Abstract
Myocardial oxidative stress leading to apoptosis and remodeling is the major consequence of ischemic heart disease. In the present study, we investigated the effect of Lagerstroemia speciosa L. leave (LS) extract containing 1 % corosolic acid in the context of cardiovascular disorder by using isoproterenol (ISO)-induced myocardial injury mouse model. Serum was analyzed for specific cardiac injury biomarkers. Cardiac tissue was examined for lipid peroxidation, protein carbonyl content, antioxidant (GSH, GR, GPx, GST, SOD, CAT, NQO1, and HO-1), and apoptosis (cleaved caspase-3, Bax, Bcl-2, p53, and DNA fragmentation) status. Myocardial protein expression of nuclear factor erythroid 2-related factor 2 (Nrf2) in different experimental groups was evaluated. Pathological changes in heart tissue and activities of matrix metalloproteinases (MMPs) were also analyzed. Our results demonstrated that LS pretreatment augmented myocardial antioxidant status and attenuated myocardial oxidative stress. Myocardial apoptosis as well as MMPs activities was significantly prevented by LS pretreatment in ISO-induced mice. In addition, the immunoblot of Nrf2 revealed that LS pretreatment enhanced the nuclear protein expression of Nrf2 when compared to ISO control group. Thus, the overall results indicate that LS has cardioprotective effect and may prevent the myocardial stress by suppressing apoptosis through up-regulation of myocardial antioxidant levels.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Myocardial infarction is the first manifestation of coronary artery disease. It may also occur, repeatedly, in patients with established disease due to imbalance between myocardial blood supply and demand resulting in the development of ischemia and induction of necrosis in myocardium [1]. Heart is an organ that is highly supplied with blood and also has very high energy utility. Hence, a little imbalance in supply of oxygenated blood immediately leads to overproduction of reactive oxygen species (ROS), which is the primary cause for major cardiovascular diseases [2]. ROS, depending on their concentration, can activate a number of key signaling molecules and transcription factors leading to myocardial hypertrophy, apoptosis, and necrosis contributing to ventricular remodeling and cardiac dysfunction [3]. It has also been documented that matrix metalloproteinases (MMP) are implicated in cardiac inflammation/injury and subsequent failure [4]. In spite of using pharmacological interventions such as anticoagulant, antiplatelet, and vasodilator therapies, surgical interventions such as coronary artery bypass grafting (CABG) and percutaneous coronary interventions such as percutaneous transluminal coronary angioplasty (PTCA), cardiovascular disorders are still leading cause of death and disability worldwide due to changes in lifestyle [5]. Therefore, it is necessary to develop potential therapies that target multiple mechanisms against cardiovascular-related disorders to stay healthy and improve the quality of life. Discovery of novel compounds, which supports endogenous antioxidant defense and suppresses apoptosis and structural remodeling, is an alternate approach with huge therapeutic prospective to attenuate myocardial stress. Documented evidence suggested that isoproterenol-induced myocardial injury model is a rapid and widely used model for evaluating myocardial consequences of ischemic disorders [6]. Isoproterenol, a synthetic catecholamine, in supramaximal doses induces local myocardial infarction-like pathological changes as seen in human myocardial infarction. Among the various mechanisms proposed to explain the isoproterenol-induced cardiotoxicity, generation of highly cytotoxic free radicals through auto-oxidation of catecholamine has been implicated as one of the important causative factors [7].
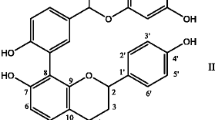
Lagerstroemia speciosa L. (Crepe myrtle, Banaba) widely cultivated as an ornamental plant in tropical and subtropical areas primarily in Southeast Asia. It is also used as popular folk medicine in Southeast Asia, and tea made from the leaves has been used for the treatment for diabetes mellitus [8]. Triterpenoids such as corosolic acid and ursolic acid, and ellagitannins such as lagerstroemin and ellagic acid represent the active constituents of Lagerstroemia speciosa leaves (LS). In Philippines, it is now commercially produced under prepared formulation in the form of tablet, capsules, extract, powder, and tea displayed in drug stores and health stores. In a randomized clinical trial involving patients with type II diabetes, the antidiabetic effect of the standardized Lagerstroemia speciosa leaf extract containing 1 % corosolic acid (Glucosol™) has been demonstrated [9]. Existing studies that have been conducted in animals, humans, and in vitro systems suggest the potent free radical scavenging, antihyperglycemic, antihyperlipidemic, antihypertensive, antioxidant, anti-inflammatory, antifungal, and antiviral activities of Lagerstroemia speciosa [10]. Various experimental data further demonstrated the anticancer effects of corosolic acid and Lagerstroemia speciosa extract (LS) on various human cancer cell lines [11, 12]. In an in vitro study, LS suppressed the TNF-induced activation of NF-κB in rat H9c2 cardiomyocytes and demonstrated the beneficial effects of LS on cardiomyocyte hypertrophy [13]. Since LS extract is able to scavenge reactive oxygen species and exerts beneficial effect on H9c2 cardiomyocyte hypertrophy, it is reasonable to hypothesize that LS may have some potential protective effect against in vivo cardiovascular disease model. Herbal medicines used for many years around the globe are of paramount importance not only as ideal therapeutics but also even represent possible alternate strategy to combat or cure many diseases. Since all free radical and oxidative substances do not get neutralized by all antioxidants, the use of bioactive compounds or antioxidant-containing plants, which contains number of antioxidant compounds, is likely to elucidate more beneficial health effects than those derived of individual function such as superoxide dismutase and vitamin C. The reason for a recent effort in introducing new herbal product as a phytomedicine into the drug development pipeline is obvious, and understanding of the molecular targets of natural products is important. Hence, the present study was aimed to investigate whether LS offers protection against experimental model of isoproterenol-induced myocardial stress in mice. To the best of our knowledge, this is the first study to demonstrate the cardioprotective effect of Lagerstroemia speciosa in mice.
