Abstract
Although extensive research has been conducted on producing sophorolipids using Candida (Starmerella) bombicola from pure sugars and various oil sources, production of this biosurfactant has not been evaluated when cells are cultivated in lignocellulosic hydrolysates. Here, we report for the first time that C. bombicola is capable of producing sophorolipids on hydrolysates derived from sweet sorghum bagasse and corn fiber. Without oil supplementation, a sophorolipid concentration of 3.6 and 1.0 g/L was detected from cultures with bagasse and corn fiber hydrolysates, respectively. With the addition of soybean oil at 100 g/L, the yield of sophorolipids from these two hydrolysates in the same order was 84.6 and 15.6 g/L. Surprisingly, C. bombicola consumed all monomeric sugars and nonsugar compounds in the hydrolysates, and cultures with bagasse hydrolysates had higher yield of sophorolipids than those from a standard medium which contained pure glucose at the same concentration.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Compared with petroleum-based detergents, surfactant produced by microorganisms, biosurfactant, is biodegradable, has low toxicity, and is compatible with the environment [18]. Several microorganisms are known as glycolipid producers. These microbes include the following: rhamnolipid-producing pathogenic bacterium Pseudomonas aerugisosa, cellobiose lipids-producing fungi Pseudozyma flocculosa and Ustilago maydis, and sophorolipid-producing Candida (Starmerella) bombicola. Among these producers, C. bombicola has attracted the greatest attention due to the following: (1) this yeast strain is nonpathogenic, (2) and it has high productivity of sophorolipids. The highest one reported is 400 g/L with a yield of 0.6 g sophorolipids/g substrate [31], and (3) sophorolipids have been commercially used in several products: Sophoron, a dishwasher detergent by the Japanese company Saraya, defatting sprayers by the Belgian company Ecover, and sophorolipid-based cosmetics by the French company Soliance [5].
Sophorolipids are a group of compounds that are composed of a sophorose head whose reducing end is connected to a terminally or subterminally hydroxylated fatty acid through a beta-glycosidic bond [6]. Sophorose is a glucose disaccharide with an unusual β-1,2 bond and can be acetylated on the 6′ and/or 6″ positions. The carboxylic end of the fatty acid is either free (acidic or open form) or internally esterified at the 4″ or, in some rare cases, at the 6′ or 6″ position (lactonic or close form). The hydroxyl fatty acid itself is generally either C16 or C18 and can have one or more unsaturated bonds. As a result, sophorolipid produced by C. bombicola is a mixture of related molecules with differences in the fatty acid part (chain length, saturation, position of hydroxylation) and the lactonization and acetylation pattern. Through using a gradient elution high-performance liquid chromatography (HPLC) and evaporative light scattering for detection, over 20 different sophorolipids are identified [11].
As unpurified and crude mixtures, besides their role as emulsifiers, sophorolipids have been used as a bactericidal agent to treat acne, dandruff, and body odors [25]. In addition, they are claimed to trigger several beneficial events in terms of protection of hair and skin, thus making them attractive as components in cosmetic, hygienic, and pharmaco-dermatological products [26]. It is believed that sophorolipids can stimulate the dermal fibroblast metabolism and collagen neosynthesis [2], inhibit free radical and elastase activity, possess macrophage-activating and fibrinolytic properties, and act as desquamating (i.e., eliminating the surface portion of the protective layer of the epidermis as part of the wound healing process) and depigmenting agents [14, 26]. Furthermore, crude sophorolipid mixtures are found to trigger cell differentiation instead of cell proliferation and inhibit protein kinase C activity of the human promyelocytic leukemia cell line HL60 [16].
Production of sophorolipids necessitates two kinds of substrates, a sugar and a lipid source. Over the years, various sugars, such as glucose, sucrose, galactose, and lactose, have been tested [3, 7, 8, 12]. A broad range of oil sources, such as corn, canola, safflower, sunflower, olive, rapeseed, grape seed, palm, coconut, fish, soybean, waste frying oil, and waste streams from biodiesel production, has been evaluated [1, 13, 15, 17, 20, 27]. The highest SL yield of 400 g/L was obtained when corn oil and honey served as the carbon sources [31].
