Abstract
In recent years, there is a growing interest in the application of nanoparticles like zirconium dioxide (zirconia <100 nm), for many purposes. Since a comprehensive study on the toxic effects of zirconia has not been done, we decided to investigate the effects of zirconia nanoparticles on cultured PC12 and N2a cells. In this study, cytotoxic effect of different concentrations of zirconia nanoparticles at three different time intervals were evaluated using MTT and ROS (reactive oxygen species) assays. Also, Lipid peroxidation, glutathione (GSH) content changes, and DNA damage were measured. Zirconia nanoparticles caused a significant reduction in cell viability and GSH content of the cells, and induce a significant increase in intracellular ROS and MDA content of PC12 and N2a cells. Moreover, it increases the percentage of DNA tail of treated cells as compared with control group. Zirconia nanoparticles have cytotoxic and genotoxic effects in PC12 and N2a cells in a time and concentration-dependent manner in concentration more than 31 µg/mL.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Recently, tendency in using different nanoparticles in many aspects of life has been increased (Ma et al. 2011). According to different physicochemical properties of nanoparticles in comparison with traditional millimeter- or micron-sized materials (mm to µm), together with the surface properties, electronic structure, coordination and other properties, nanoscale particles show their own specific and diverse features (Tahmasebpour 2008).
The name zirconium (Zr) is derived from the Persian word, zargon (golden in color), which combines two Persian words, zar (gold) and gun (color) (Piconi and Maccauro 1999).
Zirconium(IV) oxide (zirconia) compounds are utilized worldwide in biological fields such as dental implants and other restorative practices (Aramouni et al. 2008; Chang et al. 2007; Uludag et al. 2008; Vigolo and Fonzi 2008), in knee (Tsukamoto et al. 2006) and hip (Garvin et al. 2009; Lappalainen and Santavirta 2005) replacement, and in middle-ear ossicular chain reconstruction surgery (Schadel et al. 1993). Moreover, it is utilized in settings such as nephrological practice, hemofiltration (Lee et al. 2010), hemodialysis (Hansen 2006), and in the design and construction of artificial kidneys (Davenport et al. 2007; Lee and Roberts 2008).
Zirconia nanoparticles are used in the production of nanobiosensor for DNA (Yang et al. 2007), phosphoprotein measurement in cell signaling studies (Rainer et al. 2008), in producing the bio-chip and information storage device (Liu et al. 2004) and also in poisons such as parathion (Parham and Rahbar 2010), organophosphate pesticides and nerve agents (Liu and Lin 2005). Recently, it is shown that zirconia nanoparticles are utilized as efficient gene delivery vehicles with target specificity for the spleen (Tan et al. 2007) and for transfection of mammalian cells (Link et al. 2007) as well as for drug delivery targets (Singh and Lillard 2009). For example, it is employed for development of ampicillin in local controlled drug delivery system (Catauro et al. 2008), itraconazole nanosuspension for oral delivery (Nakarani et al. 2010) and in the targeting delivery of bisphosphonates-based drugs such as alendronate and zoledronate (Colilla et al. 2009), which the optimized formulation has indicated better results than the marketed one (Colilla et al. 2009; Nakarani et al. 2010).
Recent studies in nanocomponent field have revealed that zirconia nanocomposites could tolerate the severe vapor-moist environment better than zirconium component (Pezzotti et al. 2010). Then again, nanofunctionalized zirconia and barium sulfate particles as bone cement additives could improve the properties of traditional bone cements for orthopedic applications (Gillani et al. 2010). However, the introduction of these materials into clinical practice as bone cement additives has been complicated by concerns over the unknown long-term risk profile of these new structures in vivo (Gomoll et al. 2008). The large majority of people do not experience short-term side effects of metal denature. Nevertheless, prolonged exposure to dissolved metal ions released from implants can lead to poor performance of implants and may cause pathological effects (Chevalier 2006; Hallab et al. 2004; Hallab et al. 2000).
Although previously it was thought that zirconia is a neutral bioceramic metal, more recent studies have demonstrated that it is likely to cause some toxic effects. For instance, analysis of zirconium ion showed significant DNA damage induction at concentration of 5 mM and apoptosis in human (Jurkat) T-cells at concentration exceeding 0.5 mM (Caicedo et al. 2008). Similarly zirconia particles around 0.6 µm in size could induce TNF-α release and macrophage apoptosis in J774 mouse cell line (Catelas et al. 1998, 1999). As well, osteoblast-like (MG63) cells cultured on zirconia and colloidal zirconium coated disks could alter the expression of several genes which significantly up or down regulate a broad range of functional activities: such as (a) cell cycle regulation, (b) signal transduction, (c) immunity, and (d) vesicular transport (Carinci et al. 2004; Sollazzo et al. 2008). Exposure of human marrow stroma-derived mesenchymal stem cells (hMSCs) to submicron particles of zirconia, compromises cell viability through the induction of apoptosis, causing increased levels of the tumor suppressor proteins p53 and p73 (Wang et al. 2003). Additionally, exposure of zirconia (0–30 ppm) to human mesothelioma (MSTO-211H) and a rodent fibroblast (3T3) cell line for duration of 3 and 6 days inhibited the cell proliferation (Brunner et al. 2006). Zirconia was moderately apoptotic, inducing >50 % caspase-9 positive cells at 0.5 mM concentration or higher, However, zirconia did not induce any significant DNA damage below 1 mM, significant (p < 0.05) DNA damage could be observed at 5 mM, reaching 192 and 250 IDD (index of DNA damage), respectively (Caicedo et al. 2008).
