Abstract
The present study was aimed to investigate the dose-dependent effect of zinc oxide (ZnO) nanoparticles on antioxidant enzyme activities and messenger RNA (mRNA) expression in the cocultured C2C12 and 3T3-L1 cells. Coculturing experiments are 3D and more reliable compared to mono-culture (2D) experiment. Even though, there are several studies on ZnO nanoparticle-mediated cytotoxicity, but there are no studies on the effect of ZnO nanoparticle on antioxidant enzyme activities and mRNA expression in the cocultured C2C12 and 3T3-L1 cells. A cytotoxicity assay was carried out to determine the effect of ZnO nanoparticles on the C2C12 and 3T3-L1 cell viability. At higher concentration of ZnO nanoparticles, C2C12 and 3T3-L1 cells almost die. ZnO nanoparticles increased reactive oxygen species (ROS) and lipid peroxidation and reduced glutathione (GSH) levels in a dose-dependent manner in the C2C12 and 3T3-L1 cells. In addition, ZnO nanoparticles increased antioxidant enzyme activities and their mRNA expression in the C2C12 and 3T3-L1 cells. In conclusion, the present study showed that ZnO nanoparticles increased oxidative stress, antioxidant enzyme activities, and their mRNA expression in the cocultured C2C12 and 3T3-L1 cells.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Nanostructural zinc oxide (ZnO) attracts several researchers because of its potent biomedical applications in the modern world. ZnO nanoparticles were synthesized by several methods including physical and chemical methods, and their properties are determined by the size, chemical composition, and surface chemistry [1]. ZnO is used in cosmetic lotions as UV blockers [2], increases the antibacterial activity [3], and used in the cotton fabric, rubber, and food packaging industry [4]. Cytotoxicity of nanoparticles and their interaction with biological systems is still unclear [5]. ZnO nanoparticles showed its distribution in the mice tissue such as the liver, spleen, kidneys, and adipose tissue [6]. Zinc level increased in the liver, adipose tissue, and pancreas because ZnO nanoparticles were systemically absorbed [7]. DNA damage and cell death are reported to occur due to oxidative stress induced by ZnO nanoparticles [8].
However, there are no studies on the effect of ZnO nanoparticles on the cocultured C2C12 and 3T3-L1 cells. The present study was aimed to investigate the dose-dependent effect of ZnO nanoparticles on reactive oxygen species (ROS), lipid peroxidation, reduced glutathione, antioxidant enzyme activities, and messenger RNA (mRNA) expression in the C2C12 and 3T3-L1 cells.
Materials and Methods
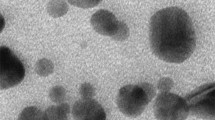
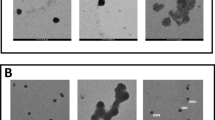
ZnO nanoparticles were synthesized and characterized by SEM and TEM analysis. All chemicals and laboratory wares were purchased from Sigma-Aldrich Chemical Co. (St. Louis, MO, USA) and Falcon Labware (Becton-Dickinson, Franklin Lakes, NJ, USA), respectively.
Cell Culture
C2C12 and 3T3-L1 cells were incubated at a density of 7000 cells/cm2 and grown in Dulbecco’s modified Eagle’s medium (DMEM) containing 10 % fetal bovine serum (FBS) and 1 % antibiotics at 37 °C in 5 % CO2. Confluent 3T3-L1 cells were induced to differentiate with a standard differentiation medium consisting of DMEM medium supplemented with 10 % FBS, 250 nM dexamethasone, 0.5 mM 3-isobutyl-1-methylxanthine, 5 μg/ml insulin, and 1 % antibiotics. Cultures were re-fed every 2 days to allow 90 % cells to reach fully differentiation before coculturing. C2C12 cells were grown to 90 % confluence and changed into differentiation medium and fed with fresh differentiation medium everyday.
Cell Coculture
C2C12 and 3T3-L1 cells were cocultured by using Transwell inserts with a 0.4-μm porous membrane to separate the cells. Each cell type was grown independently on the Transwell plates. After cell differentiation, inserts containing adipocytes were transferred to myotube plates and inserts containing myotubes were transferred to adipocyte plates [9].
