Abstract
The zinc oxide (ZnO) nanoparticle has been widely used in biomedical applications and cancer therapy and has been reported to induce a selective cytotoxic effect on cancer cell proliferation. The present study investigated the cytotoxicity of ZnO nanoparticles against co-cultured C2C12 myoblastoma cancer cells and 3T3-L1 adipocytes. Our results showed that the ZnO nanoparticles could be cytotoxic to C2C12 myoblastoma cancer cells than 3T3-L1 cells. The messenger RNA (mRNA) expressions of p53 and bax were significantly increased 114.3 and 118.2 % in the C2C12 cells, whereas 42.5 and 40 % were increased in 3T3-L1 cells, respectively. The mRNA expression of bcl-2 was reduced 38.2 and 28.5 % in the C2C12 and 3T3-L1 cells, respectively, whereas the mRNA expression of caspase-3 was increased 80.7 and 51.6 % in the C2C12 and 3T3-L1 cells, respectively. The protein expressions of p53, bax, and caspase-3 were significantly increased 40, 81.8, and 80 % in C2C12 cells, whereas 20.3, 28.2, and 37.9 % were increased in 3T3-L1 cells, respectively. The mRNA expression of bcl-2 was significantly reduced 32.2 and 22.7 % in C2C12 and 3T3-L1 cells, respectively. Caspase-3 enzyme activity and reactive oxygen species (ROS) were increased in co-cultured C2C12 cells compared to 3T3-L1 cells. Taking all these data together, it may suggest that ZnO nanoparticles severely induce apoptosis in C2C12 myoblastoma cancer cells than 3T3-L1 cells.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
ZnO nanoparticles have been used in the biomedical applications and various therapy [1]. The manipulation of ZnO at the nanoscale levels enables the precision engineering to control the physicochemical properties of nanoparticles and their interactions with cellular systems [2, 3]. ZnO has been widely used in the cosmetic lotions [4] and increases the anti-bacterial activity [5]. It is also utilized in the cotton fabric, rubber, and food packaging industry [6]. Nanoparticles have been known as a promising agent for cell imaging, biosensing, gene delivery, and cancer therapy. ZnO nanoparticles have attracted researchers for their implications in the cancer therapy and have been reported to induce cytotoxicity at in vitro and in vivo level [7–10].
The uncontrolled proliferation and regulated cell death are called apoptosis that plays a role in the development of cancer and therapy. The suppressor gene p53 controls apoptosis, DNA repair, and activation of cell cycle checkpoints [11]. The p53 protein triggers cell cycle arrest to provide time for recovery from damage and self-medicated apoptosis in the presence of DNA damage [12, 13]. The bcl-2 protein is anti-apoptotic, whereas bax is pro-apoptotic. The bax/bcl-2 protein ratio is a crucial factor in determining cell death in response. The increased bax/bacl-2 ratio has been known to reduce the resistance to apoptotic stimuli [14]. The role of caspases has been well known in the apoptotic process [15, 16].
The present study investigates the cytotoxicity of ZnO nanoparticles against co-cultured C2C12 myoblastoma cancer cells and 3T3-L1 adipocytes. Quantitative real-time polymerase chain reaction (qPCR) and Western blotting analysis were used to measure the apoptosis-related gene expressions. Reactive oxygen species (ROS) and increased oxidative stress could play a crucial role in apoptosis [17, 18]. Therefore, the levels of ROS, lipid peroxidation, glutathione, and anti-oxidant enzyme activity were determined. There is no study on the effect of ZnO nanoparticles against the co-cultured cells. Cell co-culturing has been considered more reliable and three-dimensional compared to the monoculture of cells [19].
Materials and Methods
ZnO nanoparticles (<100-nm particle size (DLS), Sigma-Aldrich), Dulbecco’s modified Eagle’s medium (DMEM), fetal bovine serum (FBS), and antibiotics were purchased from Sigma. Primers were purchased from Macrogen Inc. (South Korea). C2C12 and 3T3-L1 cells were purchased from ATCC (10801 University Blvd, Manassas, USA).
