Abstract
Nickel (Ni) is a heavy metal element and a pollutant that threatens the organism’s health. Melatonin (Mel) is an antioxidant substance that can be secreted by the organism and has a protective effect against heavy metals. Selenoprotein M (SelM) is a selenoprotein widely distributed of the body, and its role is to protect these tissues from oxidative damage. To study the mechanism of Ni, Mel, and SelM in mouse spleen, 80 SelM+/+ wild-type and 80 SelM−/− homozygous mice were divided into 8 groups with 20 mice in each group. The Ni group was intragastric at a concentration of 10 mg/kg, while the Mel group was intragastric at 2 mg/kg. Mice were injected with 0.1 mL/10 g body weight for 21 days. Histopathological and ultrastructural observations showed the changes in Ni, such as the destruction of white and red pulp and the appearance of pyroptosomes. SelM knockout showed more severe injury, while Mel could effectively interfere with Ni-induced spleen toxicity. The results of antioxidant capacity determination showed that Ni could cause oxidative stress in the spleen, and Mel could also effectively reduce oxidative stress. Finally, Ni exposure increased the expression levels of the pyroptotic genes, including apoptosis-associated speck protein (ASC), absent in melanoma-2 (AIM2), NOD-like receptor thermal protein domain-associated protein 3 (NLRP3), Caspase-1, interleukin- (IL-) 18, and IL-1β (p < 0.05). Loss of SelM significantly increased these (p < 0.05), while Mel decreased the alleviated impact of Ni. In conclusion, the loss of SelM aggravated Ni-induced pyroptosis of the spleen via activating oxidative stress, which was alleviated by Mel, but the effect of Mel was not obvious in the absence of SelM, which reflected the important role of SelM in Ni-induced pyroptosis.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Selenium is a non-metallic element and an indispensable trace element for normal physiological activities of the body (Xue et al. 2022; Zhang et al. 2021a), which is linked to apoptosis (Qing et al. 2022), oxidative stress (Li et al. 2022a), autophagy (Zheng et al. 2021b), and inflammation (Zhang et al. 2021b). The biological function is mainly through the antioxidant effect in the form of selenoprotein and selenozyme, and selenoprotein M (SelM) is one of them (Cai et al. 2022a, b). Moreover, SelM is one of the executive agents of selenium in vivo, which has antioxidant, neuroprotective, and intracellular calcium regulation effects, and distributes widely, especially highly expressed in brain tissue (Chen et al. 2013). Selenoproteins are closely related to body damage caused by toxic substances (Hofstee et al. 2022). A study showed that exposure to mercuric chloride significantly reduced SelM expression in chicken kidneys (Chu et al. 2020). Furthermore, it was confirmed that Se antagonized Pb-induced apoptosis in chicken neural tissue through selenoproteins, including SelM (Zhu et al. 2017).
Nickel (Ni) is a heavy metal widely distributed in the environment, which has become an important pollutant due to the pollution of Ni manufacturing and the burning of fossil fuels. Long-term high-dose exposure has various toxicities to humans and animals. It is a kind of human carcinogen (Guo et al. 2021). Heavy metal poisoning damages immune cells (Xu et al. 2021), especially Ni, and damages the immune system of animals. It was demonstrated that NiCl2 inhibited T-cell function (Harkin et al. 2003). The total number of spleen cells in female mice decreased after 24 h of systemic exposure to Ni (Buxton et al. 2021).
Lead, a heavy metal, can cause oxidative stress in rat nerve tissue (Shaban et al. 2021) and liver (Shaban et al. 2020), and thioacetamide can cause oxidative stress in rat liver (Shaban et al. 2022a). Ni induced increased levels of oxidative stress in rats and inhibited the activities of glutathione peroxidase, catalase, and other antioxidant defense systems in the spleen (Boulila et al. 2014).
Melatonin (Mel) is a hormone secreted by the pineal gland, which has a protective effect on metal toxicity (Romero et al. 2014). Recently, it showed that Mel alleviated the pyroptosis caused by TMT-induced excessive oxidative stress (Cai et al. 2021). At the same time, Mel improved brain autophagy in mice by reducing oxidative stress (Qiao et al. 2022) and improved kidney cell apoptosis in fish by Mir-140-5p/TLR4/NF-κB axis (Li et al. 2022e). However, it has not been reported whether SelM has a role in Ni-induced animal toxicity.
Pyroptosis is a Caspase-11 or Caspase-1-dependent inflammasome-dependent programmed cell death by lysis of cells or, in some cases, by the endosomal sorting complexes required for transport (Du et al. 2021). On the other hand, selenoproteins are closely related to pyroptosis. Thioredoxin reductase 3 is a selenoprotein that inhibits pyroptosis and necrosis (Liu et al. 2022b). Current research has proved that pyroptosis is related to arteriosclerosis (Lin et al. 2022), rheumatoid arthritis (Zhao et al. 2021), diabetic nephropathy (Zuo et al. 2021), central system diseases (Hu et al. 2022), cancer (Al Mamun et al. 2021), and other diseases. Among them, chromium induces pyroptosis of grass carp spleen lymphocytes through oxidative stress and NOD-like receptor thermal protein domain-associated protein 3 (NLRP3) signaling pathway (Zhang et al. 2020). The spleen is an important immune organ in the body. It is the principal place for the production of lymphocytes and the immune response. Studies have confirmed that pyroptosis occurs in the spleen under certain stimuli, and Ni exposure could cause damage to spleen tissue (Boulila et al. 2014; Buxton et al. 2021). However, the role of SelM in Ni exposure-induced spleen pyroptosis was uncertain.
In this study, to explore the role of SelM in the effects of Ni exposure on immune organs in mice and whether Mel could act as a relevant antagonist, we established a SelM knockout mouse model of Ni poisoning and a Mel recovery group model. Our study is aimed at probing the role and mechanism of Ni exposure-induced spleen injury and the antagonism of Mel on Ni through various molecular biology and histomorphological experiments and at clarifying the effect of SelM on Mel antagonism of the Ni-induced spleen in mice tissue pyroptosis. According to the above experiments, it is expected to supply a reference for clinical medication and disease therapy.
Materials and methods
Ethics
All the procedures were approved by the Academic Committee of Northeast Agricultural University.