Treatment of Cells
C2C12 and 3T3-L1 cells were treated with various concentrations of ZnO nanoparticles ranging from 10 to 30 μg/ml. ZnO nanoparticle suspension was sonicated for 10 min prior to start of each experiment and freshly diluted in the medium before treatment. C2C12 and 3T3-L1 cells were incubated with medium containing ZnO nanoparticles for 48 h at 37 °C in 5 % CO2 prior to harvesting.
MTT Assay
Cytotoxicity of ZnO nanoparticle was determined by MTT assay [10]. C2C12 and 3T3-L1 cells were seeded at seeding density of 2.5 × 104 cells/ml into 96-well microplates and allowed to adhere for 24 h and treated with various concentrations of ZnO nanoparticles ranging from 10 to 30 μg/ml. Cells were observed under light microscope to determine cellular morphology after 48-h incubation. Cells were labeled with 3-(4,5-Dimethylthiazol-2-yl)-2,5-Diphenyltetrazolium Bromide (MTT) solution for 4 h, and the resulting formazan was solubilized in DMSO. The absorption was measured at 570 nm by plate reader.
Determination of Cellular ROS
Cellular level of ROS was determined based on the measurement of 2,7-dichlorofluorescein diacetate [11]. After treatment, cells were washed twice with HBSS and then incubated in 1 ml of 2,7-dichlorofluorescein diacetate at 37 °C for 30 min. C2C12 and 3T3-L1 cells were lysed in alkaline solution and centrifuged for 10 min. Supernatant was transferred to a 96-well plate, and fluorescence was measured at 485-nm excitations and 520-nm emissions with a microplate reader. Values were expressed as a percentage of intensity.
Determination of MDA and GSH
Malondialdehyde (MDA) was determined by the method of Umrani and Paknikar [7] in the C2C12 and 3T3-L1 cells. Reduced glutathione (GSH) was determined by the method of Owen and Allan Butterfield [11].
Antioxidant Enzymes
Glutathione peroxidase, glutathione reductase, superoxide dismutase (SOD), and catalase were assayed as by Weydert and Cullen [12]. Lactate dehydrogenase (LDH) enzyme activity was measured by the method of Dawei et al. [13].
qPCR
Total RNA was isolated from the control and treated C2C12 and 3T3-L1 cells with Trizol reagent according to the manufacturer’s protocol. The first-strand complementary DNA (cDNA) was synthesized from 1 μg of the total RNA using the M-MLV reverse transcriptase with the anchored oligo d(T)12–18 primer. qPCR was performed using a cDNA equivalent of 10 ng of total RNA from each sample with primers specific for glutathione reductase (GenBank NM_053906.1), glutathione peroxidase (GenBank NM_030826.3), SOD (GenBank NM_017051.2), catalase (GenBank NM_012520.1), and a housekeeping gene GAPDH. The reaction was carried out in 10 μl using SYBR Green Master Mix (Invitrogen) according to the manufacturer’s instructions. Relative ratios were calculated based on the 2−△△CT method [14]. PCR was monitored using the MiniOpticon Real-Time PCR System (Bio-Rad).
Statistical Analysis
All values are expressed as means ± SEM. Student’s t test was performed to determine the differences between control and treatments. p < 0.05 was considered to be significant.
Results
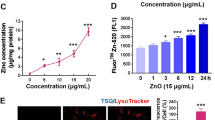
C2C12 and 3T3-L1 cells exposed up to 20 μg/ml ZnO nanoparticles for 48 h showed no significant changes in cellular architectures. However, when cells were exposed to greater than 30 μg/ml of ZnO nanoparticles for 48 h, cells rounded up and lost their morphological features. C2C12 and 3T3-L1 cells incubated with different concentrations (10, 20, and 30 μg/ml) of ZnO nanoparticles for 48 h showed a dose-dependent effect that was evident from the MTT assay. C2C12 and 3T3-L1 cells exhibited 90 % viability up to 20 μg/ml of ZnO nanoparticle exposed for 48 h (Fig. 1).
