Abstract
The current study investigates the therapeutic and curative effect of Ulva lactuca polyphenolic extract (ULPE) in general and particularly polyphenolics compounds against heavy metal mixture (HME). The toxicity behind heavy metal is due to oxidative stress resulted from heavy metals pollution or administration through contaminated food (vegetables, water, and fish). Heavy metal toxicity plays a major role in different cardiovascular diseases. The objective of this study is aimed to examine the protective effect of ULPE against heavy metal mixture induced cardiovascular diseases through oxidative/antioxidant and inflammatory pathways. Sixty male rats (Sprague-Dawley) were assigned to six groups. Group I served as the control, group II served as the induced group receiving subcutaneously for 7 days 0.25 mg/100 gm body weight/day heavy metal mixtures (Equal concentration of Ni, Cd, Co and Hg chloride, and Pb acetate), group III received (i.p.) ULPE of dose 30 mg for 15 days, group IV served as the protected group pretreated with ULPE for 15 days as a protection dose, and then treated with the heavy metal-mixture, group V served as protected standard group pretreated with vitamin C (VitC ) (50 mg/Kg) and then treated with the heavy metal-mixture, and group VI served as standard group treated with VitC (50 mg/Kg). The main pathological changes within the heart revealed heart inflammation after heavy-metal mixtures administrations. On contrast to the protected group treated with ULPE (group IV), the protection group (group II) showed a significant increase in the antioxidant as well as anti-inflammatory biomarker. The cardiovascular biomarkers (Troponin T, CRP, and BNP) showed similar attitude elevations in induction group and decreased greatly in protection and VitC group. The antioxidant and the anti-inflammatory activities of ULPE are a consequence of their higher polyphenolic contents as well as marine secondary metabolites which are confirmed using qualitative and quantitative analysis. From the current result, we concluded that ULPE possesses a cardiovascular protective agent as a result of highly contents of different bioactive secondary metabolites which have antioxidant as well as free-radical scavenging and anti-inflammatory activates.
Graphical abstract

Showed the mechanism of ULPE as cardioprotective against heavy metal mixture
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
There are many substances in our body that is exposed to various xenobiotic compounds which need to be protected against different polluted substances including heavy metals from natural (e.g., earth’s crust, volcanic eruptions, dusts, soil, and aerosolized) and anthropogenic (e.g., agricultural, industrial practices, mining, smoking, and traffic) sources (Fathy et al. 2010; Shreadah et al. 2014, 2019). Xenobiotic in general and heavy metals particularly have been extensively used from years by human which resulted various side effects and diseases on human as well animal health exposure as well as administration of heavy metals (air, water and food) through different administration route in continues manner, Additionally, some parts of the world have increasing exposure to toxic metals, particularly in less developed countries (Abdel-Fatah et al. 2003; Järup 2003; Shakweer et al. 2006; Shobier et al. 2011; Abdel Ghani et al. 2013). Increase as well as accumulation of xenobiotic in general and toxic metals particularly in the body lead to metal toxicity (poisoning) and increase free radical’s production due to oxidative stress (Fathy et al. 2012). Many studies were reported and confirmed the important role of environmental metals in different cardiovascular disease. The awareness increased worldwide towards association between cardiovascular diseases and heavy metal contaminations to elevate the protection level against cardiovascular diseases incidence (Tellez-Plaza et al. 2018). Different metals which enter the human or the animal bodies from different sources are distributed to nearly all tissues. The xenobiotic metals inter to our bodies, replace the essential minerals, for examples, magnesium and calcium, disrupt normal cellular processes, and increase inflammation. Additionally, several of these metals participate in a variety of various pathophysiological and physiological processes and are biologically active (Lamas et al. 2016). Recently, the environmental exposures to toxic metals, for examples, nickel, mercury arsenic, lead, copper, and cadmium, have great focus in many research programs and become a major public health concern because they are highly and potentially harmful on human health. The potential effect between continuous and chronic heavy metal exposure in association with the increased heart and cardiovascular disease risk is regularly investigated by international entities, for examples, the WHO (Solenkova et al. 2014). Exposure to xenobiotic metal pollution is associated with various cardiovascular (CV) diseases. Many studies confirmed that these various effects of heavy metals don’t happen only after exposure to high levels of heavy metals but also due to low or safe exposure levels which are recorded in many negative cardiovascular outcomes. The mechanism of heavy metal toxicity is due to their electron-sharing, which can bind to sulfhydryl groups of the antioxidants enzymes systems, change and modifying their activity and function. Different xenobiotic metals such as nickel mercury, cadmium, arsenic, and lead can interact and bind to different antioxidant proteins such as glutathione, which lead to depleting its levels and ultimately leading to ROS generation and oxidative stress (Zwolak 2020). Cadmium, nickel, lead, mercury, and other metals can deactivate paraoxonase enzymes and other antioxidant enzymes, which furthermore lead to increase in free radical’s level and oxidative stress (Sharma et al. 2014). Toxicity of heavy metals leads to increase in the level of inflammation biomarkers such as IL-6, C-reactive protein, VCAM-1, and TNF-alpha (Machoń-Grecka et al. 2018). Cardiovascular disease (CVD), considered one of the most leading cause of death worldwide, is usually attributed to various reasons such as hypertension, tobacco use, diabetes, dietary factors, lack of physical activity, and environmentally exposure, but pollutants and toxic chemicals are the main contributors to CVD mortality. The cardiovascular system specifically appears to be a notable target for the actions of various metal pollutions. There are many evidences that report the association between acute exposure and cardiovascular events (Burroughs Peña and Rollins 2017). The exact mechanism of heavy metals which lead to increase cardiovascular risk still unknown, although one possible mechanism explains the impaired of antioxidants metabolism system and oxidative stress confirmed and investigated in different reports and may play a role. Inflammation and oxidative stress are likely to act in synergistically way to amplify each other’s role in the cardiovascular diseases (Engwa et al. 2019). Metals are known as non-biodegradable; they can produce different effects and persist in the environment. The maximum exposure levels for heavy metals in food have been set and recorded by different studies and international organization such as WHO (Emara and Shriadah 1991; Said et al. 2006; Shreadah et al. 2008, 2015; Thompson and Darwish 2019). Although contaminated food may contain environmental toxins, they are also a very important source of normal nutrients, such as omega 3 fatty acids, which reported in different studies as protective agent against chronic diseases, among which is CVD (Awuchi et al. 2020). So the main issue is to obtain the beneficial health content of natural normal food without increase exposure to xenobiotic contaminants particularly heavy metals. Unfortunately, not all people are able to revoke environmental pollution and exposure to toxic metals; the government should have more effort to put rules against industrial and agriculture sector which penetrate the safe level of heavy metals in their work practices, for example, sewage discharge and effluents which cause contamination because of hazardous heavy metals. Biologically active substances, on the other hand, having a potential to act as pharmaceuticals can be identified and extracted from a very heterogeneous group in the oceans (Hegazy et al. 2015a, b; Nabil-Adam et al. 2020a, b), the marine organisms, which are powerful and excellent reservoirs multi-target activity against different xenobiotic substances (Shreadah et al. 2018; Shreadah et al. 2020a, b). The unique as well as effective treasures of the ocean which are the algae produce a vast and a variety of remarkable bioactive compounds in both primary and secondary metabolites like polyphenols, flavonoids, and sulfated polysaccharides that have now received much attention among scientist and researchers as natural antioxidants and anti-inflammatory secondary metabolites (Abdel Moneam et al. 2017a, b, c, d; Abdel Moneam et al. 2018; Pereira 2018; Andrade et al. 2018; Bule et al. 2018). Seaweeds are photosynthetic similar to the plants that form biomass in seabed and intertidal zones. More than 10,000 species are recorded as well as being identified and classified in the three main phyla according to their pigmentation (Terme et al. 2018). Seaweeds are very useful agent for the prevention as well as protection and treatment of various diseases as they are considered important natural resources marine secondary metabolites of antioxidants and anti-inflammatory effect (Zhao et al. 2018). Ulva lactuca, which is a green macro alga observed worldwide, showed different bioactive activities as antioxidant immunomodulatory effects, hepatoprotective and antimicrobial activities against different pathogens (bacteria and fungi) (Shreadah et al. 2017).
Although there is confirmation about association between heavy metals and cardiovascular diseases, there is a shortage about toxicities and associated mechanisms information, so the main objective of the present study is to understand (a) to which extent the exposure of heavy metal affects and induces the cardiovascular diseases, exerts and shows an adverse health effects through investigating the causal link between heavy metals exposure and the cardiovascular disease, and (b) to investigate the role of Ulva marine polyphenolic extract against heavy metal mixture induced cardiovascular using oxidative/antioxidant pathways and hematological parameters as biomarkers.
Material and methods
Study area and seaweeds collection
Seaweed samples (Ulva lactuca) were collected from Abu-Qir Bay at Alexandria of Egypt during the summer of 2018. The site located about 36 km east of Alexandria, between longitudes 30° 5′ and 30° 22′ E and latitude 31° 16′ and 31° 21′ N. (Fig. 1).