Cell Culture
C2C12 and 3T3-L1 cells were incubated at a density of 8000 cells/cm2 and grown in DMEM containing 10 % FBS and 1 % antibiotics at 37 °C in 5 % CO2. Confluent 3T3-L1 cells were induced to differentiate with a standard differentiation medium consisting of DMEM medium supplemented with 10 % FBS, 250 nM dexamethasone, 0.5 mM 3-isobutyl-1-methylxanthine, 5 μg/ml insulin, and 1 % antibiotics. 3T3-L1 cells were maintained in this differentiation medium for 3 days. C2C12 cells were grown up to 90 % confluence and transferred into the differentiation medium.
Co-Culture of C2C12 and 3T3-L1 Cells
C2C12 and 3T3-L1 cells were co-cultured by using Transwell inserts with a 0.4-μm porous membrane to separate the cells. Both cell types were grown independently and separately on the Transwell plates. After cell differentiation, inserts containing 3T3-L1 cells were transferred to C2C12 cell-containing plates, and inserts containing C2C12 cells were transferred to a 3T3-L1 cell-containing plate [20]. Cells in the lower well were utilized for analysis.
MTT Assay
Cytotoxicity of ZnO nanoparticle was determined by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay [21]. C2C12 and 3T3-L1 cells were seeded at a seeding density of 2 × 104 cells/ml into 96-well microplates. Cells were allowed for 24 h to adhere and treated with various concentrations of ZnO nanoparticle ranging 0.2, 0.4, 0.8, 1.6, 3.2, 6.4, and 12.8 mg/l. Then, the cells were labeled with MTT solution for 4 h, and absorption was measured at 570 nm.
Lipid Peroxidation
Malondialdehyde (MDA) was determined in the co-cultured C2C12 and 3T3-L1 cells [22]. Cells were treated with 0.8, 1.6, and 3.2 mg/l of ZnO nanoparticles for 36 h. A mixture of 0.1 ml supernatant and 1.9 ml of 0.1 M sodium phosphate buffer (pH 7.4) was incubated for 1 h at room temperature. After precipitation with 5 % trichloroacetic acid (TCA), it is centrifuged, and the supernatant was collected. Then, 1 ml of 1 % of thiobarbituric acid (TBA) was added and boiled for 15 min. After cooling to room temperature, the absorbance was measured at 532 nm and expressed in nanomole per milligram protein.
Determination of Cellular Reactive Oxygen Species
The cellular level of ROS was determined based on the measurement of 2,7-dichlorodihydrofluorescein diacetate (DCF-DA) in the co-cultured C2C12 and 3T3-L1 cells (2 × 104 cells/ml) [23]. Cells were treated with 0.8, 1.6, and 3.2 mg/l of ZnO nanoparticles for 36 h. Cells were incubated with 5 μM of DCFH-DA in the growth medium for 30 min at 37 °C and 5 % CO2. The fluorescence was measured using a fluorescent plate reader at excitation⁄emission wavelengths of 490 and 525 nm, respectively, and images were taken using a fluorescence microscope (Axiovert 2000, Carl Zeiss, Germany).
Quantitative Real-Time Polymerase Chain Reaction
Total RNA was isolated from the co-cultured C2C12 and 3T3-L1 cells (2 × 104 cells/ml) with TRIzol reagent according to the manufacturer’s protocol. The qPCR was performed using a cDNA equivalent of 10 ng of total RNA from each sample with primers mouse specific for p53 (forward primer: 5′-CACGTACTCTCCTCCCCTCAAT-3′, reverse primer: 5′-AACTGCACAGGGCACGTCTT-3′), bax (forward primer: 5′-CCAGGATGCGTCCACCAAGA-3′, reverse primer: 5′-GGTGAGGACTCCAGCCACAA-3′), bcl-2 (forward primer:5′-TGAGTACCTGAACCGGCATCT-3′, reverse primer: 5′-GCATCCCAGCCTCCGTTAT-3′), caspase-3 (forward primer: 5′-CAAACTTTTTCAGAGGGGATCG-3′, reverse primer: 5′-GCATACTGTTTCAGCATGGCAC-3′), and a housekeeping gene GAPDH (forward primer: 5′-AGAACATCATCCCTGCCTC-3′, reverse primer: 5′-GCCAAATTCGTTGTCATACC-3′). PCR was monitored using the Mini Opticon Real-Time PCR System (Bio-Rad) [24].
