Abstract
Lead (Pb) is an environmental oncogenic metal that induces immunotoxicity and anaemia. Emerging evidence has linked Pb toxicity with endoplasmic reticulum-driven apoptosis and autophagy. Glucose-regulated protein of 78 kDa (Grp78 or binding immunoglobulin protein (BiP)), a master endoplasmic reticulum chaperone, drives macrophage activation and regulates protein folding and calcium flux in response to heavy metals. The spleen may be involved in Pb poisoning due to its crucial role in erythrocatheresis and immune response, although there are no data to support this theory. Here, we found haematic and histopathological changes in the spleen of mice exposed to medium doses of Pb acetate (200 ppm–1 mM) in drinking water for 45 days. Pb deposition was also detected in organs such as the liver, kidney, brain, bone, blood and faeces, indicating an accumulation of this metal despite relatively short exposure time. Blood Pb content (BBL) reached 21.6 μg/dL; echinocytes and poikilocytes were found in Pb smears of treated group. Inside the spleen, higher Fe(II) and Fe(III) deposits inside macrophages were observed. Grp78 immunostaining, weakly expressed in spleen cells of control mice, after Pb exposure was specifically restricted to macrophages and megakaryocytes of the marginal zone of red pulp. Furthermore, Pb exposure induced superoxide dismutase 1 (SOD1) expression, cleaved caspase-3 and p62/SQSTM1, consistent with oxidative stress, apoptosis and dysregulated autophagy in spleen compartments. We suggest that even at a middle dose, oral Pb intake induces oxidant iron deposition in the spleen and that this may trigger sustained Grp78 redistribution to cells, thus leading to oxidative and autophagy dysfunction as early local reactions to this dangerous metal.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Lead (Pb) is a widespread environmental heavy metal that can cause damage to many organs and tissues such as the brain, heart, liver and kidney. It mimics essential minerals such as calcium and zinc, inducing chronic cardiovascular, renal, immunological and neurological diseases as well as cancer in humans and animals [1–3]. Pregnant women, children and city habitants are particularly at risk of Pb intoxication [4, 5].
People are exposed to Pb through air, water and food/ingestion, despite restrictions of additives in both gasoline and paintings, which have serious consequences for human health in industrialized regions. Toxic effects are usually due to long-term exposure. The atmospheric concentration of lead varies greatly, with highest levels found near stationary sources such as lead smelters. The EPA national environmental air quality standard for lead is 1.5 μg/m3 [6]. Unfortunately, Pb does not degrade and is highly absorbed in the soil, as previous lead use remains.
The major routes of occupational lead exposure are inhalation and ingestion of lead-bearing dusts and fumes [6]. According to Italy’s National Statistical Institute, in 2001, a total of 2082 Italian workers were employed in the production of zinc, lead, tin and their semi-finished products (website: http://www.istat.it). Levels of lead in environmental air range between about 7.6 × 10–5 μg/m3 in remote areas such as Antarctica and >10 μg/m3 near point sources. Pb absorbed due to air or water/soil contamination enters the blood stream, where it deposits into erythrocytes (RBCs) and induces anaemia [7, 8]. From an occupational point of view, blood lead level (BLL) is considered the principal index of Pb exposure [9].
Acute toxicity is related to occupational exposure and is quite uncommon. Chronic toxicity, on the other hand, is much more common. In adults and children, symptoms can occur at levels above 40 μg/dL but are more likely to occur only above 50–60 μg/dL [10, 11].
Pb toxicity occurs at BBL above 40 μg/dL, even if Pb-induced damage is often underestimated in children and adolescents, and there is no real threshold for safety as even BBL below 10 μg/dL can be toxic [12]. Interestingly, Pb induces phosphatidylserine externalization in erythrocytes which attracts macrophages [13] and intensifies pro-coagulant and thrombotic events [14]. Cellular mechanisms involved in Pb poisoning include indirect oxidative stress due to its link with glutathione and anti-oxidant enzymes, calcium dysregulation and altered endoplasmic reticulum (ER) homeostasis [8, 15].
Grp78, the glucose-regulated protein 78 kDa, also known as binding immunoglobulin protein (BiP) or master resident ER chaperone [16], is an important regulator of ER function. Several in vitro studies have reported Grp78 induction by Pb in astrocytes and glioma [17], hepatoma HepG2 [18], renal NRK52 cells [19] and endothelial cells [20]. Intriguingly, Grp78 has also been described in other intracellular sites aside from ER, such as mitochondria and plasma membrane [21, 22].
The Pb impact on the spleen has rarely been assessed, probably due to the fact that the spleen can be removed without much problem if it is affected. In any case, we believe that it is important to analyse Pb effects on this organ, due its crucial role in erythrocatheresis and immune response modulation [23, 24]. Recently, Kasten-Jolly and Lawrence [24] indicated that Pb affected innate immunity and induced helper type 2 T cells and macrophages in the spleen. Nevertheless, Grp78’s role and relevance in the spleen after sub-chronic Pb intoxication has not yet been reported.
So, the principal aim of this paper is to investigate whether sub-chronic medium doses of Pb assumed in drinking water for 45 days can determine early oxidative effects and ER stress on the spleen. We therefore performed the study on young mice orally administered with 200 ppm Pb. Blood and serum parameters, Pb levels in the spleen and other organs such as the kidney, brain, bone, liver, blood and faeces, as well as splenic iron accumulation, Grp78, apoptotic and autophagy markers, were all analysed.
Materials and Methods
Drugs and Chemicals
Pb-acetate trihydrate, buffers, poly-l-lysine and iron stains were of the highest purity grade, produced by Sigma-Aldrich Chemical Co. (Milan, Italy). DPEX synthetic mounting medium was suppled from BDH. Polyclonal anti-rabbit anti-Grp78 antibody (sc1050) was from Santa Cruz Biotechnology (CA); anti-p62/SQSTM1 (ab64134) and anti-caspase-3 (ab4051) was from Abcam (UK) and ABC-peroxidase Elite kit was from Santa Cruz Biotechnology.