Mouse model
To construct the model, 160 male C57BL/6 N mice weighing 25–30 g at 8 weeks of age, 80 SelM+/+ wild-type, and 80 SelM−/− homozygous mice were randomly divided into 8 groups of 20 mice each, including control, Ni, Mel, Ni + Mel, KO + C, KO + Ni, KO + Mel, and KO + Ni + Mel groups. SelM−/− homozygous mice were generated by a commercial supplier (Cyagen Biosciences, Santa Clara, CA, USA) using CRISPR/Cas-mediated genome engineering, which is used to design single-guide RNA (sgRNA), and then high-throughput electro-transfer fertilized eggs to obtain SelM−/− homozygous mice. At 9:00 a.m. and 5:00 p.m., the stomach was perfused twice daily with an interval of 8 h, a total of 21 days. The eight groups include control group mice (morning saline, afternoon saline), Ni group mice (morning Ni, afternoon saline), Mel group mice (morning saline, afternoon Mel), Ni + Mel group mice (morning Ni, afternoon Mel), KO + C group mice (morning saline, afternoon saline), KO + Ni group mice (morning Ni, afternoon saline), KO + Mel group mice (morning saline, afternoon Mel), and KO + Ni + Mel group (Ni in the morning, Mel in the afternoon). It is necessary to ensure that each group has the same gastric perfusion time to eliminate the error caused by the gastric perfusion operation. Normal saline (0.9%) is used as the control solution. Ni (Macklin, Shanghai, China) is a 10 mg/kg nickel chloride solution, and the concentration of Mel (Sangon Biotech, Shanghai, China) is 2 mg/kg. Each mouse was given 0.1 mL/10 g body weight by gavage. With regard to Ni and Mel concentrations, according to the previous experiment of the research group and references (Cai et al. 2021; Liu et al. 2022c; Qiao et al. 2022), we selected the minimum concentration of Ni chloride that caused injury to mice and the maximum concentration of Mel that alleviated Ni injury without harming mice. All animals consumed enough food and water for 21 days (Zhang et al. 2022c). The status of mice was observed daily after establishing the model successfully.
Spleen sample collection
The mice were put into clean containers and euthanized by slowly injecting carbon dioxide (CO2) when the heart and breathing stopped, and the reflex disappeared. After the mice were euthanized, the scalpel and surgical scissors were disinfected. The abdominal cavity was opened, the intestinal tissue was removed, and the spleen was fully exposed and collected. Spleen tissues were harvested for histopathological examination, and remaining spleen tissues were immediately stored at − 80 °C for subsequent experiments (− 80 °C ultra-low temperature preservation box, Haier Company, Qingdao, China) (Chen et al. 2022).
Histological analysis
Fresh spleen tissue was reduced into small 5-μm squares and fixed in a 10% neutral formalin solution (Servicebio, Wuhan, China) for 1 day. The tissues were trimmed, dehydrated, embedded, and sectioned (Xu et al. 2022). These sections were stained with hematoxylin–eosin (H&E), dehydrated, dried, pressed (Zhu et al. 2021), and magnified with a normal optical microscope (Eclipse Ni/Ci, Nikon Eclipse-TI Inc., Tokyo, Japan).
Transmission electron microscopy (TEM)
Spleen samples were left overnight in 2.5% glutaraldehyde and subsequently post-fixed with osmium tetroxide. Afterward, being reduced into thin sections, washed, gradient dehydrated, and resin-soaked, these samples were stained with uranyl acetate (Zhang et al. 2022a). Then, sections were watched and photographed by TEM (JEM-1200ES, JEOL, Tokyo, Japan).
Detection of oxidative stress and antioxidant indicators
Spleen tissues were homogenized in normal sterile saline and centrifuged at 5000 r for 13 min. Next, the supernatant was used (Li et al. 2022b). Several important oxidative and antioxidant indicators were measured by assay kits. Superoxide dismutase (SOD, Jiancheng Bioengineering Institute, Nanjing, China) was determined by the xanthine oxidase method; total antioxidant capacity (T-AOC, Nanjing Jiancheng Bioengineering Institute, Nanjing, China) and malondialdehyde (MDA, Nanjing Jiancheng Bioengineering Institute, Nanjing, China) were determined by the colorimetric method; oxidized glutathione disulfide (GSSG, Geruisi Bio, Suzhou, China) and reduced glutathione (GSH, Geruisi Bio, Suzhou, China) were determined by the spectroscopic method.
RNA isolation and determination of messenger RNA (mRNA) expression of pyroptosis factors
Total RNA was segregated from the spleen using TRIzol reagent (Takara Bio, Dalian, China). Total RNA was synthesized into complementary DNA (cDNA) using superscript II reverse transcriptase (BIOER, Hangzhou, China), depending upon the manufacturer’s instructions (Zheng et al. 2021a). The synthesized cDNA was stored at – 80 °C. A quantitative real-time polymerase chain reaction (qRT-PCR) was performed in the LightCyclers@480 II system (FQD-96A, BIOER, Hangzhou, China). Pyroptosis pathway-related genes, such as nicotinamide adenine dinucleotide phosphate oxidase 2 (NOX2), apoptosis-associated speck protein (ASC), absent in melanoma-2 (AIM2), NLRP3, Caspase-1, interleukin 18 (IL-18), and interleukin 1-beta (IL-1β), were detected. The housekeeping gene (β-actin) was used as an internal reference (Li et al. 2022c, d; Zhang et al. 2022b). The sequences of the relevant specific primers (Sangon Biotechnology, Shanghai, China) are shown in Table 1.
Protein extraction and Western blot (WB) analysis
The spleen tissue was removed in a 100:1 ratio of RIPA lysate (Biosharp, Beijing, China) and phenylmethylsulfonyl fluoride (PMSF, Biosharp, Beijing, China) and centrifuged at 4 °C for 15 min at 12,000 r/min. The supernatant was aspirated and quantified by bicinchoninic acid (BCA, Mei5bio, Beijing, China). One-fourth of the supernatant volume of sodium dodecyl sulfate (SDS) sample buffer (Biosharp, Beijing, China) was added and boiled for 10 min, followed by protein immunoblot analysis. Prepared sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) gels at 12% and/or 10% concentrations were separated from the proteins by SDS–polyacrylamide gel electrophoresis and transferred to nitrocellulose (NC) membranes at a constant current of 200 mA (Yang et al. 2021). NC membranes were closed with 5% bovine serum albumin (BSA) prepared in TBST for 2 h and incubated with diluted β-actin, ASC, NLRP3, Caspase-1, and IL-1β diluted, respectively. The specific dilution ratio of the antibody (Wanleibio, Shenyang, China) is shown in Table 2 for 12 h at 4 °C. Afterward, the membranes were washed 2–3 times with a test for 15 min each. Subsequently, the membrane was incubated with an anti-rabbit immunoglobulin G (IgG) antibody for 1 h at 37 °C and washed 3 times with TBST for 10–15 min each time. β-Actin content was used as an internal reference. Finally, bands with the chemiluminescence imaging system (Azure Biosystems C300, Azure Biosystems Inc., California, USA) were detected with the ECL kit (Biosharp, Beijing, China).
Statistical analysis
All data were analyzed using GraphPad Prism (GraphPad Software Inc., California, USA) for an unbalanced one-way analysis of variance (ANOVA) combined with Tukey’s test (Wu et al. 2022). The data were expressed as the mean ± standard deviation (SD) of triplicate independent experiments. Those with different letters were different from the corresponding groups (p < 0.05). Identical letters represented non-significant values (p > 0.05).
Results
Loss of SelM aggravated Ni-induced pathological damage, whereas Mel alleviated damage
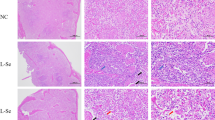
To observe the effects of Ni and effect of Mel on the action of Nil on wild-type and or Selm knockout mice, histopathological damage by H&E staining for microscopic examination was given in Fig. 1. In the control group, the spleen tissue was neatly arranged. The lymphocytes (LY), spleen cord (SP), and spleen sinus (SS) structures were complete, and the coloration of the white pulp (WP) and the red pulp (RP) was clear. In the Ni group, WP was scattered, and lymphocytes were partially stained. SS was enlarged, and there was slight inflammatory congestion in RP. In the Ni + Mel group, WP was poorly demarcated, and lymphocytes were heavily stained, less severe than those in the Ni group. The KO + C group behaved normally, which was similar to the first group. In the KO + Ni group, WP was scattered; RP was atrophied; SP was broken. There were many red blood cells (RBCs) in the SS, which was aggravated by comparing with the Ni group. In the KO + Ni + Mel group, the WP was atrophied; the lymphocytes were heavily stained; the SS was enlarged. There were a few RBCs in the SS. Mel alleviated the pathological damage in the spleen of mice induced by Ni exposure, and SelM knockout aggravated the damage. However, the remission of Mel in the absence of SelM was limited.