However, the current production of sophorolipids through use of glucose and other pure sugars is not truly sustainable and renewable. Thus, for this study, we aimed to evaluate the feasibility of producing sophorolipids from lignocellulosic feedstocks. We chose sweet sorghum bagasse and corn fiber in light of (1) their abundance globally and (2) they are both preprocessed during extraction of juice and oil/carbohydrates/proteins, respectively. Based on our previous study, a simple pretreatment using dilute sulfuric acid (0.5 %) at 121 °C for 1 h releases 83.2 and 86.5 % of theoretically available sugars out of corn fiber and sorghum bagasse, respectively [21]. Considering the complicated compositions of bagasse and corn fiber hydrolysates which contain both sugar and nonsugar compounds, we sought to answer these four questions: (1) Could C. bombicola grow on the two hydrolysates? (2) If yes, how would the cells handle five-carbon sugars and the potentially toxic degradation products in the hydrolysates? (3) What would be the yield of sophorolipids on these two hydrolysates? (4) Could lignocellulosic hydrolysates replace pure glucose in producing sophorolipids?
Material and Methods
Microorganism and Inoculum Preparation
C. bombicola (ATCC 22214) was maintained on agar plates containing (per liter): glucose, 100 g; yeast extract, 10 g; urea, 1 g; and agar, 20 g. Colonies were transferred to fresh plates every 6 weeks and were used to start inoculum in the same medium but without agar. After 2-day growth at 25 °C in a shaking incubator set at 120 rpm, the inoculum culture was used to inoculate different samples as described below.
Pretreatment of Sorghum Bagasse and Corn Fiber
The same sorghum bagasse and corn fiber that were studied in our previous works [4, 22–24, 35] were used in this research. Based on National Renewable Energy Laboratory (NREL)’s protocol [33], the sorghum bagasse contained approximately 36.9 ± 1.6 % cellulose, 17.8 ± 0.6 % hemicellulose, and 19.5 ± 1.1 % lignin [4]. The corn fiber consisted of 17.2 ± 1.8 % of cellulose, 27.6 ± 1.7 % of xylan and galactan, and 12.1 ± 0.7 % of arabinan [22]. Pretreatment of both biomass materials followed our published procedure exactly [21]. Briefly, bagasse or corn fiber (10 g dry weight) mixed with 100 mL 0.5 % sulfuric acid was autoclaved at 121 °C for 1 h. Following pretreatment, the slurry was centrifuged at 4,000 × g for 10 min. The resulting liquid phase after pH adjustment to 5.5 through use of NaOH was used in the experiments detailed below.
Fermentation of C. bombicola on Cellulosic Hydrolysates
C. bombicola inoculum was used to inoculate hydrolysates at two different conditions: (1) only hydrolysates derived from pretreatment without any oil supplements—for this testing condition, corn fiber hydrolysates added with yeast extract (10 g/L) were also studied and (2) hydrolysates supplemented with soybean oil (100 g/L) and pure glucose to ensure a total glucose concentration of 100 g/L—for this second condition, controls with standard medium which contained (per liter): glucose, 100 g; yeast extract, 10 g; urea, 1 g; and soybean oil, 100 g were also investigated. All experiments were conducted in Erlenmeyer flasks comprising 10 % of the inoculum with a total volume of 40 mL for the first test and 50 mL for the second. All flasks were cultivated in a shaking incubator set at 25 °C and 120 rpm. At different time points, a volume of 1-mL sample was withdrawn and used for observing cells under microscope to check for contamination and measurements of cell dry weight and sophorolipid concentrations.
Analysis
Sophorolipid Extraction
For 1-mL samples taken at different time points, the whole sample was subjected to extraction using equal volume of ethyl acetate twice. The top (ethyl acetate) layer was then extracted by two volumes of hexane for residual oil. The remaining ethyl acetate fraction was proceeded with solvent evaporation. Sophorolipids were determined gravimetrically. Following ethyl acetate extraction, the water phase in the original samples was centrifuged at 10,000g for 5 min. The liquid layer was used for HPLC analysis. The pellet was freeze-dried and used to determine cell dry weight.