Since a comprehensive study based on the probable toxic effects of zirconia nanoparticles has not been carried out and regarding the increasing production and usage of this nanoparticle, and as PC12 and N2a cell lines are widely used in neurotoxicological study and oxidative stress induced by nanoparticles (Perreault et al. 2012; Wang et al. 2011), we decided to investigate the oxidative stress induced by zirconia nanoparticles on PC12 and N2a cells.
Materials and methods
Cell lines and reagents
Commercial zirconium (IV) oxide nanopowders (Zirconia) <100 nm particle size (TEM), were purchased from Sigma-Aldrich (544760-25G, St Louis, MO). PC12 and N2a cell lines, derived from rat pheochromocytoma and mouse neuroblastoma, respectively, were purchased from Pasteur Institute (Tehran, Iran). High glucose (4.5 g/L) Dulbecco’s Modified Eagles Medium (DMEM) and fetal calf serum (FCS) were purchased from Gibco (Carlsbad, CA). 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium (MTT), 2′,7′-dichlorodihydrofluorescein diacetate (H2DCF-DA), monochlorobimane (mBCl), and other cell culture materials were purchased from Sigma (St Louis, MO). Low melting point (LMP) and normal melting point (NMP) agarose were obtained from Fermentas (Glen Burnie, MD). Other chemicals mainly ethylene diaminetetraacetic acid disodium salt (Na2EDTA), Tris (hydroxymethyl) aminomethane (Trizma base), t-octylphenoxy poly-ethoxyethanol (Triton X-100), dimethyl sulfoxide (DMSO), sodium lauroylsarcosinate (sarkosyl, SLS), and ethidium bromide were purchased from Merck (Darmstadt, Germany). 2-Thiobarbituric acid (TBA) was purchased from Sigma-Aldrich (St Louis, USA). Trichloroacetic acid (TCA) and malondialdehyde bis-(dimethyl acetal) (MDA) were obtained from Merck (Darmstadt, Germany).
Dispersion and characterization of the zirconia nanoparticles
The zirconia nanoparticles were suspended in media and dispersed by an ultrasonic bath for 60 min, and then the suspension was characterized by the TEM using one drop of sample on a carbon coated grid and high voltage (120 kV) imaging. The size of particles was tested using Transmission Electron Microscopy (TEM, LEO 912AB, Germany).
Cell culture and nanoparticle treatment
PC12 and N2a cell lines were maintained in DMEM containing 10 % FCS, penicillin 100 IU/mL, and streptomycin 100 mcg/mL. Cells were grown and maintained in 75 cm2 cell culture flasks at 37 °C in a 5 % CO2 humidified incubator. The suspension of zirconia nanoparticles was prepared using the culture media and dispersed for 20 min using a sonicator (Bandelin electronic, DT 510, Germany) to prevent aggregation. The cells were treated with various concentrations of nanoparticles for 12, 24, and 48 h.
Test concentration
Different concentration of nanoparticle suspension was used for various tests. For MTT and ROS assays, cells were treated with 2000, 1000, 500, 250, 125, 62, 31, 15, and 7.8 µg/mL final concentrations of zirconia nanoparticles suspension and For remaining assays cells exposed to 2000, 1000, 500, 250, 62, 31 µg/mL of nanoparticle suspension all for 12, 24, and 48 h.
Cell viability test
MTT was used to identify viable cells which could reduce this compound to a violet formazan (Mosmann 1983). Cells were seeded on 96-well tissue culture plates with 5 × 103 cells in 100 µL media per well. After allowing the cells to be seeded for 24 h, nanoparticle suspension (100 µL) were added to each well. At the end of exposure, MTT solution in phosphate-buffered saline (PBS, 5 mg/mL) was added to a final concentration of 0.05 %. After 2 h, the formazan precipitate was dissolved in DMSO containing 10 % glycine buffer (pH 10.5). The microplates were then gently shaken in the dark for 30 min, and the absorbance at 570 and 620 nm (background) was measured using a StatFAX303 plate reader.
All experiments were carried out as triplicate; the number of viable cells was determined as mean ± SEM and is presented as a percentage of non-treated control groups, which was assumed to be 100 %.
Measurement of reactive oxygen species
A fluorometric assay using intracellular oxidation of DCFH-DA was performed to measure ROS generation (Zhang et al. 2010). H2DCF-DA readily diffuses through the cell membrane and is enzymatically hydrolyzed by intracellular esterase to non-fluorescent H2DCF, which is then rapidly oxidized to highly fluorescent DCF (2′,7′-dichlorofluorescin) in the presence of ROS. The DCF fluorescence intensity is believed to be parallel to the amount of intracellular ROS. Cells were seeded on 96-well tissue culture plates with 5 × 103 cells in 100 µL media per well and cultured so that about 70 % confluency was reached after 24 h. then they were pre-treated with different concentrations of nanoparticles, the cells were incubated with 10 µm H2DCF-DA at 37 °C for 30 min in the dark space. Cells were washed twice with warm PBS and collected. The DCF fluorescence intensity was quantified using a FLUO-star galaxy fluorescence plate reader (Perkin Elmer 2030, Multi label reader, Finland) with excitation wavelength set at 485 nm and emission wavelength set at 530 nm. The results are given as percent’s relative to the ROS content of the control cells which set as 100 %. All experiments were performed in triplicate.
MDA assay
The thiobarbituric acid reactive substances (TBARS) assay measures lipid hydroperoxides and aldehydes, such as malondialdehyde (MDA), both, in the cell culture media and cell lysate. MDA combines with thiobarbituric acid (TBA) in 1:2 ratio to form a fluorescent adduct that is measured at ex. 530 nm and em. 550 nm. TBARS are expressed as MDA equivalents.
