The present study was designed to assess the phytochemical contents as well as the in vitro biological activities fruit of Rosa pisiformis and possible in vivo cardioprotective and hepatoprotective properties of the aqueous extract of R. pisiformis fruit on trace elements (cadmium, selenium, nickel, zinc, iron, copper, cobalt, chromium, manganese, lead), minerals (sodium, calcium, potassium, magnesium), glutathione and malondialdehyde in heart and liver tissue samples, serum vitamin (retinol, cholecalciferol, phylloquinone, α-tocopherol), total sialic acid, and lipid-bound sialic acid in a rat model of isoproterenol-induced oxidative damage. In the study, 40 Wistar albino rats were divided into four groups of ten each: control, isoproterenol 100 mg/kg bodyweight; isoproterenol 100 mg/kg bodyweight; then R. pisiformis 300 mg/kg bodyweight, and R. pisiformis 300 mg/kg bodyweight. Rats were given isoproterenol twice at an interval of 24 h for two days (on days 28 and 29) subcutaneously. The experimental period was maintained at 30 days. According to analysis results, caffeic acid and p-coumaric acid were found to be the high contents of the fruit extracts at 6.01 ± 0.0006 and 3.93 ± 0.007 mg/100 g dry weight. It showed that R. pisiformis (300 mg/kg bodyweight) aqueous extract had a potent action on oxidative damage. The R. pisiformis (300 mg/kg bodyweight) treatment significantly alleviated toward normalcy on the zinc, manganese, cobalt, magnesium, and sodium values in the heart, and zinc and magnesium values in liver tissue samples. These positive effects may be related to the action of p-coumaric acid and caffeic acid present in the R. pisiformis 300 mg/kg and it has hypolipidemic and antioxidant properties that could protect from myocardial damage.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cardiovascular disease is the leading cause of mortality globally [1]. The most important risk factors for cardiovascular disease include hypercholesterolemia, hypertension, smoking, and diabetes mellitus. All of these risk factors induce oxidative stress in the vessel wall [2]. Free radicals, especially reactive oxygen species (ROS), play a major role in the pathogenesis of oxidative myocardial damage with a consequence of cardiac dysfunction [3]. It has been suggested that the pathway of oxidative stress is an essential etiological factor in the development of coronary heart disease and atherosclerosis in experimental and prospective human studies [4]. Isoproterenol (ISP), a synthetic β-adrenoceptor agonist, has been observed to cause myocardial injury in rats owing to a disturbance of the physiological balance between free radical generation and the antioxidant defense system [5].
Medicinal plants have been used for centuries in the world for the treatment of diseases. Owing to their low toxicity and their good therapeutic performance, people are more interested in medicinal plants [6]. As is known, phenolic compounds are secondary metabolites derived from the pentose phosphate, phenylpropanoid, and shikimate pathways in plants [7]. These compounds are broadly found in almost all plant species and have important biological effects [8]. Rosa pisiformis (R.PS) fruit have strong antioxidant and antihyperlipidemic properties owing to their phytochemical composition. R.PS fruit has been associated with a protective effect on human health, such as systolic blood pressure and chronic chest pain for the risk of cardiovascular and liver diseases.
Our study was aimed at investigating the phytochemical contents and biological activity of R.PS fruits, which are publicly used against cardiovascular disease and are one of the endemic rosehip species, used both as a food as well as for therapeutic purposes. Their natural antioxidant compounds are used to make humans healthy. We also tried to see its protective effects on trace elements (Cd, Se, Ni, Zn, Fe, Cu, Co, Cr, Mn, Pb), minerals (Na, K, Ca, Mg), GSH and MDA in heart and liver tissue samples, vitamins (α-tocopherol, retinol, cholecalciferol, phylloquinone), TSA and LSA, and some biochemical parameters (LDH, AST, ALT, albumin, total cholesterol, glucose, HDL, LDL, triglyceride) in serum samples in a rat model that was subjected to isoproterenol-induced oxidative damage.
Experimental Chemical Part
Plant Material
Rosa pisiformis (Christ.) D. Sosn. (R.PS) fruits were collected in Van Hoşap, Güzelsu, Bahçecik village, Turkey. R.PS was identified by Dr. Fevzi Ozgokce who works in the Department of Biology. Aspecimen was stored in the Botany Herbarium of Van Yuzuncu Yil University, VANF-F13827.
