Abstract
The extracellular surface-active glycolipids produced by the yeast, Candida bombicola when grown on glucose and α-linolenic acid, were analyzed by HPLC with electro-spray ionization (ESI−MS) and collision-induced dissociation mass spectrometry. The analysis confirmed that the sophorolipid (SL) mixture contained three different forms of C18:3 SL molecules: free acid, lactone and a diacetylated lactone, which has not been reported previously. Also a minor amount of diacetylated lactone form of C18:1 SL was detected. Further, the SL mixture was subjected to chemical esterification reaction with sodium methoxide. The reaction product was analyzed with ESI−MS and confirmed to be the single homogenous esterified product containing C18:3 moieties in its fatty acid chain.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
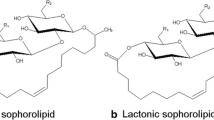
Sophorolipids (SLs) are extracellular surface-active agents produced by yeasts such as Candida bombicola. The sophorolipid structure is composed of a disaccharide and a hydroxy fatty acid. The disaccharide is a sophorose (2′-O-β-d-glucopyranosyl-β-d-glucopyranose) typically with the 6′- and 6″-acetylated hydroxy groups (Gorin et al. 1961). The sophorose moiety is linked to the fatty acid through a glycosidic bond between carbon-1′ and the terminal (ω) or sub-terminal (ω-1) carbon of a long chain fatty acid. SLs possess surfactant type properties due to their amphipathic structure and are industrially important for various purposes. Modification of the lipophilic portion of the SL molecule can alter their physicochemical properties (Glenns and Cooper 2006). Lipophilic substrates such as fatty acids and vegetable oils have been used for SL production (Prabhune et al. 2002; Daverey and Pakshirajan 2009; Parekh and Pandit 2011). There are several reports on the analysis of SLs produced using oleic acid and different vegetable oils with the help of analytical methods such as FAB-MS, APCI-MS and ESI−MS techniques (Asmer et al. 1988; Koster et al. 1995; Nunez et al. 2001, 2004). SLs also have antiviral, anticancerous and antibacterial activities (Shah et al. 2005; Chen et al. 2006; Sleiman et al. 2009). To our knowledge, there is no such report on the analysis of individual SL molecule produced using pure α-linolenic acid (ALA) as a lipophilic substrate. SL production using ALA as the lipophilic substrate may become a valuable product of interest. For the exploitation of this kind of SL, there must be some analytical methods to identify chemically distinct forms present in the heterogeneous mixture of SL. In this paper, the analysis of SL mixture produced using C. bombicola grown on glucose and ALA with the help of HPLC and mass spectrometry techniques such as electro-spray ionization (ESI) and collision-induced dissociation (CID) is presented. Further, the SL mixture was converted to the single homogenous esterified product following chemical esterification reaction (Bisht et al. 1999) which was also confirmed by ESI−MS analysis. Possible structures of SLs with ALA are shown in Fig. 1 and the scheme for chemical esterification reaction is shown in Fig. 2.
Materials and methods
α-Linolenic acid
α-Linolenic acid (≥99%, purity) was obtained from Sigma-Aldrich.
Production and isolation of SLs
C. bombicola ATCC 22214 was pre-cultivated at 28°C in 100 ml MGYP medium containing (g l−1): malt extract, 3; glucose, 100; yeast extract, 3; peptone, 5; pH 6.0 and shaken at 200 rpm. After 72 h, 100 ml seed culture was inoculated into 1 l MGYP medium supplemented with 4 ml ALA and then incubated at 28°C and 200 rpm for SL production. After 168 h, culture medium was centrifuged at ~4000×g for 20 min. The supernatant was extracted twice with equal volumes of ethyl acetate, the organic layer was dried over anhydrous sodium sulphate and the solvent was removed by rotary-evaporation. The brownish semi-crystalline product was washed twice with n-hexane to remove un-reacted fatty acid and was stored at 4°C.
Liquid chromatography
Crude SL mixture was analyzed by HPLC using an analytical symmetry C18, 5 μm column (250 × 4.6 mm2). The gradient solvent elution profile used was as follows: water/acetonitrile (95:5, v/v) holding for 10 min; to a final composition of water/acetonitrile (5:95, v/v) with a linear gradient over 50 min and holding for 10 min. The flow rate was 0.5 ml min−1. The peaks were detected at 220 nm. Fractions from different peaks were collected and pooled separately in many runs.