Cells were processed as above in 6 well plates (106 cells per well) and after seeding were pre-treated with different concentrations of nanoparticles.
Cell media sample preparation
After collecting the Cell media from each well, 500 µL of sample media were added to 400 µL TCA 15 % plus 800 µL TBA 0.67 %/BHT 0.01 % in 5 mL amber vials. Then samples were vortexed and heated for 20 min in a 95 °C water bath. Thereupon 3 mL butanole were added to the samples’ vials and the attained phases gently mixed. Finally, 200 µL of the butanole phase (top) were transferred to 96 well plates.
Cell lysate sample preparation
The 96-well plates were washed with cold PBS. Cells were scraped into 1 mL TCA2.5 %. Then cell suspensions were centrifuged at 13000 g for 2 min and samples were processed as above.
Cell media and cell lysate plates were read in fluorescence mode, ex = 530 nm, em = 550 nm. The MDA amounts calculated according to the standard curve which was obtained with various concentration of MDA from 400 to 0.007 nmol/mL.
GSH determination
Cells were seeded in 96-well tissue culture plates containing 5 × 103 cells in 200 µL media per well. After treating the cells with different concentrations of nanoparticles, fluorescent probe (mBCl, monochlorobimane) was dissolved in dimethyl sulfoxide. Working solutions in PBS with concentration equivalent to 20 times the required final concentration were prepared immediately before use and added directly to the cultures in 96-well plates. Fluorescence was recorded before (blank) and after adding the probe in a FLUO-star galaxy fluorescence plate reader at the following excitation/emission wavelengths ± band pass: 360 ± 40/460 ± 40 nm. Data were obtained by subtracting blank values and were presented as a percentage of control cells fluorescence (Sebastia et al. 2003).
Single cell gel electrophoresis (SCGE, Comet) assay
The alkaline SCGE assay was conducted based on the method described previously (Asadpour et al. 2014a; Ghorbani et al. 2015). Briefly, PC12 and N2a cells (3 × 105) were incubated with five different concentrations (2000, 1000, 500, 250, 62, 31 µg/mL) of zirconia nanoparticles. After removing the medium, the cells were washed three times with cold PBS, harvested and centrifuged at 3000 rpm for 5 min at 4 °C. The pellets were then re-suspended in PBS at a cell density of 1 × 105. For the comet assay, 100 μL NMP agarose was quickly layered on conventional slides, covered with a cover slip, and then the slides were placed on ice to allow agarose to get form of gel. 10 μL of the nucleus suspension, prepared as above, was mixed with 100 μL LMP agarose, and the mixture was quickly layered over the NMP agarose layer after removal of the cover slip. Finally, another layer of LMP agarose was added on top. The slides were immersed immediately in a chilled lysing solution (pH 10) made up of 2.5 M NaCl, 100 mM Na2EDTA, 10 mM Trizma, 1 % sarkosyl, 10 % DMSO, and 1 % Triton X-100, and kept at 0 °C in the dark overnight. Then, the slides were placed on a horizontal gel electrophoresis platform and covered with a prechilled alkaline solution made up of 300 mM NaOH and 1 mM Na2EDTA (pH > 13). They were left in the solution in the dark at 0 °C for 40 min, and then electrophoresed at 0 °C in the dark for 30 min at 25 V and approximately 300 mA. The slides were rinsed gently three times with 400 mM Trizma solution (adjusted to pH 7.5 by HCl) to neutralize the excess alkali, stained with 50 μL of 20 µg/mL ethidium bromide, and covered with a cover slip. For comet analysis, 150 nuclei were randomly selected from three replicated slides (50 nuclei per slide), examined and photographed through a fluorescence microscope (Nikon, Kyoto, Japan), at ×400 magnification equipped with an excitation filter of 520–550 nm and a barrier filter of 580 nm. Undamaged cells resemble an intact nucleus without a tail, and damaged cells have the appearance of a comet. The percent of DNA in the comet tail (% tailed DNA), which is an estimation of DNA damage, was analyzed using the computerized image analysis software (CASP software). The experiments were carried out in triplicate.
Statistical analysis
The results are presented as the mean ± SEM. The values were compared using the one- and two-way analysis of variance (ANOVA) followed by Bonferroni post tests for multiple comparisons. The p < 0.05 were considered to be statistically significant.
Results
Characterization of zirconia nanoparticles
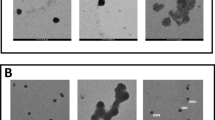
The size of the zirconia nanoparticles was varied <100 nm as shown in the images of Transmission Electron Microscopy (TEM) with a size distribution between 8 and 50 nm, multivariate shapes and molecular weight of 123.22 g/mol (Fig. 1).
Cytotoxicity of the zirconia nanoparticles
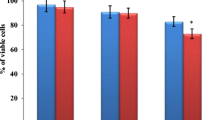
Assessment of zirconia nanoparticles cytotoxicity through the MTT assay exhibited the dose-dependent toxic effects on cell viability in a concentration range of 31–2000 µg/mL (with IC50 value for the PC12 cell line of about 66.68, 53.32, and 40.82 µg/mL and IC50 value for the N2a cell line of about 45.49, 33.06, and 26.27 µg/mL following 12, 24, and 48 h exposure, respectively). Comparison of PC12 cells viability between 12 h and the two other time exposures showed a significant difference from 62 to 2000 µg/mL (p < 0.05 for 62 µg/mL between 12 and 24 h and p < 0.001 for others), but this significant differences were not seen between 24 and 48 h except for the 15 and 31 µg/mL (p < 0.001). These results were different in N2a cells since the difference between 12 and 24 h was started from 250 µg/mL (p < 0.05 for 250 and 500 µg/mL and p < 0.001 for upper concentrations), between 12 and 48 h from 31 µg/mL (p < 0.001 for 31–125 µg/mL and p < 0.05 for upper concentrations) and between 24 and 48 h from 31 µg/mL (p < 0.001) (Fig. 2).