α-Glucosidase, α-Amylase and Pancreatic Lipase Inhibitory Activities
Inhibitory enzyme activity of α-glucosidase was carried out using a modification of the method used by Lam, et al. [9] and recorded at 405 nm by using a microplate reader (VersaMax). Enzyme inhibitory activity of R.PS fruit extract was assayed at a concentration of 2000 μg/mL, which was used as the reference. Inhibitory enzyme activity of α-amylase of the extracts from the fruits of R.PS was determined by modifying the method used by Ali, et al. [10] determined at a wavelength of 505 nm. Acarbose was used as a reference. Inhibitory enzyme activity of pancreatic lipase of R.PS fruit extracts was conducted by the modified method proposed by Lee, et al. [11] estimated at 405 nm. Orlistat was used as the reference.
Phenolic Contents of Rosa pisiformis Fruits
The extract was prepared at a concentration of 5 mg/mL for the quantitative analysis of phenolic compounds. The phenolic compounds were measured by RP-HPLC on ACE 5 μm, C18 column (150 × 4.6 mm). The temperature of the column was maintained at 25°C. The mobile phase included formic acid (0.2%) in acetonitrile (80%; solvent A) and formic acid (0.2%) in water (solvent B). The gradient system was as follows: 0 – 10 min, from 5% A to 15% A at 0.8 mL/min flow rate, 10 – 15 min 15% A at from 0.8 mL/min to 0.6 mL/minute flow rate, 15.01/17 min 15% Aat 0.6 mL/min flow rate, 17.01/32 min from 15% to 30% at 0.8 mL/min flow rate, 32/35 min from 30% A to 100% A at 0.8 mL/min flow rate. The analysis time was 45 min. The sample injection volume was 20 μL. Within 5 min, it was returned to the initial conditions. The caffeic acid, sinapic acid, chlorogenic acid, ferulic acid, and p-coumaric acid were detected with a UV detector at 320 nm; whereas 2-hydroxycinnamic acid, vanillic acid, rutin, and protocatechuic acid were measured with a UV detector at 265 nm. For calibration, six different phenolic compound concentrations were prepared in acetonitrile (25%). For analysis of reproducibility, the phenolic compounds of standard solution were injected three times at each level of concentration. Calibration plots were prepared by reading the peak areas against the concentration. To measure the spike recovery, three different phenolic compound concentrations (1, 5, and 10 ppm) were added to the extracts. At each addition level, six assays were performed and the average was taken to calculate the percentage recovery.
Experimental Pharmacological Part
Experimental Animals
A total of 40 male Wistar albino rats (weight 200 ± 50 g, 4 weeks of age) were used for the present study. Rats were provided by the Van Yuzuncu Yıl University, Faculty of Medicine Animal Care Center. The rats were housed at normal temperature (22 ± 2°C) and normal daylight with a 12-h light/dark cycle and provided with drinking water and food ad libitum. Experiments were performed in accordance with the general principles of the Van Yuzuncu Yil University animal ethics committee (YUHADY-EK, 30.06.2011/Decision no: 06).
Experimental Design
The rats were randomly assigned to four equal groups of ten rats each. Group 1 rats were regarded as the control group and given 0.9% NaCl. Rats in group II were given ISP 100 mg/kg bodyweight dissolved in saline and subcutaneously administered twice with an interval of 24 h for two consecutive days (on days 28 and 29). Rats in group III subcutaneously received ISP 100 mg/kg bodyweight with an interval of 24 h for two following days (on days 28 and 29) and R.PS fruits 300 mg/kg bodyweight with an intragastric tube. Rats in group IV were given R.PS fruits 300 mg/kg bodyweight with an intragastric tube throughout the experimental period. The duration of treatment was 30 days for all groups.
Plant Material and Preparation of Extract
The fruits of R.PS (3 g) were infused in 300 mL of boiled distilled water for 15 min. The filtrate was then dried at 38°C in an incubator. The aqueous extracts were then prepared daily in physiological saline. The prepared extract was used to assess cardioprotective and hepatoprotective properties.