Materials and Methods
Chemicals and Reagents
Antibodies against cleaved caspase-3, Bax, Bcl-2, p53, Nrf2, HO-1, β-actin, Lamin-B, and secondary antibody were purchased from Cell Signaling Technology (Boston, MA). MMP-9 and MMP-2 fluorimetric drug discovery kits were purchased from Enzo Life Sciences Inc., Farmingdale, NY. Isoproterenol, superoxide dismutase assay kit, Bradford reagent, gelatin, reduced glutathione (GSH), glutathione oxidized (GSSG), 5,5-dithio-bis (2-nitrobenzoic acid) (DTNB), 2-thiobarbituric acid (TBA), glutathione reductase, catalase, etc., were purchased from Sigma-Aldrich Co, St. Louis, MO, USA. All other chemicals were of analytical grade. Standardized methanolic Lagerstroemia speciosa leaves extract containing 1 % corosolic acid was supplied by Laila Impex Pvt. Ltd., Vijayawada, India, as gratis.
DPPH (2-2-Diphenyl-1-picrylhydrazyl) Radical Scavenging Assay
In a 96-well microplate, 25 μL of different concentrations of test sample, 100 μL of 0.1 M Tris–HCl buffer (pH 7.4), and 125 μL DPPH solution (0.5 mM in methanol) were added. The reaction mixture was shaken well, incubated for 30 min in dark at room temperature, and reading was recorded at 517 nm. Ascorbic acid (Sigma-Aldrich), a stable antioxidant, was used as a synthetic reference. IC50 values denote the concentration required to scavenge 50 % of the free radicals generated by DPPH inhibition (%) and was calculated by applying the formula [(ODcontrol − ODtest)/ODcontrol] × 100.
Experimental Animals
The study was conducted according to the ethical norms approved by CPCSEA, Government of India, and by the Institutional Animal Ethical Committee (IAEC) of Indian Institute of Chemical Technology (IICT), Hyderabad, India. Male Swiss albino mice of 22–25 g were obtained from National Institute of Nutrition (NIN), Hyderabad, India, and acclimatized for 1 week prior to initiation of experiment. Mice were housed under controlled climatic conditions with artificial 12-h light/dark cycle. They were fed a standard rodent diet and water ad libitum.
Study Design
Isoproterenol was administered twice at a dose of 150 mg/kg subcutaneously (sc) at an interval of 24 h to induce myocardial stress in mice. The concentration of corosolic acid in the LS extract was preferred based on the previous studies where corosolic acid was found to elicit antioxidant and anti-inflammatory property in hypertensive rats [8] and produce significant hypoglycemic effect in humans [14].
A pilot study was conducted with four different doses of LS (25, 50, 100, and 200 mg/kg) to determine the dose-dependent effect of LS in ISO-induced cardiotoxic mice. During the 14 days of LS (oral) treatment, ISO (150 mg/kg, sc) was administered on 12th day and 13th day at an interval of 24 h. After 48 h of first dose of ISO administration, blood was collected and serum-specific cardiac biomarkers (CK-MB, LDH, and AST) were determined. It was observed that LS pretreatment at the dose of 25 mg/kg did not produce significant effect compared to ISO control group. A significant dose-dependent inhibition of cardiac biomarkers was observed between 50- and 100 mg/kg-treated group compared to ISO-treated group (data not shown). At the same time, no significant difference was observed between 100 and 200 mg/kg. Hence, 50 and 100 mg/kg dose levels were chosen for further study.
For main study, animals were randomly selected and divided into five groups of eight mice in each and were treated as follows:
-
1.
Normal control (NC): Gum acacia (2 %) suspension was administered per os for 14 days;
-
2.
LS control (LS): LS (100 mg/kg) suspension in gum acacia (2 %) was administered per os for 14 days and a subcutaneous (sc) injection of normal saline at an interval of 24 h on 12th day and 13th day;
-
3.
Isoproterenol control (ISO): Gum acacia (2 %) suspension was administered per os for 14 days, and a subcutaneous (sc) injection of isoproterenol (150 mg/kg) was dissolved in normal saline at an interval of 24 h on 12th day and 13th day;
-
4.
Isoproterenol + LS (50 mg/kg) (ISO + LS50): Mice were fed with LS (50 mg/kg/day) suspension in 2 % gum acacia per os for 14 days and a subcutaneous (sc) injection of isoproterenol (150 mg/kg) was dissolved in normal saline at an interval of 24 h on 12th day and 13th day, 1 h prior to LS dose;
-
5.
Isoproterenol + LS (100 mg/kg) (ISO + LS100): Mice were fed with LS (100 mg/kg/day) suspension in 2 % gum acacia per os for 14 days, and a subcutaneous (sc) injection of isoproterenol (150 mg/kg) was dissolved in normal saline at an interval of 24 h on 12th day and 13th day, 1 h prior to LS dose.
At the end of the experiment (i.e., after 48 h of first dose of ISO administration), blood samples were collected through retro-orbital plexus. Mice were euthanized in CO2 asphyxiation; heart tissue was collected, weighed, immediately frozen in liquid nitrogen, and stored at −80 °C until analysis.
Assessment of Serum-Specific Biomarkers Related to Cardiac Injury
Serum levels of creatine kinase-MB (CK-MB) isoenzyme, lactate dehydrogenase (LDH), and aspartate transaminase (AST) were estimated to assess the myocardial damage using commercial kits (Siemens, India) employing auto-blood analyzer (Siemens, Dimension Xpandplus, USA). The ratio of heart weight to body weight (relative weight of heart) was assessed for each as indices of cardiac hypertrophy.
Assessment of Myocardial Oxidative Stress
Frozen heart samples were minced into small pieces, homogenized in ice-cold phosphate buffer saline (PBS) (0.05 M, pH 7) containing protease inhibitor cocktail (Sigma-Aldrich Co, St. Louis, MO, USA) to obtain (12 %) whole homogenate, and were used for the estimation of total protein (Bradford reagent, Sigma-Aldrich), thiobarbituric acid-reactive substances (TBARS) [15], protein carbonyl content [16], reduced glutathione (GSH) [17], catalase (CAT) [18], superoxide dismutase (SOD) (SOD assay kit, Sigma-Aldrich Co., St. Louis, MO, USA), glutathione reductase (GR) [19], glutathione S-transferase (GST) [20] and glutathione peroxidase (GPx) [21] activities as reported in the earlier literature. Myocardial NAD(P)H:quinine oxidoreductase 1 (NQO1) activity in heart tissue of different experimental groups was also estimated [22].