Effect of various concentrations of ZnO nanoparticles on cell viability. C2C12 and 3T3-L1 cells were seeded at seeding densities of 2.5 × 104 cells/ml into 96-well micro plates and allowed to adhere for 24 h and treated with 10–30 μg/ml of ZnO for 48 h. Cells were labeled with MTT solution for 4 h. Values are expressed means ± SEM, n = 6. Viability expressed as percentage of cell survival relative to untreated control (cell viability = 100 %)
C2C12 and 3T3-L1 cells exposed to different concentrations of ZnO nanoparticles for 48 h showed significant changes in ROS, MDA, and GSH contents depending on the ZnO nanoparticle concentrations. ROS content was increased 26, 40, and 50 % in C2C12 cells, and 18.7, 28.1, and 35.4 % in 3T3-L1 cells at 10, 20, and 30 μg/ml of ZnO nanoparticles, respectively (Fig. 2). MDA content was increased 13.3, 26.6, and 33.3 % in C2C12 cells, and 23, 38.4, and 46 % in 3T3-L1 cells at 10, 20, and 30 μg/ml of ZnO nanoparticles, respectively (Fig. 3). GSH content was increased 25, 45, and 60 % in C2C12 cells, and 31.8, 41, and 54.5 % in 3T3-L1 cells at 10, 20, and 30 μg/ml of ZnO nanoparticles, respectively (Fig. 4).
C2C12 and 3T3-L1 cells were exposed to different concentrations of ZnO nanoparticles for 48 h showed significant increase in glutathione reductase, glutathione peroxidase, SOD, catalase, and LDH activity in a dose-dependent manner. Glutathione reductase enzyme activity was increased 17.3, 30.4, and 43.4 % in the C2C12 cells, and 13.3, 33.3, and 54 % in the 3T3-L1 cells at 10, 20, and 30 μg/ml of ZnO nanoparticles, respectively (Fig. 5). Glutathione peroxidase enzyme activity was increased 27, 53, and 73 % in the C2C12 cells, and 30, 50, and 80 % in the 3T3-L1 cells at 10, 20, and 30 μg/ml of ZnO nanoparticles, respectively (Fig. 6). SOD enzyme activity was increased 18.5, 42.5, and 57 % in the C2C12 cells, and 12, 14, and 30 % in the 3T3-L1 cells at 10, 20, and 30 μg/ml of ZnO nanoparticles, respectively (Fig. 7). Catalase enzyme activity was increased 15.3, 38.4, and 53.8 % in the C2C12 cells, and 20, 50, and 70 % in the 3T3-L1 cells at 10, 20, and 30 μg/ml of ZnO nanoparticles, respectively (Fig. 8). LDH enzyme activity was increased 10.7, 27, and 32 % in the C2C12 cells, and 11.3, 20, and 26.6 % in the 3T3-L1 cells at 10, 20, and 30 μg/ml of ZnO nanoparticles, respectively (Fig. 9).
C2C12 and 3T3-L1 cells exposed to 10, 20, and 30 μg/ml of ZnO nanoparticle for 48 h showed significant changes in the mRNA expression of antioxidant enzymes in a dose-dependent manner. Glutathione reductase mRNA expression was increased 0.4-, 0.9-, and 1.3-fold in the C2C12 cells, and 0.5-, 1.5-, and 1.75-fold in 3T3-L1 cells at 10, 20, and 30 μg/ml of ZnO nanoparticles, respectively. Glutathione peroxidase mRNA expression was increased 0.44-, 0.66-, and 1-fold in the C2C12 cells, and 0.43-, 1-, and 1.14-fold in the 3T3-L1 cells at 10, 20, and 30 μg/ml of ZnO nanoparticles, respectively. SOD mRNA expression was increased 0.25-, 0.45-, and 0.5-fold in the C2C12 cells, and 0.35-, 0.47-, and 0.58-fold in the 3T3-L1 cells at 10, 20, and 30 μg/ml of ZnO nanoparticles, respectively. Catalase mRNA expression was increased 0.37-, 0.75-, and 1.5-fold in the C2C12 cells, and 0.6-, 0.7-, and 1-fold in the 3T3-L1 cells at 10, 20, and 30 μg/ml of ZnO nanoparticles, respectively (Figs. 10 and 11).