Determination of Metals in Liver and Heart Tissues
Lead (Pb), nickel (Ni), cadmium (Cd), selenium (Se), manganese (Mn), zinc (Zn), iron (Fe), cobalt (Co), copper (Cu), chromium (Cr), magnesium (Mg), sodium (Na), calcium (Ca), potassium (K) measurements in liver and heart tissue samples were performed using ICP-OES (Thermo-iCAP, 6300). Metal concentrations were measured with wet weight. Metal determination was performed with reference materials of multi-element (IV Stock 8 inorganic ventures).
Preparation of Heart and Liver Homogenates
Liver and heart tissues were washed with physiological saline (sodium chloride 0.9%), and the tissue samples were kept at –64°C until determination. Tissue homogenates was prepared according to the method of Xia, et al. [12]. Supernatant was used to determine GSH and MDA analysis.
Serum Sample Preparation
Blood samples were obtained by the intracardiac method. The serum was separated by centrifugation at 500 g at 4°C for 15 min and collected serum samples kept at –64°C until all experimental procedures were carried out.
Biochemical Parameters
Biochemical parameter measurements, namely, glucose, lactate dehydrogenase (LDH), albumin (ALB), alanine transaminase (ALT), aspartate transaminase (AST), total cholesterol (TC), triglyceride (TG), low-density lipoprotein cholesterol (LDL-C), HDL-C (high-density lipoprotein cholesterol) were determined at the laboratory of the Van YYU, Medicine Faculty, Department of Pharmacology by kit methods using a biochemical autoanalyzer (Vitros DT 60 II) in accordance with the manufacturer’s instructions.
GSH and MDA in Heart and Liver Tissue
The GSH levels of the heart and liver tissues were measured at 412 nm according to the method of Rizzi, et al. [13]. Data are presented as μmol/g protein. Malondialdehyde (MDA) levels of the heart and liver tissues were determined using the modified method by Jain, et al. [14], and recorded at 532 nm. Values were presented as μmol/g protein.
Total Protein Concentration Determination
Total protein levels of heart and liver tissue homogenates was measured according to the standard method of Lowry, et al. [15] and estimated at 695 nm.
Analysis of Vitamins (A, E, D, and K) in Serum
The serum samples were prepared according to the method determined by Su, et al. [16]. The chromatographic system used consisted of ALS autosampler G1329 (–8°C) and HP-Agilent (1100) with a diode array detector (G-1328). Separation was performed using a GL-Science, 5-μm C18 reversed phase column (250 × 4.6 mm). The mobile phase of the methanol-tetrahydrofuran mixture (80/20) was described by Siluk, et al. [17]. The chromatogram was observed at 325, 290, 265, and 248 nm for the simultaneous measuring of retinol, cholecalciferol, α-tocopherol, and phylloquinone.
TSA and LSA Analysis
Serum concentration of TSA were measured according to the method reported by Sydow [18]. The optical density was measured at 525 nm. Serum LSA level was measured using the method described by Katopodis, et al. [19], as 580 nm.
Statistical Evaluation
The results are presented as means ± standard error of the means (\(\overline{X }\) ± SEM). The Kruskal–Wallis or ANOVA test was performed for statistical analysis, and then, Tukey’s test was performed for post hoc comparisons of means. Statistical significance was regarded as p < 0.05. The statistical evaluation was conducted using the statistical software SPSS v23, (Chicago, USA).
Results and Discussion
The present study was conducted to determine the content of phytochemicals (caffeic acid and p-coumaric acid) as well as the in vitro biological activity of R.PS fruits and the possible in vivo cardioprotective and hepatoprotective properties of aqueous extract of R.PS fruits on trace elements (Cd, Se, Ni, Zn, Fe, Cu, Co, Cr, Mn, Pb), minerals (Na, K, Ca, Mg), GSH, and MDA in heart and liver tissue samples, serum vitamin (retinol, α-tocopherol, phylloquinone, cholecalciferol), LSA and TSA, and some biochemical parameters (LDH, AST, ALT, albumin, total cholesterol, glucose, HDL, LDL, triglyceride) in a rat model of isoproterenol-induced oxidative damage.