Effects of zirconia nanoparticles on a PC12, b N2a cell viability after 12, 24, and 48 h treatment. The cell viability (quantified by MTT assay) is shown and discussed as percentage of control group (zirconia nanoparticles 0 µg/mL). Mean and SEM of three independent experiments are shown. *p < 0.05, **p < 0.01 and ***p < 0.001 as compared to control
Measurement of reactive oxygen species
ROS generation was elucidated in PC12 and N2a cell lines at all three exposure times from concentration of 15 µg/mL and continues up to concentration of 2000 µg/mL (p < 0.01 for 15–31 µg/mL of N2a after 48 h exposure and p < 0.001 for all other concentrations). Comparison between different exposure times did not show a time-dependent manner for ROS generation since in PC12 cell line it was obvious only at concentration of 7, 15, and 2000 µg/mL between 12 h and the two other exposure times (p < 0.0001) and in N2a cell line between 12 and 24 h was significant for 62–1000 µg/mL (p < 0.01), between 12 and 48 h only for 7 and 2000 µg/mL (p < 0.01) and between 24 and 48 h only for 500 µg/mL (p < 0.01). This increase was not significantly different between various duration of exposure but only it showed a concentration-dependent manner (Fig. 3).
Effects of zirconia nanoparticles on intracellular reactive oxygen species (ROS) content in a PC12, b N2a cell line after 12, 24 and 48 h treatment. The cell ROS content is shown and discussed as percentage of control group (zirconia nanoparticles 0 µg/mL). Mean and SEM of three independent experiments are shown. *p < 0.05, **p < 0.01, and ***p < 0.001 as compared to control
Effect of zirconia nanoparticles on MDA generation in PC12 and N2a cells
Zirconia nanoparticles lipid peroxidation effect was increased in both cell lines gradually from lower concentration toward upper ones. But the amount of MDA in the cell lysate is almost 10 times lower than the cell culture media. This effect is also time dependent especially between 12 and 48 h exposure times. The significant difference between 12 and 48 h, at PC12 cell line sample of culture media, started from 31 µg/mL (p < 0.01) but in cell lysate sample the difference is seen between 12 and 24 h from 62 µg/mL (p < 0.001) and between 12 and 48 h from 31 µg/mL (p < 0.001). At N2a cell line sample of culture media, the lipid peroxidation effect made a significant difference between 12 and 48 h of culture media from 31 µg/mL (p < 0.01) and in cell lysate between 12 h and the two other exposure times from 62 µg/mL (p < 0.01) (Fig. 4).
Effects of zirconia nanoparticles on malondialdehyde (MDA), in a PC12 cell culture media content, b N2a cell culture media content, c PC12 cell lysate content and d N2a cell lysate content. Cells were treated with different concentrations of zirconia nanoparticles for 12, 24 and 48 h. The MDA content was measured against the standard curve provided with different concentration of MDA (zirconia nanoparticles 0 µg/mL). *p < 0.05, **p < 0.01, and ***p < 0.001, as compared with control group of 12, 24, and 48 h, respectively
GSH changes induced by zirconia nanoparticles
As a reference for the fluorescence studies, GSH was analyzed by Tietze’s method. These data indicated that PC12 and N2a cells are practically devoid of GSH after different times of exposure to zirconia nanoparticles. Both cell lines showed almost same level of GSH depletion during three different exposure times from almost 85 % at 12 h exposure in concentration of 31 µg/mL to nearly 35 % at 48 h exposure in 2000 µg/mL. The GSH depletion seen here was concentration dependent without any remarkable time-dependent manner (Fig. 5).
Fluorescence labeling curves of a PC12, b N2a cell cultures with mBCl at the three indicated exposure duration with different concentrations of zirconia nanoparticles. Fluorescence values are shown as a percentage of control cells fluorescence. N = 9, from three independent cultures. First column indicates control cells, and after that, columns for each exposure time indicate cells exposed to the GSH-depleting media. (mBCl, monochlorobimane), *p < 0.05, **p < 0.01, and ***p < 0.001, as compared with control group of 12, 24, and 48 h respectively
Effect of zirconia nanoparticles on DNA damage in PC12 and N2a cells
DNA tail percentage was measured by SCGE as an indicator of DNA damage. The results showed that exposure of PC12 and N2a cells to zirconia nanoparticles significantly increased DNA fragmentation (DF), comparing to the control cells. Similar to GSH, here, both cell lines showed parallel manner regarding treatment with zirconia nanoparticles since the DNA tail percentage was enlarged from almost 10 % at 12 h exposure in concentration of 31 µg/mL to nearly 70 % at 48 h exposure in 2000 µg/mL. DNA damage measurement revealed a concentration-dependent mode in both cell lines (Fig. 6).