Animals
The experiments were conducted in accordance with the European Communities Council Directive of November 24, 1986 (86/609/EEC), and approved by the Committee Responsible for Animal Care and Use (Organismo Preposto per il Benessere Animale (OPBA)) of the Brescia University and by the Italian Ministry of Health (decree no. 176/2013-B, 2013) and complied with the National Animal Protection Guidelines. Sixteen male C57BJ mice (1-month old) (Harlan Laboratories, Italy) were used, eight animals for each group. The mice were housed separately in clean polypropylene cages and divided into two experimental groups: (1) one was exposed to Pb (as Pb-acetate trihydrate, mol. wt. 379.33) through its drinking water for 45 days (Pb group). The Pb doses were 200 ppm and were chosen to simulate middle-dose lead exposure over a short period of time. Following Teijon et al. [25], a small amount (1 mL/L) of acetic acid was added to the water to avoid salt precipitation. (2) Mice drinking fresh tap water, added with acetic acid (1 mL/L), were used as a control.
The water was filtered and did not contain any detectable amounts of Pb or other heavy metals. The animals were placed in a quiet, temperature and humidity-controlled room and were kept on a 12-/12-h light/dark cycle (lights on from 7 a.m. to 7 p.m.). All mice were fed a standard rodent diet ad libitum (19.4% rough protein content; metabolizable energy 2668 kcal/kg, Mucedola srl, Milan, Italy).
Experimental Protocol
Body weight (bw), food (g) and water (mL) consumption for each animal were monitored every 2 days, and the concentrations of drinking solutions were adjusted accordingly. Water consumption was measured by weighing the bottle, with bw measured using an electronic balance to an accuracy of 0.1 g. These data allowed us to detect Pb intake in terms of mg Pb/kg bw/day.
At the end of treatments, the animals were killed by cervical dislocation. Blood smears were obtained for each mice and stained by May-Grünwald and Giemsa methods to visualize the red and white elements. Blood samples from the right heart in the Pb-treated and control groups were used to analyse the haematocrit and haemoglobin content and serum proteins. The spleen from each animal was quickly removed, weighed, split and fixed with immune fix for 12 h at 4 °C. The samples were then washed in phosphate-buffered saline (PBS) buffer (0.2 M, pH 7.4) for 12 h and processed according to standard procedures for paraffin embedding. Thick sections (about 5 μm) by a Leica microtome, collected on poly-l-lysinated slides, were used for histochemical (HC) and immunohistochemical (IHC) procedures.
Histopathology
Paraffin sections of spleen were stained by haematoxylin and eosin (H&E); Perls’Prussian blue or Turnbull’s blue staining were used to check general feature, the presence of fibrosis and free iron accumulation such as ferric [Fe(III)] or ferrous [Fe(II)] ions, respectively [26].
Immunohistochemistry
Paraffin sections were stained according to ABC-peroxidase method as previously described [27]. Paraffin sections were incubated overnight at 4 °C with primary polyclonal Grp78 (sc1050) (1:100) from Santa Cruz Biotechnology (CA), p62/SQSTM1 (ab64134) (1:50) from Abcam (UK) and active/cleaved caspase-3 (NB100-56113 from Novus Bio) antibodies. In order to exclude any incorrect interpretation of immunostaining due to endogenous biotin, we also used the peroxidase-anti-peroxidase detection system.
We obtained similar results with both methods. The IHC control (negative control) was performed by omitting the primary antibody in presence of isotype-matched IgGs. The sections were processed in accordance with the manufacturers’ protocol, visualized with a rabbit ABC-peroxidase staining system kit and mounted with DPEX. The reaction product was visualized using 0.3% hydrogen peroxide and diaminobenzidine at room temperature. Each set of experiment was carried out in triplicate, with each replicate carried out strictly under the same experimental conditions.
Splenic parenchyma was described in three zones: white pulp (WP) comprising follicles; marginal zone (MZ), at the boundary between white and red area and red pulp (RP). Different researchers, blinded to the samples, independently analysed all slides. Staining intensified in HC and IHC methods was semi-quantitatively evaluated using an optical Olympus BX50 light microscope. According to Hall et al. [28], the evaluating scale adopted for HC was − undetectable; +/− barely detectable; + weak (10–30% positive cells); 2+ evident (30–50% positive cells); 3+ moderate (50–70% positive cells) and 4+ intense/strong staining (>70% positive cells). The staining intensity in IHC slides was evaluated using light microscope equipped with an image analysis program (Image-Pro Plus 4.5.1). The integrated optical density (IOD) was calculated for arbitrary areas, by measuring at least five fields for each sample.
Pb Measurement
Pb concentration in the blood, spleen, faeces, brain, bone, liver and kidney was performed by the Laboratory of Food Chemistry of the National Reference Centre for Animal Welfare (IZSLER-Bs), a national institution for animal health, according to the certified analytical IZSLER procedure for the determination of heavy metals. Briefly, the samples were weighed and subjected to mineralization by wet way with nitric acid and concentrated hydrogen peroxide in the open digester. The mineralized samples were brought to the appropriate volume with the aqueous solution of nitric acid and were analysed using inductively coupled plasma mass spectrometer (ICP-MS, Agilent 7700, Agilent Technologies, Santa Barbara, CA, USA) with computerized control (software Agilent MassHunter Workstation). As reference, solutions for the calibration curves used commercial standard Pb solutions (1000 mg/L).
Blood Analysis
The analysis was performed by Division of Laboratory Animals of IZSLER-Bs. Blood samples from each animal were split and collected into tubes containing K3-EDTA anti-coagulant (BD Vacutainer 1.5 mL) for haemochromocytometric analysis and in a 1.5-mL vial (Eppendorf srl, Milan, Italy) without anti-coagulant for serum analysis. Haemochromocytometric values were measured by CELL-DYN 3700 laser-impedance cell counter (Abbott Diagnostics Division, Abbott Laboratories, IL, USA). From the blood serum, urea, albumin, creatinine and haptoglobin concentrations were measured using a biochemical automatic analyser ILab Aries (Instrumentation Laboratory, Lexington, MA, USA) and its ready-to-use kits.