Upregulation of glutathione reductase, glutathione peroxidase, catalase, SOD, and LDH mRNA expression by ZnO nanoparticles in C2C12 cells. Expressions of glutathione reductase, glutathione peroxidase, catalase, SOD, and LDH mRNA are related to GAPDH and presented as fold. Relative expression values are the normalized mean ± SEM, n = 6. *P < 0.05
Upregulation of glutathione reductase, glutathione peroxidase, catalase, SOD, and LDH mRNA expression by ZnO nanoparticles in 3T3-L1 cells. Expressions of glutathione reductase, glutathione peroxidase, catalase, SOD, and LDH mRNA are related to GAPDH and presented as fold. Relative expression values are the normalized mean ± SEM, n = 6. *P < 0.05
Discussion
ZnO nanoparticles are widely used in medicine, cosmetics, sunscreens, and food products [15]. Sudden growth of the nanotechnology has led to huge production and application of nanoparticles. Reduced zinc concentration is associated with several metabolic and antioxidant enzymes such as glutathione peroxidase and glutathione reductase [8]. ZnO nanoparticles were absorbed, which leads to increased zinc levels in the liver, adipose tissue, and pancreas [7]. ZnO nanoparticle induces oxidative stress, which leads to DNA damage and cell death [8].
Exposure of copper oxide (CuO) nanoparticles to airway epithelial cells shows a dose-dependent cytotoxicity and oxidative stress. TiO2 nanoparticles have been reported to induce oxidative stress and apoptosis [16]. Yakimovich et al. [17] reported that the antioxidant activity of the gold (Au) nanoparticles with respect to hydroxy radicals significantly depends on the specific surface of the particles. Cerium oxide (CeO) nanoparticles exhibit potent antioxidant activity in brain cells, which depends on the size, composition, and particle surface area. Shen et al. [18] demonstrated that there is a strong correlation between ZnO nanoparticle-induced cytotoxicity and free intracellular zinc concentration in human immune cells, indicating a requirement for nanoparticle dissolution to precede cytotoxicity. ZnO nanoparticle decreases the mitochondrial membrane potential and increase the production of ROS [19]. ZnO nanoparticles selectively induce apoptosis in human cancer cells through ROS production [20].
Peroxidation of fatty acids leads to the generation of the ROS [21], and MDA is the by-products of cell membrane lipid peroxidation. ROS derived from mitochondria and endoplasmic reticulum of cells [22] and cell would be injured when exposed to a higher concentration ROS [13], even though ZnO nanoparticles are not toxic at low concentration. However, ROS content increases at higher concentration of ZnO nanoparticles [23]. In our study, ROS and MDA contents were significantly altered even at low concentration of ZnO nanoparticles in the C2C12 and 3T3-L1 cells.
Airway epithelial cells exposed to CuO nanoparticles showed significant increase in the ratio of GSSG to total GSH [24]. Redox metabolism corresponds to a complex interacting network involving the generation of reactive oxygen species and enzymatic/non-enzymatic cellular antioxidant defenses. Changes in this balance induces redox signaling, and the result is an oxidative stress in which free radicals may exert their deleterious effects [25]. In our study, ZnO nanoparticle increases GSH content in the cocultured C2C12 and 3T3-L1 cells and this agreed with the result of Shyama et al. [23]. Metabolic diseases are associated due to altered expression of NADPH-oxidase and antioxidant enzymes in the adipose tissue [26]. Glutathione reductase and glutathione peroxidase are indirectly associated with oxidative stress [27]. Seung Ho Lee et al. [28] reported that the ZnO nanoparticles increased glutathione peroxidase enzyme activity in keratinocytes. Shyama et al. [23] reported that the effect of ZnO nanoparticles on glutathione reductase and glutathione peroxidase in the liver tissue. Our results also showed dose-dependent increase of glutathione reductase and glutathione peroxidase activities and their mRNA expression in the cocultured C2C12 and 3T3-L1 cells.