Mass spectrometry
Positive and negative ESI and CID mass spectra were obtained with an API QSTAR PULSAR hybrid MS/MS quadrupole TOF system (Applied biosystems). ESI mass spectra were obtained with time-of-flight mass spectrometry (TOF MS). Ion source was set at 3.8 kV. Tandem mass spectra (MS/MS) were acquired by selecting the desired precursor ion with Q1, which fragments ions in a collision cell (Q2). N2 gas was used in the collision cell as collisionally activated dissociation gas. These fragment ions were detected in the time of flight section. 1 μl samples from different fractions (collected from HPLC) were injected separately into mass spectrometer for analysis.
Synthesis of SL methyl ester
Dry crude SL, 100 mg, and methanol was held in 100 ml round-bottom flask equipped with a reflux condenser. To this mixture, a small piece of Na metal was added to form sodium methoxide. The reaction assembly was protected from atmospheric moisture by a CaCl2 guard-tube. The reaction mixture was refluxed for 3 h, cooled to room temperature and acidified with glacial acetic acid. The reaction mixture was concentrated by rota-evaporation. The concentrated product was taken in ethyl acetate and extracted with chilled de-ionized water. Organic layer was separated and extracted in two steps with chilled saturated solution of NaHCO3 and then with chilled brine solution. After extraction, organic layer containing the desired compound was separated and rota-evaporated (Bisht et al. 1999). The yield of SL methyl ester was 80% (w/w). Scheme for synthesis is shown in Fig. 2.
Results and discussion
3.98 g of SL was produced by C. bombicola when grown for 168 h in 1 l MGYP media containing 4 ml ALA and 100 g glucose as described in “Materials and methods” section. The SL mixture was analyzed by HPLC which revealed three major peaks (Fig. 3).
Mass spectrometric analysis of SL mixture
Mass spectra of first fraction
The positive mode ESI mass spectrum of the first fraction (eluting at 6.8 min) is shown in Fig. 4. Ions corresponding to protonated and sodiated SL molecules were observed at m/z 619 [M+H]+ and m/z 664 [M+2Na]+ respectively. Some pseudo-molecular ions of higher masses due to the formation of solvent adducts were also detected at m/z 655 [M+H+2H2O]+, m/z 679 [M+2H+H2O+CH3CN]+ and m/z 700 [M+2CH3CN]+ (Fig. 4). The protonated and sodiated molecular ions as well as pseudo-molecular ions confirmed the presence of free acid form of C18:3 SL molecules in the SL mixture (Fig. 1, compound 2).
Positive mode ESI spectra of first fraction from 6.8 min showing presence of free acid form of C18:3 SL molecules (Fig. 1, compound 2)
The negative mode ESI mass spectrum was also recorded for this fraction. The spectrum consists of [M−H]− ion at m/z 617 with no apparent fragmentation (Fig. 5). CID-MS analysis of m/z 617 [M−H]– ion gave sufficient information on the molecular structure of free acid form (Fig. 6). CID mass spectrum of [M−H]– ion showed a rich diversity of fragment ions and consists of a uniform pattern of 14 a.m.u. equidistant peaks (Fig. 6). This phenomenon is known as charge remote fragmentation because bond cleavage takes place at a site in the ion remote from the charge site and is well recognized in the CID spectra of fatty acid carboxylate anions (Adams 1990). Characteristic charge-remote fragmentation of ions containing a saturated hydrocarbon chain involve losses of the elements of C n H 2n+2 from the alkyl terminus resulting in a regular series of peaks with a spacing of 14 mass units. The presence of a double bond and its position are established by the absence of specific C n H 2n+2 losses, namely those which would arise as a result of vinylic hydrogen transfer or double bond cleavages (Tomer et al. 1983). As shown in Fig. 6 the charge remote fragment ion series results from collisionally induced losses of C n H2n+1COO− from m/z 617, yielding peaks at m/z 558, 544, 530, and so on. The series is interrupted between m/z; 488 and 434, 434 and 394, 394 and 355, indicative of the presence of unsaturation between C9 and C10, C12 and C13, C15 and C16 of the fatty acid alkyl chain. The low abundances of peaks at interruption sites are most likely caused by the relatively high energy requirement for vinylic hydrogen transfers and double bond cleavages.