Comet assay images and %Tail DNA induced by zirconia nanoparticles in a PC12, b N2a after 12, 24, and 48 h exposure time, including Comet assay images. All data are represented as the mean ± SEM of three independent experiments *p < 0.05, **p < 0.01, and ***p < 0.001, as compared with control group of 12, 24, and 48 h, respectively
Discussion
In the present study for the first time, we have mechanistically shown that zirconia nanoparticles persuade cytotoxic and genotoxic effects on PC12 and N2a cell lines at specific concentration. Recently, there are growing interest in the application of nanometal oxides such as zirconia in tissue engineering and implants especially orthopedic and dental implants. It has been shown that metal-based nanoparticles could penetrate through the blood brain barrier into the central nervous system of exposed animals, via blood circulation or the nervous olfactorius after intravenous injection or deposition on the olfactory mucosa following intranasal instillation (Kreyling et al. 2002; Liu et al. 2010; Lockman et al. 2004; Oberdorster et al. 2004). Recently the potential risk of copper oxide nanoparticles on the function of nervous system has been investigated (Xu et al. 2009; Zhao et al. 2009). It also stated that nanoparticle-based exposure of PC12 and N2a cells resulted in dose-dependent neurotoxicity in cultured cells (Perreault et al. 2012; Wang et al. 2011).
The in vitro cytotoxicity of zirconia nanoparticles exhibited the dose-dependent reduction in cell viability over a concentration range of 31–2000 µg/mL which is in consistent with the previous results quantified its cytotoxicity by MTS assay (Asadpour et al. 2014b). Our findings suggest the toxicity difference of zirconia nanoparticles with respect to the cell types. PC12 cells were found to be more tolerant to zirconia-induced cytotoxicity than N2a cells. The reduction in cell viability is more evident in N2a than PC12 cell line since N2a cell viability decreased to lower amount in all three different assessed time durations. In N2a cell line the cytotoxic effect after 24 h was stayed almost constant, this could prove the more sensitivity of this line comparing to PC12 which the cytotoxic effect is continued up to 48 h exposure. The cell viability reduction could be the consequence of physical or chemical characteristics of nanoparticles that persuade to cell injury or death by disturbing effect on the cell membrane or the intracellular organelles (Ghasempour et al. 2015). These nanoparticles’ adverse effects can be ATP decline through mitochondrial respiratory chain damage, formation of reactive oxygen species (ROS), and oxidative stress contributing to elevated chance of cell injury or death (Xia et al. 2006).
Our results also indicated that the cytotoxicity appears to be mediated by oxidative stress, a common mechanism which has been well documented for nanoparticles-induced toxic effects (Jeong et al. 2011; Wu et al. 2010). This ROS generation could provoke inflammation or pro-apoptotic cell signaling through caspase activation and cytochrome c release from mitochondria and the following oxidative stress produced by ROS could lead to external or internal cell damage (Jeong et al. 2011; Simon et al. 2000). More studies in this area are warranted to discover the cellular mechanism of ROS damage. Here, we have considered that the cellular oxidative stress induced by zirconia nanoparticles were indicated by elevated ROS generation, reduced GSH levels, increased lipid peroxidation and oxidative DNA damage.
Lipid peroxidation has been considered as a crucial key in the nanoparticles-induced cytotoxicity and DNA damage (Asadpour et al. 2014a; Blair 2008) as it started from 31.25 µg/mL. An increase in the level of thiobarbituric acid reactive species that correlated reversely with the cell viability of the cells, is suggesting that zirconia nanoparticles could induce membrane damage through lipid peroxidation. The glutathione redox cycle has an important role in detoxifying the generated ROS and other free radicals (Khodagholi et al. 2012). From the results, the GSH depletion was seen from 31.25 µg/mL after 12 h exposure, but this effect was more obvious at two highest concentrations and following 48 h exposure. Since the GSH is markedly reduced by zirconia nanoparticles’ exposure, it is reasonable to deem that cytotoxic effect of this nanoparticle, at least partially, is mediated via oxidative properties. Moreover, cell membrane injury triggered by lipid peroxidation can induce continuous enzyme release like lactate dehydrogenase which in exchange initiate a chain reaction of more cell loss (Wang et al. 2011). This is in agreement with previous studies which revealed the lactate dehydrogenase level boost in cell medium after the exposure of cells with nanoparticles (Hussain et al. 2005; Lin et al. 2006).
it is well documented that excessive generation of ROS could induce DNA damage by changing the mitochondrial membrane permeability or by oxidative alteration of DNA molecule, directly (Circu and Aw 2010). Our investigation showed that great DNA damage is evident following treatment of the cells by concentrations above 31.25 µg/mL. Amorphous zirconia increases both cytotoxicity and genotoxicity in human osteosarcoma cell line but in another study it is indicated nano zirconia reveals no genotoxic effect on human peripheral blood lymphocytes and cultured human embryonic kidney cells (Demir et al. 2013; Di Virgilio et al. 2014). This could suggest that particular physical characteristics of zirconia nanoparticles may not be the more vigorous DNA damage mediator and its effect on diverse cell lines is poles apart.
In the present study, we have evaluated the cytotoxic and genotoxic effect of zirconia nanoparticles without the existence of any positive control like hydrogen peroxide for comparing possibility of cytotoxic effect and with no facility for comparing. Moreover, we did not have access to any standard drugs to counteract with probable toxic effects of zirconia nanoparticles.
These data highlight the prominence of using numerous assays to cover the wide-ranging interactions between cell and nanoparticles and to define nontoxic nanoparticles concentrations especially in neuropathic disease like Parkinson and Alzheimer.
Conclusion
In conclusion, the present study addressed the potential toxicity of zirconia nanoparticles in PC12 and N2a cells. The results showed that zirconia nanoparticles could induce cell damage via oxidative stress induction which found to be dependent on the concentration used and time of exposure. Further studies are required to address the molecular mechanisms of the cell death induced by zirconia nanoparticles, in vitro or in vivo.