The presence of the caffeic acid, p-coumaric acid, chlorogenic acid, ferulic acid, 2-hydroxycinnamic acid, protocatechuic acid, rutin, sinapic acid, and vanillic acid was investigated in the R.PS fruit extract by using HPLC. The chlorogenic acid, p-coumaric acid, ferulic acid, caffeic acid have been identified in the extract, but, owing to the fact that the amount of chlorogenic acid and ferulic acid was below the LOQ, their amount cannot be calculated. Retention times, linear relations between concentrations and peak areas, test ranges, LOD, LOQ, RSD%, spike recovery values, and the caffeic acid and p-coumaric acid levels were given in Table 1.
Figures 1, 2, and 3 illustrate at 320 nm the chlorogenic acid, caffeic acid, sinapic acid, ferulic acid, and p-coumaric acid and at 265 nm 2-hydroxycinnamic acid, protocatechuic acid, vanillic acid, and rutin chromatograms.
Among the investigated phenolics, 2-hydroxycinnamic acid, protocatechuic acid, rutin, sinapic acid, and vanillic acid were not found in the R.PS fruit, whereas caffeic acid and p-coumaric acid were detected using the RP-HPLC method. The HPLC chromatograms of phenolic compounds are shown in Figs. 1, 2, and 3. In the results of RP HPLC analysis, caffeic acid and p-coumaric acid were found to be the important components of R.PS fruit extracts as 6.01 ± 0.0006 and 3.93 ± 0.007 mg/100 g dry weight. When the results were assessed, it was observed that R.PS fruit consist of caffeic acid and p-coumaric acid at significant levels.
α-Glucosidase and α-amylase were compared with the reference drug acarbose (97.62 ± 0.30% at 2000 μg/mL) and (83.35 ± 1.66%, at 2000 μg/mL), and for pancreatic lipase inhibitory activity orlistat was used (71.16 ± 3.56% at 1 μg/mL). α-Glucosidase inhibitory activity was found to be 62.58 ± 2.78%, at 2000 μg/mL. Pancreatic lipase and α-amylase inhibitory activity were not detected in R.PS fruit, at 2000 μg/mL. The inhibitory activity on α-glucosidase enzyme was about two times lower than that of acarbose. While assessing the results, it was shown that R.PS fruit have low inhibitory activity (IC50 = 1311 μg/mL) in α-glucosidase enzyme in comparison with the reference, acarbose (IC50 = 0.510 μg/mL). Our findings that showed R.PS fruit have a strong inhibitory effect on the enzyme α-glucosidase.
Table 2 demonstrates retinol, α-tocopherol, phylloquinone, cholecalciferol, TSA, LSA, LDH, AST, ALT, albumin, total cholesterol, glucose, HDL, LDL, and triglyceride in controls, and administration with ISP 100 mg/kg bodyweight, ISP 100 mg/kg bodyweight + R.PS 300 mg/kg bodyweight, and R.PS 300 mg/kg bodyweight groups in serum samples.
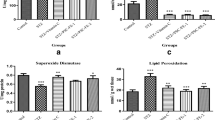
The effects of ISP and R.PS administration on trace elements (Cd, Se, Ni, Zn, Fe, Cu, Co, Cr, Mn, Pb), minerals (Na, K, Ca, Mg), GSH, and MDAlevels in heart and liver tissue samples of controls and ISP 100 mg/kg bodyweight, ISP 100 mg/kg bodyweight and R.PS 300 mg/kg bodyweight, and (300 mg/kg bodyweight R.PS) groups were presented in Table 3.
Atherosclerosis is considered a chronic inflammatory process and is affected by several events, such as oxidative stress caused by the excessive ROS generation [20]. These ROS caused a direct injury to vascular cells and cardiomyocytes and may trigger a series of local chemical reactions that increase the initial ROS-mediated cardiac myocyte dysfunction [3]. The metabolism of ISP produces quinones that react with oxygen to form hydrogen peroxide and superoxide anions, causing oxidative stress and degradation of the endogenous antioxidant system [21].
Lim, et al. [22] examined the cardioprotective effect of Korean red ginseng extracts (500 mg/kg) on serum samples taken from rats. They observed that serum LDH, AST, ALT, and heart tissue MDA levels were significantly elevated in ISP-administered alone group (for all p < 0.01). Another study by Suchal, et al. [23] evaluated serum LDH and heart tissue MDA, and GSH levels in rats administered ISP and reported that the concentrations of serum LDH and heart tissue MDA significantly (p < 0.001, p < 0.001) higher than those of the control group and GSH lower than the control group (p < 0.001). Othman, et al. [24] examined the serum samples taken from total cholesterol, triglycerides, LDL, LDH, and ALT in ISP-induced rats and observed that they were significantly (p < 0.05, p < 0.05, p < 0.05, p < 0.05, and p < 0.05) increased when comparing with control groups, whereas there was a significant decrease in HDL-C level (p < 0.01).