Mass spectra of second fraction
The positive mode ESI mass spectrum of second fraction (eluting at 14.8 min) is shown in Fig. 7. The protonated molecular ion was observed at m/z 602 [M+2H]+ that corresponds to the lactone form of SL (Fig. 1, compound 1). The loss of 2H2O from the protonated [M+H]+ molecular ion generated the peak at m/z 565 in the same spectra (Fig. 7) that gave the evidence for the presence of lactone form of C18:3 SL molecule in the SL mixture (Fig. 1, compound 1). Further structural evidence on this form was obtained by the CID-MS analysis of the protonated molecular ion [M+H]+. There is a report on the CID-MS analysis of isolated fractions of SLs produced by C. bombicola using glucose and oleic acid as substrates by Koster et al. (1995). In positive CID-MS analysis, he reported the characteristic consecutive loss of 3 H2O from protonated molecular ion and an important ion generated by the loss of C6H10O5 fragment. They proposed that the loss of C6H10O5 fragment occurs from the reducing end of the disaccharide that leads to the formation of oxonium ion. The similar kind of ions was observed in the CID-MS spectra of protonated molecular ion (m/z 601) using our conditions (Fig. 8). The base peak in the spectrum at m/z 439 results from the fragmentation of [M+H]+ ion by the loss of a C6H10O5 molecule which follows the scheme given by Koster et al. (1995). Fragment ion peaks at m/z, 421, 403, 385 and 367 were obtained as the result of consecutive losses of H2O molecules from m/z 439. The other less intense peak at m/z 277 (C18H29O2) indicates the loss of H2O molecule from the protonated hydroxy fatty acid (C18H31O3, m/z 295). The protonated hydroxy fatty acid peak at m/z 295, which is the characteristic peak of acidic form is absent in the spectra of this lactonic form. Spectrum also contains fragment ion peaks at m/z, 565 and 547 which were the results of losses of two and three H2O molecules from m/z 601 respectively.
Positive mode ESI spectra of second fraction from 14.8 min showing presence of lactone form of C18:3 SL molecules (Fig. 1, compound 1)
Mass spectra of third fraction
The positive mode ESI mass spectrum of third fraction (eluting at 27.6 min) is shown in Fig. 9. The protonated molecular ion peak was observed at m/z 685 [M+H]+ that corresponds to the diacetylated SL lactone having C18:3 in its fatty acid chain (Fig. 1, compound 7). In the same spectra, one more peak was observed at m/z 711 [M+Na]+ (Fig. 9) that corresponds to the diacetylated SL lactone having C18:1 in its fatty acid chain.
Positive mode ESI spectra of third fraction from 27.6 min showing presence of diacetylated lactone of C18:3 SL molecules (Fig. 1, compound 7) and diacetylated lactone of C18:1 SL molecules
The CID-MS spectrum of m/z 685 molecular ion gave more evidence for its presence (Fig. 10). Fragment ion peaks at m/z 667, 649 and 631 were generated by consecutive losses of H2O molecules from m/z 685. The fragment ion peak at m/z 409 was obtained by the loss of hydroxy fatty acid moiety from m/z 685. Less intense peak observed at m/z 205 is the characteristic of the compound with an acetate group at C-6″ atom of the nonreducing component. Fragment ion peak at m/z 277 (C18H29O2) indicates the loss of H2O molecule from the protonated hydroxy fatty acid (C18H31O3, m/z 295).
ESI–MS analysis of SL methyl ester
The positive mode ESI mass spectrum of sophorolipid methyl ester is shown in Fig. 11. Two peaks were observed at m/z 633 [M+H]+ and m/z 655 [M+Na]+ respectively. These two peaks gave the evidence for the presence of SL methyl ester (m/z 632). The fatty acid chain of this compound contains C18:3 moieties.
Few reports are available in which linolenic acid has been used as a lipophilic substrate. Tulloch et al. (1962) used several free fatty acids and showed the incorporation of stearic, oleic, palmitic and linoleic acid into the SL structure while no incorporation could be observed for ALA. The work published by Davila et al. (1994) with vegetable oils and their corresponding methyl or ethyl esters, among them, two of the tested lipophilic substrates were rapeseed oil/esters and linseed oil/esters, containing 10 and 54% ALA respectively. No incorporation was observed for ALA although analytical techniques such as HPLC and GC–MS were used. In the present work, ESI and CID mass spectral analysis confirmed that the C. bombicola when grown on glucose and ALA produces a mixture of glycolipids consisting of free acid, lactone and diacetylated lactone forms of C18:3 SLs as well as diacetylated lactone form of C18:1 SL. The presence of different lipophilic carbon sources (secondary carbon sources) influences the composition of SLs or in other words the fatty acids incorporated into the SL structure reflects the secondary carbon source on which the microorganism is grown (Tulloch et al. 1962; Nunez et al. 2001). Presence of the C18:3 SLs in the mixture indicates the direct incorporation of this fatty acid into the SL structure. Candida (Torulopsis) bombicola produces C18:1 SLs during growth on glucose and nitrogen source (Gorin et al. 1961). So, it may be justified that the C18:1 SL is produced from the yeast itself by utilizing glucose and nitrogen sources from the medium. The approximate composition of the fermentation product (SL mixture) was 7.5% free acid, 80% lactone and 4.5% diacetylated lactone of C18:3 molecules and 8% of diacetylated lactone of C18:1 SL molecules. This composition was determined from the initial crude SL loaded on the column. Further, for the ease of purification, we followed the chemical esterification reaction from the literature in order to get a single homogenous product (Bisht et al. 1999). We observed the conversion of SL mixture into the SL methyl ester and ESI–MS analysis confirmed the presence of C18:3 moieties in the fatty acid chain of this product.
