA, Ejelonu BC, Nwosu EO, Olayiwola MA (2005) Heavy metal and macronutrient content in selected herbal plants of Nigeria. Int J Chem 15:147–154
Ibrahim MA (2014) Determination of some mineral and heavy metals in Saudi Arabia popular herbal drugs using modern techniques. Afr J Pharm Pharmacol 8(36):893–898
Filipiak-Szok A, Kurzawa M, Szlyk E (2015) Determination of toxic metals by ICP-MS in Asiatic and European medicinal plants and dietary supplements. J Trace Elem Med Biol 30:54–58
Guil JL, Torija ME, Gimenez JJ, Rodriguez-Garcia I, Gimenez A (1996) Oxalic acid and calcium determination in wild edible plants. J Agric Food Chem 44:1821–1823
Jia L-H, Liu Y, Li Y-Z (2011) Determination of wholesome elements and heavy metals in safflower (Carthamus tinctorius L.) from Xinjiang and Henan by ICP-MS/ICP-AES. J Pharm Anal 1(2):100–103
Hicsonmez U, Erees FS, Ozdemir C, Ozdemir A, Cam S (2009) Determination of major and minor elements in the Malva sylvestris L. from Turkey using ICP-OES techniques. Biol Trace Elem Res 128:248–257
Prince PS (2011) A biochemical, electrocardiographic, electrophoretic, histopathological and in vitro study on the protective effects of (−) epicatechin in isoproterenol-induced myocardial infarcted rats. Eur J Pharmacol 671:95–101
Patel V, Upaganlawar A, Zalawadia R (2010) Cardioprotective effect of melatonin against isoproterenol-induced myocardial infarction in rats: a biochemical, electrocardiographic and histoarchitectural evaluation. Euro J Pharmacol 644:160–168
Kasa JK, Singh TU, Parida S et al (2015) Assessment of Indian rosewood (Dalbergia sissoo) standardized leaf extract on isoproterenol-induced myocardial injury in rats. Cardiovasc Toxicol 15(3):250–260
El-Tantawy WH (2014) Biochemical effects of Solidago virgaurea extract on experimental cardiotoxicity. J Physiol Biochem 70:33–42
Jay NC, Peter RK, Paul KW, Prisant M (2000) New guidelines for potassium replacement in clinical practice: a contemporary review by the National Council on Potassium in Clinical Practice. Arch Intern Med 160:2429–2436
Nurminen ML, Krpela R, Vapattalo H (1998) Dietary factors in the pathogenesis and treatment of hypertension. Ann Med 30(2):1433–1450
Nagoor Meeran MF, Stanely Mainzen Prince P (2012) Protective effects of N-acetyl cysteine on membrane bound adenosine triphosphatases and minerals in isoproterenol-induced myocardial-infarcted rats: an in vivo and in vitro study. J Biochem Molecular Toxicology 26:7
Al-Numair KS, Chandramohan G, Alsaif MA (2011) Pretratement with morin, a flavonoid, ameliorates adenosine triphosphates and glycoproteins in isoproterenol induced myocardial infarction in rats. J Nat Med 48:1083–1090
Ahmed K, Thomas BS (1971) The effect of long chain fatty acids on sodium plus potassium ion stimulated adenosine triphosphatase of rat brain. J Biol Chem 246:103–109
Ferraro JM, Saiz J Jr, Ferraro JM, Thakor NV (1996) Simulation of action potentials from metabolically impaired cardiac myocytes. Role of ATP sensitive K+ current. Circ Res 79:208–221
Gubdjorson S, Hallgrimson J, Skuldottir G (1983) Properties of transport adenosine triphosphatase. In: Peter H, Geshaw GA, Paoethi R (eds) Arterial pollution. Plenum Publishing Corporation, New York, p 103
Varadi G, Mori Y, Mikala G, Schwartz A (1995) Molecular determinants of Ca2+ channel function and drug action. Trends Pharmacol Sci 16:43–49
Chambers DE, Parks DA, Patterson G, Roy R, McCord JM, Yoshida S, Parmley LF, Downey JM (1985) Xanthine oxidase as a source of free radical damage in myocardial ischemia. J Mol Cell Cardiol 17:145–152
Raghuvanshi R, Chandra M, Misra PC, Misra MK (2005) Effect of vitamin E on the platelet xanthine oxidase and lipid peroxidation in the patients of myocardial infarction. Ind J Clin Biochem 20:26–29
Prasad K, Kalra J, Bharadwaj L (1993) Cardiac depressant effects of oxygen free radicals. Angiology 44:257–270
Sangeetha T, Darlin Quine S (2009) Preventive effect of S-allyl cysteine sulphoxide (Alliin) on mitochondrial dysfunction in normal and isoproterenol induced cardiotoxicity in male Wistar rats: a histopathological study. Mol Cell Biochem 328:1–8
Townsend DM, Tew KD, Tapiero H (2003) The importance of glutathione in human disease. Biomed Pharmacother 57:144–155
Ji LL, Stratman FW, Lardy HA (1988) Antioxidant enzyme systems in rat liver and skeletal muscle. Influences of selenium deficiency, chronic training, and acute exercise. Arch Biochem Biophys 263:150–160
Dhana RK, Gurusamy K (2014) Antioxidant effect of Garcinia indica Linn fruit extract against isoprenaline hydrochloride-induced myocardial necrosis in rats. International Journal of Pharmaceutical Sciences and Drug Research 6(3):220–223
Paritha Ithayarasi A, Shymala devi CS (1997) Effect of α-tocopherol on lipid peroxidation in isoproterenol induced myocardial infarction in rats. Indian J Physiol Pharmacol 41:369
Sahu BD, Madhusudana K, Shyam SR, Ramakrishna S (2015) Lagerstroemia speciosa L. attenuates apoptosis in isoproterenol-induced cardiotoxic mice by inhibiting oxidative stress: possible role of Nrf2/HO-1. Cardiovasc Toxicol 15:10–22
Kameswara Rao B, Renuka Sudarshan P, Rajasekhar MD, Nagaraju N, Appa rao C (2003) Antidiabetic activity of Terminalia pallida fruit in alloxan induced diabetic rats. J Ethnopharmacol 85:169–172
Gupta M, Mazumder UK, Manikandan L, Bhattacharya S, Haldar PK, Roy S (2002) Antibacterial activity of Terminalia pallida. Fitoterapia 73:165–167
Priscilla DH, Prince PS (2009) Cardioprotective effect of gallic acid on cardiac troponin-T, cardiac marker enzymes, lipid peroxidation products and antioxidants in experimentally induced myocardial infarction in Wistar rats. Chem Biol Interact 179:118–124
Acknowledgments
This project was supported by King Saud University, Deanship of Scientific Research, College of Science Research Center, Saudi Arabia.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The animal experimental study use was approved by Sri Krishnadevaraya University, Institutional Animal Ethics Committee, India (Reg. No. 470/01/a/CPCSEA).
Competing Interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Althaf Hussain, S., Kareem, M.A., Rasool, S.N. et al. Trace Element Determination and Cardioprotection of Terminalia pallida Fruit Ethanolic Extract in Isoproterenol Induced Myocardial Infarcted Rats by ICP-MS. Biol Trace Elem Res 181, 112–121 (2018). https://doi.org/10.1007/s12011-017-1037-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-017-1037-8