DNA Fragmentation
Proteinase K digestion with phenol/chloroform/isoamyl alcohol extraction method was employed to isolate DNA from heart tissue. Gel electrophoresis (Bio-Rad, Gel Electrophoresis Unit) using 1.5 % agarose containing 0.5 µg/mL ethidium bromide at 80 V for 2 h in TBE buffer was performed to separate DNA fragments. Gel was illuminated with UV light using UV transilluminator (BioDoc-It, Imaging system).
Western Blot Analysis
Radioimmunoprecipitation (RIPA) buffer (Pierce Biotechnology, Rockford, IL, USA) and NE-PER nuclear and cytoplasmic extraction kit (Pierce Biotechnology, Rockford, IL, USA) containing 1 % protease inhibitor assay cocktail (Sigma-Aldrich Co., St. Louis, MO, USA) were used to prepare total and nuclear protein extract, respectively, from left ventricular heart tissue. In total protein extract, protein expressions of cleaved caspase-3, Bax, Bcl-2, p53, HO-1, and Nrf2 were determined, and in nuclear protein extract, protein expression of Nrf2 was determined. The protein concentration was estimated using BCA reagent (Sigma-Aldrich Co., St Louis, MO, USA), and equal amounts of the proteins (40 μg) were separated on 10 % SDS–PAGE and transferred to nitrocellulose membrane. Membranes were blocked in 5 % nonfat dry milk (Blotto, Santa Cruz) in TBST solution (137 mM NaCl, 3 mM KCl, 25 mM Tris, and 0. 05 % Tween 20) for 1 h at room temperature and probed with primary antibodies such as cleaved caspase-3 (1:1,000 dilution; Cell Signaling Technology), Bax (1:1,000 dilution; Cell Signaling Technology), Bcl-2 (1:1,000 dilution; Cell Signaling Technology), p53 (1:1,000 dilution; Cell Signaling Technology), HO-1 (1:1,000 dilution; Cell Signaling Technology), and Nrf2 (1:1,000 dilution; Cell Signaling Technology) for overnight at 4 °C. β-actin (1:1,000 dilution; Cell Signaling Technology) and Lamin-B (1:1,000 dilution; Cell Signaling Technology) were used as internal control for equal loading of total and nuclear protein extract, respectively. Protein bands were visualized by enhanced chemiluminescence (Supersignal West Pico, Pierce Biotechnology, Rockford, IL, USA) following incubation with horseradish peroxidase (HRP)-conjugated secondary antibodies (1:3,000 dilution; Cell Signaling Technology) for 2 h at room temperature. The densitometry analysis was performed by employing ImageJ software, NIH, USA.
Assessment of MMP-9 and MMP-2 Activities
Matrix metalloproteinase (MMP-9 and MMP-2) activities in heart tissue homogenates were determined using fluorimetric MMP-9 and MMP-2 drug discovery assay kits (Enzo life sciences Inc., Farmingdale, NY), respectively, according to the manufacturer’s specifications and were expressed as relative fluorescence unit (RFU). The assay is based on the hydrolysis of the fluorogenic peptide substrate Mca-Pro-Leu-Gly-Leu-Dpa-Ala-Arg-NH2 [Mca = (7-methoxycoumarin-4-yl)-acetyl; Dpa = N-3-(2, 4-dinitrophenyl)-l-α-β-diaminopropionyl]. Mca fluorescence is quenched by the Dpa group until cleavage by MMPs at the Gly-Leu bond separates the two moieties.
Gelatin Zymography for MMP-2 and MMP-9 Expressions Assessment
Zymography using 0.1 % gelatin gel was performed in left ventricular tissue homogenates as described elsewhere [23] to assess the MMP-9 and MMP-2 activities. Heart homogenates were assayed for protein concentration using Bradford assay. Samples normalized for protein concentration were mixed with sample buffer and loaded onto 10 % SDS–PAGE containing 0.1 % gelatin as MMP substrate under non-reducing conditions. After SDS–PAGE, the gel was washed in 2.5 % Triton X-100 for 30 min, rinsed in water, and was incubated for 16 h in developing buffer (50 mmol/L Tris–HCl, 5 mmol/L CaCl2, and 0.02 % NaN3, pH 7.5) at 37 °C. Gel was stained with 0.25 % Coomassie blue R-250 (Sigma) and destained appropriately. Proteolytic activity was detected as clear bands against the background stain of undigested substrate in the gel.
Histopathology
Heart tissue (N = 3/group) was excised and fixed in 10 % neutral buffered formalin solution, processed, embedded in paraffin, and cut into 5-μm slices. The slides were then stained with Hematoxylin–Eosin (H&E) and were evaluated for histological changes under light microscopy (Nikon E800).
Statistical Analysis
The intergroup variations were analyzed by one-way analysis of variance (ANOVA) (GraphPad Prism, version 5.0) followed by Dunnett’s multiple comparison test (DMCT). All data were expressed as the mean and standard errors or as percent activity compared to normal control mice. Results were considered statistically significant when P < 0.05.
Results
DPPH Scavenging Activity
To assess the antioxidant potency of LS, in vitro DPPH free radical scavenging activity was performed. Lagerstroemia speciosa extract was found to effectively scavenge free radicals generated by DPPH, and concentration exhibiting 50 % scavenging effect was 0.128 mg/mL. In contrast, DPPH scavenging activity of ascorbic acid was 0.11 mg/mL (Fig. 1).
Effect of LS on ISO-Induced Myocardial Injury
Isoproterenol administration significantly (P < 0.01) increased the cardiac injury biomarkers as indicated by increase in CK-MB (Fig. 2a), LDH (Fig. 2b) and AST (Fig. 2c) activity compared with those of normal control mice. Pretreatment with LS, at both doses, significantly (P < 0.001 for CK-MB and LDH; P < 0.05 for AST) attenuated the ISO-induced myocardial injury. Notably, in LS control group, LS administration alone for 14 days significantly (P < 0.05) decreased the LDH and AST activity compared to normal control, which indicates a protective mechanism. No significant difference in CK-MB levels was observed between normal control and LS control mice. A significant (P < 0.001) increase in heart-to-body weight ratio (Fig. 2d) (as an index of cardiac hypertrophy) was observed in ISO control group exhibiting the evidence of myocardial injury. Pretreatment with LS significantly (P < 0.05) attenuated the cardiac hypertrophy compared to ISO control group. No statistical significant difference was observed in body weight changes upon LS pretreatment, when compared to normal control mice (data not shown). Histopathological study showed no apparent difference in the cardiac morphology between normal control (Fig. 3a) and LS-treated (Fig. 3b) mice. However, ISO-administered mice revealed extensive cytoplasmic vacuolization, loss of myofibrils, and myocardial degeneration with diffused infiltration of inflammatory cells (Fig. 3c). Though LS treatment at 50 mg/kg produced moderate protection (Fig. 3d), at higher dose (100 mg/kg body weight), LS pretreatment clearly ameliorated the ISO-induced myocardial damage as evidenced by normal myofibrillar structure with occasional infiltration of inflammatory cells (Fig. 3e).