Haematologic indices were determined according to standard methods. Tests included counts of red blood cells (RBCs), white blood cells (WBCs) and PLT; differential counts of neutrophils, lymphocytes, monocytes and eosinophils and the concentrations of haemoglobin (Hb), mean corpuscular volume (MCV), mean corpuscular haemoglobin (MCH), mean corpuscular haemoglobin concentration (MCHC), MPV, packed cell volume (PCV/haematocrit) and red cell distribution width (RDW).
Statistics
Organ weight was normalized as a ratio of final body weight. Statistical analysis was performed by a Student’s t test for paired samples (t). A value of p < 0.05 was considered statistically significant.
Results
Food and Water Intake and Body Weight/Organ Weight Ratio
The daily food and water intake did not vary between groups. The daily amount of Pb ingested by each animal was about 1 mg/day of Pb (39 mg/kg/day). The weekly lead intake/mouse was calculated by the formula water consumption (mL) × Pb concentration (ppm) was rather constant; on average, each animal ingested 7.3 ± 0.4 mg Pb/week. On the whole, after 45 days, each animal ingested about 45 mg of Pb. The bw did not vary between groups. Pb-treated animals significantly increased the ratio between spleen weight and bw compared to control ones (0.31% ± 0.03 vs 0.28% ± 0.01, respectively, t = 2.32, p = 0.04), whereas other organs like liver and kidney did not show any change.
Pb Level Distribution
Blood and biological samples from Pb-treated animals showed significant Pb levels compared to controls. The higher amount of Pb were eliminated by faeces (98.6%), and the remaining was distributed in the blood (1%), bone (0.14%), spleen (0.12%), kidney (0.1%), liver (0.05%) and brain (0.02%) (Table 1).
Pb Exposure and Effect on the Blood
Microscopic analyses of the blood smears from Pb-treated animals showed abnormal echinocytes, with speckles in their membrane (Fig. 1a), and poikilocytes, in a tennis racket-like shape (Fig. 1b). Control animals did not show any erythrocyte alteration (normocytes). Even if in Pb-treated animals, there were no differences in haemoglobin and in RBC count compared to controls; in the haematocrit, lymphocyte values (L) increased. Moreover, neutrophils (N), the neutrophil/lymphocyte ratio (N/L), together with monocytes and eosinophils, drastically decreased. In the serum, haptoglobin, an inflammation marker, significantly increased about six times. Blood values are summarized in Table 2.
Pb Exposure Changes Spleen Architecture and Iron Deposits
Pb administration altered the WP architecture of the spleen which appeared larger than in the control animals. Furthermore, the germinative center of the inner peri-arteriolar lymphoid sheath (or PALS) was not easily identifiable. The MZ did not show any significant differences vs control mice.
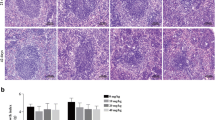
Pb-treated animals showed a high amount of ferric (Fe3+) and ferrous (Fe2+) ions accumulated in the macrophages in different splenic compartments (Fig. 2). Inside the RP of control spleen, peri-MZ macrophages showed the intense blue staining of ferritin (Fe3+) deposits and sometimes, also, WP resident macrophages were stained. However, Pb exposure increased ferritin deposits in all RP macrophages and outer and inner PALS (Fig. 2a, b). Similarly, the ferrous (Fe2+) staining was mainly located inside the peri-MZ macrophages of untreated spleen. Pb exposure increased Fe2+ staining of all RP macrophages, and sometimes, also, macrophages of inner PALS were also intensely stained. Occasionally, follicular and MZ macrophages were stained (Fig. 2c, d). In addition, the Pb exposure strongly increased hemosiderin deposits inside the macrophages of the RP and less of PALS (Fig. 2e, f). Semi-quantitative analysis of iron deposition in different experimental groups is resumed in Table 3.
Iron histochemistry. a, b Perls-Fe(III). The spleen of the control animals shows scarce macrophages of peri-MZ of RP stained with Perls (a and insert). Conversely, RP, outer and inner PALS of Pb-treated animals showed a lot of Perl-stained splenocytes (b and insert). c, d Turnbull-Fe(II). In control, the intense ferrous staining is located mainly inside the macrophages of peri-MZ of RP (c and insert). The Pb exposure increases the staining intensity of RP macrophages (insert) and sometimes also macrophages of inner PALS (d). e, f Emosiderin staining (arrows) in control (e) and Pb-treated (f) animals. The emosiderin deposits increase with Pb treatment. WP white pulp, RP red pulp, MZ marginal zone. Scale bar 200 μm
Pb Exposure Stimulates Endoplasmic Reticulum Stress, Anti-oxidant and Pro-Apoptotic Systems and Inhibits Autophagy of Splenocytes
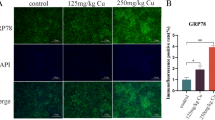
In the spleen of untreated animals, Grp78 was constitutively but moderately expressed in the macrophages of MZ as well as in some WP lymphocytes and macrophages (Fig. 3a). Occasionally, some RP macrophages also showed immunostaining. On the contrary, Pb-treated spleen showed the strong Grp78 reactivity, particularly inside MZ macrophages. However, both WP macrophages and lymphocytes and macrophages of RP were also moderately stained (Fig. 3b). Furthermore, the megakaryocytes showed intense cytoplasmic immunostaining. (Fig. 3b insert). The IOD value of Grp78 staining is resumed in Fig. 3c.
