Abstract
Caspase-9 (CASP9) and caspase-10 (CASP10) polymorphisms were associated with human cancers; however, the results remain controversial. In this meta-analysis, we aimed to estimate the relationship among CASP9 (rs1052576, rs1052571, rs4645978, rs4645981, rs4645982, rs2308950) and CASP10 (rs13006529, rs13010627, rs3900115) polymorphisms and the overall risk of cancers. Relevant studies were obtained from Web of Science, MEDLINE, PubMed, Scopus, and Google scholar databases (updated January 1, 2021). Odds ratio (OR) and 95% confidence intervals (CIs) were measured to estimate the strength of association. Our meta-analysis included 40 studies. The rs4645981 significantly enhanced the risk of cancer under TT vs. CC (OR = 2.42), TC vs. CC (OR = 1.55), TT+ TC vs. CC (OR = 1.66), TT vs. TC + CC (OR = 1.91), and T vs. C (OR = 1.57) inheritance models. As for the rs1052571 variant, increased risk of cancer was observed under TT vs. CC (OR =1.22), TC vs. CC (OR = 1.17), and TT+ TC vs. CC (OR = 1.18) models. The stratified analysis showed a significant correlation between rs4645978 or rs4645981 polymorphisms and cancer risk, while in Asians rs4645978 conferred an increased risk of colorectal, lung, and prostate cancer. Both rs4645981 and rs1052576 polymorphisms were correlated with an enhanced risk of lung cancer. In conclusion, our meta-analysis suggested that CASP9 rs4645981 and rs1052571 polymorphisms are associated with overall cancer risk. More studies on larger populations are warranted to validate these associations.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cancer is ranked as the second leading cause of death among adults in the USA and is considered a global public health concern with great importance [1]. Only in the USA, a total of 1,806,590 new cancer cases and more than six hundred thousand cancer-related deaths are projected to occur in 2020 [2]. Moreover, it has been approximated that the worldwide incidence of cancer will exceed 25 million by 2032 [3]. As a multifactorial disease, genetics and environmental factors serve pivotal roles in cancer etiology [4].
Apoptosis is a programmed cell death mechanism that modulates tissue hemostasis in different organisms [5]. The escape from apoptosis is known to be a hallmark of malignancy, and it regulates the development and progression of tumors [5,6,7]. Historically, major pathways of apoptosis have been characterized that lead to activation of effector caspases and cell death: the intrinsic (mitochondrial) pathway and the extrinsic (receptor-mediated) pathway. Apoptotic pathways converge at the activation of effector caspases (CASP-3, -6, and -7) [5, 8]. CASP 8 and 10 are initiator caspases activated after ligand binding to death receptors (i.e., tumor necrosis factor receptor superfamily) [9]. Downregulation of CASP9 and CASP10 is frequently observed in cancer patients and correlates with resistance to chemotherapy and/or poor clinical outcome [10, 11].
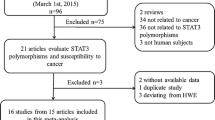
The gene encoding human CASP9 is mapped on chromosome 1p36.2, spans ~33 kb in length, and consists of 8 introns and 9 exons. The human CASP10 gene resides on chromosome 2q33.1 with ~46 kb length, 13 exons, and 11 introns and located approximately 20–30 kb to the 5′ untranslated region of the CASP8 gene [12]. Genomic mutations in CASP10 and allelic imbalance and epigenetic modifications in the CASP9 gene have been reported in multiple human tumors [13, 14]. Single-nucleotide polymorphisms (SNPs) are the most common type of single-base variations that predicts the risk of multiple diseases, including cancer [15]. Among the candidate SNPs, variations mapped in the promoteric regions of genes are well-studied because they most probably influence gene expression and might affect cancer susceptibility [5]. Figure 1 and Table 1 illustrate the genetic location of CASP9 and CASP10 polymorphisms.
Several reports have studied the relationship between CASP9 and CASP10 polymorphisms and cancer [5, 15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53]. However, the implication of these two initiator caspases and the risk of developing human cancers remains ill-defined. Hence, gaining a better understanding of the role of CASP9 and CASP10 variations in cancer incidence will expand our horizons for designing such curative strategies. Therefore, given the amount of accumulated data, we conducted a comprehensive meta-analysis by including the most relevant and recent publications (updated January 1, 2021) to identify statistical evidence.
Methods
Literature Search
We retrieved a list of the case–control studies through a comprehensive Internet-based literature search of Web of Knowledge, PubMed, Scopus, Google Scholar, and Embase databases. The used keywords were as follows: (“cancer” OR “carcinoma” OR “tumor” OR “neoplasm” OR “neoplasia”) AND (“caspase-10” OR “caspase-9” OR “CASP 10” OR “CASP 9” OR “caspase10” OR “caspase9” OR “CASP10” OR “CASP9” OR “caspase 10” OR “caspase 9” OR “CASP-10” OR “CASP-9”) AND (“gene polymorphism” OR “polymorphism” OR “SNP” OR “gene mutation” OR “gene variant” OR “mutation” OR “variant”). No language, country, or ethnicity restrictions were imposed. Additional publications were retrieved using a hand search. If the results of studies on different tumors or gene polymorphisms were reported in the same literature, they were regarded as a separate study to report. The included studies met the following criteria; [1] original case–control study about CASP9 and CASP10 polymorphisms and cancer susceptibility; [2] studies with sufficient published data to enable the estimation of odds ratios (ORs) with confidence intervals (CIs); [3] the frequency distribution of genotypes in the control group conformed to Hardy–Weinberg equilibrium (HWE). Review articles, case reports, duplicate publications, and studies with too little information were excluded.
Document Quality Assessment
Quality assessment (QA) of the included publications was carried out by two researchers as described previously [54]. Each publication was scored carefully. Low-quality studies were scored equal to or less than 9, while high-quality studies were scored more than 9 (Table 1). In case of disagreement, the two researchers would settle through information exchange and ultimately reached an agreement.