Effect of Lagerstroemia speciosa (LS) on isoproterenol (ISO)-induced changes in serum-specific cardiac injury biomarkers. a Creatine kinase-MB (CK-MB), b lactate dehydrogenase (LDH), and c aspartate transaminase (AST). d Heart-to-body weight ratio was measured as index of myocardial hypertrophy. Values are expressed as mean ± SEM, N = 8, where * P < 0.05, ** P < 0.01, and *** P < 0.001 versus normal control, ### P < 0.001 and # P < 0.05 versus ISO control
Effect of Lagerstroemia speciosa (LS) and isoproterenol (ISO) on the histo-architecture of the heart. Photomicrographs of the heart sections depicting histological finding of a normal control group exposed to only vehicle, b LS control group exposed to only Lagerstroemia speciosa L., with intact normal cardiac morphology (H and E, 20×), c ISO control group, showing extensive cytoplasmic vacuolization (black arrow), loss of myofibrils, and myocardial degeneration with diffused infiltration of inflammatory cells (red arrow) (H and E, 20×), d ISO + LS50 group, showing moderate protection as evidenced by the presence of mild myocardial degeneration with decreased infiltration of inflammatory cells (red arrow) (H and E, 20×), e ISO + LS100 group, showing nearly normal cardiac morphology with occasional infiltration of inflammatory cells (H and E, 20×) (Color figure online)
Effect of LS on Myocardial Oxidative Stress and Nrf2 Nuclear Translocation
Isoproterenol administration significantly (P < 0.01) decreased the myocardial content of GSH (Fig. 4a), GR (Fig. 4b), and GPx (Fig. 4c) activities when compared to normal control mice, whereas GST activity (Fig. 4d) was unaltered in ISO-treated mice compared to normal. Pretreatment of LS at both doses (50 mg/kg and 100 mg/kg body weight) significantly attenuated the ISO-induced decrease in GSH- and GSH-linked enzymes. Furthermore, ISO-treated mice showed a significant (P < 0.05) decrease in SOD (Fig. 5a) as well as catalase (Fig. 5b) activities compared to normal control mice. LS pretreatment, at both doses, significantly increased the SOD (P < 0.05) and catalase (P < 0.05) activities compared to ISO-treated mice. Myocardial TBARS (Fig. 5c) and protein carbonyl contents (Fig. 5d) were significantly (P < 0.001) increased in ISO-induced mice when compared to normal control. LS pretreatment at high dose (100 mg/kg) followed by ISO significantly (P < 0.01 for TBARS; P < 0.05 carbonyl content) attenuated the increase in TBARS and carbonyl contents when compared to ISO control group. LS alone had no effect on lipid peroxidation as well as protein oxidation.
Effect of Lagerstroemia speciosa (LS) on isoproterenol (ISO)-induced changes in myocardial antioxidant status in mice. a Reduced glutathione (GSH), b glutathione reductase (GR), c glutathione peroxidase (GPx), d glutathione S-transferase (GST). Values are expressed as mean ± SEM, N = 8, where # P < 0.05 versus normal control; * P < 0.05, ** P < 0.01, and *** P < 0.001 versus ISO control
Effect of Lagerstroemia speciosa (LS) on isoproterenol (ISO)-induced changes in myocardial. a Superoxide dismutase (SOD), b catalase (CAT), c thiobarbituric acid-reactive substances (TBARS), and d protein carbonyl content. Values are expressed as mean ± SEM, N = 8, where # P < 0.05 and ### P < 0.001 versus normal control; * P < 0.05, ** P < 0.01, and *** P < 0.001 versus ISO control
Myocardial NQO1 activity was significantly (P < 0.05) decreased in ISO control group compared to normal control (Fig. 6a). In addition, mice treated with ISO showed a significant (P < 0.05) decrease in HO-1 protein expression (Fig. 6b) level compared to normal control. LS pretreatment at high dose (100 mg/kg body weight) significantly (P < 0.05 for NQO1; P < 0.01 for HO-1) increased both the NQO1 activity as well as HO-1 protein expression levels compared to only ISO-treated mice. Though there was no difference in Nrf2 protein expression in total myocardial extract among different control and experimental groups, immunoblot of nuclear Nrf2 revealed that LS pretreatment augmented the nuclear translocation of Nrf2 protein when compared to only ISO-treated mice (Fig. 6b).
Effect of Lagerstroemia speciosa (LS) pretreatment on ISO-induced changes in myocardial a NAD(P)H:quinine oxidoreductase 1 (NQO1) activities. Values are expressed as mean ± SEM, N = 8. b Representative immunoblot showing the effect of Lagerstroemia speciosa (LS) pretreatment on ISO-induced changes in myocardial heme oxygenase-1 (HO-1) as well as nuclear translocation of nuclear factor erythroid 2-related factor 2 (Nrf2) protein expressions. Graphical representation of the intensity of c nuclear Nrf2, d total Nrf2, and e HO-1 protein expression. Data represent the means (3 animals per group), with their standard error (SEM) represented by vertical bars, where NS normal control, LS Lagerstroemia speciosa control, ISO isoproterenol control, ISO + LS50 LS (50 mg/kg body weight) followed by ISO, ISO + LS100 LS (100 mg/kg body weight) followed by ISO, where # P < 0.05 versus normal control (NS); * P < 0.05 and ** P < 0.01 versus ISO control
Effect of LS on Myocardial Apoptosis
As shown in Fig. 7, the protein expression of cleaved caspase-3, Bax, and p53 in left ventricular tissue was significantly (P < 0.05) increased after ISO administration and was significantly attenuated by LS pretreatment. ISO administration also significantly (P < 0.001) reduced the levels of the anti-apoptotic protein Bcl-2, which was significantly (P < 0.05) improved by LS pretreatment. Furthermore, there is substantial increase in internucleosomal DNA smearing and fragmentation as evident from both DNA laddering and smearing pattern in the agarose gel induced by ISO treatment. Pretreatment with LS at a low dose (50 mg/kg) did not prevent DNA fragmentation induced by ISO. However, high-dose LS (100 mg/kg) treatment prevented DNA fragmentation substantially as evident by the absence of DNA laddering as well as smearing pattern.