To measure concentrations of sophorolipids and residual oil in the final-day cultures, the remaining samples at the termination of different experiments were freeze-dried first. The dried material was then extracted by ethyl acetate for 5 days in a shaking incubator set at 120 rpm. Following this extraction, ethyl acetate was evaporated from the liquid phase through use of a rotovap. Once ethyl acetate disappeared, hexane was added to extract remaining oil. Both sophorolipids and residual oil were quantified gravimetrically.
Sophorolipid Characterization
Detection of sophorolipids was performed by liquid chromatography-tandem quadrupole mass spectrometry (LC-MS/MS). Separation of sophorolipid compounds was achieved using an Agilent 1260 HPLC equipped with a Phenomenex Luna® C18(2) column (100 mm, 2-mm i.d., 3-μm particle diameter). The mobile phases consisted of water spiked with 0.5 % formic acid (v/v) (A) and acetonitrile (B). The mobile phase flow rate was 0.2 mL/min, and the following gradient was employed: 5 % B ramped to 70 % B in 3 min (linear) and then ramped to 80 % B in 12 min (linear), followed by a linear increase to 95 % B in 3 min (held for 8 min) and then a change to 5 % in 1 min (held for 3 min). The HPLC was interfaced with a 3200 Q Trap® triple quadrupole/linear ion trap mass spectrometer (Applied Biosystems/MDS Sciex; Toronto, Canada) equipped with a TurboIonSpray® electrospray ionization (ESI) probe operated in multiple reaction monitoring (MRM) mode for quantitative determination.
High-Performance Liquid Chromatography
Concentrations of monomeric sugars (glucose, xylose, arabinose) and nonsugar compounds (formic acid, acetic acid, levulinic acid, and 5-hydroxymethylfurfural (5-HMF)) in samples were determined by HPLC (Shimadzu Scientific Instrument, Inc. Columbia, MD, USA) with a refractive index detector. An Aminex HP87 column (5 μm, 30 cm × 4.6 mm, Bio-Rad, CA, USA) was used in an oven set at 50 °C. Sulfuric acid at 0.005 M was used as the mobile phase with a flow rate of 0.6 mL/min. The injection volume was 20 μL. Concentrations of the aforementioned chemicals were calculated based on calibration curves built for each compound using external standards. Before HPLC analysis, all samples were filtered through 0.2-μm filters to remove any potential particles.
Results and Discussion
Extensive discussions of dilute acid pretreatment of sorghum bagasse and corn fiber have been provided in our previous study [21]. According to results revealed by that research, the pretreatment condition of using 0.5 % sulfuric acid at 121 °C for 1 h yields the highest sugar recovery compared with pretreatment using 2 % sulfuric acid and/or at 134 °C. Thus, for this work, we adopted the best pretreatment scheme but focused on understanding the behavior of C. bombicola on cellulosic hydrolysates.
Fermentation of C. bombicola on Cellulosic Hydrolysates Without Oil Supplementation
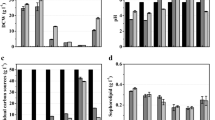
As shown by Fig. 1, C. bombicola grew well on both hydrolysates tested. By day 6, the cell density was 9.2, 9.8, and 10.8 g/L for hydrolysate derived from sorghum bagasse, corn fiber, and corn fiber with the addition of yeast extract during fermentation, respectively. We tested the effect of adding yeast extract since it was found that an oleaginous yeast strain, Cryptococcus curvatus, does not grow on corn fiber hydrolysate alone and requires the presence of a minimal medium which contains yeast extract [22]. But for C. bombicola, the addition of this nutrient resulted in slightly higher cell yield. The overall cell density for the three tested samples, however, did not differ significantly from a statistical standpoint.