GRP-78 immunostaining in control (a) and Pb-treated (b) mice. The Pb-treated spleen shows the intense staining mainly in MZ and RP. Stained megakaryocyte are common in Pb-treated group (b insert). c IOD value of immunostaining according to groups and zones of parenchyma. WP white pulp, RP red pulp, MZ marginal zone. **p < 0.000. Scale bar 50 μm
The spleen of untreated animals showed diffuse superoxide dismutase 1 (SOD1) expression mainly inside the RP cells (Fig. 4a). The macrophages of MZ and outer and inner PALS did not show any staining or occasionally faint to moderate staining. Pb administration greatly increases SOD1 expression in RP, MZ and less so in WP. In particular, the MZ and many cells of the inner PALS were intensely stained (Fig. 4b). The IOD value of anti-SOD1 staining is resumed in Fig. 4c.
SOD1 immunostaining. a In control spleen, the immunostaining is present in RP and less in WP. The MZ (arrow) shows very faint staining. b The Pb-treated spleen shows the strong and diffuse staining in all spleen compartment. c IOD value of immunostaining according to groups and zones of parenchyma. WP white pulp, RP red pulp, MZ marginal zone. **p < 0.000. Scale bar 50 μm
Apoptotic fluxes were analysed by cleaved caspase-3 immunostaining. Pb exposure strongly increased immune reactivity inside the cytoplasm and nuclei of splenocytes in RP and WP. Commonly, the MZ cells and outer PALS were more intensely stained than RP and inner PALS cells. We also observed immune reactivity inside the megakaryocytes. The IOD value of cleaved caspase-3 staining is resumed in Fig. 5a. Finally, autophagy was analysed by p62/SQSTM1 immunostaining. All spleen compartments from Pb-treated animal increased p62 immunoreaction. Frequently RP, MZ and germinative center of inner PALS showed a more intense reaction. In contrast, there was no p62/SQSTM1 immunostaining in control mice. The IOD value of p62/SQSTM1 staining is resumed in Fig. 5b.
Discussion
Here, we have reported that young male mice, with orally ingested sub-chronic doses of Pb (200 ppm) in drinking water for 6 weeks, developed haematic changes such as neutropenia, eosinophilopenia and increased inflammation markers. Interestingly, the spleen displayed a disorganized architecture, iron deposition, strong ER stress and compromised anti-oxidant and cell survival markers.
The BLL value of 30 μg/dL, which we used to classify Pb exposure, is the current biological exposure index (BEI) of the American Conference of Governmental Industrial Hygienists (ACGIH) [29] and is also recommended as the biological limit value (BLV) by the European Union Scientific Committee on Occupational Exposure Limits (SCOEL) [30]. Centers of Diease Control and Prevention (CDC—USA) considers children to have a high level of lead whenever the amount of lead in the blood is at least 10 μg/dL (http://www.cdc.gov/nceh/lead/). Many states or local programs will intervene for individual children with BLL equal to or greater than 10 μg/dL. Medical evaluation and environmental investigation and remediation should be carried out for all children with blood lead levels equal to or greater than 20 μg/dL [6]. The BLL adopted in our work (21.6 μg/dL) was lower than BLV. However, after only a short time acting early on the spleen, Pb favoured ER stress, apoptosis, autophagy, the Fe2+ and Fe3+ turnover and immune changes as well. We believe that this may explain or favour the auto-immune diseases frequently observed throughout the population, especially in children and the elderly exposed to Pb.
The adverse haematological effects of Pb are mainly a result of its perturbation of the haeme biosynthesis pathway. It is known that Pb reduces the lifespan of circulating erythrocytes by increasing the fragility of their cell membranes. The combined effect of these two processes leads to anaemia [8]. However, our haematological data did not show any alterations in haemoglobin concentrations or RBC counts.
Furthermore, our data also disagree with those of Ray and Sarkar [7], who reported abnormal haemoglobin levels and erythrocyte counts after i.p. Pb-nitrate administration (8 mg/kg) in mice. Perhaps the different routes of Pb administration, the different treatment times and the susceptibility of animals could explain these differences.
Unfortunately, we were unable to measure ALAD, an enzyme occurring early in the heme synthesis pathway. ALAD activity is negatively correlated with Pb blood concentration between 5 and 95 μg/dL [6]. However, although inhibition of ALAD occurs at low Pb exposure levels (10–20 μg/dL), there is some controversy as to the toxicological significance of a depression in ALAD activity without any detectable effect on haemoglobin levels [6]. Indeed, anaemia occurred only when BBL is significantly elevated for prolonged periods [31]. Haeme biosynthesis does not decrease until ALAD activity is inhibited by 80–90%, which only occurs at a much higher blood Pb concentration of about 55 μg/dL [32]. In light of data from the literature, we speculate that the BLL measured in our experiments did not significantly inhibit ALAD, which could explain the normal values of haemoglobin and RBC concentrations.
Altered RBC counts and eryptosis are common in occupationally Pb-exposed workers [33–35]. Intriguingly, due to its adverse effects on RBCs, Pb exposure has recently been considered a pathogenetic factor for chronic cardiovascular and renal diseases [36, 37].
The effect of Pb, at blood low doses (from 5 μg/dL) but environmentally relevant, deregulated immune response and macrophage defence against bacterial agents by reducing toll-like receptor affinity [38]. Furthermore, Pb affects cytokine production in a dose-dependent manner according to hormesis [39]. By using microarray analysis, La Breche et al. [40] sustained that low Pb dose transiently stimulated IFNγ. Our haematic data showed a strong reduction of neutophils and eosinophils, consequent to Pb administration and abnormal N/L ratio. These data agree with alterations in the haemogram in rodent Meriones libycus as indicator of contamination [41]. In addition, human workers with low BLL have a primary impairment of the neutrophil chemotactic and phagocytic activities [42, 43]. According to Adham et al. [41], the decrease in N/L ratio in Pb-treated mouse mostly refers to increased stress and decreased immune response of various insults [44].