Catalase is an antioxidant enzyme abundant in various tissues, and it protect cells against potentially harmful effects of the hydrogen [29]. Okuno et al. [30] reported that adipose tissue expresses catalase on the level comparable to liver and kidney. Our results showed that the ZnO nanoparticle increases catalase activity and mRNA expression in the cocultured C2C12 and 3T3-L1 cells.
SOD plays a role in modulating ROS and highly sensitive to Zn [31]. Faraci [32] reported that the SOD is a major antioxidant enzyme that protects cells from the harmful effects of superoxide by accelerating the dismutating superoxide. ZnO nanoparticles altered SOD enzyme activity in keratinocytes and liver tissue [23]. Our results showed that the ZnO nanoparticles increase SOD enzyme activity in the cocultured C2C12 and 3T3-L1 cells. LDH is present in five isozymic forms in adipose tissue of rat and their relative distribution of the LDH was significantly altered by metabolic stress [33]. ZnO nanoparticles increased LDH activity in H1355 cells [34]. Our results also showed that the ZnO nanoparticle increases LDH enzyme activity in the cocultured C2C12 and 3T3-L1 cells.
Conclusion
The cells remain viable at lower concentration of ZnO nanoparticles. However, C2C12 and 3T3-L1 cells lost their shape and features at higher concentration of ZnO nanoparticles. ROS, MDA, and GSH contents were significantly altered in a dose-dependent manner. ZnO nanoparticles significantly increased mRNA expressions of glutathione reductase, glutathione peroxidase, catalase, SOD, and LDH in the C2C12 and 3T3-L1 cells. In addition, antioxidant enzymes levels were also significantly altered in the cocultured C2C12 and 3T3-L1 cells. Therefore, the present study concludes that the ZnO nanoparticles significantly altered antioxidant defense system in the cocultured C2C12 and 3T3-L1 cells in a dose-dependent manner.
References
Zhao, J., & Castranova, V. (2011). Journal of Toxicology and Environmental Health B, 14, 593–632.
Becheri, A., Durr, M., Nostro, P. L., & Baglioni, P. (2008). Journal of Nanoparticle Research, 10, 679–689.
Nagarajan, P., & Rajagopalan, V. (2008). Science and Technology of Advanced Materials, 9, 035004.
Qun, L., Shui-Lin, C., & Wan-Chao, J. (2007). Journal of Applied Polymer Science, 103, 412–416.
Snyder-Talkington, B. N., Qian, Y., Castranova, V., & Guo, N. L. (2012). Journal of Toxicology and Environmental Health B, 15, 468–492.
Li, C. H., Shen, C. C., Cheng, Y. W., Huang, S. H., Wu, C. C., Kao, C. C., Liao, Y. W., & Kang, J. J. (2012). Nanotoxicology, 6, 746–756.
Umrani, D.R., & Paknikar, K.M. (2013). Nanomedicine, 1–16.
Kumar, A., Pandey, A. K., Singh, S. S., Shanker, R., & Dhawan, A. (2011). Free Radical Biology & Medicine, 51, 1872–1881.
Sun, X., & Zemel, M. B. (2008). Journal of Nutritional Biochemistry, 19, 392–399.
Muthuraman, P., Ramkumar, K., & Kim, D. H. (2014). Applied Biochemistry and Biotechnology. doi:10.1007/s12010-014-1231-5.
Owen, J. B., & Butterfield, D. A. (2010). Methods in Molecular Biology, 648, 269–277.
Weydert, C. J., & Cullen, J. J. (2010). Nature Protocols, 5, 51–66.
Dawei, A. I., Zhisheng, W., & Anguo, Z. (2010). World Journal of Agricultural Sciences, 6, 149–153.