References
Aramouni P, Zebouni E, Tashkandi E, Dib S, Salameh Z, Almas K (2008) Fracture resistance and failure location of zirconium and metallic implant abutments. J Contemp Dent Pract 9:41–48
Asadpour E, Ghorbani A, Sadeghnia HR (2014a) Water-soluble compounds of lettuce inhibit DNA damage and lipid peroxidation induced by glucose/serum deprivation in N2a cells. Acta Pol Pharm 71:409–413
Asadpour E, Sadeghnia HR, Ghorbani A, Boroushaki MT (2014b) Effect of zirconium dioxide nanoparticles on glutathione peroxidase enzyme in PC12 and N2a cell lines. Iran J Pharm Res 13:1141–1148
Blair IA (2008) DNA adducts with lipid peroxidation products. J Biol Chem 283:15545–15549. doi:10.1074/jbc.R700051200
Brunner TJ et al (2006) In vitro cytotoxicity of oxide nanoparticles: comparison to asbestos, silica, and the effect of particle solubility. Environ Sci Technol 40:4374–4381
Caicedo M, Jacobs JJ, Reddy A, Hallab NJ (2008) Analysis of metal ion-induced DNA damage, apoptosis, and necrosis in human (Jurkat) T-cells demonstrates Ni2+ and V3+are more toxic than other metals: Al3+, Be2+, Co2+, Cr3+, Cu2+, Fe3+, Mo5+, Nb5+, Zr2+. J Biomed Mater Res A 86:905–913. doi:10.1002/jbm.a.31789
Carinci F, Pezzetti F, Volinia S, Francioso F, Arcelli D, Farina E, Piattelli A (2004) Zirconium oxide: analysis of MG63 osteoblast-like cell response by means of a microarray technology. Biomaterials 25:215–228
Catauro M, Raucci M, Ausanio G (2008) Sol-gel processing of drug delivery zirconia/polycaprolactone hybrid materials. J Mater Sci Mater Med 19:531–540. doi:10.1007/s10856-007-3065-y
Catelas I, Huk OL, Petit A, Zukor DJ, Marchand R, Yahia L (1998) Flow cytometric analysis of macrophage response to ceramic and polyethylene particles: effects of size, concentration, and composition. J Biomed Mater Res 41:600–607. doi:10.1002/(SICI)1097-4636(19980915)41:4<600:AID-JBM12>3.0.CO;2-I
Catelas I, Petit A, Zukor DJ, Marchand R, Yahia L, Huk OL (1999) Induction of macrophage apoptosis by ceramic and polyethylene particles in vitro. Biomaterials 20:625–630
Chang PP, Henegbarth EA, Lang LA (2007) Maxillary zirconia implant fixed partial dentures opposing an acrylic resin implant fixed complete denture: a two-year clinical report. J Prosthet Dent 97:321–330. doi:10.1016/S0022-3913(07)60020-1
Chevalier J (2006) What future for zirconia as a biomaterial? Biomaterials 27:535–543. doi:10.1016/j.biomaterials.2005.07.034
Circu ML, Aw TY (2010) Reactive oxygen species, cellular redox systems, and apoptosis. Free Radic Biol Med 48:749–762. doi:10.1016/j.freeradbiomed.2009.12.022
Colilla M, Manzano M, Izquierdo-Barba I, Vallet-Regí M, Boissiére C, Sanchez C (2009) Advanced drug delivery vectors with tailored surface properties made of mesoporous binary oxides submicronic spheres. Chem Mater 22:1821–1830. doi:10.1021/cm9033484
Davenport A, Gura V, Ronco C, Beizai M, Ezon C, Rambod E (2007) A wearable haemodialysis device for patients with end-stage renal failure a pilot study. Lancet 370:2005–2010. doi:10.1016/S0140-6736(07)61864-9
Demir E, Burgucu D, Turna F, Aksakal S, Kaya B (2013) Determination of TiO2, ZrO2, and Al2O3 nanoparticles on genotoxic responses in human peripheral blood lymphocytes and cultured embyronic kidney cells. J Toxicol Environ Health A 76:990–1002. doi:10.1080/15287394.2013.830584
Di Virgilio AL, Arnal PM, Maisuls I (2014) Biocompatibility of core@shell particles: cytotoxicity and genotoxicity in human osteosarcoma cells of colloidal silica spheres coated with crystalline or amorphous zirconia. Mutat Res Genet Toxicol Environ Mutagen 770:85–94. doi:10.1016/j.mrgentox.2014.05.009
Garvin KL, Hartman CW, Mangla J, Murdoch N, Martell JM (2009) Wear analysis in THA utilizing oxidized zirconium and crosslinked polyethylene. Clin Orthop Relat Res 467:141–145. doi:10.1007/s11999-008-0544-5
Ghasempour S, Shokrgozar MA, Ghasempour R, Alipour M (2015) Investigating the cytotoxicity of iron oxide nanoparticles in vivo and in vitro studies. Exp Toxicol Pathol. doi:10.1016/j.etp.2015.07.005
Ghorbani A, Sadeghnia HR, Asadpour E (2015) Mechanism of protective effect of lettuce against glucose/serum deprivation-induced neurotoxicity. Nutr Neurosci 18:103–109. doi:10.1179/1476830513y.0000000107
Gillani R, Ercan B, Qiao A, Webster TJ (2010) Nanofunctionalized zirconia and barium sulfate particles as bone cement additives. Int J Nanomed 5:1–11
Gomoll AH, Fitz W, Scott RD, Thornhill TS, Bellare A (2008) Nanoparticulate fillers improve the mechanical strength of bone cement. Acta Orthop 79:421–427. doi:10.1080/17453670710015349