Western Blot Analysis
The protein levels were determined in the co-cultured C2C12 and 3T3-L1 cells (2 × 104 cells/ml). Control and treated samples were lysed in lysis buffer, and equal amounts of protein samples were run on SDS-polyacrylamide gel and transferred to PVDF membrane. After blocking, the membranes were probed with primary antibodies p53, bax, bcl-2, and caspase-3 overnight and incubated with secondary antibody for 1 h. The proteins levels were determined by chemiluminescence kit (Bioscience Technology, South Korea) [25].
Caspase-3 Assay
Caspase-3 enzyme activity was measured in the co-cultured C2C12 and 3T3-L1 cells (2 × 104 cells/ml) based on the method of Muthuraman [26]. Cells were lysed in caspase assay buffer and incubated with caspase-3 substrate Ac-DEVD-AMC for 30 min at 37 °C. Caspase-3 activity was determined at excitation 380 nm and emission at 440 nm in a Verso fluorometer.
Statistical Analysis
All the values were expressed as means ± SEM. The statistical analysis was carried out using SPSS 17 (590 Madison Avenue, New York, USA). The difference between control and test was determined using Student’s t test. A p < 0.05 was considered to be significant.
Results
Cytotoxicity
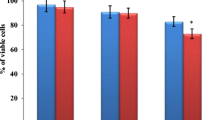
C2C12 and 3T3-L1 cells were incubated with different concentrations (0.2–12.8 mg/l) of ZnO nanoparticles for 36 h showing the dose-dependent effect that was evident from the MTT assay (Fig. 1). ZnO nanoparticle was highly toxic to normal cells at 6.4 and 12.8 mg/l. Therefore, we selected 0.8, 1.6, and 3.2 mg/l for further investigation.
Malondialdehyde and Reactive Oxygen Species Content
MDA content was increased 33.3, 80, and 140 % at 0.8, 1.6, and 3.6 mg/l of ZnO nanoparticles in C2C12 cells, whereas it was 38.4, 74.9, and 92.3 % in 3T3-L1 cells, respectively (Fig. 2). ROS content was increased 25, 36.7, and 83.3 % at 0.8, 1.6, and 3.6 mg/l of ZnO nanoparticles in C2C12 cells, whereas it was increased 10.7, 26.8, and 48 % in 3T3-L1 cells, respectively (Fig. 3).
Messenger RNA Expression
C2C12 and 3T3-L1 cells exposed to 3.6 mg/l of ZnO nanoparticles showed changes in the messenger RNA (mRNA) expression of apoptotic-related genes such as p53, bax, bcl-2, and caspase-3. The mRNA expressions of p53 and bax were increased, whereas the expression of bcl-2 was significantly decreased in ZnO nanoparticle-treated cells. The mRNA expressions of p53 and bax were increased 114.3 and 118.2 % in the C2C12 cells, whereas it was increased 42.5 and 40 % in 3T3-L1 cells, respectively. The mRNA expression of the bcl-2 was reduced 38.2 and 28.5 % in the C2C12 and 3T3-L1 cells, respectively. The mRNA expression of the caspase-3 enzyme was significantly increased 80.7 and 51.6 % in the C2C12 and 3T3-L1 cells, respectively (Fig. 4).
Protein Expression
Western blot analysis determined the effect of ZnO nanoparticles on p53, bax, bcl-2, and caspase-3 protein expression in the co-cultured C2C12 and 3T3-L1-L1 cells. The protein expressions of p53, bax, and caspase-3 were increased 40, 81.8, and 80 % in the C2C12 cells, whereas 20.3, 28.2, and 37.9 % were increased in the 3T3-L1 cells, respectively. The mRNA expression of the bcl-2 was decreased 32.2 and 22.7 % in the C2C12 and 3T3-L1 cells, respectively (Fig. 5).