Data Extraction
Two independent researchers (S.S and A.Z.A) abstracted the relevant data according to the standard protocol and the study’s criteria. A third author (H.S) joined the study later to settle possible discrepancies. The following information were extracted from each included study: the first author’s name, publication date, ethnicity, country, cancer type, the method for genotyping of CASP9 and CASP10 polymorphisms, allele, and genotype distribution in the studied groups, and results of the HWE test (Table 1).
Statistical Analysis
Data analysis was carried out by both Stata15.0 statistical software and MetaGenyo web tool [55]. Deviation from HWE was examined in controls via a χ2 test. Pooled ORs with 95% CIs were calculated to estimate the strength of association between CASP9 and CASP10 variants and susceptibility to cancer under allelic, homozygous/heterozygous codominant, dominant, and recessive contrasted genetic models. P < 0.05 was considered statistically significant. Heterogeneity between-studies was evaluated via I2 statistics. We applied a fixed-effects model if I2 < 50%, and if heterogeneity was present (I2 > 50%), analyses were repeated using a random-effects model. Publication bias was determined via Egger’s test and visual inspection of funnel plots. The sensitivity analysis was done by sequentially omitting each study and calculating the pooled OR to investigate the effect of each study on overall estimates.
TSA and FPRP Analyses
In this study, trial sequential analysis (TSA) was used to enhance the robustness of the conclusion and decrease the random errors caused by sparse data and repetitive testing. We used the TSA software version 0.9.5.10 (http://www.ctu.dk/tsa/) to calculate the required information size (RIS) (meta-analysis sample size) [56] under the assumption of a plausible relative risk of 10% with low-risk bias, and the significance of 5% for type I error and 20% for type II error (power 80%). The TSA monitoring boundaries were plotted based on the required information size and the risk for type I and type II errors. The robustness of the conclusion is confirmed when the cumulative Z-curve (blue line) passes the TSA monitoring boundary (dotted red lines sloping inward) before the required information size is obtained. Otherwise, the data is insufficient to get a robust conclusion, and more trials are required. False-positive report probability (FPRP) values were assessed with different prior probabilities (0.25, 0.1, 0.01, 0.001, and 0.0001) [57]. An FPRP value < 0.2 indicated a significant correlation.
Result
Basic Information of Research Data
A total of 40 articles published between 2004 and 2020, including 15 case–control studies on CASP9 rs4645978, 13 studies on CASP9 rs1052576, 8 studies on CASP rs4645981, 7 studies on CASP9 rs4645982 and CASP10 rs13006529, 5 studies on CASP9 s1052571, 4 studies on CASP10 rs3900115, and 3 studies on either CASP9 rs2308950 or CASP10 rs13010627 were included in this study (Table 1). Figure 1 illustrates the specific screening process of the retrieved studies. Figure 2 and Supplementary Table 1 show the position of analyzed SNPs within the CASP9 and CASP10 genes. The basic information of the included studies and their QA scores are represented in Supplementary Table 2.
Main Analysis Results
Table 2 demonstrates the main results of the meta-analysis on the association of CASP9 and CASP10 variants with cancer susceptibility. Our pooled analysis revealed no significant association between CASP9 rs4645978, rs1052576, rs4645982, and rs2308950 polymorphisms and cancer incidence under different inheritance patterns. However, the pooled OR from 6 studies showed that CASP9 rs4645981 enhanced the risk of developing cancer under allelic [OR = 1.57; 95% CI, 1.25–1.97; P < 0.001, T vs. C], codominant homozygous [OR = 2.42; 95% CI, 1.46–4.00; P < 0.001, TT vs. CC], codominant heterozygous [OR =1.56; 95% CI, 1.21–2.00; P < 0.001, TC vs. CC], dominant [OR = 1.66; 95% CI, 1.26–2.17; P < 0.001, TT+ TC vs. CC], and recessive [OR = 1.92; 95% CI, 1.42–2.58; P < 0.001, TT vs. TC + CC] genetic patterns. Figure 3 demonstrates the forest plot for the relationship between CASP9 rs4645981 polymorphism and cancer risk using different genetic contrasted models. Regarding CASP9 rs1052571, an increased risk of developing cancer was observed under codominant homozygous [OR = 1.22; 95% CI, 1.00–1.50; P = 0.046, TT vs. CC], codominant heterozygous [OR = 1.17; 95% CI, 1.00–1.38; P = 0.049, TC vs. CC], and dominant [OR = 1.18; 95% CI, 1.00–1.38; P = 0.032, TT+ TC vs. CC] modes of inheritance. No significant association was found between CASP10 SNPs and overall risk of cancer.
Stratified Analysis Results
The subgroup analyses of CASP9 and CASP10 variants on risk of cancer are shown in Tables 3 and 4. Regarding ethnicity of cancer patients, the CASP9 rs4645978 variant conferred an increased risk of cancer in Asians, under codominant homozygous [OR = 1.26; 95% CI, 1.06–1.50; P = 0.008, AA vs. GG], recessive [OR = 1.16; 95% CI, 1.02–1.31; P = 0.015, AA vs. AG + GG], and allelic [OR = 1.12; 95% CI, 1.03–1.23; P = 0.006, A vs. G] genetic patterns. With respect to CASP9 rs4645981, the findings from five studies suggested a noteworthy association between this variant and increased risk of cancer in the Asian population (P < 0.001 for all the assessed genetic models) (Table 3).
Stratifying according to cancer type indicated that CASP9 rs4645978 significantly enhanced the risk of developing colorectal cancer (under all examined genetic models), and lung and prostate cancer (under codominant heterozygous and dominant genetic patterns). We also observed an enhanced risk of lung cancer (n = 4 studies) regarding codominant homozygous (OR = 2.25), codominant heterozygous (OR = 1.34), dominant (OR = 1.39), and recessive (OR = 1.92) models of CASP9 rs4645981 polymorphism. The T allele of this polymorphism increased susceptibility to lung cancer by 1.43-fold. Interestingly, the G allele of CASP9 rs1052576 was associated with a diminished risk of developing lung cancer [OR = 0.70; 95% CI, 0.56–0.90; P = 0.004] (Table 4).