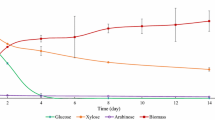
As cells grew, concentrations of both sugar and nonsugar compounds decreased with time (Fig. 2). The sorghum bagasse hydrolysate had a total monomeric sugar concentration of 42.9 g/L. In the fermentation culture, the total concentration of glucose, xylose, and arabinose was 38.6 g/L due to dilution caused by the addition of inoculum at 10 % of the final volume. Among the three sugars, glucose was consumed at a rate of 9.1 g/L-day and disappeared after 2 days (Fig. 2a). Xylose was used simultaneously with glucose, but the utilization rate of 1.8 g/L-day was much lower than that of glucose. Arabinose which had a very small concentration of 0.98 g/L in the cultures was consumed by C. bombicola, too. Thus, this is the first study to demonstrate that five-carbon sugars, xylose, and arabinose can be utilized by this special yeast strain. Besides sugars, the fermentation cultures also contained nonsugar compounds introduced by the bagasse hydrolysate: 5-HMF, 1.6 g/L; acetic acid, 1.0 g/L; levulinic acid, 0.2 g/L; and formic acid, 0.16 g/L (Fig. 2b). Interestingly, in 8 days, C. bombicola utilized all of these compounds which are often found to cause significant inhibition to other fermenting microorganisms, such as ethanol-producing yeast and bacteria [19, 28–30].
Compound utilization by C. bombicola during fermentation. a Sorghum bagasse: sugar compounds. b Sorghum bagasse: nonsugar compounds. c Corn fiber: sugar compounds. d Corn fiber: nonsugar compounds. Xylose (■), glucose (♦), arabinose (Δ) (a, c); HMF (Δ), acetic acid (♦), levulinic acid (■), formic acid (×)
The corn fiber hydrolysates after pretreatment had a total sugar concentration of 43.2 g/L which was similar to that of the bagasse hydrolysate. In the fermentation cultures, the total sugar concentration of 38.9 g/L was contributed by glucose, 12.2 g/L; xylose, 19.0 g/L; and arabinose, 7.7 g/L (Fig. 2c). The high concentration of arabinose was due to the high percentage of arabinan in corn fiber. Similar to results demonstrated in Fig. 2a, C. bombicola consumed glucose and xylose at the same time with glucose being completely depleted in 2 days. The only nonsugar compound in corn fiber hydrolysate was acetic acid, which was in agreement with our previous study [21]. This acid at a concentration of 2.2 g/L was exhausted in 4 days (Fig. 2d).
After 8-day fermentation, sophorolipids were observed in all tested cultures. The concentration was 3.6 ± 0.6, 1.0 ± 0.1, and 0.4 ± 0.05 g/L for cultures with bagasse hydrolysates, corn fiber hydrolysates, and corn fiber hydrolysates supplemented with yeast extract, respectively (Fig. 3). Between hydrolysates from bagasse and corn fiber, sorghum bagasse gave the highest yield of sophorolipids. Adding yeast extract did not promote sophorolipid production although it led to a slightly better cell growth (Fig. 1). These results are consistent with previous observations that (1) C. bombicola does produce sophorolipids on sugars only and synthesizes fatty acids through the de novo synthetic pathway and (2) the yield of sophorolipids is low under this condition [34].
Fermentation of C. bombicola on Cellulosic Hydrolysates Supplemented with Soybean Oil
To increase sophorolipid yield, a hydrophobic carbon source, such as oil, fatty acid, or alkane, needs to be supplemented. Throughout literature, the majority of researchers use an oil source at 100 g/L and a hydrophilic carbon sources, generally glucose, at the same concentration. Besides these two carbon sources, yeast extract (10 g/L) and urea (1 g/L) are also included in the fermenting medium. In order to evaluate whether the complex nature of cellulosic hydrolysates, especially the degradation products and many other unidentified compounds by HPLC can inhibit sophorolipid production, we tested the performance of C. bombicola in three setups as described above. All samples had a total glucose and soybean oil concentration of 100 g/L. For the standard medium which served as controls, yeast extract (10 g/L) and urea (1 g/L) were also added.