Profitable fishing areas, however, it is subjected to major threats which are related to land-based activities including urbanization and coastal development. The bay is continuously exposed to various loads of chemicals from natural and anthropogenic sources. These factors affect the physical, chemical, and biological characteristics of the bay waters and consequently on the marine ecosystem biodiversity (Shriadah and Abdel Ghani 2007; Emara and Shriadah 2009; Shreadah et al. 2012). The fresh marine algae were collected from the bay, and identified by the hydrobiological lab at the National Institute of Oceanography and Fisheries, Alexandria, Egypt, as Ulva lactuca.
Preparation and extraction of Ulva lactuca polyphenolic extract (ULPE)
Extraction of polyphenolic compounds from Ulva lactuca were carried out using ethanol as organic solvent, the Ulva lactuca were air dried and then grinding o obtain the powder (500 g) after that samples were soaked in ethanol for 1 h in sonicator and remain overnight in dark in refrigerator (4 °C), the previous step repeated 3 times to obtain all metabolites in polyphenolic extracts (ethanol 3 × 500 mL) then solvent removed at reduced pressure and 35 °C.
Phytochemical analyses of ULPE
The quantitative analysis of ULPE of phytochemical was investigated using total phenolic total flavonoids, total tannic, sulfated polysaccharides, and total carbohydrates. Total phenolic compounds in the ULPE was investigated by the method of Taga et al. (1984) as mg gallic acid equivalent in 1 mL of the extract using the standard curve of the gallic acid, and total flavonoid compositions in the extracts were investigated by a colorimetric method (Zhishen et al. 1999), and the results were expressed as mean mg/mL of (+)-Quercetin equivalents, whereas the assessment of tannins compounds compositions was investigated according to Sun et al. (1998). Sulfate content was assayed turbidmetrically with barium chloride (Dodgson 1961), and total carbohydrates were investigated by the methods of Agrawal et al. (2015).
Screening of polyphenolic compounds using high performance liquid chromatography (HPLC)
The screening of phenolic as well as flavonoid compounds were determined using HPLC. Analytical analyses were conducted using Agilent 1260 (Agilent, USA). Chromatographic system with Kinetex 5 μm EVO C18 100 HPLC Column 150 × 4.6 mm. The separations are achieved using tertiary liner elution gradient with HPLC grade 0.2 H2PO4 (v/v), (B) methanol, and (c) acetonitrile. The injection volume is 20 μL and the detection was carried out by using WWD 284 nm according to the method described by Uddin et al. (2014).
In vitro—biochemical broad bench assay
Primary screening assay
2.2/-Diphenyl–α-picrylhydrazyl (DPPH) radical scavenging effect of the UPLE
DPPH radical scavenging assay of the UPLE was performed using modified previously established methodology by Amarowicz et al. (2007). The mixture was measured at 490 nm. Scavenging activity % was calculated using the following equation:
Experimental animals, experimental design, and tissue preparation
Rat models of cardiovascular diseases have been used to addresses the effects of chronic exposure to metals on cardiovascular system. To explore the health effects of multi-heavy metal exposure, sixty male Sprague-Dawley rats were assigned into six groups. The first group, was the negative control, (-ve control group), the second group II was induction group (toxicity group) receiving for 7 days subcutaneously 0.25 mg/100 gm body weight/day of heavy metal mixtures composed of equal concentration of Ni, Cd, Co, and Hg chloride and Pb acetate, the third group (extract group) received (i.p.) for 15 days ULPE of 30-mg dose, and the fourth group was the protected group (Protection group) pretreated with ULPE for 15 days as a protection dose, and then treated with the heavy metal-mixture group. The fifth group served as protected standard group pretreated with VitC (50 mg/Kg) and then treated with the heavy metal mixture, and group VI served as standard group treated with VitC (50 mg/kg). After the experiment protocol ended, the rats were anesthetized using the isoflurane. The blood samples were collected, sera were centrifugation at 3000 rpm for 20 min and then stored at − 20 °C for further analyses according to Abdel Moneam et al. (2017a, b, c). The heart tissues were cleaned from blood and adhering matters by washing in cold isotonic saline. The heart of each animal was quickly removed, washed, and homogenized with phosphate-buffered saline (PBS, pH 7.4/1:10 w/v). The heart homogenate was centrifuged for 30 min at 4 °C at 17,000 g, and the supernatant was used for different biochemical assays (Panda et al. 2017). The crude heart extract homogenate was used for the determinations and assessment of MDA, antioxidant, MPO, and NO.
Biochemical measurements and assessment of lipid profiling tests
Serum lipid was determined by the method of Zöllner and Kirsch (1962), and serum triglyceride was determined enzymatically by the method of Bucolo and David (1973). On the meantime, serum cholesterol was determined enzymatically by the method of Allain et al. (1973).
Biochemical measurements and assessment of cardiovascular biomarkers
Serum C-reactive protein (CRP) was determined by the method of Manack and Richards 1971, the Troponin T was determined by the methods of Herman et al. 1999, and the BNP was determined by the methods of Li et al. 2013.
Antioxidant activity and oxidative stress biomarkers in heart tissue
The oxidative stress in heart tissue was determined using lipid peroxidation assay (LPO) by measuring malondialdehyde (MDA) according to the methods of Rehman (1984). The total-reduced glutathione was determined according to the method described by Salbitani et al. (2017), and the catalase activity was determined according to Hadwan (2018).
Determination of total antioxidant capacity (TAC) in heart tissue
Determination of total antioxidant capacity in heart tissue was determined spectrophotometrically at 510 nm according to Koracevic et al. (2001).
Assessment of inflammatory biomarker
The MPO was determined according to peroxidase activity with 3, 3′, 5, 5′-tetramethylbenzidine (TMB, Sigma) and was measured according to Pulli et al. (2013). The NO was determined according to the method described by Green et al. (1982).
Histopathological study
The heart histology was investigated according to Griffith and Farris (1942).
Ethical for animal experimentation
The ethical animal treatment was according to the guideline of ethical animal treatment in the National Institute of Health (NIH) followed in adherence to established protocols, and all animal protocols were approved and accomplished by the Institutional Animal Care and Use Committee (IACUC) in Alexandria University (ethical approval reference number: AU- 0304862 ).
Online software
The current study uses different online software in graphical abstract (Bio-Render.com) and heat map based on multi-principal component analysis (Metsalu and Metsalu 2015)
Statistical analysis
The data were given as individual values and as means (X) ± standard deviation (SD) for 7 animals in each group. All statistical calculations were analyzed using SPSS and prism statistical software.
Results
The qualitative screening of UPLE
The qualitative phytochemical screening showed that the ULPE have higher contents of phytochemical compounds such as phlobatannins, saponins, and flavonoids (Table 1).
The quantitative bioactive screening for UPLE
The phytochemical analyses for Ulva lactuca are shown in Fig. 2a. The UPLE showed higher contents of total carbohydrates, total phenolic compounds, total flavonoids, total tannic acids, and total sulfated polysaccharide. The phytochemical assays for Ulva lactuca extract showed higher contents of total phenolic, flavonoid, carbohydrates, and total sulfated polysaccharide, while total tannic acids were very low compared to other phytochemical parameters (Fig. 2a).
The phytochemical screening of ULPE. a Showed the total phenolic, flavonoids, carbohydrates, sulfated polysaccharide, and tannins. b showed the total antioxidant capacity using DPPH model. c showed the total anti-inflammatory using NO assays. d The figure showed the heat map and principal component analysis using phytochemicals contents, antioxidant, and anti-inflammatory results
HPLC profiling for phenolic and flavonoids compounds in ULPE
The HPLC profiling of UPLE showed total polyphenolic contents with a variety of different phenolic and flavonoids, e.g., gallic acids, caffeine, vanillin acids, synergic acids, vanillin, ferulic acids, rutin, ellagic acids, benzoic acids, salicylic acids, and cinnamic acids (Table 2 and Figs. 3 and 4). The polyphenolic screening for UPLE showed higher total polyphenolic compounds with 4.05755 μg/mL. The UPLE showed an increase in the polyphenolic concentration which is a total of 87.91%.
Total in vitro antioxidant capacity of UPLE
The determination of total antioxidant capacity using DPPH assay revealed high total antioxidant capacity for UPLE at all concentrations from 1 up to 6 mg compared to standard drugs VitC (Fig. 2c).