Heterogeneity and Publication Bias
The heterogeneity results of all the studied polymorphisms are summarized in Tables 2 and 3. As shown in Fig. 4, a symmetrical-shaped funnel plot was generated for the association between CASP9 rs4645981 polymorphism and cancer risk, which indicates no publication bias. Regarding CASP9 rs4645981 and rs1052571 polymorphisms, Egger’s linear regression analysis detected no publication bias for the current meta-analysis under different genetic models (P values for bias > 0.05). No publication bias was also detected for significant findings of the stratified analysis, except for the relationship between three high-quality studies on CASP9 rs4645981 and cancer susceptibility under the codominant homozygous model (P value for bias = 0.008).
Sensitivity Analysis
Regarding TT vs. CC and TT vs. TC + CC models of CASP9 rs4645981, Lee et al.’s study had the most profound impact on pooled ORs (Fig. 5); thus, these findings should be interpreted carefully. As for the other significant results of this meta-analysis, the relevant pooled ORs indicated no significant change in the assessed genetic models (data not shown). Except for TT vs. CC and TT vs. TC + CC models of CASP9 rs4645981, the final summary ORs are reliable and stable.
Results of TSA and FPRP Analyses
Our meta-analysis indicated a significant association between CASP9 polymorphisms (rs4645981 and rs1052571) and overall cancer risk. For rs1052571, rs4645978, and rs1052576, the cumulative Z-curve did not pass the TSA boundary lines illustrating that the cumulative evidence is insufficient. More trials are warranted to confirm the effect of these SNPs on cancer susceptibility. However, the TSA analysis of rs4645981 indicated the crossing of cumulative Z-curve (blue line) over the trial sequential monitoring boundary (dotted red line) (P < 0.05), suggesting reliable evidence for the rs4645981 effect on cancer risk.
Table 5 represents the calculated FPRP values regarding the main significant findings in the present meta-analysis. With the assumption of a prior probability of 0.25, most FPRP values were less than 0.2, indicating the observed significant associations were notable.
Discussion
As the role of cell death in the pathophysiology of cancer is gaining ground, it appears crucial to study the genetic variations of the apoptosis-associated gene in human malignancies. For example, variations in apoptosis-related genes (i.e., death receptors and TNF superfamily ligands) have been investigated in hematological malignancies as well as solid tumors [58, 59]. Apoptosis, a genetically mediated cell suicide program and an essential physiological response, serves an indispensable role in maintaining tissue hemostasis and discarding harmful and/or unnecessary cells [60]. Characterization of the intrinsic and extrinsic pathways of apoptosis has straightened out how apoptosis is deregulated in most human malignancies [61]. Some studies have recommended that restoring the function of these caspases by overexpressing them could be a beneficial curative approach toward cancer [62, 63].
It has been shown that CASP9 is necessary for p53-dependent apoptosis [64]. Failure of CASP9 activation also induced a higher threshold for apoptotic cell death [65]. Horn and coworkers have shown that CASP10 negatively regulates death receptor-mediated activation of CASP8, and therefore perturbing cell death [66]. Based on another hypothesis, CASP10 makes cancer cells more susceptible to TRAIL-induced apoptosis following a CASP3-dependent fashion [67]. It is assumed that any alteration in the genetic formation of caspases could potentially impact the rate of apoptosis [68]. Nevertheless, the mechanisms by which caspases, including CASP9 and 10, get regulated are not fully understood. Moreover, CASP10 mutations have been associated with impaired apoptotic function, suggesting that CASP10 deficiency might be the reason why cancer cells evade apoptosis [13].
Few SNPs (i.e., rs1052576, rs1052571, and rs2308950) are mapped within the coding region of CASP9 gene. Ulybina et al. had suggested that genetic variations in this region might not be linked to the risk of developing breast cancer [23]. Instead, most studied SNPs in the CASP9 gene have resided in non-coding regions, i.e., promoter site and intronic regions. The rs4661636, rs6685648, and rs2020902 polymorphisms (located within introns of CASP9) or rs4645982 (an insertion/deletion SNP situated in a splice donor site) were shown to be functional and with the potential to alter CASP9 mRNA splicing patterns [69, 70]. Besides, alternative splicing of CASP9 might have different impacts on apoptosis and affect cancer cells’ tumorigenicity, as this gene produces two protein isoforms (CASP9a and CASP9b) through inclusion/exclusion of four exons [71, 72]. Promoteric SNPs of CASP9 (e.g., rs4645981 and rs4645978) were more intensively studied. This evidence shed light on the relevance of transcriptional regulation of this gene [31, 33] since the Grs4645978 Crs4645981 haplotype showed an elevated promoter activity compared with Grs4645978 Trs4645981 and Ars4645978 Crs4645981 combinations [5]. We then pursued the hypothesis suggesting the SNPs within the CASP10 gene might affect overall cancer susceptibility. It has been shown that the rs13006529 polymorphism impacts the very last amino acid of the protein [73]. However, this SNP was not correlated with breast cancer incidence [48]. The rs13010627 resides 5 amino acids upstream of the cleavage site of mature CASP10; hence, this variation could impact CASP10 activation and disrupt its function [45, 74].