By the end of the 10-day fermentation, cell density, sophorolipid concentration, and residual oil concentration were determined. As indicated by Fig. 4, (1) cultures with bagasse hydrolysates, corn fiber hydrolysates, and standard medium had a cell content of 7.7 ± 0.1, 7.9 ± 0.2, and 8.9 ± 0.2 g/L, respectively. Time series data for cell growth revealed no statistically significant difference among the samples (Fig. 4a). (2) The sophorolipid concentration for the three setups in the same order was 84.6 ± 5.6, 15.6 ± 0.7, and 24.1 ± 0.1 g/L, and (3) the residual oil concentration was 52.3 ± 1.5, 41.0 ± 1.8, and 58.0 ± 3.0 g/L for the three sets of samples (Fig. 4b). Thus, apparently, the bagasse hydrolysates led to the highest production of sophorolipids while corn fiber hydrolysates resulted in a less yield of sophorolipids compared to those of the controls.
Closer look of the samples containing bagasse hydrolysates demonstrated that (1) C. bombicola again utilized all three sugars simultaneously (Fig. 5a). Glucose was consumed at a rate of 9.5 g/L-day which was higher than 9.1 g/L-day that we observed previously. Utilization rates of xylose and arabinose as 1.04 and 0.08 g/L-day were much lower than those of our previous results described above. This could be due to the presence of glucose at high concentrations. (2) Accumulation of sophorolipids did not start until cells enter stationary phase which was after day 2 (Fig. 5b). This agrees with other researchers’ report that sophorolipid production is not growth associated [32]. During this phase, cell density basically remained the same while sophorolipid concentration increased dramatically. Thus, it can be assumed that all sugars consumed during this phase were used to synthesize sophorolipids.
Although we were able to obtain time series curves for cell growth, sugar utilization, and sophorolipids accumulation, it was impossible to generate such a curve for soybean oil in the cultures. This impossibility lies in the fact that soybean oil tends to float at the surface of the cultures and it was difficult to withdraw representative samples at different time points. Thus, only residual oil concentrations in the final-day samples can be used for analysis. Based on these concentrations and considering all sugars consumed, the yield of sophorolipids was 0.55 g/g carbon (sugars plus oil) for cultures with bagasse hydrolysates. Higher yields of 0.64 [9], 0.68 [10], and 0.73 g/g [32] substrate have been reported for C. bombicola. But, it needs to be noted that all of these numbers are from studies conducted at fed-batch fermentation mode which aims to enhance production of sophorolipids. In light of the fact that higher sophorolipids were produced from bagasse hydrolysates than those from pure glucose, we expect a much higher production when the fermentation process is optimized.
Sophorolipids extracted from cultures containing bagasse hydrolysates were analyzed by HPLC-MS-MS. As shown by Fig. 6, the recovered sophorolipids was a mixture and consisted of acidic sophorolipids with C18 fatty acid chains and lactonic sophorolipids with either C16 or C18 fatty acids. Detailed characterization of each sophorolipid molecule is ongoing.
Conclusion
This is the first study to demonstrate that sophorolipids can be produced from sorghum bagasse and corn fiber through the process of pretreatment and fermentation. C. bombicola was able to consume all monomeric sugars and nonsugar compounds. Cultures with bagasse hydrolysates had the highest concentration of sophorolipids of 84.6 g/L, which was higher than that from cultures containing corn fiber hydrolysates or a standard medium. The overall yield of sophorolipids on bagasse hydrolysates was 0.55 g/g carbon. Results from this study open the door for producing a biosurfactant from truly sustainable resources and warrant further efforts to increase the yield of sophorolipids from lignocellulosic biomass.
References
Ashby, R. D., & Solaiman, D. K. (2010). The influence of increasing media methanol concentration on sophorolipid biosynthesis from glycerol-based feedstocks. Biotechnology Letters, 32, 1429–1437.
Borzeix, C. F. (1999). Use of sophorolipids comprising diacetyl lactones as agent for stimulating skin fibroblast metabolism. World patent 99/62479.
Casas, J., & García-Ochoa, F. (1999). Sophorolipid production by Candida bombicola: medium composition and culture methods. Journal of Bioscience and Bioengineering, 88, 488–494.