Hussain, et al. [25] determined that there was a significant increase in serum LDH, ALT, AST enzyme activities, and heart tissue Ca and Na (p < 0.05, p < 0.05, p < 0.05, p < 0.05, p < 0.05) in ISP-treated rats groups when compared with control groups. By contrast, there were decreased levels of K (p < 0.05) and reduced glutathione (p < 0.05) in the ISP rat group. Khan, et al. [26] reported that serum LDH, cholesterol, triglyceride, LDL, and heart MDA levels were significantly higher (p < 0.01, p < 0.01, p < 0.01, p < 0.01, p < 0.01) in ISP-administered rat groups than in the control group; in contrast, GSH and HDL level had decreased significance (p < 0.01, p < 0.01) in ISP-caused myocardial infarction in rats compared with the control group.
In the present study, the statistical analysis demonstrated clearly that Na and GSH levels of the heart tissue were lower in the ISP group than in the control group (p < 0.001, p < 0.05). Otherwise, the ISP group was also significantly elevated compared with the control group regarding MDA, LDH, AST, cholesterol, triglyceride, and LDL level (p < 0.01, p < 0.01, p < 0.01, p < 0.001, p < 0.01, p < 0.01), whereas the R.PS group had an increased level of Na compared with the ISP group (p < 0.001). Similarly, the ISP+R.PS group had a significantly higher level of MDA and cholesterol levels than the control group (p < 0.05, p < 0.01). The ISP + R.PS group was also significantly lower than the ISP group with regard to triglyceride (p < 0.01). However, the ISP group had a significantly higher level of MDA, LDH, ALT, and triglyceride than the R.PS group (p < 0.01, p < 0.01, p < 0.05, p < 0.05) in heart tissue and serum samples of rats. The level of AST concentrations was significantly elevated between the R.PS and control groups (p < 0.05).
In this study, the significant increase in triglyceride, LDL-C, total cholesterol, LDH, AST, ALT, and heart tissue MDA levels were consistent with those of other studies [22,23,24,25,26]. Our present results, the significant reduction in GSH and HDL-C levels in ISP-induced rats were agreement with the values obtained by other studies [23,24,25,26]. Another publication [25] did not support the findings of elevated Na and Ca values in an ISP-administered rat group. However, the decrease in K does agree with the data of Hussain, et al. [25].
As a result of in vivo studies on cardiac function negative effects, isoproterenol (ISP), a synthetic adrenoreceptor agonist properties is a substance on the study application with ISP, compared with controls, statistically increase TSA, LSA, MDA, LDH, AST, total cholesterol, LDL, triglyceride, cholesterol/HDL and decrease Cu, Zn, Se, Mn, Co, Mg, Na, GSH, retinol, albumin shown to have negative effects of the ISP significant in terms of statistical results. The increase in cholesterol, total lipids, and triglycerides in the serum lipid profile can also be attributed to an increase in lipid peroxidation. Our study clearly demonstrated that rats treated with the R.PS fruit extract have hypolipidemic potency.
In rats ISP myocardial infarction, there was a reduction in the marker enzymes LDH, CPK, and AST activities in the heart homogenate, followed by an elevation in their serum levels. These results confirmed the beginning of myocardial necrosis and leaking of the marker enzymes from the heart into the blood [27]. Hyperlipidemia, in particular, increased serum LDL-C levels and has been demonstrated to be a risk factor for cardiovascular disease [28]. In the current study, ISP increased oxidative damage in the heart of rats, resulting in a reduction in tissue GSH, and an increase in tissue MDA, serum LDL-C, AST, and LDH. Hyperglyceridemia and increased total cholesterol/HDL ratio indicate an increased risk of cardiovascular disease.