Total in vitro anti-inflammatory
The anti-inflammatory activities using NO inhibition model showed that the VitC has higher anti-inflammatory activity for NO inhibition compared to UPLE at different concentrations from 1 up to 6 mg (Fig. 2b)
The lipid profiling
The current study showed that the injection of rats in induction group with toxicant heavy metal mixtures (HEM) caused a highly significant (P < 0.01) elevation in the level of serum TG (141.0 ± 15.95 and 469.3 ± 38.34 mg/dL), and in contrast, the administration of rats with the of UPLE revealed a highly significant (P < 0.01) decrease in the level of serum TG compared to control group (141.0 ± 15.95 and 115.0 ± 10.34 mg/dL). Additionally, in protection group, prior treatment with UPLE revealed a highly significant (P < 0.01) decrease in the level of serum TG of UPLE + HEM (469.3 ± 38.34 and 184.6 ± 6.335 mg/dL) compared to the induction group II (HEM-treated group) (Fig. 5). Furthermore, the VitC group showed a significant decrease (P < 0.01) in serum TG compared to HEM-treated groups, i.e., Ind. and VitC + HEM (469.3 ± 38.34 and 266.2 ± 14.98).
The induction group (HEM-injection group) revealed a highly significant (p < 0.01) elevation in the level of total cholesterol (TC) and that with compared to the -ve control group (group I) (162.6 ± 6.689 and 476.6 ± 75.16 mg/dL); in contrast to protection group (in rats pretreated with UPLE), non-significant elevation was observed (168.7 ± 5.344 mg/dL) compared to that of control group (162.6 ± 6.689 mg/dL). Interestingly, there was a highly significant (P < 0.01) reduction in the total cholesterol level of the group, i.e., UPLE + HEM group (226.7 ± 12.19 mg/dL) compared to the induction group (HEM treated) (Fig. 5), while the HEM-administrated group (induction group) resulted in a significant (P < 0.0001) reduction in the HDL level (31.17 ± 4.556 mg/dL) and a nonsignificant (P > 0.05) decrease in HDL in protection group (treated with UPLE prior to HEM injection) (50.88 ± 2.663 mg/dL) compared to controls (49.36 ± 1.387 mg/dL), whereas pretreatment with UPLE prior to HEM led to a highly significant (P < 0.001) elevation in HDL level (47.64 ± 4.908 mg/dL) compared to the induction group (HEM group) (Fig. 9). Additionally, our results revealed a highly significant (P < 0.001) increase in the level of serum VLDL of the Induction group.
Induction group (HEM-injected rats) was compared to negative control group I (28.58 ± 3.327 and 93.88 ± 7.712 mg/dL), while there was a nonsignificant decrease found in the level of serum VLDL in rats pretreated with UPLE (23.01 ± 2.078 mg/dL) compared to that of the control group I (28.58 ± 3.327 mg/dL) (Fig. 5). Furthermore, the pretreatment of rats with UPLE prior to HEM injection resulted in a highly significant (P < 0.001) decrease in the VLDL level compared to the induction group (HEM-injection) (36.09 ± 4.364 and 93.88 ± 7.712). On the meantime, the standard-treated group (VitC + HEM) revealed a significant decrease in the level of VLDL compared to HEM induction group (56.13 ± 3.009 and 93.88 ± 7.712). Additionally, the VitC group showed a nonsignificant increase in the VLDL levels compared to control group (31.84 ± 3.590 and 28.58 ± 3.327 mg/dL).
Effects of UPLE on the heart LPO and DPPH levels
The present results (Fig. 7) showing the effects of the UPLE on the heart LPO s and DPPH levels indicated that the group II (animals that received HEM only) revealed a significant reduction (P < .001) in the antioxidant capacity (71.66 ± 4.882 and 90.12 ± 0.4740) and an elevation in the heart LPO levels compared to the control group (0.0630 ± 0.008 and 0.1793 ± 0.0116). Treatment with UPLE (groups treated) and VitC (standard drugs) reflected a nonsignificant decreased or increased in the LPO and DPPH and levels compared to the control group. However, in the pretreated group, i.e., UPLE + HEM (0.09417 ± 0.013) and VitC + HEM (0.1203 ± 0.008) a remarkable reduction in the LPO levels (P < 0.05) compared to the HEM group (induction group).
Effects of UPLE on heart GSH and CAT activities
The induction of rats with heavy metal mixture (HEM) revealed a significant reduction (P < 0.05) in the levels of heart GSH and CAT activities in the induction group II (HEM group) compared to the control group (Fig. 7a and b). Treatment with UPLE and VitC led to a considerable increase (P < 0.05) in the heart GSH and CAT activities compared to group II (HEM-only group). Increase in the levels of GSH and CAT activities in the extract group that received of UPLE was significantly higher than (P < 0.05) that of the other injected groups with UPLE and VitC (Fig. 7). Moreover, in group VI (group orally treated with 30 mg/kg UPLE), remarkable elevations (P < 0.05) in the heart GSH and CAT activities compared to group II (treated with HEM) (Fig. 7).
Effects of Ulva lactuca marine extract on level of MPO and NO
The current study (Fig. 8) showing the effects of the Ulva lactuca marine extract on the MPO and NO levels indicated that the induction group II (treated with HEM) showed a significant increase (P < 0.001) in the levels of MPO (1.321 ± 0.373) as well as NO (0.8052 ± 0.11) compared their values to the control group (MPO: 0.1456 ± 0.02156 and NO: 0.1456 ± 0.02156). On contrast, treatment of Ulva lactuca marine extract and VitC led to a significant reduction (P < .001) in the levels of MPO and NO (0.552 ± 0.044 and 0.552 ± 0.044) compared to group II (HEM-only group).
Effect of UPLE on cardiovascular biomarkers (troponin T, CRP, and BNP) and mitochondrial enzymes succinate dehydrogenase (SDH)
The levels of troponin T showed significant increases ( P < 0.0001) in serum of HEM group ( 1.798 ± 0.4214) compare to normal control group (0.1450 ± 0.045) (Fig. 6), while the protection group pretreated with ULPE was significantly decreased (P < 0.0001) compared to induction group (treated with HEM). Furthermore, the pretreated VitC group showed significant decreased in troponin T compared to induction group (0.63 ± 0.1354 vs 1.798 ± 0.4214, P < 0.0001). The levels of BNP and CRP in induction group significantly increased (43.16 ± 8.45 P < 0.0001 and 14.5 ± 4.20, P < 0.0001) comparing to control group (3.97 ± 0.90 and 0.1550 ± 0.01871) in contrast to protection group which showed a significant decrease (11.63 ± 1.288, P < 0.0001; and 1.23 ± 0.419, P < 0.0001 ); additionally, the VitC + HEM (standard protection group) showed also a significant decreased in BNP and CRP (14.68 ± 1.708, P < 0.0001).
The level of heart mitochondrial enzyme activity (SDH) showed a significant decrease (P < 0.01) in the heart mitochondria of HEM-treated rats compared to the normal control rats (0.2137 ± 0.01260 and 0.0885 ± 0.01046) (Fig. 6). On the meantime, the pre-treated of ULPE to HEM toxicity rats significantly (P < 0.001) were increased the activity of SDH enzyme compared to HEM treated rats (0.1977 ± 0.0101 and 0.0885 ± 0.01046). Additionally, the pre-treated VitC to HEM toxicity rats significantly increased the activity of SDH enzyme compared to the induction group (0.1622 ± 0.006 and 0.0885 ± 0.01046). The activity of SDH heart mitochondrial enzyme in UPLE rats revealed no significant difference compared to the control rats (0.1313 ± 0.0034). Similarly, the rats treated with standard drugs (VitC) showed a non-significant decrease (0.1252 ± 0.005)
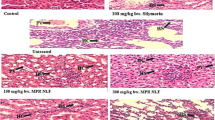
The effect of UPLE on cardiac histopathology of cardiac tissues
Control rats (Fig. 9a) show normal cardiac fibers; and the extract group is represented in Fig. 9d. On the meantime, the protective group (Fig. 9c) of UPLE and heavy metal mixtures administered rats revealed recovered architecture of cardiac myofibrils, while induction group-treated rats (Figs.1 and 2b) revealed disruption as well as degeneration of the cardiac myofibrils and highly marked necrosis in the tissue of heart organ.