Choudhary, R., Umagiliyage, A. L., Liang, Y., Siddaramu, T., Haddock, J., & Markevicius, G. (2012). Microwave pretreatment for enzymatic saccharification of sweet sorghum bagasse. Biomass and Bioenergy, 39, 218–226.
Ciesielska, K., Li, B., Groeneboer, S., Van Bogaert, I., Lin, Y. C., Soetaert, W., Van de Peer, Y., & Devreese, B. (2013). SILAC-based proteome analysis of Starmerella bombicola sophorolipid production. Journal of Proteome Research, 12, 4376–4392.
Ciesielska, K., Van Bogaert, I. N., Chevineau, S., Li, B., Groeneboer, S., Soetaert, W., Van de Peer, Y., & Devreese, B. (2014). Exoproteome analysis of Starmerella bombicola results in the discovery of an esterase required for lactonization of sophorolipids. Journal of Proteomics, 98, 159–174.
Daniel, H.-J., Reuss, M., & Syldatk, C. (1998). Production of sophorolipids in high concentration from deproteinized whey and rapeseed oil in a two stage fed batch process using Candida bombicola ATCC 22214 and Cryptococcus curvatus ATCC 20509. Biotechnology Letters, 20, 1153–1156.
Daverey, A., & Pakshirajan, K. (2010). Kinetics of growth and enhanced sophorolipids production by Candida bombicola using a low-cost fermentative medium. Applied Biochemistry and Biotechnology, 160, 2090–2101.
Davila, A.-M., Marchal, R., & Vandecasteele, J.-P. (1992). Kinetics and balance of a fermentation free from product inhibition: sophorose lipid production by Candida bombicola. Applied Microbiology and Biotechnology, 38, 6–11.
Davila, A.-M., Marchal, R., & Vandecasteele, J.-P. (1997). Sophorose lipid fermentation with differentiated substrate supply for growth and production phases. Applied Microbiology and Biotechnology, 47, 496–501.
Davila, A. M., Marchal, R., Monin, N., & Vandecasteele, J. P. (1993). Identification and determination of individual sophorolipids in fermentation products by gradient elution high-performance liquid chromatography with evaporative light-scattering detection. Journal of Chromatography, 648, 139–149.
Gao, R., Falkeborg, M., Xu, X., & Guo, Z. (2013). Production of sophorolipids with enhanced volumetric productivity by means of high cell density fermentation. Applied Microbiology and Biotechnology, 97, 1103–1111.
Gupta, R., & Prabhune, A. A. (2012). Structural determination and chemical esterification of the sophorolipids produced by Candida bombicola grown on glucose and α-linolenic acid. Biotechnology Letters, 34, 701–707.
Hillion, G., Marchal, R., Stoltz, C. and Borzeix, F. (1998). Use of a sophorolipid to provide free radical formation inhibiting activity or elastase inhibiting activity. US patent 5756471.
Imura, T., Kawamura, D., Morita, T., Sato, S., Fukuoka, T., Yamagata, Y., Takahashi, M., Wada, K., & Kitamoto, D. (2013). Production of sophorolipids from non-edible jatropha oil by Stamerella bombicola NBRC 10243 and evaluation of their interfacial properties. Journal of Oleo Science, 62, 857–864.
Isoda, H., Kitamoto, D., Shinmoto, H., Matsumura, M., & Nakahara, T. (1997). Microbial extracellular glycolipid induction of differentiation and inhibition of the protein kinase C activity of human promyelocytic leukemia cell line HL60. Bioscience Biotechnology and Biochemistry, 61, 609–614.
Kim, Y. B., Yun, H. S., & Kim, E. K. (2009). Enhanced sophorolipid production by feeding-rate-controlled fed-batch culture. Bioresource Technology, 100, 6028–6032.
Kitamoto, D., Isoda, H., & Nakahara, T. (2002). Functions and potential applications of glycolipid biosurfactants—from energy-saving materials to gene delivery carriers. Journal of Bioscience and Bioengineering, 94, 187–201.
Klinke, H. B., Thomsen, A., & Ahring, B. K. (2004). Inhibition of ethanol-producing yeast and bacteria by degradation products produced during pre-treatment of biomass. Applied Microbiology and Biotechnology, 66, 10–26.