According to the statistical analysis, Cu, Se, Zn, Mg, and GSH levels were significantly lower in the heart tissue of the ISP group than in that of the control group (p < 0.01, p < 0.01, p < 0.01, p < 0.05, p < 0.05 respectively), whereas, Zn, Cu, and Mg were increased in the R.PS group compared with the ISP group (p < 0.05, p < 0.01, p < 0.05). Otherwise, Mg level was significantly higher in the ISP + R.PS group than in the ISP group (p < 0.05). The result of this study indicated that the R.PS fruit has positive effects on the levels of Mg and triglycerides, when there is a statistically significant difference between the ISP and the ISP + R.PS groups. In conclusion, our study has clearly shown that the fruits of R.PS may be used to prevent and treat various cardiovascular diseases.
It was found that R.PS fruit aqueous extract (300 mg/kg bodyweight) had a strong effect on oxidative damage. The results show that the R.PS (300 mg/kg bodyweight) aqueous extract had a significant anti-cardiotoxic and anti-hepatotoxic effect on ISP-induced heart and liver toxicity in vivo. Treatment with R.PS (300 mg/kg bodyweight) also resulted in significant normalization of the Zn, Mn, Co, Mg, and Na levels in the heart and the Zn and Mg levels in liver tissue samples.
Trace elements such as Cu and Co may contribute to myocardial dysfunction. It has been shown that the onset of cardiac dysfunction in Cu deficiency is rapid, when the Cu level of the liver has decreased [29]. Several experimental, epidemiological, and clinical studies have established the role of Mg2+ in the pathogenesis of cardiovascular disorders [30].
In the chronically developed CHD, decreased Se levels have led to inadequate prevention of LDL oxidation through uptake by macrophages and endothelial cells [31]. Zn deficiency correlates directly with oxidative stress. Therefore, control and regulation of the intracellular Zn content is essential with involvement of different transporter and Zn-binding proteins, such as metallothionein [32].
A reduction in α-tocopherol may result from the increased consumption of α-tocopherol in scavenging the produced oxyradicals, or endogenous antioxidants, because of an interaction between α-tocopherol and these compounds [33]. In the present study, the statistical analysis clearly shows that comparing between ISP and R.PS fruit groups indicated that the ISP group had a significantly lower level of α-tocopherol than the R.PS fruit group (p < 0.05).
The fruit extracts of R.PS had an apparently high enzyme inhibitory activity of α-glucosidase. To clarify the important antioxidant status of the plant, phenolic compounds such as p-coumaric acid and caffeic acid were quantitatively measured using RP-HPLC. The results indicated that caffeic acid and p-coumaric acid were the important constituents of the fruit extracts. As a result of an in vivo study, it was found that R.PS fruits (300 mg/kg bodyweight) may have a positive effect on rat cardiac tissue, with the damage caused by ISP having a deleterious effect on cardiac and hepatic functions. We can thus suggest that the constituents of the fruits of R.PS, particularly caffeic acid and p-coumaric acid might be in charge of the altered biochemical variables in the heart and liver tissues, as well as the biological activity of R.PS observed in this study.
In the present study, ISP caused increased oxidative damage in the hearts of rats, which was confirmed by an increase in LDL-C, LDH, total cholesterol, triglyceride, and cholesterol/HDL ratio. Owing to the treatment with R.PS fruits, these parameters were reduced. It was found that R.PS shows lipid-lowering potential. Furthermore, R.PS (300 mg/kg bodyweight) treatments successfully resulted in an improvement of Zn, Mn, Co, Mg, and Na content in heart tissue. The phytochemical composition is responsible for the pharmacological effects of R.PS fruit, containing caffeic and p-coumaric acid. R.PS (300 mg/kg bodyweight) has antioxidant and hypolipidemic properties that could protect against myocardial damage.
Conflicts of Interest Statement
The authors declare that there are no conflicts of interest.