Discussion
Microalgae are getting importance as a source of unique secondary bioactive metabolites with great and very interesting biological activities including chemopreventive effects against breast carcinogenesis, anti-inflammatory, antimicrobial, antidiabetic, antivirus, antioxidant, etc. which suggest it a potent source against various ailments (Abd-Ellatef et al. 2017; Shreadah et al. 2018, 2020b; Rosa et al. 2019). On the other hand, humans are generally exposed to a cocktail of different heavy metal mixtures, even if the concentration of heavy metals lower than the level documented and reported in no observable effect concentration (NOEC) (Anyanwu et al. 2018). Toxicities resulted from exposure to heavy metals are very important and underscore issue because of their nature they do not decompose or degrade easily in the nature and they bio-accumulate as well as biomagnifying lead to dangerous and damage effects in different organs and causes several diseases such as Alzheimer’s rheumatic diseases carcinogenesis, depression and different mutations (Ali et al. 2019). Their mechanism of action is initiated by the interaction of the heavy metals (toxicant) with the membrane and cell receptor in different organisms (Abdel Moniem et al. 2013). Oxidative stress and ROS production result from an imbalance between the detoxification and production of free radicals. The toxicity mechanism of free radicals and ROS is depending on their capability to oxidize biological molecules in intracellular as well as extracellular structure for several molecules such as nucleic acids, proteins, lipids, various enzyme, and proteins. Enzymatic scavengers for free radicals are one of the most effective detoxification systems in which various enzymes are involved such as superoxide dismutase’s (SOD), glutathione S-transferases, catalase as well as glutathione peroxidase, and the non-enzymatic system such as sulfated polysaccharides, polyphenolic (phenolic and flavonoids) vitamins (A, B, C and E), lipoic acid, glutathione (GSH), iron chelators, and carotenoids (Napierska et al. 2018). Previous studies showed that UPLE have varieties of bioactive secondary metabolites with highly activities and multi-target diseases and function such as gallic, tannic rosmarinic acid, caffeic acid, and other phenolic compounds, in addition to flavonoids that have the ability to inhibit oxidative stress and lipid peroxidation (Alagan et al. 2017; Shreadah et al. 2017). The current study results agree well with these findings as the levels of total antioxidant capacity were greatly diminished in the toxicity group HEM (induction group) compared to the -ve control group I (Fig. 7). Additionally, the current study revealed that the administration with UPLE and VitC revealed a significant elevation in anti-oxidants levels and that can be explained due to their higher contents of antioxidant secondary metabolites compounds (Fig. 2). This also can explain the capability of UPLE to reduce lipid peroxidation by decreasing the production of MDA in both the extract and protection groups due to the high contents of phenolic and flavonoids compounds in the UPLE. In this study, there was a remarkable increase in serum TG, TC, VLDL, and a reduction in serum HDL in group II (Fig. 5). In most cases of toxicity by metals, there are main general mechanisms focus on oxidative stress result from the imbalance of free radicals and reactive oxygen species (ROS) and defect detoxification system (enzymatic and non-enzymatic) of different species of free radicals. Additionally, there are many studies and evidence confirmed the associations between the cardiovascular toxicity and modification in the lipid profiles (Okediran et al. 2018) which is in agreement with the current observations revealing an increase in the lipid peroxidation and levels of MDA heart tissue and a decrease in antioxidants capacity (Fig. 7). Previous research studies showed that the exposure to hazard heavy metals such as arsenic, lead, cadmium, mercury, and nickel are linked with a raise risk of heart and cardiovascular diseases which are also accompanied with a change in the lipid profiles (Chowdhury et al. 2018). This is matching well with the present findings as the lipid profiles were significantly elevated in induction group (treated with HEM). Furthermore, it was found that increased serum levels of triglycerides in the rats treated with HEM induced that caused cardiovascular toxicity in induction group II may be due to the inhibition of lipase activity and/or elevation of serum inflammatory biomarkers (Pirahanchi and Sharma 2020). Additionally the increase in lipid peroxidation formation lead to modification and damage in HDL receptor and that in sequence result in elevation in the level of blood cholesterol (Marín et al. 2019). However, treatment with UPLE decreases the serum levels of TG, TC, and VLDL compared to the induction group II (toxicity group, HEM-only group), whereas UPLE administration leads to significant elevation in the level of HDL (Figs. 7, 8, 9). Previous studies from different researchers confirmed that flavonoids and other antioxidant compounds enzymatic and non-enzymatic in UPLE are important bioactive secondary metabolites compounds in pharmacological and medicinal studies in reducing serum TC, TG, HDL, and VLDL and exhibiting various protective, preventive, and therapeutic properties against different diseases such as in neurodisorder, renal protective, anti-tumor, hepatoprotective, and especially lipid-lowering activities (Karimi-Khouzan et al. 2017; Messyasz et al. 2018). In the current study, HEM administration lead to an increase of free radicals and oxidative stress with increase of MDA which is in agreement with many previous reports (Ahmadi-Naji et al. 2017). Furthermore, treatment with UPLE and VitC leads to a significant decrease in MDA. The enzymatic system antioxidants such as CAT and a non-enzymatic system such as antioxidant molecule GSH ameliorate the free radicals. CAT save also cells as it prevents oxidative stress free radicals’ molecules (hydrogen peroxide) and transfers them to water and oxygen (Sharifinasab et al. 2016). In the present study, administration of HEM caused a significant reduction of enzymatic GSH and CAT contents in the HEM group compared to the negative group (control group) (Fig. 7) which is in agreement with previous reports (Sharifinasab et al. 2016). Various enzymatic systems protect the body against free radicals and ROS. On the meantime, many xenobiotic metals have electron sharing and are able of forming covalent bonds with the sulfhydryl groups of antioxidants proteins (e.g., cysteine, glutathione, albumin, metallothionein, and homocysteine). By binding to glutathione molecules, these metals deplete its levels and, therefore, increase the intracellular concentration of free radicles as well as ROS and that was in agreement with the current study as the levels of these antioxidants biomarkers were greatly decreased in the induction group compared to the control group. Furthermore, the great decrease in the levels of glutathione (Fig. 7) in the present study was in agreement with the finding of Kumar and Trivedi (2018) who confirmed that the heavy metals toxicity may be due to the binding of toxic (xenobiotic) metals to sulfhydryl groups in different proteins such as glutathione (GSH). These interaction and binding lead to suppress and inhibit the activities of antioxidant enzyme, resulting in displacement of important essential metal elements such as copper and zinc and leading to subsequent interference in the antioxidants protein confirmation and structure, causing great deficiency in antioxidant capacity. Jan et al. (2015) reported that xenobiotic heavy metals continuous exposure in addition to their metabolism as well as excretion from the body depends on the existence of antioxidants such as glutathione, α-tocopherol, and ascorbate. The excess of ROS which attacks biomembranes propagates lipid peroxidation chain reactions, and subsequently induces different types of cell death (Su et al. 2019). On the other hand, the consequences include elevation of lipid peroxidation; damage in cell membrane, DNA damage; oxidation of amino acids in proteins and, therefore, changes in their structural conformation which affect protein activity and function and lead to inactivation (Ayala et al. 2014). One of the possible and reported mechanism explains that cardiovascular diseases are oxidative stress which promotes oxidative damage including the destruction of microtubule, damage and disruption of mitochondrial membrane potential, suppress and inhibition of adenosine triphosphate production, and then dysfunction of ion transporters such as Ca–adenosine triphosphatase and Na–K–adenosine triphosphatase causing alteration in the calcium homeostasis (Siasos et al. 2018). The current study investigated the elevation of NO which is consider as one of inflammatory biomarkers and interact and bind to sulfhydryl groups of proteins which in sequence decrease and inhabit the detoxification of oxidative stress of different species. Metals are responsible for biological impairments such as endothelial dysfunction through inhabiting endothelial nitric oxide synthase via binding with xenobiotic metals and diminishing the production of nitric oxide as well as through binding to sulfhydryl (SH) groups of NF-kB that affects the level of gene expression. Malondialdehyde (MDA), which is a breakdown product of lipid peroxidation, can be produced from oxidation of fatty acid and protein (Karimi-Khouzan et al. 2017). However, the administration of UPLE and VitC showed a significant increase in cardio-antioxidants system such as GSH and CAT (Fig. 7) which significantly reduce heart injury in groups treated with UPLE due to its high contents of polyphenolic (phenolic and flavonoids) compounds (Karimi-Khouzan et al. 2017). Myeloperoxidase, which is inflammatory biomarkers that usually increase in oxidative stress, can be generated during inflammation of heart and cardiac system as pre-inflammatory biomarkers (Khan et al. 2018). Recent findings showed also that the expression of MPO in heart increased significantly during heart damages (Ahmadi-Naji et al. 2017; Karimi-Khouzan et al. 2017). In the current study, injection of rats in induction group II with HEM significantly increased the level of heart MPO which is associated with lymphocytes infiltration in heart tissue of the induction group (Fig. 9b). It is documented that polyphenolics have different biological activities like anti-inflammatory, anti-tumor, antioxidants, and cytotoxic activity (Zhang et al. 2020). Therefore, the anti-inflammatory roles of UPLE which was observed in the present study may be attributed to existence of polyphenolic compounds in UPLE (Fig. 2). However, the MPO and histopathological examinations of heart tissues in the sixth group (HEM + UPLE) showed that the inflammatory biomarkers (MPO) and infiltration of lymphocyte cell increased compared to fifth group (Fig. 9b). The investigation of change and modification of the enzyme activities are considered one of the most important tools for pollution toxicity study and powerful biomarkers since these organic cellular catalysts control the production of the biochemical intermediates which are main effector in the normal physiological state. Moreover, the decreased activity of SDH as a physiological measure of the degree of inhibition of succinate-fumarate conversion indicates the depressed oxidative metabolism at the level of mitochondria resulting in decrease in oxidative metabolism. The decrease in the level of SDH activities can be associated with the enzyme dysfunction as a result of lipid peroxidation activations. This perhaps due to the increase in ROS and free radical production to counter these toxic effects (Rajeswarareddy et al. 2012). In the current study, the activity of the SDH enzyme is greatly decreased in in the heart tissues of the induction group (treated with heavy metal mixture) (Fig. 6). This can be attributed to the fact that exposure to heavy metals can lead to various disorders and can also result in excessive damage due to oxidative stress induced by free radical formation which is drastically affect by the action of SDH (Tretter et al. 2016).