In 2012, a meta-analysis was carried out by Yan and coworkers on 7 case–control studies, including 3962 subjects. They reported a positive association between the A allele carriers of CASP9 rs1052576 polymorphism and cancer incidence in American and Chinese populations (OR = 0.63); thus, A allele and A allele carriers of this polymorphism had established protective roles against cancer [75]. In our study, we included 14 studies and enrolled 4877 subjects to enhance statistical power. In contrast, we found no relationship between this variant and the overall risk of cancer in Asians or Caucasians. In the same year, another comprehensive meta-analysis was performed by Xu et al. on 9 studies with 5528 subjects for rs4645978, 6 studies with 2403 subjects for rs105276, and 2 studies with 2304 subjects for rs4645981. By pooling the results of included studies, they observed a protective effect for CASP9 rs1052576 under AA vs. GG (OR = 0.75) and A vs. G models (OR = 0.85) against cancer susceptibility. As for CASP9 rs4645981, they found an increased incidence of lung cancer among Asians under allelic (OR = 1.23, T vs. C) and recessive (OR = 1.22, CC vs. CT+TT) contrasted genetic models. However, they found no evidence of the association between CASP9 rs4645978 and overall cancer incidence [76]. In our updated meta-analysis, we enrolled 5910 subjects for examining the link between CASP9 rs4645981 polymorphism and cancer risk and observed significant results under different contrasted models. Still, by including 14 studies for each SNP, we found no association between CASP9 rs1052576 and rs4645978 and the overall risk of cancer.
In 2012, Yan and colleagues reviewed and conducted a meta-analysis on the relationship between CASP10 variants and cancer incidence. They pooled the results from 8 studies with 29,936 cases of cancer and 34,041 healthy subjects. Concerning CASP10 rs13006529, they included 3751 subjects and found that the T allele was associated with a 1.17-fold increased risk of cancer. Simultaneously, the other two CASP10 polymorphisms, rs3900115 and rs13010627, were not associated with cancer risk. Moreover, by performing a stratified analysis, they observed a positive association of CASP10 rs13006529*T carriers with breast cancer incidence (OR=1.17) [77]. In our study, we enrolled 5648 subjects and found no noteworthy association between this variant and cancer incidence.
Dysregulated apoptosis might lead to tumorigenesis [78]. In this respect, somatic or non-somatic mutations within caspase genes are frequent in a wide range of malignancies [79]. In 2019, Hashemi et al. showed that CASP3 polymorphisms are associated with the overall risk of cancer [80]. One year later, Hashemi and colleagues performed a meta-analysis and showed that common SNPs within CASP8, including rs3769818, rs3769821, rs3769825, rs3834129, and rs1045485, are also correlated with susceptibility to cancer [68]. In 2013, Yan and colleagues reported that variations in CASP7, another caspase that contributed to cell proliferation and cytokine maturation, are involved in the pathogenesis of cancer [81]. The possible association between variations in other caspases and cancer risk is currently being investigated.
Heterogeneity partly determines the difficulty in drawing overall conclusions, and it might affect the results of our meta-analysis. Hence, our results should be interpreted with attention because of some limitations. This might happen because of differences in the genetic background of the subjects or allele frequencies between the studied populations. Moreover, the observed heterogeneity might be due to the lifestyle and age of cancer patients, dissimilar methods of diagnosis, and cancer types. On the other hand, most of the included studies were conducted on a limited number of populations. Cancer is a multifactorial disorder, and environmental factors play crucial roles in its susceptibility. Despite these limitations, the findings of the pooled analysis provided a conclusive estimate for the impact of CASP9 and CASP10 polymorphisms on cancer susceptibility.
Conclusion
In conclusion, our meta-analysis suggested that CASP9 rs4645981 and rs1052571 polymorphisms are associated with overall cancer risk. However, more studies on larger populations are warranted to validate these associations.
Change history
16 September 2021
A Correction to this paper has been published: https://doi.org/10.1007/s12010-021-03647-0
References
Cao, J.-L., Yuan, P., Abuduwufuer, A., Lv, W., Yang, Y.-H., & Hu, J. (2015). Association between the TERT genetic polymorphism rs2853676 and cancer risk: meta-analysis of 76 108 cases and 134 215 controls. PloS one., 10(6), e0128829.
Siegel, R. L., Miller, K. D., & Jemal, A. (2020). Cancer statistics. A Cancer Journal for Clinicians., 70(1), 7–30.
Bender, E. (2014). Developing world: Global warning: Much of the world is ill-equipped to cope with its rising cancer burden and are pushing prevention and screening. Nature, 509, 7502.
Marahatta, S. B., Sharma, N., Koju, R., Makaju, R. K., Petmitr, P., & Petmitr, S. (2005). Cancer: Determinants and progression. Nepal Medical College journal, 7(1), 65–71.
Park, J. Y., Park, J. M., Jang, J. S., Choi, J. E., Kim, K. M., Cha, S. I., Kim, C. H., Kang, Y. M., Lee, W. K., Kam, S., Park, R. W., Kim, I. S., Lee, J. T., & Jung, T. H. (2006). Caspase 9 promoter polymorphisms and risk of primary lung cancer. Human Molecular Genetics, 15(12), 1963–1971.
Qin, Q., Zhang, C., Zhu, H., Yang, X., Xu, L., Liu, J., Lu, J., Zhan, L., Cheng, H., & Sun, X. (2014). Association between survivin-31G> C polymorphism and cancer risk: Meta-analysis of 29 studies. Journal of Cancer Research and Clinical Oncology., 140(2), 179–188.
Hajra, K., & Liu, J. (2004). Apoptosome dysfunction in human cancer. Apoptosis., 9(6), 691–704.
Degterev, A., Boyce, M., & Yuan, J. (2003). A decade of caspases. Oncogene., 22(53), 8543–8567.
Wilson, N. S., Dixit, V., & Ashkenazi, A. (2009). Death receptor signal transducers: Nodes of coordination in immune signaling networks. Nature Immunology., 10(4), 348–355.
Shen, X. G., Wang, C., Li, Y., Wang, L., Zhou, B., Xu, B., Jiang, X., Zhou, Z. G., & Sun, X. F. (2010). Downregulation of caspase-9 is a frequent event in patients with stage II colorectal cancer and correlates with poor clinical outcome. Colorectal Disease., 12(12), 1213–1218.