References
B. J. Lee, Y. F. Tseng, C. H. Yen, et al., J. Nutr., 12(1),142 (2013).
H. Li, S. Horke, U. Förstermann, Trends Pharmacol. Sci., 34(6), 313 – 319 (2013).
A. J. Almzaiel, J. Contemp. Med. Sci., 1(2),18 – 22 (2015).
S. Yang, M. K. Jensen, E. B. Rimm, et al., Am. J. Epidemiol., 180(9), 901 – 908 (2014).
S. Goyal, M. K. Siddiqui, K. M. Siddiqui, et al., Exp. Toxicol. Pathol., 62(1), 61 – 74 (2010).
P. Nisha, P. A. Nazar, P.A. Jayamurthy, Food Chem. Toxicol., 47(10), 2640 – 2644 (2009).
N. Balasundram, K. Sundram, S. Samman, Food Chem., 99(1), 191 – 203 (2006).
M. P. Kähkönen, A. I. Hopia, H. J. Vuorela, et al., J. Agric. Food Chem., 47, 3954 – 3962 (1999).
S. H. Lam, J. M. Chen, C. J. Kang, et al., Phytochem., 69(5), 1173 – 1178 (2008).
H. Ali, P-J. Houghton, A. Soumyanath, J. Ethnopharmacol., 107(3), 449 – 455 (2006).
Y-M. Lee, Y. S. Kim, Y. Lee, et al., Phytother. Res., 26(5), 778 – 782 (2012).
E. Xia, G. Rao, H. Van Remmen, et al., J. Nutr., 125(2), 195 – 201 (1995).
R. Rizzi, A. Caroli, P. Bolla, et al., J. Dairy Res., 55(3), 345 – 353 (1988).
S. K. Jain, R. McVie, J. Duett, et al., Diabetes, 38(12), 1539 – 1543 (1989).
O. Ulmer Verlag Lowry, N. Rosebrough, A. Farr, et al., J. Biol. Chem., 193(1), 265 – 275 (1951).
Q. Su, K. G. Rowley, N. D. Balazs, J. Chromatogr. B. Analyt. Technol. Biomed. Life Sci., 781(1 – 2), 393 – 418 (2002).
D. Siluk, R. V. Oliveira, M. Esther-Rodriguez-Rosas, et al., J. Pharm. Biomed. Anal., 44(4), 1001 – 1007 (2007).
G. A. Sydow, Biomed. Biochim. Acta, 44(11-12), 1721 – 1723 (1985).
N. Katopodis, Y. Hirshaut, N. L. Geller, et al., Cancer Res., 42(12), 5270 – 5275 (1982).
X. B. Wang, Y. D. Han, S. Zhang, et al., J. Cell. Mol. Med., 20(12), 2362 – 2373 (2016).
A. Jyoti Roy, P. P. Stanely Mainzen, Eur. J. Pharmacol., 699(1 – 3), 33 – 39 (2013).
K. H. Lim, D. Ko, J. H. Kim, J. Ginseng Res., 37(3), 273 – 282 (2013).
K. Suchal, S. Malik, N. Gamad, et al., Phytomedicine, 23(12), 1401 – 1408 (2016).
A. I. Othman, M. M. Elkomy, M. A. El-Missiry, et al., Eur. J. Pharmacol., 794, 27 – 36 (2017).
S. A. Hussain, M. A. Kareem, S. N. Rasool, et al., Biol. Trace Elem. Res., 181(1), 112 – 121 (2018).
V. Khan, S. Sharma, U. Bhandari, et al., Life Sci., 194, 205 – 212 (2018).
V. S. Panda, S. R. Naik, Exp. Toxicol. Pathol., 60(4 – 5), 397 – 404 (2008).
A. Nakamura, Y. Monma, S. Kajitani, et al., Heart Vessels, 31(9), 1446 – 1455 (2016).
Y. Q. Yan, X. C. Liu, W. B. Jing, et al., Biol. Trace Elem. Res., 151(3), 344 – 349 (2013).
D. Kolte, K. Vijayaraghavan, S. Khera, et al., Cardiol. Rev., 22(4), 182 – 192 (2014).
C. Benstoem, A. Goetzenich, S. Kraemer, et al., Nutrients, 7(5), 3094 – 3118 (2015).
V. Kloubert, L. Rink, Food. Funct., 6(10), 3195 – 3204 (2015).
M. M Kannan, S. D Quine, Eur. J. Pharmacol., 659(1), 45 – 52 (2011).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ekin, S., Akkoyun, M.B., Kiziltas, H. et al. Phenolic Contents, Enzyme Inhibitory Activities, and Protective Effect of Aqueous Extract of Rosa Pisiformis Fruits. Pharm Chem J 57, 1799–1806 (2024). https://doi.org/10.1007/s11094-024-03081-6
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11094-024-03081-6