The current study showed varieties of bioactive compounds (Figs. 2, 3, and 4 and Table 1) in the UPLE such as gallic acids (GA) that has antihyperglycemic and lipid homeostasis actions (Huang et al. 2016). In the present study, the protected group (pretreated with UPLE prior to heavy metal injection) cleared out a significant decrease in the lipid profile (Fig. 5) and revealing that GA is a potent cardioprotective agent (Jin et al. 2017). Previous studies reported that pretreatment with GA diminished the levels of cardiovascular enzymes such as ALT, AST, VLDL, CK-MB, LDH, CRP, BNP, and cardiac troponin T possibly due to the decrease of myocardial damage and thereby limiting the leakage of these enzymes from myocardium. This matches well with the current study as the VLDL was observed to be greatly decrease (Priscilla and Prince 2009). GA protects the heart through inhibiting lipid peroxidation and that because it scavenges the free radicals such as superoxide, and hydroxyl radicals (Stanely Mainzen Prince et al. 2009; Jadon et al. 2007). Moreover, GA was reported to suppress and downregulates the cardiac Nox2 expression and Nox2-induced oxidative stress response via suppression of GATA6 (which increased in cardiac diseases) or by affecting DNA-binding activity of GATA6 (Jin et al. 2017). According to Akbari (2020), study the levels of various cardiac proteins such as ECM proteins, cTnT,, CK-MB,LDL-c, , LDH, VLDL, TG and MDA levels were significantly decrease revealing that GA is a potent cardioprotective agent. Additionally, GA prevents histopathological alterations and increasing levels of HDL, GSH, SOD, and CAT as confirmed in the current study (Figs. 7, 8, 9). Polyphenolic compounds such as vanillic acid, which is known as antioxidant agent (Nabil-Adam et al. 2020a, b), have potent activities to neutralize the free radicals and active oxygen species. Radmanesh et al. (2017) reported that vanillic acid pretreatment revealed significant therapeutic and protective effects on the antioxidant enzymes, electrocardiogram, cardiac troponins, lipid peroxidation, as well as interleukin-6, stimulation of genes expression of interleukin-1β, and TNF-α in the heart of group treated with isoproterenol (toxicity group) which cause cardiotoxic rats. Similar results were obtained in the current study where the antioxidants capacity in the protected group was increased and the inflammatory biomarkers such as NO as well as MPO in addition to the oxidative stress biomarker LPO were significantly increased with a change in lipid profile. Vanillic acid, which is a product resulted from oxidation of vanillin, has protective role against lipid peroxidation, indicated by reduction in malondialdehyde (MDA), and increases in the endogenous antioxidant enzymes, which indicated by increased catalase (CAT) glutathione (GSH), and total antioxidant capacity (TAC) in rat hearts of the protected group. Similar effect was observed also for syringic acid (SA) as it is an excellent compound to be used as a therapeutic agent in various diseases such as diabetes, CVDs, cancer, cerebral ischemia, neurodisorder and liver damage. It possesses antioxidant, antimicrobial, anti-inflammatory, and anti-endotoxic activities (Srinivasulu et al. 2018). Moreover, SA has the ability to decrease the area of myocardial infarct and save the heart from xenobiotic which induces heart damage as it showed a significant decrease in the size infarcted region. In the histopathological study a characteristic damage resulted in myocardium tissue changes in the induction group which treated with heavy metal mixture (HEM treated) were observed such as inflammatory infiltrate, edema, necrosis, and collagen deposition myofibrillary separation. On contrast to the induction group, the control and extract groups showed no changes indicating the absence of any cardiotoxicity (Fig. 9). However, the clear and obvious myocardium changes and damage found in the induction group after injection with heavy metals were greatly decreased by UPLE pre-treatment due to the presence of phenolic and flavonoids compounds such as SA which has been in previous studies confirmed to have a myocardial protective effect by maintaining the architecture of cardiomyocytes tissue. The scavenging of ROS and free radicals is one of the important key functions of SA mechanisms of action which confirmed by total antioxidant capacity using DPPH radical scavenging in vitro assay (Fig. 2c). On the meantime, UPLE exhibited similar effect against NO (Fig. 2b). Thus, the mechanism of SA as cardiac protective is due to its ability to quenching free radical. Additionally, ellagic acid were reported to have cardioprotective effect via diminishing the calcium level in the vascular tissue near to normal level in rats which was induced by hypertension and in rats treated with EA which is attributed to their action as vasoprotective (Al-Obaidi et al. 2016). Song et al. (2013) showed that cinnamic acid (CA) decrease the ST-segment elevation. Administration of CA was also reported in several studies to decrease CK-MB, LDH, TNF-α, and IL-6 levels, and diminished NO and MPO level which agrees well with the current study (Fig. 8). Moreover, treatment with CA increased the level as well as the activity of CAT, GSH, and total antioxidant capacity activity and decreased MDA levels in the heart tissue (Fig. 7) (Song et al. 2013). In previous studies, CA also showed a cardioprotective effects especially in myocardial ischemia as it had a therapeutic effect via their antioxidative as well as anti- inflammatory roles and increased NO concentrations (Cebova and Pechanova 2020). Another phenolic compound found in UPLE in the present study is hydroxybenzoic acids which are phytochemicals bioactive compounds capable of scavenging free radicals and oxidants through hydroxyl groups in their structures (Gulcin 2020). Their biological activity is mainly due to their ability to change and modify the cellular signaling processes which are responsible for a cascade and multiplier role in activation of the Nrf2 metabolic pathway which stimulates antioxidant mechanisms (multiple endogenous) (Tu et al. 2019). Salicylic acids, which were commonly known as aspirin, have also similar activities against inflammation. Spite and Serhan (2010) reported that aspirin is able to inhibit the mode of action of cyclooxygenase (COX) through inhibiting the formation of eicosanoids pro-inflammatory. Aspirin was known to promote the acetylation process of COX2 resulting in the synthesis of 15-hydroxyeicosatetraenoic acid that is then transforms into the eicosanoid 15-epi-lipoxin A4 anti-inflammatory (Spite and Serhan 2010). It is used for a various of illnesses and diseases such as pain, fever, inflammation, and for its anti-platelet agent which happens via inhibition of COX-1, whereas its anti-inflammatory and analgesic effects occur first via COX-2 inhibition (Kojok et al. 2019 ).In addition, salicylic acids have been reported to bind to a number of cellular proteins which known as SABPs (salicylic acid binding proteins) such as IκB kinase (IKK), which is one of NF-κB complex components (Kojok et al. 2019).
Conclusion
The current study confirmed that the marine secondary metabolite extracted from UPLE has an extraordinary effect as well as cardioprotective role against HEM-induced cardiotoxicity in rats. The therapeutic effect of UPLE can be due to its highly antioxidant and anti-inflammatory effects on the diminished the level of inflammatory as well as oxidative stress biomarkers and finally cardiovascular biomarkers.
Data Availability
All data are available in the paper.