Shen, X.-G., Wang, C., Li, Y., Zhou, B., Xu, B., Yang, L., Zhou, Z. G., & Sun, X. F. (2011). Downregulation of caspase-10 predicting poor survival after resection of stage II colorectal cancer. International Journal of Colorectal Disease., 26(12), 1519–1524.
Grenet, J., Teitz, T., Wei, T., Valentine, V., & Kidd, V. J. (1999). Structure and chromosome localization of the human CASP8 gene. Gene., 226(2), 225–232.
Park, W. S., Lee, J. H., Shin, M. S., Park, J. Y., Kim, H. S., Lee, J. H., Kim, Y. S., Lee, S. N., Xiao, W., Park, C. H., Lee, S. H., Yoo, N. J., & Lee, J. Y. (2002). Inactivating mutations of the caspase-10 gene in gastric cancer. Oncogene., 21(18), 2919–2925.
Allan, L. A., & Clarke, P. R. (2009). Apoptosis and autophagy: Regulation of caspase-9 by phosphorylation. The FEBS Journal., 276(21), 6063–6073.
Liamarkopoulos, E., Gazouli, M., Aravantinos, G., Tzanakis, N., Theodoropoulos, G., Rizos, S., & Nikiteas, N. (2011). Caspase 8 and caspase 9 gene polymorphisms and susceptibility to gastric cancer. Gastric Cancer., 14(4), 317–321.
Fang, C.-Q., Liu, S. L., Lou, Y., & Li, J. H. (2007). Expression of the caspase 9 gene and its polymorphism distribution in gastric cancer. World Chinese Journal of Digestology., 15(30), 3190.
Lan, Q., Zheng, T., Chanock, S., Zhang, Y., Shen, M., Wang, S. S., Berndt, S. I., Zahm, S. H., Holford, T. R., Leaderer, B., Yeager, M., Welch, R., Hosgood, D., Boyle, P., & Rothman, N. (2007). Genetic variants in caspase genes and susceptibility to non-Hodgkin lymphoma. Carcinogenesis., 28(4), 823–827.
Lou, Y., Fang, C., & Li, J. (2007). A study on the expression of CASP9 gene and its polymorphism distribution in non-small cell lung cancer. Zhonghua yi xue yi Chuan xue za zhi= Zhonghua Yixue Yichuanxue Zazhi=. Chinese Journal of Medical Genetics, 24(1), 59–62.
He, X., Wang, L., Fang, C., Liu, S., Lou, Y., & Li, J. (2008). Expression of CASP9 gene and its polymorphism distribution in colon cancer. Shijie Huaren Xiaohua Zazhi., 16, 2371–2375.
Hosgood III, H. D., Baris, D., Zhang, Y., Zhu, Y., Zheng, T., Yeager, M., Welch, R., Zahm, S., Chanock, S., Rothman, N., & Lan, Q. (2008). Caspase polymorphisms and genetic susceptibility to multiple myeloma. Hematological oncology., 26(3), 148–151.
Ulybina, Y. M., Kuligina, E. S., Mitiushkina, N. V., Rozanov, M. E., Ivantsov, A. O., Ponomariova, D. N., Togo, A. V., Levchenko, E. V., Shutkin, V. A., Brenister, S. I., Devilee, P., Zhivotovsky, B., Hirvonen, A., & Imyanitov, E. N. (2009). Coding polymorphisms in Casp5, Casp8 and DR4 genes may play a role in predisposition to lung cancer. Cancer letters., 278(2), 183–191.
Wu, H., Yang, Z., Xie, Y., Kuang, Z., Luo, X., Liang, A., et al. (2009). Correlation between DNA repair gene XRCC1 Arg280His polymorphism and susceptibility to hepatocellular carcinoma in Fusui county of Guangxi. China Journal of Modern Medicine., 19(18), 2737–2743.
Ulybina, Y. M., Kuligina, E. S., Mitiushkina, N. V., Sherina, N. Y., Baholdin, D. V., Voskresenskiy, D. A., Polyakov, I. S., Togo, A. V., Semiglazov, V. F., & Imyanitov, E. N. (2011). Distribution of coding apoptotic gene polymorphisms in women with extreme phenotypes of breast cancer predisposition and tolerance. Tumori Journal., 97(2), 248–251.
Cingeetham, A., Vuree, S., Dunna, N. R., Gorre, M., Nanchari, S. R., Edathara, P. M., Mekkaw, P., Annamaneni, S., Digumarthi, R. R., Sinha, S., & Satti, V. (2014). Association of caspase9 promoter polymorphisms with the susceptibility of AML in south Indian subjects. Tumor Biology., 35(9), 8813–8822.
Ozdogan, S., Kafadar, A., Yilmaz, S. G., Timirci-Kahraman, O., Gormus, U., & Isbir, T. (2017). Role of caspase-9 gene Ex5+ 32 G> A (rs1052576) variant in susceptibility to primary brain tumors. Anticancer Research., 37(9), 4997–5000.
Yilmaz, S. G., Yencilek, F., Yildirim, A., Yencilek, E., & Isbir, T. (2017). Effects of caspase 9 gene polymorphism in patients with prostate cancer. International Institute of Anticancer Research., 31(2), 205–208.
Ercan, S., Arinc, S., Yilmaz, S. G., Altunok, C., Yaman, F., & Isbir, T. (2019). Investigation of caspase 9 gene polymorphism in patients with non-small cell lung cancer. Anticancer Research., 39(5), 2437–2441.
Edathara, P. M., Gorre, M., Kagita, S., Cingeetham, A., Annamaneni, S., Digumarti, R., et al. (2019). Influence of Caspase-9 polymorphisms on the development of chronic myeloid leukemia-a case-control study. Gene X., 1, 100002.
Lavender, N. A., Rogers, E. N., Yeyeodu, S., Rudd, J., Hu, T., Zhang, J., et al. (2012). Interaction among apoptosis-associated sequence variants and joint effects on aggressive prostate cancer. BMC Medical Genomics., 5(1), 11.