Protein expression of p53, bax, bcl-2, and caspase-3 in ZnO nanoparticle-treated C2C12 myoblastoma cancer cells (a) and 3T3-L1 adipocytes (b). Western blot analysis of C2C12 myoblastoma cancer cell and 3T3-L1 adipocyte extracts probed with anti-p53, bax, bcl-2, and caspase-3. Quantitation analysis was carried out using densitometry. Values were expressed as means ± SEM, n = 6, *p < 0.05
Caspase-3 Activity
Caspase-3 activity was increased 21.4, 35.7, and 71.4 % at 0.8, 1.6, and 3.6 mg/l of ZnO nanoparticles in C2C12 cells, whereas it was 14.3, 33.3, and 58.3 % in 3T3-L1 cells, respectively (Fig. 6).
Discussion
The rapid growth of the nanotechnology leads to the huge production and application of nanoparticles and its wide use in medicine, cosmetics, sunscreens, and food products [27]. Nanoparticles have been utilized in the treatment of human diseases [28]. The size, shape, crystal structure, purity, hydrodynamic size, agglomeration, and aqueous stability of nanoparticles are critical for the better interpretation of results in biomedical research [17]. ZnO nanoparticles could act as novel photosensitizers of the conventional photosensitizing drugs in the photodynamic therapy of cancer [29]. UV irradiation enhances the ZnO nanoparticle’s ability to suppress the cancer cell proliferation [30].
The selective action of anti-cancer drugs is one of the major challenges [31]. The ability of most of cancer cells to avoid apoptosis and propagate rapidly could be the target of several anti-cancer drugs. Our study shows that ZnO nanoparticles could selectively induce severe toxicity in cancer cells than normal cells. ZnO nanoparticles have induced severe cytotoxicity in human glioma cells than normal human astrocytes [32]. Our results agreed with these findings.
The shape of ZnO nanoparticles plays a significant role in cancer cell inhibition. The rod-shaped ZnO nanoparticles induce less cytotoxicity in osteoblast cancer cells than spherical ZnO nanoparticles [33]. In our study, we have observed that spherical ZnO nanoparticles seem to be much more cytotoxic to cancer cells. The stability of the particles in cell culture and toxicity of dissolved Zn2+ ions are also to be concerned. ZnO nanoparticles release Zn2+ when they are suspended in the medium [34]. However, the released levels of Zn2+ were insufficient to promote toxicity to cells unless the particulate matter is in contact with the cells [35].
The clinical importance of ZnO nanoparticles is its selectivity in cancer cell inhibition. The greatest challenge for anti-cancer drugs is the differentiation of normal and cancer cells. ZnO nanoparticles selectively inhibit human myeloblastic leukemia cells compared to the normal peripheral blood mononuclear cells [36] and selectively inhibit cancerous T cells than normal cells [37]. In the present study, we have used the co-cultured mouse myoblastoma cancer cells and mouse adipocytes to investigate the selective effect of ZnO nanoparticles. Co-culture experiments are believed to be more reliable and have three-dimensional view compared to the monoculture experiments.
The mRNA and protein expressions of p53, bax, and caspase-3 were significantly increased in C2C12 cancer cells than normal 3T3-L1 cells. The expression of bcl-2 was significantly decreased in C2C12 cancer cells than normal 3T3-L1 cells. Under cellular stress, p53 triggers cell cycle arrest to provide time for the recovery and self-medicated apoptosis [11]. The role of p53 is to up-regulate the expression of bax. The bax is up-regulated by p53 [38], and in our study, the expression of bax was increased. The formation of DNA fragments was regarded as a biochemical hallmark of apoptosis [39]. Increased expression of caspase-3 could activate autocatalysis and activates other members of the caspase family leading to irreversible apoptosis [40]. In the present study, caspase-3 activity was increased in C2C12 cancer cells than 3T3-L1 cells.