References
Abdel Ghani SA, Shobier AH, Shreadah MA (2013) Assessment of arsenic and vanadium pollution in surface sediments of the Egyptian Mediterranean Coast. J Environ Technol Manag 16(1/2):82–101
Abdel Moneam NM, Yacout GA, Aboul-Ela HM, Shreadah MA (2017a) Hepatoprotective activity of chitosan nanocarriers loaded with the ethyl acetate extract of astenotrophomonas sp. bacteria associated with the red sea sponge Amphimedon ochracea in ccl4 induced hepatotoxicity in rats. Adv Biosci Biotechnol 8(1):27–50
Abdel Moneam NM, Al-Assar SA, Shreadah MA, Nabil-Adam A (2017b) Isolation, identification and molecular screening of psudomance sp metabolic pathways NRPs and PKS associated with the Red Sea sponge, Hyrtios aff. Erectus, Egypt. J Pure Appl Microbiol 11(3):1299–1311
Abdel Moneam NM, Shreadah MA, Al-Assar SA, Nabil-Adam A (2017c) Protective role of antioxidants capacity of Hyrtios aff. Erectus sponge extract against mixture of persistent organic pollutants (POPs)-induced hepatic toxicity in mice liver: biomarkers and ultrastructural study. Environ Sci Pollut Res 24(27):22061–22072. https://doi.org/10.10071/s11356-017-9805-8
Abdel Moneam NM, Al-Assar SA, Shreadah MA, Nabil-Adam A (2017d) The hepatoprotective effect of Hyrtios aff. Erectus sponge isolated from the Red sea extract against the toxicity of Persistent organic pollutants (POPs) from Sediments of Lake Mariout. J Biotechnol Biotechnol Equip 32(3):734–743. https://doi.org/10.1080/13102818.2018.1441747
Abdel Moneam NM, Shreadah MA, Al-Assar SA, De Voogd NJ, Nabil-Adam A (2018) Hepatoprotective effect of Red Sea sponge extract against the toxicity of a real-life mixture of persistent organic pollutants. Biotechnol Biotechnol Equip 32(3):734–743
Abdel Moniem NM, Abdel-Azeem AM, El-Ashry ESH, Ghareeb D, Nabiel-Adam A (2013) Pretreatment hepatoprotective effect of the marine fungus derived from sponge on hepatic toxicity induced by heavy metals in rats. Open J Med Chem 03:60–73. https://doi.org/10.4236/ojmc.2013.33009
Abdel-Fatah L, Fahmy MA, Shriadah MA (2003) Zn, Cu, Cd, Pb and Hg in the Egyptian coastal sediments along the Mediterranean Sea Ass. Mod. & Simul. Enterpr (AMSE) (France) 64(3):55–69
Abd-Ellatef GF, Ahmed OM, Abdel-Reheim ES, Abdel-Hamid AZ (2017) Ulva lactuca polysaccharides prevent Wistar rat breast carcinogenesis through the augmentation of apoptosis, enhancement of antioxidant defense system, and suppression of inflammation. Breast Cancer (Dove Med Press) 9:67–83. https://doi.org/10.2147/BCTT.S125165
Agrawal N, Minj DK, Rani K (2015) Estimation of total carbohydrate present in dry fruits. IOSR Journal of Environmental Science, Toxicology. Food Technol 1(6):24–27
Ahmadi-Naji R, Heidarian E, Ghatreh-Samani K (2017) Evaluation of the effects of the hydroalcoholic extract of Terminalia chebula fruits on diazinon-induced liver toxicity and oxidative stress in rats. Avicenna J Phytomed 7(5):454–466
Akbari G (2020) Molecular mechanisms underlying gallic acid effects against cardiovascular diseases: an update review. Avicenna journal of phytomedicine 10(1):11–23
Alagan V, Rajesh NV, Rajesh KD (2017) Bioactive chemical constituent analysis, in vitro antioxidant and antimicrobial activity of whole plant methanol extracts of Ulva lactuca Linn. Br J Pharm Res 15:1–14. https://doi.org/10.9734/BJPR/2017/31818
Ali H, Khan E, Ilahi I (2019) Environmental chemistry and ecotoxicology of hazardous heavy metals: environmental persistence, toxicity, and bioaccumulation. J Chem. Article ID 6730305 | 14 pages. https://doi.org/10.1155/2019/6730305
Allain CC, Poon LS, Chan CSG, Richmond W, Fu PC (1973) Enzymatic determination of total serum cholesterol. Clin Chem 20(4):470–475. https://doi.org/10.1093/clinchem/20.4.470
Al-Obaidi MM, Al-Bayaty FH, Al Batran R, Ibrahim OE, Daher AM (2016) Ellagic acid increases osteocalcin and alkaline phosphatase after tooth extraction in nicotinic-treated rats. Curr Pharm Des 22(16):2403–2410. https://doi.org/10.2174/138161282216160428002842
Amarowicz R, Naczk M, Zadernowski R, Shahidi F (2007) Antioxidant activity of condensed tannins of beach pea, Canola hulls, evening primrose, and faba bean. J Food Lipids 7:195–205
Andrade LM, Andrade CJ, Dias M, Nascimento CAO, Mendes MA (2018) Chlorella and spirulina microalgae as sources of functional foods, nutraceuticals, and food supplements; an overview. MOJ Food Process Technol 6(1):45–58. https://doi.org/10.15406/mojfpt.2018.06.00144.
Anyanwu BO, Ezejiofor AN, Igweze ZN, Orisakwe OE (2018) Heavy metal mixture exposure and effects in developing nations: an update. Toxics 6(4):65. https://doi.org/10.3390/toxics6040065
Awuchi C, Victory I, Ikechukwu A (2020) Nutritional diseases and nutrient toxicities: a systematic review of the diets and nutrition for prevention and treatment. Int J Adv Acad Res 6:1–46
Ayala A, Muñoz MF, Argüelles S (2014) Lipid peroxidation: production, metabolism, and signaling mechanisms of malondialdehyde and 4-hydroxy-2-nonenal. Oxidative Med Cell Longev:360438. https://doi.org/10.1155/2014/360438
Bucolo G, David H (1973) Quantitative determination of serum triglycerides by the use of enzymes. Clin Chem 19(5):476–482. https://doi.org/10.1093/clinchem/19.5.476
Bule MH, Ahmed I, Maqbool F, Bilal M, Iqbal HMN (2018) Microalgae as a source of high-value bioactive compounds. Front Biosci 10:197–216
Burroughs Peña MS, Rollins A (2017) Environmental exposures and cardiovascular disease: a challenge for health and development in low- and middle-income countries. Cardiol Clin 35(1):71–86. https://doi.org/10.1016/j.ccl.2016.09.001
Cebova M, Pechanova O (2020) Protective effects of polyphenols against ischemia/reperfusion injury. Molecules (Basel, Switz) 25(15):3469. https://doi.org/10.3390/molecules25153469
Chowdhury R, Ramond A, O’Keeffe LM, Shahzad S, Kunutsor SK, Muka T, Gregson J, Willeit P, Warnakula S, Khan H, Chowdhury S, Gobin R, Franco OH, Di Angelantonio E (2018) Environmental toxic metal contaminants and risk of cardiovascular disease: systematic review and meta-analysis. BMJ 362:k3310
Dodgson KS (1961) Determination of inorganic sulphate in studies on the enzymic and non-enzymic hydrolysis of carbohydrate and other sulphate esters. Biochem J 78:312–319
Emara HI, Shriadah MA (1991) Manganese, Iron, Cobalt, Nickel, and Zinc in the Eastern harbor and El-Mex Bey Waters (Alexandria). Inter Proc Symp Mar Chem. In the Arab Region, Suez, April 1991:97–112
Emara HI and Shriadah MA (2009). Distribution and composition of aliphatic aromatic petroleum hydrocarbons at some hot spots of Alexandria coastal water, Egypt. International Workshop on Integrated Coastal Zone Management (Izmir- Turkey 20-22 Oct, (2009), 201-217.
Engwa GA, Ferdinand PU, Nwalo FN, Unachukwu MN (2019) Mechanism and health effects of heavy metal toxicity in humans, poisoning in the modern world - new tricks for an old dog?, Ozgur Karcioglu and Banu Arslan, IntechOpen, https://doi.org/10.5772/intechopen.82511. Available from: https://www.intechopen.com/books/poisoning-in-the-modern-world-new-tricks-for-an-old-dog-/mechanism-and-health-effects-of-heavy-metal-toxicity-in-humans
Fathy SA, Abdel Hamid FF, Shreadah MA, Mohamed LA, El-Gazar MG (2010) Application of principal component analysis for developing water quality index for selected coastal areas of Alexandria Egypt. Resour Environ J 2(6):297–305
Fathy SA, Abdel Hamid FF, Shreadah MA, Mohamed LA, El-Gazar MG (2012) Effect of some environmental pollutants on enzymatic and total antioxidant activities in Tilapia Niloticus. Blue Biotechnol J 1(3):433–443
Green LC, Wagner DA, Glogowski J, Skipper PL, Wishnok JS, Tannenbaum SR (1982) Analysis of nitrate, nitrite and (15N) nitrate in biological fluids. Anal Biochem 126:131–138
Griffith JQ, Farris EJ (1942) The rat in laboratory investigation. (Lippincott, Philadelphia, Ed.) Google Scholar
Gulcin İ (2020) Antioxidants and antioxidant methods: an updated overview. Arch Toxicol 94:651–715. https://doi.org/10.1007/s00204-020-02689-3
Hadwan MH (2018) Simple spectrophotometric assay for measuring catalase activity in biological tissues. BMC Biochem 19(1):7. https://doi.org/10.1186/s12858-018-0097-5
Hegazy MF, Mohamed TA, Elshamy AMI, Hassanien AA, Abdel Azimd NS, Shreadah MA, Gawad A II, Elkady EM (2015a) A new steroid from the red sea soft coral Lobophytum lobophytum. Nat Prod Res 30:340–344
Hegazy MF, Gamal-Eldeen AM, Mohamed TA, Alhammady MA, Hassanien AA, Shreadah MA, Gawad A II, Elkady EM (2015b) Cytotoxic constituents from the red sea soft coral Nephthea Sp. Nat Prod Res 30:1266–1272
Herman EH, Zhang J, Lipshultz SE, Rifai N, Chadwick D, Takeda K, Yu ZX, Ferrans VJ (1999) Correlation between serum levels of cardiac troponin-T and the severity of the chronic cardiomyopathy induced by doxorubicin. J Clin Oncol 17(7):2237–2243. https://doi.org/10.1200/JCO.1999.17.7.2237
Huang DW, Chang WC, Wu JS, Shih RW, Shen SC (2016) Gallic acid ameliorates hyperglycemia and improves hepatic carbohydrate metabolism in rats fed a high-fructose diet. Nutr Res 36:150–160 Google Scholar
Jadon A, Bhadauria M, Shukla S (2007) Protective effect of Terminalia belerica Roxb and gallic acid against carbon tetrachloride induced damage in albino rats. J Ethnopharmacol 109:214–218 Google Scholar
Jan AT, Azam M, Siddiqui K, Ali A, Choi I, Haq QM (2015). Heavy metals and human health: mechanistic insight into toxicity and counter defense system of antioxidants. Int J Mol Sci; 16(12):29592-29630. doi: https://doi.org/10.3390/ijms161226183.