Azevedo, A. P., Silva, S. N., Reichert, A., Lima, F., Júnior, E., & Rueff, J. (2019). The role of caspase genes polymorphisms in genetic susceptibility to philadelphia-negative myeloproliferative neoplasms in a Portuguese population. Pathology & Oncology Research., 25(3), 961–969.
Gangwar, R., Mandhani, A., & Mittal, R. D. (2009). Caspase 9 and caspase 8 gene polymorphisms and susceptibility to bladder cancer in North Indian population. Annals of Surgical Oncology., 16(7), 2028–2034.
Theodoropoulos, G. E., Michalopoulos, N. V., Panoussopoulos, S.-G., Taka, S., & Gazouli, M. (2010). Effects of caspase-9 and survivin gene polymorphisms in pancreatic cancer risk and tumor characteristics. Pancreas., 39(7), 976–980.
Kesarwani, P., Mandal, R. K., Maheshwari, R., & Mittal, R. D. (2011). Influence of caspases 8 and 9 gene promoter polymorphism on prostate cancer susceptibility and early development of hormone refractory prostate cancer. BJU international., 107(3), 471–476.
Lee, S. Y., Choi, Y. Y., Choi, J. E., Kim, M. J., Kim, J.-S., Jung, D. K., Kang, H. G., Jeon, H. S., Lee, W. K., Jin, G., Cha, S. I., Kim, C. H., Jung, T. H., & Park, J. Y. (2010). Polymorphisms in the caspase genes and the risk of lung cancer. Journal of Thoracic Oncology., 5(8), 1152–1158.
Theodoropoulos, G. E., Gazouli, M., Vaiopoulou, A., Leandrou, M., Nikouli, S., Vassou, E., Kouraklis, G., & Nikiteas, N. (2011). Polymorphisms of caspase 8 and caspase 9 gene and colorectal cancer susceptibility and prognosis. International Journal of Colorectal Disease., 26(9), 1113–1118.
Theodoropoulos, G. E., Michalopoulos, N. V., Pantou, M. P., Kontogianni, P., Gazouli, M., Karantanos, T., Lymperi, M., & Zografos, G. C. (2012). Caspase 9 promoter polymorphisms confer increased susceptibility to breast cancer. Cancer Genetics., 205(10), 508–512.
George, G. P., Mandal, R. K., Kesarwani, P., Sankhwar, S. N., Mandhani, A., & Mittal, R. D. (2012). Polymorphisms and haplotypes in caspases 8 and 9 genes and risk for prostate cancer: A case–control study in cohort of North India. Urologic Oncology: Seminars and Original Investigations.
Wang, Y.-X., Zhao, L., Wang, X.-Y., Liu, C.-M., & Yu, S.-G. (2012). Role of Caspase 8, Caspase 9 and Bcl-2 polymorphisms in papillary thyroid carcinoma risk in Han Chinese population. Medical Oncology., 29(4), 2445–2451.
Wu, Z., Li, Y., Li, S., Zhu, L., Li, G., Yu, Z., Zhao, X., Ge, J., Cui, B., Dong, X., Tian, S., Hu, F., & Zhao, Y. (2013). Association between main Caspase gene polymorphisms and the susceptibility and prognosis of colorectal cancer. Medical Oncology., 30(3), 565.
Costa, E. F. D., Lopes-Aguiar, L., Nogueira, G. S., Lima, T. R. P., Rinck-Junior, J. A., Lourenço, G. J., & Lima, C. S. P. (2019). CASP9 c.-1339A> G and CASP3 c.-1191A> G polymorphisms alter susceptibility and clinical aspects of head and neck squamous cell carcinoma. Head & Neck., 41(8), 2665–2670.
Altamemi, I. A., Aubaid, A. H., & Hussein, T. A. (2020). Role of IL-18 and caspas-9 polymorphism in disease susceptibility in prostate cancer. EurAsian Journal of BioSciences., 14, 671–676.
Cao, S., Wang, C., Huang, X., Dai, J., Hu, L., Liu, Y., Chen, J., Ma, H., Jin, G., Hu, Z., Xu, L., & Shen, H. (2013). Prognostic assessment of apoptotic gene polymorphisms in non-small cell lung cancer in Chinese. Journal of Biomedical Research., 27(3), 231–238.
Javid, J., Mir, R., Masroor, M., Farooq, S., & Ahamad, I. (2013). Biological and clinical implications of functional promoter polymorphism of CASPASE 9 gene in non small cell lung cancer patients. J Mol Biomark Diagn S., 8, 007.
Ghasemi, M., Hoseini, V., Alizadeh, A., & Farzad, F. (2013). Caspase 9 promoter polymorphisms in gastric cancer, Mazandaran Province. Journal of Mazandaran University of Medical Sciences., 23(98), 2–7.
Frank, B., Hemminki, K., Wappenschmidt, B., Meindl, A., Klaes, R., Schmutzler, R. K., Bugert, P., Untch, M., Bartram, C. R., & Burwinkel, B. (2006). Association of the CASP10 V410I variant with reduced familial breast cancer risk and interaction with the CASP8 D302H variant. Carcinogenesis., 27(3), 606–609.
Gaudet, M. M., Milne, R. L., Cox, A., Camp, N. J., Goode, E. L., Humphreys, M. K., Dunning, A. M., Morrison, J., & Giles, G. G. (2009). Five polymorphisms and breast cancer risk: Results from the Breast Cancer Association Consortium. Cancer Epidemiology and Prevention Biomarkers., 18(5), 1610–1616.
Meyer, A., Coinac, I., Bogdanova, N., Dubrowinskaja, N., Turmanov, N., Haubold, S., et al. (2013). Apoptosis gene polymorphisms and risk of prostate cancer: A hospital-based study of German patients treated with brachytherapy. Urologic Oncology: Seminars and Original Investigations.