Enzymatic peroxidation of fatty acids leads to the generation of ROS [41]. Recent research on cancer demonstrates that several apoptotic stimuli share a common mechanistic pathway characterized by the ROS generation and oxidative stress [42]. ROS includes superoxide radical (O2−), hydrogen peroxide (H2O2), and hydroxyl radical (OH), which cause damage to DNA and proteins [43]. ROS has been generated from mitochondria and endoplasmic reticulum [44]. Cells could be injured when exposed to a higher concentration of ROS [45]. Higher concentration of ZnO nanoparticles could increase ROS through increased MDA content [46]. ROS and MDA content were significantly increased in C2C12 cancer cells than normal 3T3-L1 cells. Increased intracellular ROS and MDA in ZnO nanoparticle-treated C2C12 cancer cells might be a key mechanism for increased apoptosis in cancer cells.
Conclusion
In summary, the present study shows that the ZnO nanoparticle induces apoptosis in C2C12 myoblastoma cancer through the activation of ROS, p53, bax/bcl-2 ratio, and caspase-3 pathways. Selective cytotoxicity of ZnO nanoparticles on cancer cells against co-cultured cancer and normal cells suggests that ZnO nanoparticles are a promising drug in the cancer research and therapy.
References
Muthuraman P, Kim DH (2015) In vitro toxicity of zinc oxide nanoparticles: a review. J Nanoparticle Res 17:158
Jae-Hyun L, Yong-Min H, Young-wook J, Jung-wook S, Jung-tak J, Ho-Taek S, Sungjun K, Eun-Jin C, Ho-Geun Y, Jin-Suck S, Jinwoo C (2007) Artificially engineered magnetic nanoparticles for ultra-sensitive molecular imaging. Nat Med 13:95–99
Wang ZL (2008) Splendid one-dimensional nanostructures of zinc oxide: a new nanomaterial family for nanotechnology. ACS Nano 2:1987–1992
Becheri A, Dürr M, Nostro PL, Baglioni P (2008) Synthesis and characterization of zinc oxide nanoparticles: application to textiles as UV-absorbers. J Nanoparticle Res 10: 679–689.
Padmavathy N, Vijayaraghavan R (2008) Enhanced bioactivity of ZnO nanoparticles-an antimicrobial study Sci. Technol Adv Mater 9:035004
Snyder-Talkingtona BN, Qiana Y, Castranovaa V, Guob NL (2012) New perspectives for in vitro risk assessment of multiwalled carbon nanotubes: application of Co-culture and bioinformatics. J Toxicol Environ Health B 15:468–492
Hanley C, Layne J, Punnoose A, Reddy KM, Coombs I, Coombs A, Feris K, Wingett D (2008) Preferential killing of cancer cells and activated human T cells using ZnO nanoparticles. Nanotechnology 19:295103
Premanathan M, Karthikeyan K, Jeyasubramanian K, Manivannan G (2011) Selective toxicity of ZnO nanoparticles toward Gram-positive bacteria and cancer cells by apoptosis through lipid peroxidation. Nanomedicine 7:184–192
Akhtar MJ, Ahamed M, Kumar S, Khan MM, Ahmad J, Alrokayan SA (2012) Zinc oxide nanoparticles selectively induce apoptosis in human cancer cells through reactive oxygen species. Int J Nanomedicine 7:845–857
Rasmussen JW, Martinez E, Louka P, Wingett DG (2010) Zinc oxide nanoparticles for selective destruction of tumor cells and potential for drug delivery applications. Expert Opin Drug Deliv 7:1063–1077
Sherr CJ (2004) Principles of tumor suppression. Cell 116:235–246
Ahamed M, Karns M, Goodson M, Rowe J, Hussain SM, Schlager JJ, Hong Y (2008) DNA damage response to the different surface chemistry of silver nanoparticles in mammalian cells. Toxicol Appl Pharmacol 233:404–410