Järup L (2003) Hazards of heavy metal contamination. Br Med Bull 68(1):167–182. https://doi.org/10.1093/bmb/ldg032
Jin L, Lin MQ, Piao ZH, Cho JY, Kim GR, Choi SY, Ryu Y, Sun S, Kee HJ, Jeong MH (2017) Gallic acid attenuates hypertension, cardiac remodeling, and fibrosis in mice with NG-nitro-L-arginine methyl ester-induced hypertension via regulation of histone deacetylase 1 or histone deacetylase 2. J Hypertens 35:1502–1512 Google Scholar
Karimi-Khouzan O, Heidarian E, Amini SA (2017) Anti-inflammatory and ameliorative effects of gallic acid on fluoxetine-induced oxidative stress and liver damage in rats. Pharmacol Rep 69:830–835. https://doi.org/10.1016/j.pharep.2017.03.011
Khan AA, Alsahli MA, Rahmani AH (2018) Myeloperoxidase as an active disease biomarker: recent biochemical and pathological perspectives. Med Sci (Basel, Switz) 6(2):33. https://doi.org/10.3390/medsci6020033
Kojok K, El-Kadiry AE, Merhi Y (2019) Role of NF-κB in platelet function. Int J Mol Sci 20(17):4185. https://doi.org/10.3390/ijms20174185
Koracevic D, Koracevic G, Djordjevic V, Andrejevic S, Cosic V (2001) Method for the measurement of antioxidant activity in human fluids. J Clin Pathol 54(5):356–361. https://doi.org/10.1136/jcp.54.5.356
Kumar S, Trivedi PK (2018) Glutathione S-transferases: role in combating abiotic stresses including arsenic detoxification in plants. Front Plant Sci 2018. https://doi.org/10.3389/fpls.2018.00751
Lamas GA, Navas-Acien A, Mark DB, Lee KL (2016) Heavy metals, cardiovascular disease, and the unexpected benefits of chelation therapy. J Am Coll Cardiol 67 (20:2411–2418. https://doi.org/10.1016/j.jacc.2016.02.066
Li J, Yin FF, Hou YL (2013) Early diagnosis of rats with acute myocardial infarction by measurement of brain natriuretic peptide [J]. Exp Ther Med 5(4):1201–1205
Machoń-Grecka A, Dobrakowski M, Kasperczyk A, Birkner E, Pryzwan T, Kasperczyk S (2018) The effect of subacute lead exposure on selected blood inflammatory biomarkers and angiogenetic factors. J Occup Health 60(5):369–375. https://doi.org/10.1539/joh.2017-0307-OA
Manack JR, Richards CB (1971) J Immunol 20:1019
Marín M, Moya C, Máñez S (2019) Mutual influences between nitric oxide and paraoxonase. Antioxidants. 8(12):619. https://doi.org/10.3390/antiox8120619
Messyasz B, Michalak I, Łęska B, Schroeder G, Górka B, Korzeniowska K, Lipok J, Wieczorek P, Rój E, Wilk R, Dobrzyńska-Inger A, Górecki H, Chojnacka K (2018) Valuable natural products from marine and freshwater macroalgae obtained from supercritical fluid extracts. J Appl Phycol 30:591–603. https://doi.org/10.1007/s10811-017-1257-5
Metsalu T, Vilo J (2015) ClustVis: a web tool for visualizing clustering of multivariate data using Principal Component Analysis and heatmap. Nucleic Acids Res 43(W1):W566–W570. https://doi.org/10.1093/nar/gkv468
Nabil-Adam A, Shreadah M, El-Assar AEMN (2020a) Pesudomance sp. Bacteria Associated with marine sponge as a promising and sustainable source of bioactive molecules. Curr Pharm Biotechnol 20(11):964–984. https://doi.org/10.2174/1389201020666190619092502
Nabil-Adam A, Shreadah M, Abd El Moneam N, El Assar S (2020b) In-vitro drug screening and biochemical analysis of sponge secondary metabolites collected from the Red Sea Egypt. Turk J Pharm Sci 17(2):127–135
Napierska D, Sanseverino I, Loos R, Cortés LG, Niegowska M, Lettieri T (2018) Modes of action of the current priority substances list under the water framework directive and other substances of interest, EUR 29008 EN. Publications Office of the European Union, Luxembourg, ISBN 978-92-79-77301-3, JRC110117. https://doi.org/10.2760/226911
Okediran BS, Adah AS, Sanusi F, Suleiman KY (2018) Lipid changes in male Albino rats exposed to graded doses of Lead. Ceylon J Sci 47(2):159–163. https://doi.org/10.4038/cjs.v47i2.7512
Panda S, Kar A, Biswas S (2017) Preventive effect of Agnucastoside C against Isoproterenol-induced myocardial injury. Sci Rep 7:16146. https://doi.org/10.1038/s41598-017-16075-0
Pereira L (2018) Seaweeds as source of bioactive substances and skin care therapy-cosmeceuticals, algotheraphy, and thalassotherapy. Cosmetics 5:68. https://doi.org/10.3390/cosmetics5040068
Pirahanchi Y, Sharma S (2020). Biochemistry, Lipoprotein Lipase. [Updated 2020 Mar 19]. In: StatPearls [Internet]. StatPearls Publishing, Treasure Island. Available from: https://pubmed.ncbi.nlm.nih.gov/30726031/
Priscilla DH, Prince PS (2009) Cardioprotective effect of gallic acid on cardiac troponin-T, cardiac marker enzymes, lipid peroxidation products and antioxidants in experimentally induced myocardial infarction in Wistar rats. Chem Biol Interact 179:118–124 Google Scholar
Pulli B, Ali M, Forghani R, Schob S, Hsieh KL, Wojtkiewicz G, Linnoila JJ, Chen JW (2013) Measuring myeloperoxidase activity in biological samples. PLoS One 8(7):e67976. https://doi.org/10.1371/journal.pone.0067976
Radmanesh E, Dianat M, Badavi M, Goudarzi G, Mard SA (2017) The cardioprotective effect of vanillic acid on hemodynamic parameters, malondialdehyde, and infarct size in ischemia-reperfusion isolated rat heart exposed to PM10. Iranian J Basic Med Sci 20(7):760–768. https://doi.org/10.22038/IJBMS.2017.9007
Rajeswarareddy S, Lavany T, Narasimhulu G, Sathyavelureddy K (2012) Effect of Pimpinellatiru patiensison oxidative enzymes in STZ-induced diabetic rat kidney. Iranian J Pharm Res 11(1):277–286
Rehman S-U (1984) Lead-induced regional lipid peroxidation in brain. Toxicol Lett 21(3):333–337. https://doi.org/10.1016/0378-4274(84)90093-6
Rosa GP, Tavares WR, Sousa PMC, Pagès AK, Seca AML, Pinto DCGA (2019) Seaweed secondary metabolites with beneficial health effects: an overview of successes in in vivo studies and clinical trials. Mar Drugs 18(1):8. https://doi.org/10.3390/md18010008
Said TO, Farag RS, Younis AM, Shreadah MA (2006) Organotin species in fish and bivalves samples collected from the Egyptian Mediterranean coast of Alexandria, Egypt. Bull Environ Contam Toxicol 77(3):451–458
Salbitani G, Bottone C, Carfagna S (2017) Determination of reduced and total glutathione content in extremophilic microalga Galdieria phlegrea. Bio-Protocol 7. https://doi.org/10.21769/BioProtoc.2372
Shakweer L, Shriadah MA, Fahmy MA, Abdel Fattah L (2006) Distributions and concentrations of trace elements along the Mediterranean coastal water of Egypt. Egypt J Aquat Res 32(2):95–127
Sharifinasab Z, Banaee M, Mohiseni M, Noori A (2016) The protective role of Vitamin C and Chitosan Against paraquat-induced oxidative stress in muscles of common carp (Cyprinus carpio). Croat J Fish 74:199–217
Sharma B, Singh S, Siddiqi NJ (2014) Biomedical implications of heavy metals induced imbalances in redox systems. Biomed Res Int / Article 2014 | 26 pages. https://doi.org/10.1155/2014/640754
Shobier AH, Abdel Ghani SA, Shreadah MA (2011) Distribution of total mercury in sediments of four semi-enclosed basins along the Mediterranean coast of Alexandria. Egypt J Aquat Res 37(1):1–11
Shreadah MA, Said TO, Abd El Ghani SA, Ahmed AM (2008) Alkyllead and Alkyltin Species in different fishes collected from the Suez Gulf, Egypt. Proceedings of the 2nd International Conference on Aquatic Res. Egypt J Aquat Res 34(4):64–73