Another interesting result of our work was the strong increases of haptoglobin (Hp), a typical marker of inflammation in Pb-treated animals. Hp is a protein that in humans is encoded by the Hp gene [45]. Hp is a haemoglobin-binding protein with immunomodulatory properties and is mainly produced by hepatocytes and to a lesser extent by cells in other tissues such as lung, skin and kidney, under inflammatory conditions. Its synthesis is induced by IL-6, IL-1β and TNF. In blood plasma, Hp binds free haemoglobin released from erythrocytes with high affinity and thereby inhibits its oxidative activity. The Hp-haemoglobin complex will then be removed by the reticuloendothelial system, mostly in the spleen [46]. Hp has also been shown to modulate several aspects of the innate and adaptive immune response and to have anti-inflammatory properties [47, 48]. We therefore suggest that the Pb-induced increase of Hp serum levels could reflect the state of the body’s global inflammation, as well as the body’s ability or inability, to activate defence mechanisms against oxidative stress.
In regard to splenic changes, we found that Pb concentration increased four times in exposed vs control mice, and an evident splenomegaly occurred as a reaction to Pb, without any evident body weight change. These data partially agree with previous observations in other rodents such as Wistar rats i.p. injected with the same our dose of Pb-acetate for 4 weeks [25] and Algerian mice (Mus spretus) drinking 0.1% Pb-acetate for 45 days [49]. However, why we did not observe any body mass decrease was probably due to the Pb route of intake and different animal species.
Considering Pb-induced blood changes, we analysed splenic iron alterations by various histochemical techniques that are able to indicate both ferrous, Fe(II), and ferric, Fe(III), deposits. Splenic resident macrophages are specific targets of non-haeme iron and are enhanced by selected histochemical staining according to [50]. Our findings demonstrated that macrophages were recruited in order to remove RBCs and iron ions. However, we visualized but did not predict whether macrophages in the MZ or RP, and near congested vessels, lost their function or not or whether prolonged iron deposits could be a stimulus for cellular oxidant reactions.
It is known that Pb causes oxidative stress by generating ROS in different organs [51–55]. Sub-chronic low Pb exposure produces nephrotoxicity in vivo and in vitro [36], as well as infertility [56] in rodents. To protect against free radical damage, cells activate anti-oxidant enzymes such as SOD1 (Cu/Zn superoxide dismutase). Pb intoxication increases SOD1 expression and activity in various parts of the brain [57, 58]. Furthermore, it has also been shown that erythrocytes of Pb-exposed workers have elevated SOD activity compared to non-exposed workers [59]. Here, we demonstrated that Pb strongly induced SOD1 in RP of mouse spleen. This finding may be explained as erythrocytes bound Pb then crossed the spleen to be destroyed, so resident RP cell population over-expressed SOD1 to protect themselves against Pb-induced oxidative stress.
Another interesting finding was the strong Grp78 immunostaining was found particularly in MZ macrophages and RP megakaryocytes of the spleen. We speculate that the intense Grp78 signal seen in MZ macrophages may be due to direct ER response or by excess iron/erythrocyte uptake in these cells. Indeed, MZ at the boundary, between WP and RP sinuses, acts as a filter against infective antigens from blood circulation and MZ resident B lymphocytes and macrophages are stimulated here to quickly produce immunoglobulins.
Grp78 (BiP) is a crucial marker of environmental exposure to heavy metals and should be considered an indicator of ER dysregulation in various cell types [15, 19, 60] When unfolded proteins accumulate in the ER, BiP is released from these complexes to assist with the folding of accumulated proteins [61] and sustained Grp78 could act as an inhibitor of macrophage adhesion [62]. Recently, Grp78 has been found to be directly involved in proper antigen binding to specific receptors in MZ macrophages [63] associated with Fe dysregulation and inflammation [64]. Moreover, Kasten-Jolly and Lawrence [24] reported that Pb both activated macrophages sub-types and decreased inflammatory responses to pathogens in mice [24]. If MZ does not work properly, inflammatory responses and auto-immune disorders may occur [65, 66]. Finally, abnormal pro-inflammatory cytokines have been documented in Pb occupationally exposed workers [67].
Interestingly, we also observed the intense Grp78 immunoreaction in megakaryocytes in the RP of Pb-treated group. These cells have been described in mouse spleen as a reaction to inhaled vanadium [68]. Furthermore, both altered platelet counts and distribution were assessed in workers in a Pb-acid battery plant [69]. As a result, here we measured a progressive decreases of platelet number after Pb exposure, to assess cell impairment with significant decrease of platelet production as also demonstrated by the increase of cleaved caspase-3.
It has been reported that Pb influenced cytochrome c release and affected Bcl-2/Bax ratio in the brain [70] and liver [71], causing cleaved caspase-3-dependent apoptosis. In agreement with these studies, we localized splenic pro-apoptotic markers in RP and WP induced by Pb exposure. Interestingly, a peculiar link between ER stress and abnormal apoptosis was reported in auto-immune disorders and in patients of systemic lupus erythematosus [72]. In light of these data, increase of ER stress and pro-apoptotic cleaved caspase-3 markers in WP, after Pb treatment, could reflect an impairment of immune functions.
Intriguingly, here we first describe both cleaved caspase-3 expression and contemporary increased p62/SQSTM1 autophagy marker. The p62 protein (also called sequestosome 1), is an ubiquitin-binding scaffold protein that colocalizes with ubiquitinated protein aggregates in many neurodegenerative diseases and proteinopathies. However, in the field of autophagy, it may link ubiquitinated proteins to the autophagic machinery to enable their degradation in the lysosome. It is assumed that since p62 accumulates when autophagy is inhibited, reduced levels can be observed when autophagy is active and increased when autophagic flux is altered [73, 74]. So, an intense p62 signal that was observed in Pb-treated spleen could reflect the autophagy impairment in resident RP and WP cells.
Overall, these histopathological alterations corroborated the adverse role of Pb on the immune cells in the spleen. Indeed, adverse effects on the immune system can be detected even at frequently occurring BBL (<50 g/dL) and BLL [43, 75]. Novel findings of Grp78 over-expression in specific splenic cells, together with dysfunctional autophagy and apoptosis markers, may provide new information on cell reactions under Pb exposure. Pathogenetic events which occurred under Pb exposure in mouse spleen are resumed in Fig. 6.