Effect of LS and ISO on apoptosis-related protein expression in left ventricular heart tissue. a Immunoblot analysis of cleaved caspase-3, Bcl-2, Bax, and p53. Data represent the means (3 animals per group), with their standard error (SEM) represented by vertical bars, where NS normal control, LS Lagerstroemia speciosa control, ISO isoproterenol control, ISO + LS50 LS (50 mg/kg body weight) followed by ISO, ISO + LS100 LS (100 mg/kg body weight) followed by ISO. b Effect of the pretreatment of Lagerstroemia speciosa on DNA fragmentation exposed to ISO in mice. Agarose gel (1.5 %) picture revealed that there is intact DNA in NC and LS groups. ISO administration increased DNA damage as evidenced by the presence of both smear and ladder pattern of DNA. Though low-dose group, ISO + LS50, showed mild DNA fragmentation, higher-dose group, ISO + LS100, completely prevented the DNA damage. Graphical representation of the intensity of c cleaved caspase-3, d Bcl-2, e Bax, and f p53 protein expressions, where ### P < 0.001 and # P < 0.05 versus normal control; * P < 0.05, ** P < 0.01, and *** P < 0.001 versus ISO control
Effect of LS on ISO-Induced Modifications on MMPs Activities
As noted in Fig. 8, ISO group presented a significant (P < 0.01) increase in tissue MMP-9 (Fig. 8a) and MMP-2 (Fig. 8b) activities compared with normal control. Gelatin zymograph picture confirmed that there is activation of pro-MMP-9 and pro-MMP-2 (Fig. 8c). In contrast, LS pretreatment, at both doses, abolished the MMP-9 as well as MMP-2 activities when compared to ISO-induced group. LS alone had no effects on the above-mentioned variables.
Effects of LS and ISO on myocardial matrix metalloproteinases (MMPs) activities. The activities of a MMP-9 and b MMP-2 were measured using fluorimetric assay and expressed as relative fluorescence units (RFU). Values are expressed as mean ± SEM of 8 animals (N = 8) in each groups. c Representative gelatin zymograph for MMP-9 and MMP-2 activities in heart homogenates of different experimental groups, where NS normal control, LS Lagerstroemia speciosa control, ISO isoproterenol control, ISO + LS50 LS (50 mg/kg body weight) followed by ISO, ISO + LS100 LS (100 mg/kg body weight) followed by ISO, where ## P < 0.01 versus normal control; * P < 0.05 versus ISO control
Discussion
Lagerstroemia speciosa L. is believed to possess anti-inflammatory, antioxidative, antihyperglycemic, antihypertensive, and hypolipidemic properties [10]. However, their cardioprotective properties and underlying mechanisms are largely unknown. In the present study, we tested the hypothesis that LS ameliorates the myocardial stress in ISO-induced myocardial injury in mice. The pivotal findings in the present study are as follows. (1) We observed for the first time that LS significantly attenuated cardiac stress associated with isoproterenol in mice. (2) We also observed that LS significantly inhibited ISO-induced necrotic damage, vacuolization, and loss of myofibrils in the heart. (3) Further studies indicated that LS augmented endogenous antioxidant mechanisms, ameliorated myocardial apoptosis, and expression of MMPs.
Over-release of catecholamine is an important factor related to myocardial impairment in many cardiovascular diseases, such as myocardial ischemia, hypertrophy, and heart failure [24]. In the present study, we induced myocardial injury mouse model employing a β-adrenoreceptor agonist, isoproterenol. Isoproterenol-induced myocardial injury involves membrane permeability alterations that bring about loss of function and integrity of myocardial membranes [25]. We found that treatment with high doses of ISO (150 mg/kg) for 2 consecutive days resulted in severe myocardial injury with extensive vacuolization in heart muscle and inflammation with diffused inflammatory cell infiltration (Fig. 3c). An increase in the heart weight-to-body weight ratio was observed in ISO-treated mice indicating cardiac hypertrophy [7]. The increase in serum-specific cardiac biomarkers such as CK-MB, LDH, and AST (Fig. 2) indicated the leakage and loss of functional integrity and/or necrotic damage of cell membrane. LS pretreatment to mice followed by ISO significantly lowered all of the above parameters to normal when compared to the individual treatment groups. The present study suggests that LS restricts the leakage of these indicative enzymes by restoring the structural and functional integrity of the cardiac membrane. It is also speculated that the observed increase in the heart weight in ISO-treated mice might be due to the increased water content, edematous intramuscular space, and extensive necrosis of cardiac muscle fibers followed by the invasion of damaged tissues by the inflammatory cells [7]. Pretreatment with LS decreased the heart weight and prevented cardiac hypertrophy in ISO-treated mice.
Reactive oxygen species (ROS) generation during auto-oxidation of ISO and subsequent oxidative stress have been proposed to be important mechanisms underlying its cardiac toxic effects [26]. Studies on the myocardial antioxidant changes and their significance during heart injury have provided a new insight into the pathogenesis of heart failure. Moreover, it has been reported that the antioxidant enzymes status is lower in the heart than in other tissues [27]. Hence, up-regulation of endogenous antioxidant and phase II enzymes is an efficient protective strategy against the deleterious effects of reactive oxygen species during myocardial injury. Decrease in these antioxidants after ISO administration may be ascribed to excessive formation of reactive oxygen species (ROS) and/or their inactivation by excessive isoproterenol-induced oxidants in heart tissues. In the present study, we documented a significant (P < 0.01) increase in antioxidants (GSH, GR, GPx, GST, SOD, and CAT) and decrease in TBARS and protein carbonyl levels in heart tissue of LS pretreated ISO-induced mice when compared to those in ISO control mice. NQO1, a well-known detoxifying antioxidant, efficiently scavenges H2O2 and superoxide radicals via its ability to maintain the cellular levels of ubiquinol and vitamin E. In our study, a significant (P < 0.05) decline in NQO1 activity was observed in ISO-treated mice. LS pretreatment significantly (P < 0.05) abolished the decrease in NQO1 activity when compared to ISO-treated mice (Fig. 6a). Heme oxygenase-1 (HO-1), a cytoprotective heme-degrading enzyme catalyzes the degradation of heme into cytoprotective HO reaction products: biliverdin and bilirubin, carbon monoxide (CO), and free iron. Number of studies has shown that cardioprotective potential of HO-1 and its up-regulation in myocardium prevented cardiomyocyte apoptosis [28]. In addition, it is also documented that overexpression of HO-1 attenuates pathological ventricular remodeling after acute myocardial infarction [29]. In the present study, a significant decrease (P < 0.05) in protein expression of HO-1 was observed in ISO control group compared to normal control. Pretreatment with LS followed by ISO significantly (P < 0.001) restored the HO-1 level compared to ISO control group (Fig. 6b).