Shreadah MA, Said TO, Othman IM, Fathallah EMI, Mahmoud ME (2012) Polychlorinated biphenyls and chlorinated pesticides in Sediments along the Semi-closed Areas of Alexandria, Egypt. J Environ Prot 3(2):141–149
Shreadah MA, Masoud MS, Khattab AM, El Zokm G (2014) Impacts of different drains on the seawater quality of El-Mex Bay (Alexandria, Egypt). J Ecol Nat Environ 8(8):287–303
Shreadah MA, Fahmy MA, Abdel Fattah L (2015) Heavy metals in some fish species and bivalves from the Mediterranean coast of Egypt. J Environ Protect 6(1):1–9
Shreadah MA, Abd El Moneam NM, Al-Assar SA, Nabil-Adam A (2017) The ameliorative role of a marine sponge extract against mixture of persistent organic pollutants induced changes in hematological parameters in mice. Expert Opin Environ Biol 6(2). https://doi.org/10.4172/2325-9655.1000143b
Shreadah MA, Abdel Moniem NM, Al-Assar SA, Nabil-Adam A (2018) Phytochemical and pharmacological screening of Sargassium vulgare from Suez Canal, Egypt. Food Sci Biotechnol 27(4):963–979. https://doi.org/10.1007/s10068-018-0323-3
Shreadah MA, El Sayed A, Taha A, Ahamed A (2019) Evaluation of different anthropogenic effluents impacts on the water quality using principal component analysis: a case study of Abu-Qir Bay-Alexandria-Egypt. Int J Environ Monit Anal 7(3):56–67
Shreadah MA, Abd El Moneam NM, El-Assar SA, Nabiel-Adam A (2020a) Metabolomics and pharmacological screening of Aspergillus versicolor isolated from Hyrtios erectus Red Sea Sponge; Egypt. Curr Bioact Compd. https://doi.org/10.2174/1573407215666191111122711
Shreadah MA, Abd El Moneam N, El-Assar S, Nabil-Adam A (2020b) Marine algae of the genus Gracilaria as a multi products source for different biotechnological and medical applications. Recent Pat Biotechnol. https://doi.org/10.2174/1872208314666200121144816
Shriadah MA, Abdel Ghani S (2007). Impacts of land based sources on water quality of Abu – Qir Drain, Egypt. Proceed 8th Int Conf Med Coastal Environ. MEDCOAST 07. E. Ozhan (Editor), 13-17 November 2007, Alexandria, Egypt. 863-872.
Siasos G, Tsigkou V, Kosmopoulos M, Theodosiadis D, Simantiris S, Tagkou NM, Tsimpiktsioglou A, Stampouloglou PK, Oikonomou E, Mourouzis K, Philippou A, Vavuranakis M, Stefanadis C, Tousoulis D, Papavassiliou AG (2018) Mitochondria and cardiovascular diseases-from pathophysiology to treatment. Ann Transl Med 6(12):256. https://doi.org/10.21037/atm.2018.06.21
Solenkova NV, Newman JD, Berger JS, Thurston G, Hochman JS, Lamas GA (2014) Metal pollutants and cardiovascular disease: mechanisms and consequences of exposure. Am Heart J 168(6):812–822. https://doi.org/10.1016/j.ahj.2014.07.007
Song M, Huang L, Zhao G, Song Y (2013) Beneficial effects of a polysaccharide from Salvia miltiorrhiza on myocardial ischemia-reperfusion injury in rats. Carbohydr Polym 98:1631–1636 Google Scholar
Spite M, Serhan CN (2010) Novel lipid mediators promote resolution of acute inflammation: impact of aspirin and statins. Circ Res 107(10):1170–1184. https://doi.org/10.1161/CIRCRESAHA.110.223883
Srinivasulu C, Ramgopal M, Ramanjaneyulu G, Anuradha CM, Suresh K (2018) Syringic acid (SA) – a review of its occurrence, biosynthesis, pharmacological and industrial importance. Biomed Pharmacother 108:547–557. https://doi.org/10.1016/j.biopha.2018.09.069
Stanely Mainzen Prince P, Priscilla H, Devika PT (2009) Gallic acid prevents lysosomal damage in isoproterenol induced cardiotoxicity in Wistar rats. Eur J Pharmacol 615:139–143 Google Scholar
Su LJ, Zhang JH, Gomez H, Murugan R, Hong X, Xu D, Jiang F, Peng ZY (2019) Reactive oxygen species-induced lipid peroxidation in apoptosis, autophagy, and ferroptosis. Oxidative Med Cell Longev 2019:5080843. https://doi.org/10.1155/2019/5080843
Sun B, Ricardo da Silva JM, Spranger I (1998) Critical factors of vannilin assay for catechins and proanthocyanidins. J Agric Food Chem 64:4267–4274
Taga MS, Miller E, Pratt D (1984) Chia seeds as a source of natural lipid antioxidants. J Am Oil Chem Soc 61:928–931
Tellez-Plaza M, Guallar E, Navas-Acien A (2018) Environmental metals and cardiovascular disease. Br Med J 362:k3435. https://doi.org/10.1136/bmj.k3435
Terme N, Boulho R, Kucma J-P, Bourgougnon N, Bedoux G (2018) Radical scavenging activity of lipids from seaweeds isolated by solid-liquid extraction and supercritical fluids. OCL 25(5):D505. https://doi.org/10.1051/ocl/2018054
Thompson LA, Darwish WS (2019) Environmental chemical contaminants in food: review of a global problem. J Toxicol Sci 2019:2345283. https://doi.org/10.1155/2019/2345283
Tretter L, Patocs A, Chinopoulosac C (2016) Succinate, an intermediate in metabolism, signal transduction, ROS, hypoxia, and tumorigenesis. Biochim Biophys Acta (BBA) Bioenergetics 1857(8:1086–1101. https://doi.org/10.1016/j.bbabio.2016.03.012
Tu W, Wang H, Li S, Liu Q, Sha H (2019) The anti-inflammatory and anti-oxidant mechanisms of the Keap1/Nrf2/ARE signaling pathway in chronic diseases. Aging Dis 10(3):637–651. https://doi.org/10.14336/AD.2018.0513
Uddin R, Saha MR, Subhan N, Hossain H, Jahan IA, Akter R, Alam A (2014) HPLC-analysis of polyphenolic compounds in Gardenia jasminoides and determination of antioxidant activity by using free radical scavenging assays. Adv Pharm Bull 4(3):273–281. https://doi.org/10.5681/apb.2014.040
Zhang Y, Lan M, Lü J-P, Li J-F, Zhang K-Y, Zhi H, Zhang H, Sun J-M (2020) Antioxidant, anti-inflammatory and cytotoxic activities of polyphenols extracted from Chroogomphus rutilus. Chem Biodivers 17(1). https://doi.org/10.1002/cbdv.201900479
Zhao C, Yang C, Liu B, Lin L, Sarker SD, Nahar L, Yu H, Cao H, Xiao J (2018) Bioactive compounds from marine macroalgae and their hypoglycemic. Trends Food Sci Technol 72:1–12
Zhishen J, Mengcheng T, Jianming W (1999) The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem 64:555–559
Zöllner N, Kirsch K (1962) Colorimetric method for determination of total lipids. J Exp Med 135:545–550. https://doi.org/10.1007/BF02045455
Zwolak I (2020) The role of selenium in arsenic and cadmium toxicity: an updated review of scientific literature. Biol Trace Elem Res 193:44–63. https://doi.org/10.1007/s12011-019-01691
Author information
Authors and Affiliations
Contributions
Dr. Asmaa Nabil-Adam: Performed the measurements, involved in planning and supervision, processed the experimental data, performed the analysis, drafted the manuscript, and designed the figures. Dr. Mohamed Attia Shreadah: Interpreted the results and worked on the manuscript. All authors discussed the results and commented on the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interest
Ethical approval
Ethical approval reference number: AU- 0304862
Additional information
Responsible Editor: Lotfi Aleya
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Nabil-Adam, A., Shreadah, M.A. Ameliorative role of Ulva extract against heavy metal mixture—induced cardiovascular through oxidative/antioxidant pathways and inflammatory biomarkers. Environ Sci Pollut Res 28, 27006–27024 (2021). https://doi.org/10.1007/s11356-020-11994-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-020-11994-4