Effects of nickel, melatonin, and loss of SleM on spleen histology. Red arrows show the specific structure of the spleen, representative photographs of mouse spleens (20 × magnification, scale bar = 50 μm). Abbreviations in the figure are as follows: lymphocytes: LY; spleen cord: SP; spleen sinus: SS; white pulp: WP; red pulp: RP; red blood cells: RSC. Results were expressed as mean ± SD (n = 20)
The ultrastructure of the spleen was observed by TEM, revealing the effects of Ni on wild-type and SelM gene knockout mice and the effects of Mel on Ni-exposed SelM gene knockout mice in Fig. 2. In the control group, the cells, nuclei, and mitochondria had normal morphology; the nuclear membrane was intact; the nucleoli and mitochondrial cristae were obvious. The chromatin in the Ni group was scattered compared to the previous group. Some mitochondrial cristae were disordered and vacuolated; a few pyroptotic bodies were formed. Compared with the Ni group, the KO + Ni group showed irregular nuclear shape, the disappearance of nucleoli, scattered chromatin in the nucleus, swelling of mitochondria, the disappearance of mitochondrial disorder, partial vacuolization, and a large number of pyroptosis bodies. Mel and SelM were protective against spleen injury.
Effects of nickel, melatonin, and loss of SleM on spleen ultrastructure. Transmission electron microscopy confirmed the changes in the ultrastructure of the spleen, the red arrow only wanted to specify the location of the injury, and the scale bar is 1 μm. Results were expressed as mean ± SD (n = 20)
Loss of SelM aggravated Ni-induced changes in oxidative stress parameters, whereas Mel alleviated oxidative stress parameters in the spleen
The levels of oxidative stress markers of antioxidant enzymes (GSH, GSSG, T-AOC, SOD, and MDA) in spleen extracts from treated mice were measured (Fig. 3). As shown in the figure, compared to the previous group, the contents of GSH and GSSG in the Ni group were decreased (p < 0.05). In contrast, the activities of T-AOC and SOD were decreased, but the content of MDA was increased, which may explain the inhibitory effect of Ni exposure on antioxidant enzymes in the mouse spleen. Compared with the Ni group, the contents of GSH and GSSG and the activities of T-AOC and SOD in the Ni + Mel group were increased, while the content of MDA was decreased. The results suggested that Mel attenuated the inhibitory effect of Ni exposure on antioxidant enzymes in the spleen of mice. Compared with the Ni group, the contents of GSH and GSSG, as well as the activities of T-AOC and SOD, were significantly decreased in the KO + Ni group, while the contents of MDA were further increased (p < 0.05). SelM knockout aggravated the inhibitory effect of Ni-exposure on antioxidant enzymes in the mouse spleen. In contrast, SelM had protective effects on mouse spleen antioxidant enzymes. Simultaneously, compared with the KO + Ni group, the GSH, GSSG contents, T-AOC, and SOD activities in the KO + Ni + Mel group increased, while the MDA contents decreased, but the changes were not enough, which suggests that Mel can alleviate but not counteract the effects of SelM deletion. Thus, these results indicated that Mel alleviated Ni-induced excessive oxidative stress, which was exacerbated by SelM knockout; however, the remission of Mel in the absence of SelM was limited.
Effects of nickel, melatonin, and loss of SleM on oxidative stress in the spleen. The contents of MDA, GSH, and GSSG and activities of SOD and T-AOC in the spleen. Different letters represent significant differences (p < 0.05), and the same letters represent insignificant differences (p > 0.05). Results were expressed as mean ± SD (n = 20)
Loss of SelM aggravated Ni-induced pyroptosis, whereas Mel alleviated pyroptosis
To test whether the effects of Mel attenuating Ni-exposed spleen toxicity and SelM knockout aggravating Ni-exposed spleen toxicity were associated with pyroptosis, mice were measured by qRT-PCR and WB analysis of pyroptosis genes and proteins in the spleen (Fig. 4). We assessed the pyroptosis in mice spleen by detecting mRNA and protein levels, including ASC, NLRP3, AIM2, Caspase-1, IL-18, IL-1β, and pyroptosis upstream genes, and oxidative stress downstream genes thioredoxin-interacting protein (TXNIP), (time-resolved x-ray imager) TRXI, ASC, NLRP3, Caspase1, AIM2, IL-1β, and IL-18 were increased in the Ni group (p < 0.05), showing spleen tissue undergoes pyroptosis, which was compared with the control group. The mRNA levels of pyroptosis-related genes were decreased in the Ni + Mel group compared to the Ni group (p < 0.05). Then, the mRNA levels of pyroptosis-related genes were increased in the KO + Ni group compared to the Ni group (p < 0.05). Furthermore, the levels in the KO + C group were similar to those in the control group. Compared with the KO + Ni group, the KO + Ni + Mel group decreased, but the mRNA levels of pyroptosis-related genes increased compared with the KO + C group (p < 0.05).
Effects of Ni, melatonin, and loss of SleM on spleen via TXNIP/NLRP3 pathway. Expression levels of NOX2, TRX1, TXNIP, ASC, NLRP3, AIM2, Caspase-1, IL-18, and IL-1β mRNA in the spleen. Different letters represent significant differences (p < 0.05), and the same letters represent insignificant differences (p > 0.05). Results were expressed as mean ± SD (n = 20)
WB analysis demonstrated the proteins of ASC, NLRP3, Caspase-1, IL-1β, and β-actin, as shown in Fig. 5. Compared with the control group, the protein levels of ASC, NLRP3, Caspase-1, and IL-1β in the spleen of the Ni group, were increased (p < 0.05). The protein levels of ASC, NLRP3, Caspase-1, and IL-1β in the spleen were increased in the KO + Ni group (p < 0.05) compared to the Ni group. In addition, the levels in the KO + C group were similar to those in the control group. Compared with the KO + Ni group, the KO + Ni + Mel group decreased, but ASC, Caspase-1, and IL-1β were more than the KO + C group (p < 0.05). Thus, the results suggested Mel attenuated Ni exposure-induced pyroptosis, while SelM knockout exacerbated it; however, the remission of Mel in the absence of SelM was limited (Fig. 6).