Limitation of the Study
A possible limitation of this study is the exclusive application of IHC to support our results. This choice was dictated by the fact that the spleen contains a very heterogeneous cell population (macrophages, lymphocytes, megakaryocytes, etc.) and was divided into well-defined morphologically recognizable areas (WP, PALS, RP, MZ). Consequently, histopathological changes and especially the exact visualization of markers are possible with IHC and HC. In contrast, molecular analysis, although much more sensitive in highlighting and quantifying proteins and very often used exclusively, does require immediate freezing and homogenization of the sample. As a consequence, it does not take into account the specificities of protein location, tissue morphology and organization. This is a major limitation in the exclusive use of molecular analysis, which we believe is comparable if not superior to IHC. For all these reasons, we pointed to HC and IHC and believed that our data are interesting.
Further studies are necessary to best understand early pathways involved in Pb poisoning in the spleen and the translation of results obtained in a mouse model to human toxicology.
References
Check L, Marteel-Parrish A (2013) The fate and behaviour of persistent, bioaccumulative, and toxic (PBT) chemicals: examining lead (Pb) as a PBT metal. Rev Environ Health 28:85–96
Pokras MA, Kneeland MR (2009) Understanding lead uptake and effects across species: a conservation medicine based approach. In: Watson RT, Fuller M, Poras M, Hunt WG (eds) Ingestion of lead from spent ammunition: implications for wildlife and humans. The Peregrine Funds, Boise. doi:10.4080/ilsa.2009.0101
Stacchiotti A, Corsetti G, Rezzani R (2012) Lead nephrotoxicity. In: Uverky VN, Kretsinger RH, Permyakov EA (eds) Encyclopedia of metalloproteins. Springer, Berlin, Edt. doi:10.1007/978-1-4614-1533-6.
Caravanos J, Chatham-Stephens K, Bret E, Landrigan P, Fuller R (2013) The burden of disease from pediatric lead exposure at hazardous waste sites in seven Asian countries. Environ Res 120:119–125
Cleveland L, Minter M, Cobb K, German V (2008) Lead hazards for pregnant women and children: part 1: immigrants and the poor shoulder most of the burden of lead exposure in this country. Part 1 of a two part article details how it affects, and the harm it can do. Am J Nurs 108:40–49
Agency for Toxic Substances and Disease Registry (ATSDR) (2007) Toxicological profile for lead. U.S. Department of Health and Human Services
Ray R, Sarkar N (2013) Lead (Pb) induced anaemia in Swiss mice—light and scanning electronmicroscopic studies. Int J Pharm Bio Sci 4:B22–B30
Flora G, Gupta D, Tiwari A (2012) Toxicity of lead: a review with recent updates. Interdiscip Toxicol 5(2):47–58. doi:10.2478/v10102-012-0009-2
Hernandez-Avila M, Smith D, Meneses F, Sanin L, Hu H (1998) The influence of bone and blood lead on plasma lead levels in environmentally exposed adults. Environ Health Perspect 106:473–477
Karri SK, Saper RB, Kales SN (2008) Lead encephalopathy due to traditional medicines. Curr drug saf 3(1):54–59. doi:10.2174/157488608783333907
Needleman H (2004) Lead poisoning. Annu Rev Med 55:209–222. doi:10.1146/annurev.med.55.091902.103653
Gidlow D (2015) Lead toxicity. Occup Med 65:348–356
Jang W, Lim K, Kim K, Noh J, Kang S, Chang Y, Chung J (2011) Low level of lead can induce phosphatidylserine exposure and erythrophagocytosis: a new mechanism underlying lead-associated anemia. Toxicol Sciences 122:177–184
Shin J, Lim K, Noh J, Bae O, Chung S, Lee M, Chung J (2007) Lead-induced procoagulant activation of erythrocytes through phosphatidylserine exposure may lead to thrombotic diseases. Chem Res Toxicol 20:38–43
Kitamura M (2013) The unfolded protein response triggered by environmental factors. Semin Immunopathol 35:259–275
Hendershot L (2004) The ER function BiP is a master regulator of ER function. Mt Sinai J Med 71:289–297
Qian Y, Zheng Y, Ramos K, Tiffany-Castiglioni E (2005) GRP78 compartimentalized redistribution in Pb-treated glia: role of GRP78 in lead-induced oxidative stress. Neuro Toxicology 26:267–275
Tully D, Collins B, Overstreet J, Smith C, Dinse G, Mumtaz M, Chapin R (2000) Effects of arsenic, cadmium, chromium, and lead on gene expression regulated by a battery of 13 different promoters in recombinant HepG2 cells. Toxicol Appl Pharmacol 168:79–90
Stacchiotti A, Morandini F, Bettoni F, Schena I, Lavazza A, Grigolato P, Apostoli P, Rezzani R, Aleo M (2009) Stress proteins and oxidative damage in a renal derived cell line exposed to inorganic mercury and lead. Toxicology 264:215–224
Shinkai Y, Yamamoto C, Kaji T (2010) Lead induces the expression of endoplasmic reticulum chaperones GRP78 and GRP94 in vascular endothelial cells via the JNK-AP-1 pathway. Toxicol Sci 114:378–386
Quinones Q, de Ridder G, Pizzo S (2008) GRP78: a chaperone with diverse roles beyond the endoplasmic reticulum. Histol Histopathol 23:1409–1416
Ni M, Zhang Y, Lee A (2011) Beyond the endoplasmic reticulum: atypical GRP78 in cell viability, signaling and therapeutic targeting. Biochem J 434:181–188
Mebius R, Kraal G (2005) Structure and function of the spleen. Nature Reviews Immunol 5:606–616