Hallab NJ, Anderson S, Caicedo M, Skipor A, Campbell P, Jacobs JJ (2004) Immune responses correlate with serum-metal in metal-on-metal hip arthroplasty. J Arthroplast 19:88–93
Hallab NJ, Jacobs JJ, Skipor A, Black J, Mikecz K, Galante JO (2000) Systemic metal-protein binding associated with total joint replacement arthroplasty. J Biomed Mater Res 49:353–361. doi:10.1002/(SICI)1097-4636(20000305)49
Hansen S (2006) Sorbent dialysis in the third millennium. Nephrol News Issues 20(43):45
Hussain SM, Hess KL, Gearhart JM, Geiss KT, Schlager JJ (2005) In vitro toxicity of nanoparticles in BRL 3A rat liver cells. Toxicol In Vitro 19:975–983. doi:10.1016/j.tiv.2005.06.034
Jeong YS, Oh WK, Kim S, Jang J (2011) Cellular uptake, cytotoxicity, and ROS generation with silica/conducting polymer core/shell nanospheres. Biomaterials 32:7217–7225. doi:10.1016/j.biomaterials.2011.06.020
Khodagholi F, Ansari N, Amini M, Tusi SK (2012) Involvement of molecular chaperones and the transcription factor Nrf2 in neuroprotection mediated by para-substituted-4,5-diaryl-3-thiomethyl-1,2,4-triazines. Cell Stress Chaperones 17:409–422. doi:10.1007/s12192-011-0316-0
Kreyling WG et al (2002) Translocation of ultrafine insoluble iridium particles from lung epithelium to extrapulmonary organs is size dependent but very low. J Toxicol Environ Health A 65:1513–1530. doi:10.1080/00984100290071649
Lappalainen R, Santavirta SS (2005) Potential of coatings in total hip replacement. Clin Orthop Relat Res 430:72–79. doi:10.1097/01.blo.0000150000.75660.ff
Lee DB, Roberts M (2008) A peritoneal-based automated wearable artificial kidney. Clin Exp Nephrol 12:171–180. doi:10.1007/s10157-008-0050-9
Lee DB, Roberts M, Bluchel CG, Odell RA (2010) Zirconium: biomedical and nephrological applications. ASAIO J 56:550–556. doi:10.1097/MAT.0b013e3181e73f20
Lin W, Huang YW, Zhou XD, Ma Y (2006) In vitro toxicity of silica nanoparticles in human lung cancer cells. Toxicol Appl Pharmacol 217:252–259. doi:10.1016/j.taap.2006.10.004
Link N, Brunner TJ, Dreesen IA, Stark WJ, Fussenegger M (2007) Inorganic nanoparticles for transfection of mammalian cells and removal of viruses from aqueous solutions. Biotechnol Bioeng 98:1083–1093. doi:10.1002/bit.21525
Liu G, Lin Y (2005) Electrochemical sensor for organophosphate pesticides and nerve agents using zirconia nanoparticles as selective sorbents. Anal Chem 77:5894–5901. doi:10.1021/ac050791t
Liu S, Xu L, Zhang T, Ren G, Yang Z (2010) Oxidative stress and apoptosis induced by nanosized titanium dioxide in PC12 cells. Toxicology 267:172–177. doi:10.1016/j.tox.2009.11.012
Liu SQ, Xu JJ, Chen HY (2004) A reversible adsorption-desorption interface of DNA based on nano-sized zirconia and its application. Colloids Surf B 36:155–159. doi:10.1016/j.colsurfb.2004.03.020
Lockman PR, Koziara JM, Mumper RJ, Allen DD (2004) Nanoparticle surface charges alter blood-brain barrier integrity and permeability. J Drug Target 12:635–641. doi:10.1080/10611860400015936
Ma Y, Zheng YM, Chen JP (2011) A zirconium based nanoparticle for significantly enhanced adsorption of arsenate: synthesis, characterization and performance. J Colloid Interface Sci 354:785–792. doi:10.1016/j.jcis.2010.10.041
Mosmann T (1983) Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods 65:55–63
Nakarani M, Misra AK, Patel JK, Vaghani SS (2010) Itraconazole nanosuspension for oral delivery: formulation, characterization and in vitro comparison with marketed formulation. Daru 18:84–90
Oberdorster G, Sharp Z, Atudorei V, Elder A, Gelein R, Kreyling W, Cox C (2004) Translocation of inhaled ultrafine particles to the brain. Inhal Toxicol 16:437–445. doi:10.1080/08958370490439597
Parham H, Rahbar N (2010) Square wave voltammetric determination of methyl parathion using ZrO2-nanoparticles modified carbon paste electrode. J Hazard Mater 177:1077–1084. doi:10.1016/j.jhazmat.2010.01.031
Perreault F, Pedroso Melegari S, Henning da Costa C, de Oliveira Franco Rossetto AL, Popovic R, Gerson Matias W (2012) Genotoxic effects of copper oxide nanoparticles in Neuro 2A cell cultures. Sci Total Environ 441:117–124. doi:10.1016/j.scitotenv.2012.09.065
Pezzotti G, Saito T, Padeletti G, Cossari P, Yamamoto K (2010) Nano-scale topography of bearing surface in advanced alumina/zirconia hip joint before and after severe exposure in water vapor environment. J Orthop Res 28:762–766. doi:10.1002/jor.21069
Piconi C, Maccauro G (1999) Zirconia as a ceramic biomaterial. Biomaterials 20:1–25
Rainer M et al (2008) Analysis of protein phosphorylation by monolithic extraction columns based on poly(divinylbenzene) containing embedded titanium dioxide and zirconium dioxide nano-powders. Proteomics 8:4593–4602. doi:10.1002/pmic.200800448