Effects of Ni, melatonin, and loss of SleM on spleen via TXNIP/NLRP3 pathway. ASC, NLRP3, Caspase-1, and IL-1β protein expression levels in the spleen. Different letters represent significant differences (p < 0.05), and the same letters represent insignificant differences (p > 0.05). Results were expressed as mean ± SD (n = 20)
Discussion
Ni is a metal that exists in nature and has been extensively used in production, which is a major pollutant. Ni poisoning gets more and more become a public health pollution problem worldwide. At the same time, its exposure may cause cancer, allergic reactions, nephroptosis, hepatotoxicity, neurotoxicity, and immunotoxicity (Guo et al. 2020). Regarding the immunotoxicity of Ni, it is mainly divided into toxicity to non-immune organs and immune organs. Ni decreased the amount of T cells in the intestinal mucosa and the cecal tonsil (Wu et al. 2015). In contrast, Ni reduced the expression levels of spleen TLR4 and TLR7 mRNA, impairing innate spleen immunity (Huang et al. 2014). In addition, Ni had a significant effect on the immune system of the Carassius auratus, resulting in a decrease in spleen lymphocytes (Kubrak et al. 2012). Pyroptosis is a novel regulated form of programmed cell death, which is closely related to inflammasome formation (Tsuchiya, 2021). Pyroptosis is related to a variety of diseases, including obstetric diseases (Yu and Li, 2021), liver diseases (Al Mamun et al. 2020), cardiovascular diseases (Wang et al. 2020), and autoimmune system diseases (Wu et al. 2021). Lithium upregulates nuclear factor kappa-light-chain-enhancer of activated B cells (NF/κB) and NLRP3 through reactive oxygen species (ROS), increasing Caspase-3, IL-1β, etc., leading to pyroptosis in mice (Jing et al. 2022). Then, we explored whether Ni could cause spleen pyroptosis in mice. We found that Ni caused spleen tissue damage in mice by H&E staining and pyroptosis by electron microscopy. Oxidative stress can occur in the liver (Shaban et al. 2013) and testis (Shaban et al. 2017). At the same time, oxidative stress can cause apoptosis (Shaban et al. 2022a). In addition, elevated levels of oxidative stress also lead to increased levels of inflammation and fibrosis (Shaban et al. 2022b). In this study, Ni reduced antioxidant capacity and increased oxidative stress in the spleen of mice. Oxidative stress increased levels of pyroptosis-related factors by upregulating TRXI/TXNIP (Song et al. 2021). During pyroptosis, cytoplasmic pattern recognition receptors (PRRs), ASC, and pro-Caspase-1 work together to form activated inflammasomes, which, after cleavage by inflammatory Caspases, release gasdermin D (GSDMD) with perforating activity N-terminal fragment performs pyroptosis, releasing large amounts of IL-1β and IL-18 (Liang et al. 2020). A study showed that chlorpyrifos via miR-124-3p papulosum cyprini cells increased ROS and the occurrence of pyroptosis (Miao et al. 2022). We found elevated levels of TRXI, TXNIP, ASC, NLRP3, AIM2, Caspase-1, IL-1β, and IL-18 (p < 0.05) and pyroptosis. To explore the relationship between oxidative stress and Ni-induced pyroptosis, we selected an antioxidant, Mel.
Mel is an indole heterocyclic compound synthesized by unicellular and multicellular organisms from the essential amino acid tryptophan, which is produced in the pineal gland, kidney, liver, brain, gastrointestinal tract, adrenal glands, and immune system cells (Kvetnoy et al. 2022). It is inextricably linked to the normal function of the body. Mel has been extensively studied due to its potent antioxidant properties in recent years. Mel is associated with treatments for Alzheimer’s disease (Park and Kim, 2022), atherosclerosis (Liu et al. 2022a), ischemia–reperfusion (Zhong et al. 2022), osteoarthritis (Zhou et al. 2022), and so on. At the same time, Mel can reduce the damage brought about by poisoning, especially heavy metal poisoning. Mel attenuated aluminum-induced spleen toxicity in mice by inhibiting oxidative stress and the Nrf2 apoptosis signaling pathway (Yu et al. 2019). We found that Mel could alleviate Ni-induced spleen tissue damage in mice by H&E staining and pyroptosis with the electron microscope in mice. However, it is unclear whether Mel could alleviate Ni-induced oxidative stress and pyroptosis in spleen tissue. It was confirmed that Mel could relieve oxidative stress, mitochondrial dysfunction, and apoptosis induced by 2,2,4,4-tetrabromodiphenyl ether (Luan et al. 2022). Regarding the opaque metal Ni related to this study, Mel reduced the oxidative stress caused by Ni and relieved mitochondrial function (Xu et al. 2010). We found that Mel attenuated Ni-induced oxidative stress in the mice spleen; enhanced antioxidant capacity; downregulated TXNIP and TRXI; decreased ASC, NLRP3, AIM2, Caspase-1, IL-1β, and IL-18 (p < 0.05); and attenuated pyroptosis. Mel relieves liver fibrosis induced by Txnrd3 knockdown and Ni activation (Liu et al. 2022c). In contrast to the control group, it still showed a damaging effect. Mel alleviated pyroptosis by reducing oxidative stress and improving antioxidant capacity.
We have found that Ni exposure induces spleen pyroptosis in mice, which can be antagonized by Mel. To explore the role of SelM in Mel antagonizing Ni, we performed experiments using SelM knockout mice. Selenoprotein M is a newly discovered selenoprotein with an amino-terminal signal peptide and a thioredoxin-like domain involved in disulfide bond formation and ultimately localizes to the endoplasmic reticulum (Fomenko and Gladyshev 2003). Selenoprotein M is widely distributed, including the cerebral cortex, muscle, liver, and kidney, and protects tissues from oxidative stress (Huang et al. 2016). In addition, there are more and more studies on the effects of selenoproteins, especially SelM, on the body. Selenoprotein M is involved in developing tumors and cancers, including breast cancer and fibrosarcoma (Varlamova et al. 2019). Previous studies have shown that SelM affects the pathogenesis of Alzheimer’s disease (Yim et al. 2009). In our previous study, selenoprotein W (SelW) (Yu et al. 2015), selenoprotein T (SelT) (Pan et al. 2018), and selenoprotein S (SelS) (Chi et al. 2021) were closely related to the immune of chickens, including spleen (Khoso et al. 2019), thymus (Khoso et al. 2015a), bursa of Fabricius (Khoso et al. 2015b), trachea (Qin et al. 2020), and neutrophils (Li et al. 2017). However, the role of SelM in Ni-induced pyroptosis of mouse spleen cells has not yet been explored, so we verified this by constructing the SelM knockout model. Through electron microscopy and H&E staining, Ni exposure induced higher pyroptosis levels in the absence of SelM. Wang et al. (2022) found that SelK protected skeletal muscle by reducing endoplasmic reticulum stress and oxidative stress. We found that the KO + Ni group showed more serious spleen damage and higher levels of oxidative stress than the Ni group. Compared with the Ni exposure group, TXNIP and TRXI were upregulated; ASC, NLRP3, AIM2, Caspase-1, IL-18, and IL-1β were increased (p < 0.05), elevated levels of pyroptosis. The level of pyroptosis, oxidative stress, and spleen damage in the KO + Ni + Mel group decreased but still showed higher changes compared with the KO + C group. In conclusion, the loss of SelM exacerbated the level of pyroptosis by increasing the degree of oxidative stress. Meanwhile, Mel’s mitigation became limited.
Conclusion
Loss of SelM aggravated Ni-induced pyroptosis of the spleen via oxidative stress, but it was alleviated by Mel, which illustrated the protective effect of SelM on mice spleen. Our study is expected to provide a new attempt to treat and study the increasingly serious Ni exposure worldwide and enrich the protective effects of SelM and Mel.