Farnebo M, Bykov VJ, Wiman KG (2010) The p53 tumor suppressor: a master regulator of diverse cellular processes and therapeutic target in cancer. Biochem Biophys Res Commun 396:85–89
Chougule M, Patel AR, Sachdeva P, Jackson T, Singh M (2011) Anticancer activity of noscapine, an opioid alkaloid in combination with cisplatin in human non-small cell lung cancer. Lung Cancer 71:271–282
Tang X, Guo Y, Nakamura K, Huang H, Hamblin M, Chang L, Villacorta L, Yin K, Ouyang H, Zhang J (2010) Nitroalkenes induce rat aortic smooth muscle cell apoptosis via activation of caspase-dependent pathways. Biochem Biophys Res Commun 397:239–244
Ahamed M, Akhtar MJ, Siddiqui J, Musarrat J, Al-Khedhairy AA, AlSalhi MS, Alrokayan SA (2011) Oxidative stress-mediated apoptosis induced by nickel ferrite nanoparticles in cultured A549 cells. Toxicology 283:101–108
Nel A, Xia T, Mädler L, Li N (2006) Toxic potential of materials at the nanolevel. Science 311:622–627
Yip NC, Fombon IS, Liu P, Brown S, Kannappan V, Armesilla AL, Xu B, Cassidy J, Darling JL, Wang W (2011) Disulfiram modulated ROS-MAPK and NFkappaB pathways and targeted breast cancer cells with cancer stem cell-like properties. Br J Cancer 104:1564–1574
Muthuraman P, Inho H (2014) Application of cell co-culture system to study fat and muscle cells. Appl Microbiol Biotechnol 98:7359–7364
Ravikumar S, Muthuraman P (2014) Cortisol effect on heat shock proteins in the C2C12 and 3T3-L1 cells. In Vitro Cell Dev Bio Anim 50:581–586
Muthuraman P, Jeong Eun P, Eunjung K (2014) Aspartame downregulates 3T3-L1 differentiation. In Vitro Cell Dev Bio Anim 50:851–857
Muthuraman P, Ramkumar K, Kim DH (2014) Analysis of the dose-dependent effect f zinc oxide nanoparticles on the oxidative stress and antioxidant enzyme activity in adipocytes. Appl Biochem Biotechnol 174:2851–2863
Muthuraman P, Kim DH, Muthuviveganandavel V, Vikramathithan J, Ravikumar S (2015) Differential bio-potential of ZnS nanoparticles to normal MDCK cells and cervical carcinoma hela cells. J Nanosci Nanotechnol 15:1–8
Muthuraman P, Muthuviveganandavel V, Kim DH (2015) Cytotoxicity of zinc oxide nanoparticles on antioxidant enzyme activities and mRNA expression in the cocultured C2C12 and 3T3-L1 cells. Appl Biochem Biotechnol 175:1270–1280
Muthuraman P, Enkhtaivan G, Bhupendra M, Chandrasekaran M, Rafi N, Kim DH (2015) Investigation of the role of aspartame in apoptosis process in Hela cells. Saudi Journal of Biological Sciences. DOI: org/10.1016/j.sjbs.2015.06.01.
Muthuraman P (2014) Effect of cortisol on caspases in the co-cultured C2C12 and 3T3-L1 cells. Appl Biochem Biotechnol 173:980–988
Tassel KAV, Goldman RH (2011) The growing consumer exposure to nanotechnology in the everyday product: regulating innovative technologies in light of lessons from the past. Conn Law Rev 44:481
Wang M, Thanou M (2010) Targeting nanoparticles to cancer. Pharmacol Res 62:90–99
Li J, Guo D, Wang X, Wang H, Jiang H, Chen B (2010) The photodynamic effect of different size ZnO nanoparticles on cancer cell proliferation in vitro. Nanoscale Res Lett 5:1063–1071
Hackenberg S, Scherzed A, Kessler M, Froelich K, Ginzkey C, Koehler C, Burghartz M, Hagen R, Kleinsasser N (2010) Zinc oxide nanoparticles induce photocatalytic cell death in human head and neck squamous cell carcinoma cell lines in vitro. Int J Oncol 37:1583–1590
Wu YN, Yang LX, Shi XY, Li IC, Biazik JM, Ratinac KR, Chen DH, Thordarson P, Shieh DB, Braet F (2011) The selective growth inhibition of oral cancer by iron core-gold shell nanoparticles through mitochondria-mediated autophagy. Biomaterials 32:4565–4573