Kasten-Jolly J, Lawrence D (2014) Lead modulation of macrophages causes multiorgan detrimental health effects. J Biochem Mol Toxicol 28:355–372
Teijon C, Olmo R, Blanco M, Romero A, Teijon J (2003) Effects of lead administration at low dose by different routes on rat spleens. Study of response of splenic lymphocytes and tissue lisozyme. Toxicology 191:245–258
Meguro R, Asano YT, Iwatsuki H, Shoumura K (2003) Perfusion-Perls and Turnbull methods supplemented by DAB intensification for non heme iron histochemistry: demonstration of the superior sensitivity of the methods in the liver, spleen, and stomach of the rat. Histochem Cell Biol 120:73–82
Corsetti G, Stacchiotti A, Tedesco L et al (2011) Essential amino acid supplementation decreases liver damage induced by chronic ethanol consumption in rats. Int J Immunopathol Pharmacol 24:611–619
Hall P, Davies W, Stamp K, Clamp I, Bigley A (2013) Comparison of computerized image analysis with traditional semiquantitative scoring of Perls’Prussian blue stained hepatic iron deposition. Toxicol Pathol 41:992–1000
American Conference of Governmental Industrial Hygienists (ACGIH) (2003) Threshold limit values and biological exposure indices. ACGIH, Cincinnati, OH
Scientific Committee on Occupational Exposure Limits (2002) Recommendations from scientific committee on occupational exposure limits for lead and its inorganic compounds. Office for Official Publication the European Communities, Luxembourg
Vij AG (2009) Hemopoietic, hemostatic and mutagenic effects of lead and possible prevention by zinc and vitamin C. Al Ameen J Med Sci 2:27–36
Ahamed M, Verma S, Kumar A, Siddiqui MK (2005) Environmental exposure to lead and its correlation with biochemical indices in children. Sci Total Environ 346:48–55
Kasperczyk A, Prokopowicz A, Dobrakowski M, Pawllas N, Masperczyk S (2012) The effect of occupational lead exposure on blood levels of zinc, iron, copper, selenium and related proteins. Biol Trace Element Res 150:49–55
Aguilar-Dorado I, Hernandez Q, Quintanar-Escorza M et al (2014) Eryptosis in lead exposed workers. Toxicol Appl Pharmacol 281:195–202
Barrett J (2015) Seeds of toxicity? Erythrocytes and lead-associated kidney damage. Envir Health Persp 123:A42
Kwon S, Bae O, Noh J, Kim K, Kang S, Shin Y, Lim K, Chung J (2015) Erythrophagocytosis of lead-exposed erythrocytes by renal tubular cells: possible role in lead-induced nephrotoxicity. Envir Health Perspect 123:120–127
Zuqui Nunes K, Oliveira Nunes D, Aparecida Silveira E, Cruz Pereira C, Broseghini Filho G, Vassallo V, Fioresi M (2015) Chronic lead exposure decreases the vascular reactivity of rat aortas: the role of hydrogen peroxide. PLoS One 10:e0120965
Luna A, Acosta-Saavedra L, Martinez M, Torres-Aviles N, Gomez R, Calderon-Aranda E (2012) TLR4 is a target of environmentally relevant concentration of lead. Toxicol Lett 214:301–306
Iavicoli I, Calabrese E (2011) Redifining low lead levels. Environ Health Perspect 119:A202
La Breche HG, Meadows SK, Nevins JR, Chute JP (2011) Peripheral blood signatures of lead exposure. PLoS One 6(8):e23043
Adham KG, Al-Eisa NA, Farhood MH (2011) Impact of heavy metal pollution on the hemogram and serum biochemistry of the libyan jird, Meriones libycus. Chemosphere 84:1408–1415. doi:10.1016/j.chemosphere.2011.04.064
Queiroz MLS, Almeida M, Gallão MI, Höehr NF (1993) Defective neutrophil function in workers occupationally exposed to lead. Pharmacol Toxicol 72:73–77
Valentino M, Governa M, Marchiseppe I, Visona I (1991) Effects of lead on polymorphonuclear leukocytes function in occupationally exposed workers. Arch Toxicol 65:685–688
Zahorec R (2001) Ratio of neutrophil to lymphocyte counts—rapid and simple parameter of systemic inflammation and stress in critically ill. Bratisl Lek Listy 102:5–14
Dobryszycka W (1997) Biological functions of haptoglobin—new pieces to an old puzzle. Eur J Clin Chem Clin Biochem 35(9):647–654
Vanuytsel T, Vermeire S, Cleynen I (2013) The role of haptoglobin and its related protein, Zonulin, in inflammatory bowel disease. Tissue Barriers 1(5):e27321. doi:10.4161/tisb.27321
Galicia G, Maes W, Verbinnen B et al (2009) Haptoglobin deficiency facilitates the development of autoimmune inflammation. Eur J Immunol 39:3404–3412. doi:10.1002/eji.200939291
Van Vlierberghe H, Langlois M, Delanghe J (2004) Haptoglobin polymorphisms and iron homeostasis in health and in disease. Clin Chim Acta 345:35–42. doi:10.1016/j.cccn.2004.03.016
Marques CC, Nunes AC, Pinheiro T, Lopes PA, Santos MC, Viegas-Crespo AM, Ramalhinho MG, Mathia ML (2006) An assessment of time-dependent effects of lead exposure in Algerian mice (Mus spretus) using different methodological approaches. Biol Trace Elem Res 109:75–89
Meguro R, Asano Y, Odagiri S, Li C, Iwatsuki H, Shoumura K (2007) Non heme-iron histochemistry for light and electron microscopy: a historical, theoretical and technical review. Arch Histol Cytol 70:1–19
Guilarte TR (1997) Glutamatergic system and developmental lead neurotoxicity. Neurotoxicology 18:665–672
Savolainen KM, Loikkanen J, Eerikainen S, Naarala J (1998) Interactions of excitatory neurotransmitters and xenobiotics in excitotoxicity and oxidative stress: glutamate and lead. Toxicol Lett 102-103:363–367
