Abstract
Corn straw, lignocellulosic biomass, is a potential substrate for microbial production of bio-butanol. Bio-butanol is a superior second generation biofuel among its kinds. Present researches are focused on the selection of butanol tolerant clostridium strain(s) to optimize butanol yield in the fermentation broth because of toxicity of bio-butanol to the clostridium strain(s) itself. However, whatever the type of the strain(s) used, pretreatment process always affects not only the total sugar yield before fermentation but also the performance and growth of microbes during fermentation due to the formation of hydroxyl-methyl furfural, furfural and phenolic compounds. In addition, the lignocellulosic biomasses also resist physical and biological attacks. Thus, selection of best pretreatment process and its parameters is crucial. In this context, worldwide research efforts are increased in past 12 years and researchers are tried to identify the best pretreatment method, pretreatment conditions for the actual biomass. In this review, effect of particle size, status of most common pretreatment method and enzymatic hydrolysis particularly for corn straw as a substrate is presented. This paper also highlights crucial parameters necessary to consider during most common pretreatment processes such as hydrothermal, steam explosion, ammonia explosion, sulfuric acid, and sodium hydroxide pretreatment. Moreover, the prospective of pretreatment methods and challenges is discussed.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The global depletion of fossil fuel resources, concerns of global climate change and increased in number of vehicles reveals the necessity of alternative of fossil fuel, particularly in developing countries [1–5]. The production of liquid transportation fuels from renewable biomass has been a long-standing research goal due to its renewability and abundant in places where other liquid fuels, mainly petroleum and its by-product are not easily available [6]. Materials corrosion and moisture absorbing properties of ethanol hinders its use in the existing fuel distribution infrastructure. However, ethanol as made from corn or sugar cane has a satisfactory energy density and can be produced from a variety of biomasses at excellent yields [6]. To eliminate the above problems, bio-butanol could be one of the most capable biofuels because of its high energy content, low miscibility with water, can be transported through existing pipeline infrastructure, and low volatility with the potential to meet the needs of sustainable and green energy systems [7–13]. In addition, energy content of 1-butanol (27 MJ/l) is similar to that of gasoline (32 MJ/l) and can replace gasoline without any modification of the current vehicle and engine technologies [7–9, 13, 14]. Butanol can be produced by Clostridium acetobutylicum and/or Clostridium beijerinckii that can utilize glucose, galactose, cellobiose, mannose, xylose, and arabinose released from agricultural residues [15].
Most abundant renewable resource on the planet, lignocellulosic biomass as corn straw, has great potential to contribute to meeting the alternative energy demand [1, 2, 5, 8, 15]. Agricultural residue is mainly composed of cellulose, hemicellulose, and lignin, where cellulose fibers are surrounded by a hemicellulose and lignin matrix [4]. Researchers are focused on the use of agricultural residue as a feed stock, clostridia culture for fermentation and developing effective bioconversion processes [7, 10, 12, 16]. However, the main barrier to the production of the butanol is lignin content on biomass, which cannot be used by the solventogenic clostridia [16]. A pretreatment step is essential before fermentation to overcome lignin barrier and liberate the sugars that can be easily utilized by microbes [5]. Pretreatment process changes the structure of biomass that increases enzyme accessible surface area and reducing the degrees of polymerization of biomass [17, 18].
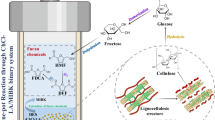
Large number of pretreatment process such as physical (hydrothermal, high pressure steam explosion, milling, and grinding), chemical (acid, alkali, oxidizing agents, organic solvents, and ammonia fiber explosion), biological (fungi, actinomycetes, and bacteria), as well as a combination of these pretreatment approaches have been investigated on a variety of feed stocks [3, 19–22]. Among them, hydrothermal, steam explosion, ammonia, sodium hydroxide, and sulfuric acid pretreatment are widely used in present researches. Hydrothermal pretreatment can be considered as an eco-friendly green pretreatment process because it contains lignocellulosic feed stock and water only [23]. To increase effectiveness of steam pretreatment, acid is used as a catalyst that increases hemicellulose recovery and the enzymatic hydrolysis [2]. It is one of the efficient pretreatments for corn stover [1]. Generally, the alkaline pretreatment as NaOH and ammonia are widely used to remove lignin without significantly carbohydrate loss [4, 5, 12]. Besides these, other alkaline pretreatments as calcium hydroxide, potassium hydroxide and sodium hydroxide in combination with hydrogen peroxide are also used for hardwood, herbaceous crops, and agricultural residues pretreatment [24]. Furthermore, sulfuric acid is one of the most utilized chemical pretreatment in the last decade. Recently, oxalic acid is also considered as an alternative to sulfuric acid [25]. However, oxalic acid is more expensive on a weight basis in comparison to sulfuric acid. Single pretreatment method may not be sufficient for enzymatic hydrolysis, although it can be enough for improvements in structure [26]. Proper choice of pretreatment process is crucial because this upstream process is also rendered the downstream microbial and enzymatic processing [2, 18]. The production of hydroxyl-methyl furfural, furfural and phenolic compounds during pretreatment process severely affects the performance of butanol fermentation process using clostridia species [3, 9, 10, 18]. Beside this, the pretreatment process also plays a vital role on economic production butanol as well as animal feed [20, 27–29]. The general flow chart of butanol production along with ongoing strategic efforts on pretreatment is shown in Fig. 1.