Data availability
All data generated or analyzed during this study are included in this published article.
Abbreviations
- Ni:
-
Nickel
- Mel:
-
Melatonin
- SelM:
-
Selenoprotein M
- H&E:
-
Hematoxylin-eosin
- SOD:
-
Superoxide dismutase
- T-AOC:
-
Total antioxidant capacity
- MDA:
-
Malondialdehyde
- GSSG:
-
Oxidized glutathione disulfide
- GSH:
-
Reduced glutathione
- qRT-PCR:
-
Quantitative real-time polymerase chain reaction
- PMSF:
-
Phenylmethylsulfonyl fluoride
- BCA:
-
Bicinchoninic acid
- NC:
-
Nitrocellulose
- BSA:
-
Bovine serum albumin
- LY:
-
Lymphocytes
- SP:
-
Spleen cord
- SS:
-
Spleen sinus
- WP:
-
White pulp
- RP:
-
Red pulp
- RSC:
-
Red blood cells
- ASC:
-
Apoptosis-associated speck protein
- AIM2:
-
Absent in melanoma-2
- NOX2:
-
Nicotinamide adenine dinucleotide phosphate oxidase 2
- NLRP3:
-
NOD-like receptor thermal protein domain-associated protein 3
- TXNIP:
-
Thioredoxin-interacting protein
- TRXI:
-
Time-resolved x-ray imager
- IL-18:
-
Interleukin- (IL-) 18
- PRRs:
-
Pattern recognition receptors
- GSDMD:
-
gasdermin D
References
Al Mamun A, Mimi AA, Aziz MA, Zaeem M, Ahmed T, Munir F, Xiao J (2021) Role of pyroptosis in cancer and its therapeutic regulation. Eur J Pharmacol 5(910):174444. https://doi.org/10.1016/j.ejphar.2021.174444
Al Mamun A, Wu Y, Jia C, Munir F, Sathy KJ, Sarker T, Monalisa I, Zhou K, Xiao J (2020) Role of pyroptosis in liver diseases. Int Immunopharmacol 84:106489. https://doi.org/10.1016/j.intimp.2020.106489
Boulila S, El Feki A, Oudadesse H, Kallel C, El Feki H (2014) Detoxification of rats subjected to nickel chloride by a biomaterial-based carbonated orthophosphate. Ann Pharm Fr 72(5):348–362. https://doi.org/10.1016/j.pharma.2014.03.004
Buxton S, Taylor MD, Weinberg JT, Randazzo JM, Peachee VL, Oller A (2021) A T-dependent antibody response evaluation in CD-1 mice after an acute whole-body inhalation exposure to nickel (II) chloride hexahydrate. J Immunotoxicol 18(1):144–153. https://doi.org/10.1080/1547691X.2021.1984618
Cai J, Guan H, Jiao X, Yang J, Chen X, Zhang H, Zheng Y, Zhu Y, Liu Q (2021) Zhang Z (2021) NLRP3 inflammasome mediated pyroptosis is involved in cadmium exposure-induced neuroinflammation through the IL-1β/IkB-α-NF-κB-NLRP3 feedback loop in swine. Toxicology 15(453):152720. https://doi.org/10.1016/j.tox.2021.152720
Cai J, Huang J, Yang J, Chen X, Zhang H, Zhu Y, Liu Q, Zhang Z (2022) The protective effect of selenoprotein M on non-alcoholic fatty liver disease: the role of the AMPKα1-MFN2 pathway and Parkin mitophagy. Cell Mol Life Sci 9 79(7):354. https://doi.org/10.1007/s00018-022-04385-0
Cai J, Yang J, Chen X, Zhang H, Zhu Y, Liu Q, Zhang Z (2022b) Melatonin ameliorates trimethyltin chloride-induced cardiotoxicity: the role of nuclear xenobiotic metabolism and Keap1-Nrf2/ARE axis-mediated pyroptosis. BioFactors 48(2):481–497. https://doi.org/10.1002/biof.1787
Chen P, Wang RR, Ma XJ, Liu Q, Ni JZ (2013) Different forms of selenoprotein M differentially affect Aβ aggregation and ROS generation. Int J Mol Sci 14(3):4385–4399. https://doi.org/10.3390/ijms14034385
Chen X, Bi M, Yang J, Cai J, Zhang H, Zhu Y, Zheng Y, Liu Q, Shi G, Zhang Z (2021) Cadmium exposure triggers oxidative stress, necroptosis, Th1/Th2 imbalance and promotes inflammation through the TNF-α/NF-κB pathway in swine small intestine. J Hazard Mater 421:126704. https://doi.org/10.1016/j.jhazmat.2021.126704
Chi Q, Zhang Q, Lu Y, Zhang Y, Xu S, Li S (2021) Roles of selenoprotein S in reactive oxygen species-dependent neutrophil extracellular trap formation induced by selenium-deficient arteritis. Redox Biol 44:102003. https://doi.org/10.1016/j.redox.2021.102003
Chu JH, Yan YX, Gao PC, Chen XW, Fan RF (2020) Response of selenoproteins gene expression profile to mercuric chloride exposure in chicken kidney. Res Vet Sci 133:4–11. https://doi.org/10.1016/j.rvsc.2020.08.020
Du T, Gao J, Li P, Wang Y, Qi Q, Liu X, Li J, Wang C, Du L (2021) Pyroptosis, metabolism, and tumor immune microenvironment. Clin Transl Med 11(8):e492. https://doi.org/10.1002/ctm2.492
Fomenko DE, Gladyshev VN (2003) Genomics perspective on disulfide bond formation. Antioxid Redox Signal 5(4):397–402. https://doi.org/10.1089/152308603768295131
Guo H, Deng H, Liu H, Jian Z, Cui H, Fang J, Zuo Z, Deng J, Li Y, Wang X, Zhao L (2021) Nickel carcinogenesis mechanism: cell cycle dysregulation. Environ Sci Pollut Res Int 28(5):4893–4901. https://doi.org/10.1007/s11356-020-11764-2
Guo H, Liu H, Jian Z, Cui H, Fang J, Zuo Z, Deng J, Li Y, Wang X, Zhao L, He R, Tang H (2020) Immunotoxicity of nickel: pathological and toxicological effects. Ecotoxicol Environ Saf 203:111006. https://doi.org/10.1016/j.ecoenv.2020.111006
Harkin A, Hynes MJ, Masterson E, Kelly JP, O’Donnell JM, Connor TJ (2003) A toxicokinetic study of nickel-induced immunosuppression in rats. Immunopharmacol Immunotoxicol 25(4):655–670. https://doi.org/10.1081/iph-120026448
Hofstee P, Perkins AV, Cuffe JSM (2022) Selenium deficiency during pregnancy in mice impairs exercise performance and metabolic function in adult offspring. Nutrients 14(5):1125. https://doi.org/10.3390/nu14051125
Hu Y, Wang B, Li S, Yang S (2022) Pyroptosis, and its role in central nervous system disease. J Mol Biol 434(4):167379. https://doi.org/10.1016/j.jmb.2021.167379
Huang J, Cui H, Peng X, Fang J, Zuo Z, Deng J, Wang X, Wu B (2014) Downregulation of TLR4 and 7 mRNA expression levels in broiler’s spleen caused by diets supplemented with nickel chloride. Biol Trace Elem Res 158(3):353–358. https://doi.org/10.1007/s12011-014-9938-2
Huang JQ, Ren FZ, Jiang YY, Lei X (2016) Characterization of selenoprotein M and its response to selenium deficiency in chicken brain. Biol Trace Elem Res 170(2):449–458. https://doi.org/10.1007/s12011-015-0486-1