Schadel A, Thun G, Stork L, Metzler R (1993) Immunodiffusion and immunohistochemical investigations on the reactivity of oxide ceramic middle-ear implants. ORL J Otorhinolaryngol Relat Spec 55:216–221
Sebastia J, Cristofol R, Martin M, Rodriguez-Farre E, Sanfeliu C (2003) Evaluation of fluorescent dyes for measuring intracellular glutathione content in primary cultures of human neurons and neuroblastoma SH-SY5Y. Cytometry A 51:16–25. doi:10.1002/cyto.a.10003
Simon HU, Haj-Yehia A, Levi-Schaffer F (2000) Role of reactive oxygen species (ROS) in apoptosis induction. Apoptosis 5:415–418
Singh R, Lillard JW Jr (2009) Nanoparticle-based targeted drug delivery. Exp Mol Pathol 86:215–223. doi:10.1016/j.yexmp.2008.12.004
Sollazzo V et al (2008) Genetic effect of zirconium oxide coating on osteoblast-like cells. J Biomed Mater Res B 84:550–558. doi:10.1002/jbm.b.30903
Tahmasebpour MBAA, Razavi Aghjeh MK (2008) Synthesis of zirconia nanopowders from various zirconium salts via polyacrylamide gel method. J Eur Ceram Soc 28:773–778
Tan K, Cheang P, Ho IA, Lam PY, Hui KM (2007) Nanosized bioceramic particles could function as efficient gene delivery vehicles with target specificity for the spleen. Gene Ther 14:828–835. doi:10.1038/sj.gt.3302937
Tsukamoto R, Chen S, Asano T, Ogino M, Shoji H, Nakamura T, Clarke IC (2006) Improved wear performance with crosslinked UHMWPE and zirconia implants in knee simulation. Acta Orthop 77:505–511. doi:10.1080/17453670610046479
Uludag B, Sahin V, Ozturk O (2008) Fabrication of zirconium primary copings to provide retention for a mandibular telescopic overdenture: a clinical report. Int J Prosthodont 21:509–510
Vigolo P, Fonzi F (2008) An in vitro evaluation of fit of zirconium-oxide-based ceramic four-unit fixed partial dentures, generated with three different CAD/CAM systems, before and after porcelain firing cycles and after glaze cycles. J Prosthodont 17:621–626. doi:10.1111/j.1532-849X.2008.00366.x
Wang F, Jiao C, Liu J, Yuan H, Lan M, Gao F (2011) Oxidative mechanisms contribute to nanosize silican dioxide-induced developmental neurotoxicity in PC12 cells. Toxicol In Vitro 25:1548–1556. doi:10.1016/j.tiv.2011.05.019
Wang ML, Tuli R, Manner PA, Sharkey PF, Hall DJ, Tuan RS (2003) Direct and indirect induction of apoptosis in human mesenchymal stem cells in response to titanium particles. J Orthop Res 21:697–707. doi:10.1016/S0736-0266(02)00241-3
Wu J, Sun J, Xue Y (2010) Involvement of JNK and P53 activation in G2/M cell cycle arrest and apoptosis induced by titanium dioxide nanoparticles in neuron cells. Toxicol Lett 199:269–276. doi:10.1016/j.toxlet.2010.09.009
Xia T et al (2006) Comparison of the abilities of ambient and manufactured nanoparticles to induce cellular toxicity according to an oxidative stress paradigm. Nano Lett 6:1794–1807. doi:10.1021/nl061025k
Xu LJ, Zhao JX, Zhang T, Ren GG, Yang Z (2009) In vitro study on influence of nano particles of CuO on CA1 pyramidal neurons of rat hippocampus potassium currents. Environ Toxicol 24:211–217. doi:10.1002/tox.20418
Yang Y, Wang Z, Yang M, Li J, Zheng F, Shen G, Yu R (2007) Electrical detection of deoxyribonucleic acid hybridization based on carbon-nanotubes/nano zirconium dioxide/chitosan-modified electrodes. Anal Chim Acta 584:268–274. doi:10.1016/j.aca.2006.11.055
Zhang S, Ye J, Dong G (2010) Neuroprotective effect of baicalein on hydrogen peroxide-mediated oxidative stress and mitochondrial dysfunction in PC12 cells. J Mol Neurosci 40:311–320. doi:10.1007/s12031-009-9285-5
Zhao J, Xu L, Zhang T, Ren G, Yang Z (2009) Influences of nanoparticle zinc oxide on acutely isolated rat hippocampal CA3 pyramidal neurons. Neurotoxicology 30:220–230. doi:10.1016/j.neuro.2008.12.005
Acknowledgment
The authors sincerely thank Dr. Helmut Kubista for his kind help in manuscript copy editing. This study is the part of the results of a research project and thesis presented for a degree of Doctor of Philosophy in pharmacology (Thesis no. A385). This project was financially supported by Vice Chancellor for Research, Mashhad University of Medical Sciences, Mashhad, Iran.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors report no conflict of interest. The authors alone are responsible for the content and writing of the paper.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Asadpour, E., Sadeghnia, H.R., Ghorbani, A. et al. Oxidative stress-mediated cytotoxicity of zirconia nanoparticles on PC12 and N2a cells. J Nanopart Res 18, 14 (2016). https://doi.org/10.1007/s11051-015-3316-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11051-015-3316-7