Pfaffl, M. W. (2001). Nucleic Acids Research, 29, e45.
Van Tassel, K. A., & Goldman, R. (2011). Connecticut Law Review, 44, 481.
Shukla, R. K., Kumar, A., Gurbani, D., Pandey, A. K., Singh, S., & Dhawan, A. (2013). Nanotoxicology, 7, 48–60.
Yakimovich, N. O., Ezhevskii, A. A., Guseinov, D. V., Smirnova, L. A., Gracheva, T. A., & Klychkov, K. S. (2008). Russian Chemical Bulletin, 57, 520–523.
Shen, C., James, S. A., de Jonge, M. D., Turney, T. W., Wright, P. F., & Feltis, B. N. (2013). Journal of Toxicological Sciences, 136, 120–130.
Guo, D., Bi, H., Liu, B., Wu, Q., Wang, D., & Cui, Y. (2013). Toxicology In Vitro, 27, 731–738.
Akhtar, M. J., Ahamed, M., Kumar, S., Khan, M. M., Ahmad, J., & Alrokayan, S. A. (2012). International Journal of Nanomedicine, 7, 845–857.
Cohen, G., Riahi, Y., & Sasson, S. (2011). Archives of Physiology and Biochemistry, 117, 131–139.
Wang, H., & Joseph, J. A. (1999). Free Radical Biology and Medicine, 27, 683–694.
Syama, S., Reshma, S. C., Sreekanth, P. J., Varma, H. K., & Mohanan, P. V. (2013). Toxicological & Environmental Chemistry, 95, 495–503.
Fahmy, B., & Cormier, S. A. (2009). Toxicology In Vitro, 23, 1365–1371.
Dröge, W. (2002). Physiological Reviews, 82, 47–95.
Peña-Orihuelaa, P., Camargoa, A., Rangel-Zuñigaa, O. A., Perez-Martineza, P., Cruz-Tenoa, C., Delgado-Listaa, J., Yubero-Serranoa, E. M., Paniaguaa, J. A., Tinahones, J., Malagon, M. M., Roche, H. M., Perez-Jimenez, F., & Lopez-Miranda, J. (2013). Journal of Nutritional Biochemistry, 24, 1717–1723.
Sies, H. (1986). Angewandte Chemie International Edition, 25, 1058–1071.
Seung Ho, L., Jae-Eun, P., Yu-Ri, K., Hee Ra, L., Sang Wook, S., & Meyoung-Kon, S. (2012). Molecular & Cellular Toxicology, 8, 113–118.
Deisseroth, A., & Dounce, A. L. (1970). Physiological Reviews, 50, 319–375.
Okuno, Y., Matsuda, M., Kobayashi, H., Morita, K., Suzuki, E., Fukuhara, A., Komuro, R., Shimabukuro, M., & Shimomura, I. (2008). Biochemical and Biophysical Research Communications, 366, 698–704.
Roberts, C. K., Won, D., Pruthi, S., Kurtovic, S., Sindhu, R. K., Vaziri, N. D., & Barnard, R. J. (2006). Journal of Applied Physiology, 100, 1657–1665.
Faraci, F. M., & Didion, S. P. (2004). Arteriosclerosis, Thrombosis, and Vascular Biology, 24, 1367–1373.
Moore, R. O., & Yontz, F. D. (1969). Journal of Nutrition, 98, 325–329.
Kao, Y.Y., Chen, Y.C., Cheng, T.J., Chiung, Y.M., & Liu, P.S. (2012). Toxicological Sciences, 125.
Acknowledgments
This paper was supported by the KU Research Professor Program of Konkuk University, Seoul, South Korea.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pandurangan, M., Veerappan, M. & Kim, D.H. Cytotoxicity of Zinc Oxide Nanoparticles on Antioxidant Enzyme Activities and mRNA Expression in the Cocultured C2C12 and 3T3-L1 Cells. Appl Biochem Biotechnol 175, 1270–1280 (2015). https://doi.org/10.1007/s12010-014-1351-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-014-1351-y