MacPherson, G., Healey, C. S., Teare, M. D., Balasubramanian, S. P., Reed, M. W., Pharoah, P. D., et al. (2004). Association of a common variant of the CASP8 gene with reduced risk of breast cancer. Journal of the National Cancer Institute., 96(24), 1866–1869.
Li, C., Zhao, H., Hu, Z., Liu, Z., Wang, L. E., Gershenwald, J. E., Prieto, V. G., Lee, J. E., Duvic, M., Grimm, E. A., & Wei, Q. (2008). Genetic variants and haplotypes of the caspase-8 and caspase-10 genes contribute to susceptibility to cutaneous melanoma. Human Mutation., 29(12), 1443–1451.
Arnaout, H. H., Khorshied, M. M., Khorshid, O. M. R., & El-Nagdy, M. H. (2012). Association of caspase 8 and caspase 10 genetic polymorphisms with B-cell non Hodgkin ' s lymphoma in Egypt: A case-control study. Journal of Cancer Science and Theraphy., 4, 249–253.
Ye, Y. (2004). Polymorphisms of caspase-8,-10 genes and their relationship with pathogenesis of non-Hodgkin lymphoma. Zhejiang University.
Liu, H., Jiang, X., Zhang, M. W., Pan, Y. F., Yu, Y. X., Zhang, S. C., et al. (2013). Association of CASP9, CASP10 gene polymorphisms and tea drinking with colorectal cancer risk in the Han Chinese population. Journal of Zhejiang University SCIENCE B., 14(1), 47–57.
Cavalcante, G. C., de Moraes, M. R., Valente, C. M. D., Silva, C. S., Modesto, A. A. C., de Assumpção, P. B., et al. (2020). Investigation of INDEL variants in apoptosis: The relevance to gastric cancer. BMC Medical Genetics., 21(1), 1–6.
He, J., Liao, X.-Y., Zhu, J.-H., Xue, W.-Q., Shen, G.-P., Huang, S.-Y., et al. (2014). Association of MTHFR C677T and A1298C polymorphisms with non-Hodgkin lymphoma susceptibility: Evidence from a meta-analysis. Scientific Reports., 4(1), 1–9.
Martorell-Marugan, J., Toro-Dominguez, D., Alarcon-Riquelme, M. E., & Carmona-Saez, P. (2017). MetaGenyo: A web tool for meta-analysis of genetic association studies. BMC Bioinformatics., 18(1), 563.
Brok, J., Thorlund, K., Gluud, C., & Wetterslev, J. (2008). Trial sequential analysis reveals insufficient information size and potentially false positive results in many meta-analyses. Journal of Clinical Epidemiology, 61(8), 763–769.
Fu, W., Zhuo, Z.-J., Chen, Y.-C., Zhu, J., Zhao, Z., Jia, W., Hu, J. H., Fu, K., Zhu, S. B., He, J., & Liu, G. C. (2017). NFKB1-94insertion/deletion ATTG polymorphism and cancer risk: Evidence from 50 case-control studies. Oncotarge., 8(6), 9806–9822.
Heredia-Galvez, B., Ruiz-Cosano, J., Torres-Moreno, D., Español, I., Morales-Lara, M. J., Pérez-Ceballos, E., González-Conejero, R., Gutiérrez-Cívicos, R., Vicente, V., Pérez-Guillermo, M., & Conesa-Zamora, P. (2014). Association of polymorphisms in TRAIL1 and TRAILR1 genes with susceptibility to lymphomas. Annals of Hematology., 93(2), 243–247.
Lee, E. B., Jeon, H.-S., Yoo, S. S., Choi, Y. Y., Kang, H.-G., Cho, S., Cha, S. I., Choi, J. E., Park, T. I., Lee, B. H., Park, R. W., Kim, I. S., Kang, Y. M., Kim, C. H., Jheon, S., Jung, T. H., & Park, J. Y. (2010). Polymorphisms in apoptosis-related genes and survival of patients with early-stage non-small-cell lung cancer. Annals of Surgical Oncology., 17(10), 2608–2618.
Kiraz, Y., Adan, A., Yandim, M. K., & Baran, Y. (2016). Major apoptotic mechanisms and genes involved in apoptosis. Tumor Biology., 37(7), 8471–8486.
Yu, J., & Zhang, L. (2004). Apoptosis in human cancer cells. Current Opinion in Oncology., 16(1), 19–24.
Mohr, A., Deedigan, L., Jencz, S., Mehrabadi, Y., Houlden, L., Albarenque, S.-M., & Zwacka, R. M. (2018). Caspase-10: A molecular switch from cell-autonomous apoptosis to communal cell death in response to chemotherapeutic drug treatment. Cell Death & Differentiation., 25(2), 340–352.
Lou, G.-G., Yao, H.-P., & Xie, L.-P. (2010). Brucea javanica oil induces apoptosis in T24 bladder cancer cells via upregulation of caspase-3, caspase-9, and inhibition of NF-κB and COX-2 expressions. The American Journal of Chinese Medicine., 38(03), 613–624.
Wu, G. S., & Ding, Z. (2002). Caspase 9 is required for p53-dependent apoptosis and chemosensitivity in a human ovarian cancer cell line. Oncogene., 21(1), 1–8.
Mueller, T., Voigt, W., Simon, H., Fruehauf, A., Bulankin, A., Grothey, A., & Schmoll, H. J. (2003). Failure of activation of caspase-9 induces a higher threshold for apoptosis and cisplatin resistance in testicular cancer. Cancer Research., 63(2), 513–521.
Horn, S., Hughes, M. A., Schilling, R., Sticht, C., Tenev, T., Ploesser, M., Meier, P., Sprick, M. R., MacFarlane, M., & Leverkus, M. (2017). Caspase-10 negatively regulates Caspase-8-mediated cell death, switching the response to CD95L in favor of NF-κB activation and cell survival. Cell Reports., 19(4), 785–797.