Jing H, Wang F, Gao XJ (2022) Lithium intoxication induced pyroptosis via ROS/NF-κB/NLRP3 inflammasome regulatory networks in kidney of mice. Environ Toxicol 37(4):825–835. https://doi.org/10.1002/tox.23446
Khoso PA, Yang Z, Liu C, Li S (2015a) Selenium deficiency downregulates selenoproteins and suppresses immune function in chicken thymus. Biol Trace Elem Res 167(1):48–55. https://doi.org/10.1007/s12011-015-0282-y
Khoso PA, Yang Z, Liu C, Li S (2015b) Selenoproteins and heat shock proteins play important roles in immunosuppression in the bursa of Fabricius of chickens with selenium deficiency. Cell Stress Chaperones 20(6):967–978. https://doi.org/10.1007/s12192-015-0625-9
Khoso PA, Zhang Y, Yin H, Teng X, Li S (2019) Selenium deficiency affects immune function by influencing selenoprotein and cytokine expression in chicken spleen. Biol Trace Elem Res 187(2):506–516. https://doi.org/10.1007/s12011-018-1396-9
Kubrak OI, Husak VV, Rovenko BM, Poigner H, Mazepa MA, Kriews M, Abele D, Lushchak VI (2012) Tissue specificity in nickel uptake and induction of oxidative stress in kidney and spleen of goldfish Carassius auratus, exposed to waterborne nickel. Aquat Toxicol 118–119:88–96. https://doi.org/10.1016/j.aquatox.2012.03.016
Kvetnoy I, Ivanov D, Mironova E, Evsyukova I, Nasyrov R, Kvetnaia T, Polyakova V (2022) Melatonin as the cornerstone of neuroimmunoendocrinology. Int J Mol Sci 23(3):1835. https://doi.org/10.3390/ijms23031835
Li J, Zhang W, Zhou P, Tong X, Guo D, Lin H (2022a) Selenium deficiency induced apoptosis via mitochondrial pathway caused by oxidative stress in porcine gastric tissues. Res Vet Sci 144:142–148. https://doi.org/10.1016/j.rvsc.2021.10.017
Li X, Bai R, Bai Y, Shi X, Yang Y, Xu S (2022b) ROS-mediated PPAR/RXR inhibition contributes to acetochlor-induced apoptosis and autophagy in Ctenopharyngodon idella hepatic cells. Fish Shellfish Immunol 128:684–694. https://doi.org/10.1016/j.fsi.2022.08.053
Li X, Bai Y, Zhu W, Shi X, Xu S (2022c) The endoplasmic reticulum-mitochondrial crosstalk is involved in the mitigation mechanism of eucalyptol on imidacloprid toxicity in Ctenopharyngodon idellus kidney cells. Fish Shellfish Immunol 127:99–108. https://doi.org/10.1016/j.fsi.2022.06.014
Li X, Xing M, Chen M, Zhao J, Fan R, Zhao X, Cao C, Yang J, Zhang Z, Xu S (2017) Effects of selenium-lead interaction on the gene expression of inflammatory factors and selenoproteins in chicken neutrophils. Ecotoxicol Environ Saf 139:447–453. https://doi.org/10.1016/j.ecoenv.2017.02.017
Li X, Yao Y, Wang J, Shen Z, Jiang Z, Xu S (2022d) Eucalyptol relieves imidacloprid-induced autophagy through the miR-451/Cab39/AMPK axis in Ctenopharyngodon idellus kidney cells. Aquat Toxicol 249:106204. https://doi.org/10.1016/j.aquatox.2022.106204
Li X, Zhang H, Qiao S, Ma W, Cai J, Zhang X, Zhang Z (2022e) Melatonin administration alleviates 2,2,4,4-tetra-brominated diphenyl ether (PBDE-47)-induced necroptosis and secretion of inflammatory factors via miR-140-5p/TLR4/NF-κB axis in fish kidney cells. Fish Shellfish Immunol 128:228–237. https://doi.org/10.1016/j.fsi.2022.08.004
Liang F, Zhang F, Zhang L, Wei W (2020) The advances in pyroptosis initiated by inflammasome in inflammatory and immune diseases. Inflamm Res 69(2):159–166. https://doi.org/10.1007/s00011-020-01315-3
Lin T, Tao J, Chen Y, Zhang Y, Li F, Zhang Y, Han X, Zhao Z, Liu G, Li H (2022) Selenium deficiency leads to changes in renal fibrosis marker proteins and Wnt/β-catenin signaling pathway components. Biol Trace Elem Res 200(3):1127–1139. https://doi.org/10.1007/s12011-021-02730-1
Liu J, Sun Q, Sun M, Lin L, Ren X, Li T, Xu Q, Sun Z, Duan J (2022a) Melatonin alleviates PM2.5-triggered macrophage M1 polarization and atherosclerosis via regulating NOX2-mediated oxidative stress homeostasis. Free Radic Biol Med 181:166–179. https://doi.org/10.1016/j.freeradbiomed.2022.02.005
Liu Q, Du P, Zhu Y, Zhang X, Cai J, Zhang Z (2022b) Thioredoxin reductase 3 suppression promotes colitis and carcinogenesis via activating pyroptosis and necrosis. Cell Mol Life Sci 79(2):106. https://doi.org/10.1007/s00018-022-04155-y
Liu Q, Sun Y, Zhu Y, Qiao S, Cai J, Zhang Z (2022c) Melatonin relieves liver fibrosis induced by Txnrd3 knockdown and nickel exposure via IRE1/NF-kB/NLRP3 and PERK/TGF-β1 axis activation. Life Sci 301:120622. https://doi.org/10.1016/j.lfs.2022.120622
Luan P, Zhang H, Chen X, Zhu Y, Hu G, Cai J, Zhang Z (2022) Melatonin relieves 2,2,4,4-tetrabromodiphenyl ether (BDE-47)-induced apoptosis and mitochondrial dysfunction through the AMPK-Sirt1-PGC-1α axis in fish kidney cells (CIK). Ecotoxicol Environ Saf 232:113276. https://doi.org/10.1016/j.ecoenv.2022.113276
Miao Z, Miao Z, Teng X, Xu S (2022) Chlorpyrifos triggers epithelioma papulosum cyprini cell pyroptosis via miR-124–3p/CAPN1 axis. J Hazard Mater 424(PtA):127318. https://doi.org/10.1016/j.jhazmat.2021.127318
Pan T, Liu T, Tan S, Wan N, Zhang Y, Li S (2018) Lower selenoprotein T expression and immune response in the immune organs of broilers with exudative diathesis due to selenium deficiency. Biol Trace Elem Res 182(2):364–372. https://doi.org/10.1007/s12011-017-1110-3
Park H, Kim J (2022) Activation of melatonin receptor 1 by CRISPR-Cas9 activator ameliorates cognitive deficits in an Alzheimer’s disease mouse model. J Pineal Res 72(3):e12787. https://doi.org/10.1111/jpi.12787
Qiao S, Sun Y, Jiang Y, Chen X, Cai J, Liu Q, Zhang Z (2022) Melatonin ameliorates nickel induced autophagy in mouse brain: diminution of oxidative stress. Toxicology 473:153207. https://doi.org/10.1016/j.tox.2022.153207