Redox-sensitive transcription factor nuclear factor erythroid 2-related factor 2 (Nrf2) has been demonstrated to be a critical transcription factor that regulates the induction of phase 2 detoxifying and antioxidant genes [30]. Zhu et al. [22] have reported that Nrf2 signaling plays a key role in preventing oxidative cardiac cell injury in vitro. Moreover, evidence has also revealed that induction of endogenous antioxidants through activation of Nrf2 has been shown to prevent oxidative stress and confers cardioprotection [31]. To explore the molecular mechanism of LS, we studied the nuclear translocation of Nrf2 in heart tissue. We observed an inhibition of nuclear translocation of Nrf2 protein in heart of ISO-induced mice compared to normal control. Pretreatment with LS significantly (P < 0.001) improved the myocardial nuclear protein expression of Nrf2 levels when compared to ISO-treated group (Fig. 6b). To summarize, in the present study, pretreatment with LS followed by ISO resulted in a significant induction of cellular antioxidants and phase 2 enzymes compared to those of ISO control. We believe that the observed effect of LS might be ascribed to its intrinsic antioxidant activity (directly neutralizing reactive species) and/or its ability to enhance antioxidant defense system through nuclear up-regulation of Nrf2 (nuclear factor erythroid 2-related factor-2) expression in heart tissues.
Evidences from experimental animal models and human cardiac disease showed that cardiomyocyte cell loss as a result of apoptosis is significant in myocardial ischemic injury [32]. As ISO-induced myocardial injury is also mediated through apoptosis of cardiomyocytes [33], we investigated the possible protective effect of LS on apoptosis. Usually, the balance between the up- and down-regulations of the members of Bax and Bcl-2 family proteins determines the fate of the cells either to undergo apoptosis or to survive in pathophysiology. In the process of apoptosis, pro-apoptotic (Bax) protein plays a dominant role in initiating cell death by disrupting the integrity of the mitochondrial membrane and further activates downstream apoptosis proteins like caspase-9/caspase-3 and PARP proteolysis followed by DNA fragmentation, whereas anti-apoptotic (Bcl-2) inhibits it [32]. Here, we examined the myocardial protein expression of cleaved caspase-3 and Bax, which has been implicated as stimulators of apoptosis, to explore the role of LS on apoptotic cell deaths caused by ISO (Fig. 7a). Both ladder and smearing pattern of DNA in agarose gel (Fig. 7b) revealed apoptotic as well as necrotic cell death in the myocardium of ISO-induced mice. Pretreatment with LS (100 mg/kg) followed by ISO attenuated myocardial protein expression of cleaved caspase-3, Bax, and DNA damage induced by ISO. Moreover, administration of LS increased the expression of the anti-apoptotic protein, Bcl-2, compared to ISO-induced group, suggesting that the LS exhibits an inhibitory effect on cell death during myocardial ischemia-induced apoptosis (Fig. 7b). The transcription factor and pro-apoptotic gene p53 induce apoptosis in response to varieties of stimuli including ischemia [34]. It documented the up-regulation of p53 and cardiomyocyte apoptosis in ischemic heart, and it has also been shown that p53 gene deletion improved cardiac function after myocardial infarction [35]. We found a significant elevation of p53 protein in ISO control group compared to normal. LS pretreatment (100 mg/kg) followed by ISO significantly decreased the protein expression of p53 compared to ISO control mice (Fig. 7b). Hence, we speculate that cardioprotective effect of LS is partly due to the inhibition of myocardial apoptosis.
Matrix metalloproteinases (MMPs) are a family of zinc-dependent endopeptidases that has been identified in the myocardium and is likely to contribute to myocardial remodeling [36]. Growing evidence from basic and clinical studies has demonstrated the important role of MMPs in the progression of left ventricular remodeling and mortality following acute myocardial infarction [37]. Studies have also shown that MMPs are able to degrade extracellular matrix and affect ventricular remodeling during the healing process after acute myocardial injury [38]. It is also reported that an abrupt increase in collagen content in conjunction with changes in MMP release and activity, mainly high levels of MMP-2 and MMP-9, has been shown in hypertrophic cardiomyopathy patients [39]. In the present study, in order to test whether LS is able to counteract the myocardial remodeling, we quantified MMP-2 and MMP-9 activities in the myocardium. Our study showed the expression and activation of pro-MMP-9 and pro-MMP-2 increased in the left ventricles of ISO-treated mice, which suggests that MMPs play a critical role in ventricular remodeling. To our surprise, LS treatment prevented the myocardial overexpressions of MMP-2 and MMP-9 activities in ISO-treated mice and restored to normal level (Fig. 8). These findings in our study demonstrate that LS is able to attenuate not only the oxidative stress and apoptosis, but also the remodeling process presented in this myocardial injury model. A proposed working model related to cardioprotective effect of Lagerstroemia speciosa in mouse heart was described in Fig. 9.