Conclusion
We have successfully demonstrated the analysis of chemically distinct forms in the SL mixture produced by C. bombicola when grown on glucose and ALA as well as its conversion into the single homogenous product by chemical esterification reaction. As mentioned in the introduction part, SLs of this fatty acid (α-linolenic acid) must have different physicochemical properties and may become potential candidates for different industrial and pharmaceutical applications.
References
Adams J (1990) Charge-remote fragmentations: analytical applications and fundamental studies. Mass Spectrom Rev 9:141–186
Asmer HJ, Lang S, Wagner F, Wray V (1988) Microbial production, structure elucidation and bioconversion of sophorose lipids. J Am Chem Soc 65:1460–1466
Bisht KS, Gross RA, Kaplan DL (1999) Enzyme-mediated regioselective acylations of sophorolipids. J Org Chem 64:780–789
Chen J, Song X, Zhang H, Qu YB (2006) Production, structure elucidation and anticancer properties of sophorolipid from Wickerhamiella domercqiae. Enzyme Microb Technol 39:501–506
Daverey A, Pakshirajan K (2009) Production, characterization and properties of sophorolipids from the yeast Candida bombicola using a low cost fermentative medium. Appl Biochem Biotechnol 158:663–674
Davila AM, Marchal R, Vandecasteele JP (1994) Sophorose lipid production from lipidic precursors: predictive evaluation of industrial substrates. J Ind Microbiol 13:249–257
Glenns RN, Cooper DG (2006) Effect of substrate on sophorolipid properties. J Am Oil Chem Soc 83(2):137–145
Gorin APJ, Spencer JFT, Tulloch AP (1961) Hydroxy fatty acid glycosides of sophorose from Torulopsis magnoliae. Can J Chem 39:846–855
Koster CG, Heerma W, Pepermans HAM, Groenevewegen A, Peters H, Haverkamp J (1995) Tandem mass spectrometry and nuclear magnetic resonance spectroscopy studies of Candida bombicola, sophorolipids and product formed on hydrolysis by cutinase. Anal Biochem 230:135–148
Nunez A, Ashby R, Foglia TA, Solaiman DKY (2001) Analysis and characterization of sophorolipids by liquid chromatography with atmospheric pressure chemical ionization. Chromatographia 53:673–677
Nunez A, Ashby R, Foglia TA, Solaiman DKY (2004) LC/MS analysis and lipase modification of the sophorolipids produced by Rhodotorula bogoriensis. Biotechnol Lett 26:1087–1093
Parekh VJ, Pandit AB (2011) Optimization of fermentative production of sophorolipid biosurfactant by Starmerella bombicola NRRL Y-17069 using response surface methodology. Int J Pharm Biol Sci 1(3):103–116
Prabhune A, Fox SR, Ratledge C (2002) Transformation of arachidonic acid to 19- hydroxy and 20-hydroxy eicosatetraenoic acid using Candida bombicola. Biotechnol Lett 24:1041–1044
Shah V, Doncel GF, Seyoum T, Eaton KM, Zalenskaya I, Hagver R, Azim A, Gross R (2005) Sophorolipids, microbial glycolipids with anti-human immunodeficiency virus and sperm-immobilizing activities. Antimicrob Agents Chemother 49:4093–4100
Sleiman JN, Kohlhoff SA, Roblin PM, Wallner S, Gross R, Hammerschlag MR, Zenilman ME, Bluth MH (2009) Sophorolipids as antibacterial agents. Ann Clin Lab Sci 39:60–63
Tomer KB, Crow FW, Gross ML (1983) Location of double bond position in unsaturated fatty acids by negative ion MS/MS. J Am Chem Soc 105:5487–5488
Tulloch AP, Spencer JFT, Gorin PAJ (1962) The fermentation of long chain compounds by Torulopsis magnoliae. Can J Chem 40:1326–1338
Acknowledgment
R.G. thanks Department of Biotechnology, Delhi for providing fellowship to carry out research.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gupta, R., Prabhune, A.A. Structural determination and chemical esterification of the sophorolipids produced by Candida bombicola grown on glucose and α-linolenic acid. Biotechnol Lett 34, 701–707 (2012). https://doi.org/10.1007/s10529-011-0818-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10529-011-0818-y