Qin L, Zhang Y, Wan C, Wang Z, Cong Y, Li S (2020) MiR-196-5p involvement in selenium deficiency-induced immune damage via targeting of NFκBIA in the chicken trachea. Metallomics 12(11):1679–1692. https://doi.org/10.1039/d0mt00164c
Qing Z, Dongliu L, Xuedie G, Khoso PA, Xiaodan H, Shu L (2022) MiR-144-3p targets STC1 to activate PI3K/AKT pathway to induce cell apoptosis and cell cycle arrest in selenium deficiency broilers. J Inorg Biochem 226:111665. https://doi.org/10.1016/j.jinorgbio.2021.111665
Romero A, Ramos E, de Los Ríos C, Egea J, Del Pino J, Reiter RJ (2014) A review of metal-catalyzed molecular damage: protection by melatonin. J Pineal Res 56(4):343–70. https://doi.org/10.1111/jpi.12132
Shaban NZ, Abd El-Kader SE, Mogahed FAK, El-Kersh MAL, Habashy NH (2021) Synergistic protective effect of Beta vulgaris with meso-2,3-dimercaptosuccinic acid against lead-induced neurotoxicity in male rats. Sci Rep 11(1):252. https://doi.org/10.1038/s41598-020-80669-4
Shaban NZ, Abdelrahman SA, El-Kersh MAL, Mogahed FAK, Talaat IM, Habashy NH (2020) The synergistic hepatoprotective potential of Beta vulgaris juice and 2,3-dimercaptosuccinic acid in lead-intoxicated rats via improving the hepatic oxidative and inflammatory stress. BMC Complement Med Ther 20(1):268. https://doi.org/10.1186/s12906-020-03056-6
Shaban NZ, Aboelsaad AM, Awad D, Abdulmalek SA, Shaban SY (2022a) Therapeutic effect of dithiophenolato chitosan nanocomposites against carbon tetrachloride-induced hepatotoxicity in rats. Environ Sci Pollut Res Int 29(6):8487–8502. https://doi.org/10.1007/s11356-021-15834-x
Shaban NZ, Ahmed Zahran AM, El-Rashidy FH, Abdo Kodous AS (2017) Protective role of hesperidin against γ-radiation-induced oxidative stress and apoptosis in rat testis. J Biol Res (thessalon) 24:5. https://doi.org/10.1186/s40709-017-0059-x
Shaban NZ, El-Kersh MA, El-Rashidy FH, Habashy NH (2013) Protective role of Punica granatum (pomegranate) peel and seed oil extracts on diethylnitrosamine and phenobarbital-induced hepatic injury in male rats. Food Chem 141(3):1587–1596. https://doi.org/10.1016/j.foodchem.2013.04.134
Shaban NZ, Zaki MM, Koutb F, Abdul-Aziz AA, Elshehawy AA, Mehany H (2022b) Protective and therapeutic role of mango pulp and eprosartan drug and their anti-synergistic effects against thioacetamide-induced hepatotoxicity in male rats. Environ Sci Pollut Res Int 29(34):51427–51441. https://doi.org/10.1007/s11356-022-19383-9
Song N, Li X, Cui Y, Zhang T, Xu S, Li S (2021) Hydrogen sulfide exposure induces pyroptosis in the trachea of broilers via the regulatory effect of circRNA-17828/miR-6631-5p/DUSP6 crosstalk on ROS production. J Hazard Mater 418:126172. https://doi.org/10.1016/j.jhazmat.2021.126172
Tsuchiya K (2021) Switching from apoptosis to pyroptosis: gasdermin-elicited inflammation and antitumor immunity. Int J Mol Sci 22(1):426. https://doi.org/10.3390/ijms22010426
Varlamova EG, Goltyaev MV, Fesenko EE (2019) Protein partners of selenoprotein SELM and the role of selenium compounds in regulation of its expression in human cancer cells. Dokl Biochem Biophys 488(1):300–303. https://doi.org/10.1134/S1607672919050065
Wang Q, Wu J, Zeng Y, Chen K, Wang C, Yang S, Sun N, Chen H, Duan K, Zeng G (2020) Pyroptosis: a pro-inflammatory type of cell death in cardiovascular disease. Clin Chim Acta 510:62–72. https://doi.org/10.1016/j.cca.2020.06.044
Wang S, Zhao X, Liu Q, Wang Y, Li S, Xu S (2022) Selenoprotein K protects skeletal muscle from damage and is required for satellite cells-mediated myogenic differentiation. Redox Biol 50:102255. https://doi.org/10.1016/j.redox.2022.102255
Wu B, Cui H, Peng X, Fang J, Zuo Z, Deng J, Wang X, Huang J (2015) Toxicological effects of nickel chloride on the cytokine mRNA expression and protein levels in intestinal mucosal immunity of broilers. Environ Toxicol 30(11):1309–1321. https://doi.org/10.1002/tox.22001
Wu H, Guo J, Yao Y, Xu S (2022) Polystyrene nanoplastics induced cardiomyocyte apoptosis and myocardial inflammation in carp by promoting ROS production. Fish Shellfish Immunol 125:1–8. https://doi.org/10.1016/j.fsi.2022.04.048
Wu J, Sun J, Meng X (2021) Pyroptosis by caspase-11 inflammasome-gasdermin D pathway in autoimmune diseases. Pharmacol Res 165:105408. https://doi.org/10.1016/j.phrs.2020.105408
Xu SC, He MD, Zhong M, Zhang YW, Wang Y, Yang L, Yang J, Yu ZP, Zhou Z (2010) Melatonin protects against nickel-induced neurotoxicity in vitro by reducing oxidative stress and maintaining mitochondrial function. J Pineal Res 49(1):86–94. https://doi.org/10.1111/j.1600-079X.2010.00770.x
Xu S, Xiaojing L, Xinyue S, Wei C, Honggui L, Shiwen X (2021) Pig lung fibrosis is active in the subacute CdCl2 exposure model and exerts cumulative toxicity through the M1/M2 imbalance. Ecotoxicol Environ Saf 1(225):112757. https://doi.org/10.1016/j.ecoenv.2021.112757
Xu Y, Li A, Li X, Deng X, Gao XJ (2022) Zinc deficiency induces inflammation and apoptosis via oxidative stress in the kidneys of mice. Biol Trace Elem Res. https://doi.org/10.1007/s12011-022-03166-x
Xue Y, Wang H, Tian B, Wang S, Gao XJ (2022) Selenium deficiency promotes the expression of lncRNA-MORC3, activating NLRP3-caspase-1/IL-1β signaling to induce inflammatory damage and disrupt tight junctions in piglets. Biol Trace Elem Res. https://doi.org/10.1007/s12011-022-03341-0
Yang J, Shi G, Gong Y, Cai J, Zheng Y, Zhang Z (2021) LncRNA 0003250 accelerates heart autophagy and binds to miR-17-5p as a competitive endogenous RNA in chicken induced by selenium deficiency. J Cell Physiol 236(1):157–177. https://doi.org/10.1002/jcp.29831