A proposed working model related to potential signaling pathways that are involved in cardioprotective effect of Lagerstroemia speciosa against isoproterenol-induced myocardial injury in mouse heart, where ROS reactive oxygen species, Nrf2 nuclear factor erythroid 2-related factor 2, Keap1 Kelch-like ECH-associated protein 1, MMPs matrix metalloproteinases, HO-1 heme oxygenase 1, NQO1 NAD(P)H:quinine oxidoreductase 1, GST glutathione S-transferase; GPx glutathione peroxidase, CAT catalase
In summary, the present study provided a convincing evidence of myocardial oxidative stress, apoptosis, and activation of MMPs in heart of isoproterenol-administered mice. Our results suggested that pretreatment with standardized Lagerstroemia speciosa leaf (LS) extract containing 1 % corosolic acid ameliorated ISO induced myocardial oxidative stress, lipid peroxidation and protein oxidation. Furthermore, LS pretreatment enhanced myocardial antioxidant enzyme status through up-regulation of Nrf2/HO-1 and suppressed myocardial apoptosis and remodeling process associated with isoproterenol in mice. Hence, our findings strongly support the therapeutic role of Lagerstroemia speciosa in the treatment for cardiovascular disorders such as myocardial infarction.
Abbreviations
- AST:
-
Aspartate transaminase
- CAT:
-
Catalase
- CK-MB:
-
Creatine kinase-MB isoenzyme
- DPPH:
-
2-2-Diphenyl-1-picrylhydrazyl
- DTNB:
-
5,5-Dithio-bis (2-nitrobenzoic acid)
- GPx:
-
Glutathione peroxidase
- GR:
-
Glutathione reductase
- GSH:
-
Reduced glutathione
- GSSG:
-
Glutathione oxidized
- GST:
-
Glutathione S-transferase
- HO-1:
-
Heme oxygenase-1
- ISO:
-
Isoproterenol
- LDH:
-
Lactate dehydrogenase
- LS:
-
Lagerstroemia speciosa leave extract
- MMPs:
-
Matrix metalloproteinases (MMPs)
- NQO1:
-
NAD(P)H:quinine oxidoreductase
- Nrf2:
-
Nuclear factor erythroid 2-related factor 2
- SOD:
-
Superoxide dismutase
- TBA:
-
2-Thiobarbituric acid
References
Thygesen, K., Alpert, J. S., Jaffe, A. S., Simoons, M. L., Chaitman, B. R., & White, H. D. (2012). Third universal definition of myocardial infarction. Nature Review Cardiology, 9, 620–633.
He, B. J., Joiner, M. A., Singh, M. V., Luczak, E. D., Swaminathan, P. D., Koval, O. M., et al. (2011). Oxidation of CaMKII determines the cardiotoxic effects of aldosterone. Nature Medicine, 17, 1610–1619.
Tavares, A. M. V., Araujo, A. S. D. R., Baldo, G., Matte, U., Khaper, N., Bello-Klein, A., et al. (2010). Bone marrow derived cells decrease inflammation but not oxidative stress in an experimental model of acute myocardial infarction. Life Sciences, 87, 699–706.
Kinugawa, S., Tsutsui, H., Hayashidani, S., Ide, T., Suematsu, N., Satoh, S., et al. (2000). Treatment with dimethylthiourea prevents left ventricular remodeling and failure after experimental myocardial infarction in mice: Role of oxidative stress. Circulation Research, 87, 392–398.
Kim, S. H., Moon, H., Kim, H. A., Hwang, K., Lee, M., & Choi, D. (2011). Hypoxia-inducible vascular endothelial growth factor-engineered mesenchymal stem cells prevent myocardial ischemic injury. Molecular Therapy, 19, 741–750.
Roy, S. J., & Prince, P. S. M. (2013). Protective effects of sinapic acid on cardiac hypertrophy, dyslipidemia and altered electrocardiogram in isoproterenol-induced myocardial infarcted rats. European Journal of Pharmacology, 699, 213–218.
Li, H., Xie, Y. H., Yang, Q., Wang, S. W., Zhang, B. L., et al. (2012). Cardioprotective effect of Paeonol and Danshensu combination on isoproterenol-induced myocardial injury in rats. PLoS ONE, 7, e48872.
Yamaguchi, Y., Yamada, K., Yoshikawa, N., Nakamura, K., Haginaka, J., & Kunitomo, M. (2006). Corosolic acid prevents oxidative stress, inflammation and hypertension in SHR/ND mcr-cp rats, a model of metabolic syndrome. Life Sciences, 79, 2474–2479.
Judy, W. V., Hari, S. P., Stogsdill, W. W., Judy, J. S., Naguib, Y. M. A., & Passwater, R. (2003). Antidiabetic activity of a standardized extract (GlucosolTM) from Lagerstroemia speciosa leaves in Type II diabetics: A dose-dependence study. Journal of Ethnopharmacology, 87, 115–117.
Stohs, S. J., Miller, H., & Kaats, G. R. (2012). A review of the efficacy and safety of banaba (Lagerstroemia speciosa L.) and corosolic acid. Phytotherapy Research, 26, 317–324.
Fujiwara, Y., Komohara, Y., Ikeda, T., & Takeya, M. (2011). Corosolic acid inhibits glioblastoma cell proliferation by suppressing the activation of signal transducer and activator of transcription-3 and nuclear-factor kappa B in tumor cells and tumor-associated macrophages. Cancer Science, 102, 206–211.
Nho, K. J., Chun, J. M., & Kim, H. K. (2013). Corosolic acid induces apoptotic cell death in human lung adenocarcinoma A549 cells in vitro. Food and Chemical Toxicology, 56, 8–17.
Ichikawa, H., Yagi, H., Tanaka, T., Cyong, J. C., & Masaki, T. (2010). Lagerstroemia speciosa extract inhibit TNF-induced activation of nuclear factor-kB in rat cardiomyocyte H9c2 cells. Journal of Ethnopharmacology, 128, 254–256.
Judy, W. V., Hari, S. P., Stogsdill, W. W., Judy, J. S., Naguib, Y. M. A., & Passwater, R. (2003). Antidiabetic activity of a standardized extract (Glucosol™) from Lagerstroemia speciosa leaves in Type II diabetics. A dose-dependence study. Journal of Ethnopharmacology, 87, 115–117.
Ohkawa, H., Ohishi, N., & Yagi, K. (1979). Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Analytical Biochemistry, 95, 351–358.
Dalle-Donne, I., Rossi, R., Giustarini, D., Milzani, A., & Colombo, R. (2003). Protein carbonyl groups as biomarkers of oxidative stress. Clinica Chimica Acta, 329, 23–38.
Ellman, G. L. (1959). Tissue sulfhydryl group. Archives of Biochemistry and Biophysics, 82, 70–77.
Aebi, H. (1974). Catalase. In H. U. Bergmeyer (Ed.), Methods of enzymatic analysis (pp. 673–677). New York: Academic Press.