Zhang J, Wang XF, ZB L, Liu NQ, Zhao BL (2004) The effects of meso-2,3-dimercaptosuccinic acid and oligomeric procyanidins on acute lead neurotoxicity in rat hippocampus. Free Radic Biol Med 37:1037–1050
Sivaprasad R, Nagaraj M, Varalakshmi P (2002) Lipoic acid in combination with a chelator ameliorates lead-induced peroxidative damages in rat kidney. Arch Toxicol 76:437–441
Jurczuk M, Moniuszko-Jakoniuk J, Brzóska MM (2007) Hepatic and renal concentrations of vitamins E and C in lead- and ethanol-exposed rats. An assessment of their involvement in the mechanisms of peroxidative damage. Food Chem Toxicol 45:1478–1486
Elgawish R, Abdebrazek H (2014) Effects of lead acetate on testicular function and caspase 3 expression with respect to the protective effect of cinnamon in albino rats. Toxicol Rep 1:795–801
Samki K, Jiyoung H, Hyunji K et al (2011) Effects of lead exposure on nitric oxide-associated gene expression in the olfactory bulb of mice. Biol Trace Elem Res 142:683–692
Bokara KK, Brown E, McCormick R et al (2008) Lead-induced increase in antioxidant enzymes and lipid peroxidation products in developing rat brain. Biometals 21:9–16
Monteiro HP, Abdaila DSP, Arcurl MS, Bechar EJH (1985) Oxygen toxicity related to exposure to lead. Clin Chem 31(10):1673–1676
Tiffany-Castiglioni E, Yongchang Qian Y (2012) ER chaperone-metal interactions: links to protein folding disorders. Neurotoxicology 33:545–557. doi:10.1016/j.neuro.2012.02.007
Gardner BM, Walter P (2011) Unfolded proteins are Ire1-activating ligands that directly induce the unfolded protein response. Science 333:1891–1894
Bai H, Li N, Zhou X et al (2014) GRP78 inhibits macrophage adhesion via SR-A. J Biomed Res 28(4):269–274. doi:10.7555/JBR.28.20130054
Choi H, Choi W, Park J, Prabagar M, Kang K, Jeon S, Park S, Shin C, Kang Y (2011) SIGN-R1, a C-type lectin, binds to Bip/GRP78 and this interaction mediates the regurgitation of T-cell-independent type 2 antigen dextran through the endoplasmic reticulum. Immunobiology 216:437–446
Zughayer S, Stauffer B, Mc Carty N (2014) Inflammation and ER stress downregulate BDH2 expression and dysregulate intracellular iron in macrophages. J Immunol Res ID140728. doi:10.1155/2014/140728
Schofield P (2005) Dementia associated with toxic causes and autoimmune disease. Int Psychogeriatr 17:S129–S147
McGaha T, Chen Y, Ravishanankar B, van Rooijen N, Karlsson M (2011) Marginal zone macrophages suppress innate and adaptive immunity to apoptotic cells in the spleen. Blood 117:5403–5412
Valentino M, Rapisarda V, Santarelli L, Bracci M, Scorcelletti M, Di Lorenzo L, Cassano F, Soleo L (2007) Effect of lead on the levels of some immunoregulatory cytokines in occupationally exposed workers. Hum Exp Toxicol 26:551–556
Fourtoul T, Pinon-Zarate G, Diaz-Bech M et al (2008) Spleen and bone marrow megakaryocytes as target for inhaled vanadium. Histol Histopathol 23:1321–1326
Barman T, Kalahashi R, Rajmohan H (2014) Effects of lead exposure on the status of platelets indices in workers involved in a lead-acid battery manufacturing plant. J Exp Sci Env Epidemiol 24:629–633
Kiran Kumar B, Prabhakara Rao Y, Noble T, Weddington K, McDowell VP, Sharada R, Rajanna B (2009) Lead-induced alteration of apoptotic proteins in different regions of adult rat brain. Toxicol Lett 184:56–60
Liu C, Zheng Y, Lu J et al (2010) Quercetin protects rat liver against lead-induced oxidative stress and apoptosis. Environ Toxicoland Pharmacol 29:158–166
Lee W, Sung M, Lee E, Yoo H, Cheon Y, Chae H, Yoo W (2014) A pathogenic role for ER stress-induced autophagy and ER chaperone GRP78/BiP in T lymphocyte systemic lupus erythematosus. J Leukocy Biol 97(2):425–433
Bjørkøy G, Lamark T, Pankiv S, Øvervatn A, Brech A, Johansen T (2009) Monitoring autophagic degradation of p62/SQSTM1. Methods Enzymol 452:181–197. In: Autophagy in mammalian systems, part B chapter 12. doi:10.1016/S0076-6879(08)03612-4
Klionsky DJ, Abdelmohsen K, Abe A et al (2016) Guidelines for the use and interpretation of assays for monitoring autophagy (3rd edition). Autophagy 12(1):1–222. doi:10.1080/15548627.2015.1100356
Singh VK, Mishra KP, Rani R, Yadav VS, Awasthi SK, Garg SK (2003) Immunomodulation by lead. Immunol Res 28:151–166
Acknowledgements
This study was supported by an ABOCA s.p.a (Arezzo, Italy) grant and University of Brescia CG fund (ex-60%). We would like to thank prof. Robert Coates (Centro Linguistico, Bocconi University, Milan, Italy), medical writer, for his linguistic revision. Furthermore, we would like to thank Dr.ssa I.L. Archetti, Dr.ssa L. Ferretti and Dr. M. Curatolo (from the National Reference Centre for Animal Welfare—IZSLER-Bs) for the blood analyses and Pb measurement in biological samples.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
All animal procedures followed the European Communities Council Directive of November 24, 1986 (86/609/EEC), and the Italian Ministry of Health and complied with The National Animal Protection Guidelines.
Conflict of Interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Corsetti, G., Romano, C., Stacchiotti, A. et al. Endoplasmic Reticulum Stress and Apoptosis Triggered by Sub-Chronic Lead Exposure in Mice Spleen: a Histopathological Study. Biol Trace Elem Res 178, 86–97 (2017). https://doi.org/10.1007/s12011-016-0912-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-016-0912-z