Engels, I. H., Totzke, G., Fischer, U., Schulze-Osthoff, K., & Jänicke, R. U. (2005). Caspase-10 sensitizes breast carcinoma cells to TRAIL-induced but not tumor necrosis factor-induced apoptosis in a caspase-3-dependent manner. Molecular and Cellular Biology., 25(7), 2808–2818.
Hashemi, M., Aftabi, S., Moazeni-Roodi, A., Sarani, H., Wiechec, E., & Ghavami, S. (2020). Association of CASP8 polymorphisms and cancer susceptibility: A meta-analysis. European Journal of Pharmacology., 173201.
Liu, C. Y., Wu, M. C., Chen, F., Ter-Minassian, M., Asomaning, K., Zhai, R., et al. (2010). A large-scale genetic association study of esophageal adenocarcinoma risk. Carcinogenesis., 31(7), 1259–1263.
Kelly, J. L., Novak, A. J., Fredericksen, Z. S., Liebow, M., Ansell, S. M., Dogan, A., Wang, A. H., Witzig, T. E., Call, T. G., Kay, N. E., Habermann, T. M., Slager, S. L., & Cerhan, J. R. (2010). Germline variation in apoptosis pathway genes and risk of non-Hodgkin ' s lymphoma. Cancer Epidemiology and Prevention Biomarkers., 19(11), 2847–2858.
Goehe, R. W., Shultz, J. C., Murudkar, C., Usanovic, S., Lamour, N. F., Massey, D. H., Zhang, L., Camidge, D. R., Shay, J. W., Minna, J. D., & Chalfant, C. E. (2010). hnRNP L regulates the tumorigenic capacity of lung cancer xenografts in mice via caspase-9 pre-mRNA processing. The Journal of Clinical Investigation., 120(11), 3923–3939.
Kwon, M., Yim, S., Kim, G., Lee, S., Jeong, C., & Lee, D. (2019). CODA-ML: Context-specific biological knowledge representation for systemic physiology analysis. BMC Bioinformatics., 20(10), 248.
Ashkenazi, A. (2002). Targeting death and decoy receptors of the tumour-necrosis factor superfamily. Nature Reviews Cancer., 2(6), 420–430.
Milhas, D., Cuvillier, O., Therville, N., Clavé, P., Thomsen, M., Levade, T., Benoist, H., & Ségui, B. (2005). Caspase-10 triggers bid cleavage and caspase cascade activation in FasL-induced apoptosis. Journal of Biological Chemistry., 280(20), 19836–19842.
Yan, S., Li, Y.-Z., Zhu, X.-W., Liu, C.-L., Wang, P., & Liu, Y.-L. (2013). Role of the CASP-9 Ex5+ 32 G> A polymorphism in susceptibility to cancer: A meta-analysis. Experimental and Therapeutic Medicine., 5(1), 175–180.
Xu, W., Jiang, S., Xu, Y., Chen, B., Li, Y., Zong, F., Zhao, W., & Wu, J. (2012). A meta-analysis of caspase 9 polymorphisms in promoter and exon sequence on cancer susceptibility. PLoS One., 7(5), e37443.
Yan, S., Li, Y., Zhu, J., Liu, C., Wang, P., & Liu, Y. (2012). Role of CASP-10 gene polymorphisms in cancer susceptibility: A HuGE review and meta-analysis. Genetology Molecular Research., 11(4), 3998–4007.
Reed, J. C. (1999). Dysregulation of apoptosis in cancer. Journal of Clinical Oncology, 17(9), 2941.
Ghavami, S., Hashemi, M., Ande, S. R., Yeganeh, B., Xiao, W., Eshraghi, M., Bus, C. J., Kadkhoda, K., Wiechec, E., Halayko, A. J., & Los, M. (2009). Apoptosis and cancer: Mutations within caspase genes. Journal of Medical Genetics., 46(8), 497–510.
Hashemi, M., Moazeni-Roodi, A., & Ghavami, S. (2019). Association between CASP3 polymorphisms and overall cancer risk: A meta-analysis of case–control studies. Journal of Cellular Biochemistry., 120(5), 7199–7210.
Yan, S., Li, Y., Zhu, X., Liu, C., Wang, P., & Liu, Y. (2013). HuGE systematic review and meta-analysis demonstrate association of CASP-3 and CASP-7 genetic polymorphisms with cancer risk. Genetology Molecular Research., 12(2), 1561–1573.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this article was revised: The original version of this article unfortunately contained a mistake in the captions of figures 1 and 2. The captions of figures 1 and 2 were interchanged.
Supplementary Information
ESM 1
(DOCX 17 kb)
Supplementary Table 1 Information of the studied polymorphisms
Supplementary Table 2 Main characteristics of the studies included in the meta-analysis
ESM 2
(DOCX 217 kb)
Supplementary Fig. 1 Trial sequential analysis of 10 studies reporting CASP9 rs4645978 and rs1052576 polymorphisms. The criteria for measuring the required information size were: α = 0.05 (two-sided), β = 0.20 (power 80%), D2 = 57.0%, a relative risk increase of 10%, and an event proportion of 38.02% in the control arm. The blue cumulative Z-curve was generated by a random-effects model
Supplementary Fig. 2 Trial sequential analysis of 10 studies reporting CASP9 rs1052571 and rs4645981 polymorphisms. The criteria for measuring the required information size were α = 0.05 (two-sided), β = 0.20 (power 80%), D2 = 57.0%, a relative risk increase of 10%, and an event proportion of 38.02% in the control arm. The blue cumulative Z-curve was constructed using a random-effects model
Rights and permissions
About this article
Cite this article
Sargazi, S., Abghari, A.Z., Sarani, H. et al. Relationship Between CASP9 and CASP10 Gene Polymorphisms and Cancer Susceptibility: Evidence from an Updated Meta-analysis. Appl Biochem Biotechnol 193, 4172–4196 (2021). https://doi.org/10.1007/s12010-021-03613-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-021-03613-w