Carlberg, I., & Mannervik, B. (1975). Glutathione reductase levels in rat brain. Journal of Biological Chemistry, 250, 5475–5480.
Habig, W. H., Pabst, M. J., & Jakoby, W. B. (1974). Glutathione S-transferases. The first enzymatic step in mercapturic acid formation. Journal of Biological Chemistry, 249, 7130.
Paglia, D. E., & Valentine, W. N. (1967). Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. Journal of Laboratory and Clinical Medicine, 70, 158–169.
Zhu, H., Itoh, K., Yamamoto, M., Zweier, J. L., & Li, Y. (2005). Role of Nrf2 signaling in regulation of antioxidants and phase 2 enzymes in cardiac fibroblasts: Protection against reactive oxygen and nitrogen species-induced cell injury. FEBS Letters, 579, 3029–3036.
Yu, Y. M., Lin, H. C., & Chang, W. C. (2008). Carnosic acid prevents the migration of human aortic smooth muscle cells by inhibiting the activation and expression of matrix metalloproteinase-9. British Journal of Nutrition, 100, 731–738.
Li, L., Zhang, L., Pang, Y., Pan, C., Qi, Y., Chen, L., et al. (2006). Cardioprotective effects of ghrelin and des-octanoyl ghrelin on myocardial injury induced by isoproterenol in rats. Acta Pharmacologica Sinica, 27, 527–535.
Sudhees, N. P., Ajith, T. A., & Janardhanan, K. K. (2013). Ganoderma lucidum ameliorate mitochondrial damage in isoproterenol-induced myocardial infarction in rats by enhancing the activities of TCA cycle enzymes and respiratory chain complexes. International Journal of Cardiology, 165, 117–125.
Vijayan, N. A., Thiruchenduran, M., & Devaraj, S. N. (2012). Anti-inflammatory and anti-apoptotic effects of Crataegus oxyacantha on isoproterenol-induced myocardial damage. Molecular and Cellular Biochemistry, 367, 1–8.
Angeloni, C., Leoncini, E., Malaguti, M., Angelini, S., Hrelia, P., & Hrelia, S. (2009). Modulation of phase II enzymes by sulforaphane: Implications for its cardioprotective potential. Journal of Agriculture and Food Chemistry, 57, 5615–5622.
Lakkisto, P., Siren, J., Kyto, V., Forsten, H., Laine, M., Pulkki, K., et al. (2011). Heme oxygenase-1 induction protects the heart and modulates cellular and extracellular remodelling after myocardial infarction in rats. Experimental Biology and Medicine, 236, 1437–1448.
Liu, X., Pachori, A. S., Ward, C. A., Davis, J. P., Gnecchi, M., et al. (2006). Heme oxygenase-1 (HO-1) inhibits post myocardial infarct remodeling and restores ventricular function. The FASEB Journal, 20, 207–2016.
Yan, D., Dong, J., Sulik, K. K., & Chen, S. Y. (2010). Induction of the Nrf2-driven antioxidant response by tert-butylhydroquinone prevents ethanol-induced apoptosis in cranial neural crest cells. Biochemical Pharmacology, 80, 144–149.
Dreger, H., Westphal, K., Weller, A., Baumann, G., Stangl, V., Meiners, S., et al. (2009). Nrf2-dependent up regulation of antioxidative enzymes: A novel pathway for proteasome inhibitor-mediated cardioprotection. Cardiovascular Research, 83, 354–361.
He, H., Xu, J., Xu, Y., Zhang, C., Wang, H., He, Y., et al. (2012). Cardioprotective effects of saponins from Panax japonicus on acute myocardial ischemia against oxidative stress-triggered damage and cardiac cell death in rats. Journal of Ethnopharmacology, 140, 73–82.
Radhiga, T., Rajamanickam, C., Sundaresan, A., et al. (2012). Effect of ursolic acid treatment on apoptosis and DNA damage in isoproterenol-induced myocardial infarction. Biochimie, 94, 1135–1142.
Matsusaka, H., Ide, T., Matsushima, S., Ikeuchi, M., Kubota, T., Sunagawa, K., et al. (2006). Targeted deletion of p53 prevents cardiac rupture after myocardial infarction in mice. Cardiovascular Research, 70, 457–465.
Naito, A. T., Okada, S., Minamino, T., Iwanaga, K., Liu, M., Sumida, T., et al. (2010). Promotion of CHIP-mediated p53 degradation protects the heart from ischemic injury. Circulation Research, 106, 1692–1702.
Moshal, K. S., Rodriguez, W. E., Sen, U., & Tyagi, S. C. (2008). Targeted deletion of MMP-9 attenuates myocardial contractile dysfunction in heart failure. Physiological Research, 57, 379–384.
Phatharajaree, W., Phrommintikul, A., & Chattipakorn, N. (2007). Matrix metalloproteinases and myocardial infarction. Canadian Journal of Cardiology, 23, 727–733.
Nie, R., Xie, S., Du, B., Liu, X., Deng, B., & Wang, J. (2009). Extracellular matrix metalloproteinase inducer (EMMPRIN) is increased in human left ventricle after acute myocardial infarction. Archives of Medical Research, 40, 605–611.
Pei, Z., Meng, R., Li, G., Yan, G., Xu, C., Zhuang, Z., et al. (2010). Angiotensin-(1–7) ameliorates myocardial remodeling and interstitial fibrosis in spontaneous hypertension: Role of MMPs/TIMPs. Toxicology Letters, 199, 173–181.
Acknowledgments
We thank the Director, CSIR-IICT, Hyderabad, India, for providing necessary facilities. We are also thankful to the CSIR 12th 5-year plan project “SMiLE” (CSC 0111) for partial financial assistance. B.D.S thanks Council of Scientific and Industrial Research (CSIR), New Delhi, India, for financial assistance in the form of Senior Research Fellowship.
Conflict of interest
The authors declare that there is no competing interest associated with this manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sahu, B.D., Kuncha, M., Rachamalla, S.S. et al. Lagerstroemia speciosa L. Attenuates Apoptosis in Isoproterenol-Induced Cardiotoxic Mice by Inhibiting Oxidative Stress: Possible Role of Nrf2/HO-1. Cardiovasc Toxicol 15, 10–22 (2015). https://doi.org/10.1007/s12012-014-9263-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12012-014-9263-1