Li, H., Ma, X. J., Wang, S., & Song, X. (2013). Production of sophorolipids with eicosapentaenoic acid and docosahexaenoic acid from Wickerhamiella domercqiae var. sophorolipid using fish oil as a hydrophobic carbon source. Biotechnology Letters, 35, 901–908.
Liang, Y., Jarosz, K., Wardlow, A. T., Zhang, J., & Cui, Y. (2014). Lipid Production by Cryptococcus curvatus on hydrolysates derived from corn fiber and sweet sorghum bagasse following dilute acid pretreatment. Applied Biochemistry and Biotechnology, 173, 2086–2098.
Liang, Y., Perez, I., Goetzelmann, K., & Trupia, S. (2014). Microbial lipid production from pretreated and hydrolyzed corn fiber. Biotechnology Progress, 30, 367–375.
Liang, Y., Tang, T., Siddaramu, T., Choudhary, R., & Umagiliyage, A. L. (2012). Lipid production from sweet sorghum bagasse through yeast fermentation. Renewable Energy, 40, 130–136.
Liang, Y., Tang, T., Umagiliyage, A. L., Siddaramu, T., McCarroll, M., & Choudhary, R. (2012). Utilization of sorghum bagasse hydrolysates for producing microbial lipids. Applied Energy, 91, 451–458.
Mager, H., Röthlisberger, R., Wzgner, F. (1987). Use of sophorolse-lipid lactone for the treatment of dandruffs and body odour. European patent 0209783.
Maingault, M. (1999). Utilization of sophorolipids as therapeutically active substances or cosmetic products, in particular for the treatment of the skin. US patent 5981497.
Otto, R. T., Daniel, H. J., Pekin, G., Müller-Decker, K., Fürstenberger, G., Reuss, M., & Syldatk, C. (1999). Production of sophorolipids from whey: II. Product composition, surface active properties, cytotoxicity and stability against hydrolases by enzymatic treatment. Applied Microbiology and Biotechnology, 52, 495–501.
Palmqvist, E., & Hahn-Hägerdal, B. R. (2000). Fermentation of lignocellulosic hydrolysates. I: inhibition and detoxification. Bioresource Technology, 74, 17–24.
Palmqvist, E., & Hahn-Hägerdal, B. (2000). Fermentation of lignocellulosic hydrolysates. II: inhibitors and mechanisms of inhibition. Bioresource Technology, 74, 25–33.
Parawira, W., & Tekere, M. (2011). Biotechnological strategies to overcome inhibitors in lignocellulose hydrolysates for ethanol production: review. Critical Reviews in Biotechnology, 31, 20–31.
Pekin, G., Vardar-Sukan, F., & Kosaric, N. (2005). Production of sophorolipids from Candida bombicola ATCC 22214 using Turkish corn oil and honey. Engineering in Life Sciences, 5, 357–362.
Rau, U., Hammen, S., Heckmann, R., Wray, V., & Lang, S. (2001). Sophorolipids: a source for novel compounds. Industrial Crops and Products, 13, 85–92.
Sluiter, A., Hames, B., Ruiz, R., Scarlata, C., Sluiter, J., Templeton, D. and Crocker, D. (2004). Determination of structural carbohydrates and lignin in biomass. NREL, Golden, CO.
Van Bogaert, I. N. A., Saerens, K., De Muynck, C., Develter, D., Soetaert, W., & Vandamme, E. J. (2007). Microbial production and application of sophorolipids. Applied Microbiology and Biotechnology, 76, 23–34.
Yesuf, J. N., & Liang, Y. (2014). Optimization of sugar release from sweet sorghum bagasse following solvation of cellulose and enzymatic hydrolysis using response surface methodology. Biotechnology Progress, 30, 367–375.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Samad, A., Zhang, J., Chen, D. et al. Sophorolipid Production from Biomass Hydrolysates. Appl Biochem Biotechnol 175, 2246–2257 (2015). https://doi.org/10.1007/s12010-014-1425-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-014-1425-x