Yim SY, Chae KR, Shim SB, Hong JT, Park JY, Lee CY, Son HJ, Sheen YY, Hwang DY (2009) ERK activation induced by selenium treatment significantly downregulates beta/gamma-secretase activity and Tau phosphorylation in the transgenic rat overexpressing human selenoprotein M. Int J Mol Med 24(1):91–96. https://doi.org/10.3892/ijmm-00000211
Yu D, Zhang Z, Yao H, Li S, Xu SW (2015) The role of selenoprotein W in inflammatory injury in chicken immune tissues and cultured splenic lymphocyte. Biometals 28(1):75–87. https://doi.org/10.1007/s10534-014-9804-x
Yu H, Zhang J, Ji Q, Yu K, Wang P, Song M, Cao Z, Zhang X, Li Y (2019) Melatonin alleviates aluminium chloride-induced immunotoxicity by inhibiting oxidative stress and apoptosis associated with the activation of Nrf2 signaling pathway. Ecotoxicol Environ Saf 173:131–141. https://doi.org/10.1016/j.ecoenv.2019.01.095
Yu SY, Li XL (2021) Pyroptosis and inflammasomes in obstetrical and gynecological diseases. Gynecol Endocrinol 37(5):385–391. https://doi.org/10.1080/09513590.2021.1871893
Zhang H, Huang J, Yang J, Cai J, Liu Q, Zhang X, Bao J, Zhang Z (2022) Zhang Z Cadmium induces apoptosis and autophagy in swine small intestine by downregulating the PI3K/Akt pathway. Environ Sci Pollut Res Int 29(27):41207–41218. https://doi.org/10.1007/s11356-022-18863-2
Zhang H, Huang J, Yang J, Cai J, Liu Q, Zhang X, Bao J, Zhang Z (2022b) Cadmium induces apoptosis and autophagy in swine small intestine by downregulating the PI3K/Akt pathway. Environ Sci Pollut Res Int 29(27):41207–41218. https://doi.org/10.1007/s11356-022-18863-2
Zhang Q, Xue Y, Fu Y, Bao B, Guo MY (2022c) Zinc deficiency aggravates oxidative stress leading to inflammation and fibrosis in lung of mice. Biol Trace Elem Res 200(9):4045–4057. https://doi.org/10.1007/s12011-021-03011-7
Zhang Y, Liu Q, Yin H, Li S (2020) Cadmium exposure induces pyroptosis of lymphocytes in carp pronephros and spleens by activating NLRP3. Ecotoxicol Environ Saf 202:110903. https://doi.org/10.1016/j.ecoenv.2020.110903
Zhang Y, Xu Y, Chen B, Zhao B, Gao XJ (2021a) Selenium deficiency promotes oxidative stress-induced mastitis via activating the NF-κB and MAPK pathways in dairy cow. Biol Trace Elem Res 200(6):2716–2726. https://doi.org/10.1007/s12011-021-02882-0
Zhang Y, Zhang J, Bao J, Tang C, Zhang Z (2021b) Selenium deficiency induced necroptosis, Th1/Th2 imbalance, and inflammatory responses in swine ileum. J Cell Physiol 236(1):222–234. https://doi.org/10.1002/jcp.29836
Zhao J, Jiang P, Guo S, Schrodi SJ, He D (2021) Apoptosis, autophagy, NETosis, necroptosis, and pyroptosis mediated programmed cell death as targets for innovative therapy in rheumatoid arthritis. Front Immunol 12:809806. https://doi.org/10.3389/fimmu.2021.809806
Zheng Y, Guan H, Yang J, Cai J, Liu Q, Zhang Z (2021a) Calcium overload and reactive oxygen species accumulation induced by selenium deficiency promote autophagy in swine small intestine. Anim Nutr 7(4):997–1008. https://doi.org/10.1016/j.aninu.2021.05.005
Zheng Y, Zhang B, Guan H, Jiao X, Yang J, Cai J, Liu Q, Zhang Z (2021b) Selenium deficiency causes apoptosis through endoplasmic reticulum stress in swine small intestine. BioFactors 47(5):788–800. https://doi.org/10.1002/biof.1762
Zhong XY, Ruan S, Wang F, Chen B, Luo J, Wang YX, Liang H (2022) Electroacupuncture ameliorates ischemic injury in cerebral ischemia-reperfusion rats by regulating endogenous melatonin and inhibiting the activation of astrocytes. Zhen Ci Yan Jiu 47(1):39–45. https://doi.org/10.13702/j.1000-0607.20210738. (Chinese)
Zhou X, Zhang Y, Hou M, Liu H, Yang H, Chen X, Liu T, He F, Zhu X (2022) Melatonin prevents cartilage degradation in early-stage osteoarthritis through activation of miR-146a/NRF2/HO-1 axis. J Bone Miner Res 37(5):1056–1072. https://doi.org/10.1002/jbmr.4527
Zhu Y, Jiao X, An Y, Li S, Teng X (2017) Selenium against lead-induced apoptosis in chicken nervous tissues via mitochondrial pathway. Oncotarget 8(64):108130–108145. https://doi.org/10.18632/oncotarget.22553
Zhu Y, Luan P, Liu X, Bao J, Liu Q, Cai J, Yang J, Zhang Z (2021) Crosstalk between autophagy and apoptosis regulates cerebral cortex and cerebellum neurodegeneration induced by cadmium in swine via the PI3K/AKT/AMPK pathway. Ecotoxicol Environ Saf 228.https://doi.org/10.1016/j.ecoenv.2021.113053
Zuo Y, Chen L, Gu H, He X, Ye Z, Wang Z, Shao Q (2021) Xue C (2021) GSDMD-mediated pyroptosis: a critical mechanism of diabetic nephropathy. Expert Rev Mol Med 27(23):e23. https://doi.org/10.1017/erm.2021.27
Funding
This work was supported by the National Natural Science Foundation of China (31872531) and Excellent Youth Foundation of Heilongjiang Province of China (YQ2021C021).
Author information
Authors and Affiliations
Contributions
Wenxue Ma: conceptualization, methodology, software, investigation, and writing—original draft. Yue Liu: formal analysis. Lihua Xu: visualization. Xiaoxue Gai: validation. Yue Sun: formal analysis. Senqiu Qiao: software. Pinnan Liu: supervision. Qiaohan Liu: data curation. Ziwei Zhang: writing—review and editing.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
All the procedures were approved by the Academic Committee of Northeast Agricultural University. They informed and agreed. The approval number is NEAUEC2021 03 22.
Consent for publication
All authors have read the manuscript and agreed to submit it in its current form for consideration for publication in the journal.
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Mohamed M. Abdel-Daim
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ma, W., Liu, Y., Xu, L. et al. The role of selenoprotein M in nickel-induced pyroptosis in mice spleen tissue via oxidative stress. Environ Sci Pollut Res 30, 34270–34281 (2023). https://doi.org/10.1007/s11356-022-24597-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-022-24597-y