Ostrovsky S, Kazimirsky G, Gedanken A, Brodie C (2009) Selective cytotoxic effect of ZnO nanoparticles on glioma cells. Nano Res 2:882–890
Nair S, Sasidharan A, Divya Rani VV, Menon D, Manzoor K, Raina S (2009) Role of size scale of ZnO nanoparticles and microparticles on toxicity toward bacteria and osteoblast cancer cells. J Mater Sci Mater Med 20:S235–S241
Bai W, Zhang Z, Tian W, He X, Ma Y, Zhao Y, Chai Z (2009) Toxicity of zinc oxide nanoparticles to zebrafish embryo: a physicochemical study of toxicity mechanism. J Nanoparticle Res 12:1645–1654
Gojova A, Guo B, Kota RS, Rutledge JC, Kennedy IM, Barakat AI (2007) Induction of inflammation in vascular endothelial cells by metal oxide nanoparticles: effect of particle composition. Environ Health Perspect 115:403–409
Premanathan M, Karthikeyan K, Jeyasubramanian K, Manivannan G (2011) Selective toxicity of ZnO nanoparticles toward Gram-positive bacteria and cancer cells by apoptosis through lipid peroxidation. Nanomedicine 7:184–192
Hanley C, Layne J, Punnoose A, Reddy KM, Coombs I, Coombs A, Feris K, Wingett D (2008) Preferential killing of cancer cells and activated human T cells using ZnO nanoparticles. Nanotechnology 19:295103
Gopinath P, Gogoi SK, Sanpui P, Paul A, Chattopadhyay A, Ghosh SS (2010) Signaling gene cascade in silver nanoparticle-induced apoptosis. Colloids Surf B Biointerfaces 77:240–245
Compton MM (1992) A biochemical hallmark of apoptosis: internucleosomal degradation of the genome. Cancer Metastasis Rev 11:105–119
Sanchez-Perez Y, Chirino YI, Osornio-Vargas AR, Morales-Barcenas R, Gutierrez-Ruiz C, Vazquez-Lopez I, Garcia-Cuellar CM (2009) DNA damage response of A549 cells treated with particulate matter (PM10) of urban air pollutants. Cancer Lett 278:192–200
Cohen G, Riahi Y, Sasson S (2011) Lipid peroxidation of polyunsaturated fatty acids in normal and obese adipose tissues. Arch Physiol Biochem 117:131–139
Ryter SW, Kim HP, Hoetzel A, Park JW, Nakahira K, Wang X, Choi AM (2007) Mechanisms of cell death in oxidative stress. Antioxid Redox Signal 9:49–89
Ott M, Gogvadze V, Orrenius S, Zhivotovsky B (2007) Mitochondria, oxidative stress and cell death. Apoptosis 12:913–922
Wang H, Joseph JA (1999) Structure-activity relationships of quercetin in antagonizing hydrogen peroxide-induced calcium dysregulation in PC12 cells. Free Radic Biol Med 27:683–694
Dawei A, Zhisheng W, Anguo Z (2010) Protective effects of nano-ZnO on the primary culture mice intestinal epithelial cells in vitro against oxidative injury. World J Agric Sci 6:149–153
Syamaa S, Reshmaa SC, Sreekanthb PJ, Varmab HK, Mohanana PV (2013) Effect of zinc oxide nanoparticles on cellular oxidative stress and antioxidant defense mechanisms in mouse liver. Toxicol Environ Chem 95:495–503
Acknowledgments
This work was supported by KU Research Professor Program, Konkuk University, Seoul, South Korea.
This research work was supported by the KU Brain Pool (2015-2016) of Konkuk University, Seoul, Republic of Korea
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chandrasekaran, M., Pandurangan, M. In Vitro Selective Anti-Proliferative Effect of Zinc Oxide Nanoparticles Against Co-Cultured C2C12 Myoblastoma Cancer and 3T3-L1 Normal Cells. Biol Trace Elem Res 172, 148–154 (2016). https://doi.org/10.1007/s12011-015-0562-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-015-0562-6