The main objective of this review is investigating contemporary status of pretreatment process particularly for corn straw and investigates the influence of process parameter on sugars production. In addition, the effect of biomass size and progress in enzymatic hydrolysis are discussed. Finally, the prospective of pretreatment for butanol production from corn straw as a substrate is also highlighted.
Structure and Choice of Lignocellulosic Biomass
Lignocellulosic biomass, agricultural residue, is composed of mainly cellulose, hemicelluloses and lignin along with smaller amounts of pectin, protein, extractives, and ash [19, 30]. In addition, the ratios between various constituents within a single plant vary with age, stage of growth, and other conditions [19]. Cellulose is the predominant polymer in lignocellulosic biomass and it consists of long homopolymer of β-(1,4) glycosidic bond linked D-glucose units. Cellulose molecules are arranged into thin hair like strands called microfibrils. These microfibrils are arranged in a mesh like pattern along with hemicelluloses and lignin, which link them together and help strengthen the plant cell wall [19, 30]. Fermentable D-glucose can be produced from cellulose through the action of either acid or enzymes breaking the β-(1,4) glycosidic linkages [19].
Hemicellulose has branches with short lateral chains consisting of different sugars such as pentoses (xylose, rhamnose, and arabinose), hexoses (glucose, mannose, and galactose), and uronic acids (e.g., 4-omethylglucuronic, D-glucuronic, and D-galactouronic acids) [19, 30]. In contrast to cellulose, the polymers present in hemicelluloses are easily hydrolysable [19]. Lignin is a complex, larger molecular structure containing cross-linked polymers of phenolic monomers. It is present in the primary cell wall, imparting structural support, impermeability, and resistance against microbial attack. Three phenyl propionic alcohols exist as monomers of lignin: coniferyl alcohol (guaiacyl propanol), coumaryl alcohol (p-hydroxyphenyl propanol), and sinapyl alcohol [19, 30]. Alkyl-aryl, alkyl-alkyl, and aryl-aryl ether bonds link these phenolic monomers together [19, 30]. Figure 2 reveals cellulose, hemicelluloses, and lignin of most common lignocellulosic agricultural residue [1, 2, 19, 23, 24, 30–32].
Agricultural residue with high cellulose and hemicelluloses content as well as low lignin content is most favorable for butanol production. Barley straw has low lignin content and obvious choice; however, single parameter cannot be used to decide the best substrate. Choice of best substrate is highly influenced with its cost and pretreatment process. Particularly for Nepal, China, and some other agricultural countries, corn stover is the most important substrate of bio-butanol due to high concentration of cellulose and hemicelluloses as well as economic.
Particle Size of Substrate
Size reduction of substrate before pretreatment is essential and an energy intensive as well as costly process [33]. High efficient heat and mass transfer is necessary for efficient and effective pretreatment process, which can be obtained from suitable substrate size before pretreatment. Smaller particle size causes high degradation of hemicelluloses, whereas a larger particle size may result in overcooked surface part and incomplete pretreatment in inner part [1]. Therefore, optimized particle size yields more sugars and subsequently reduces high preparation cost. Table 1 reflects particle sizes of past researches. Based on our knowledge, besides reference [1], none of the researchers mention about the effect of particle size on sugars and microbial inhibitors production.
The analysis of results of reference [1] is shown in Fig. 3. The steam explosion pretreatment at 200 °C for 5 min (Fig. 3) pointed out that glucose yield is increased with particle size and decreased after particle size of 2cm. The inhibitors of microorganism, hydroxyl-methyl furfural (HMF) and furfural, are increased slightly initially, almost steady before particle size of 2 cm and then increased rapidly. It is reported that particle size 2.5 cm is better for sugar conversion and particle size 1 cm as well as 0.5 cm are better for sugar recovery [1].
Effect of biomass particle size on glucose and inhibitors. This graph is prepared based on result obtained from steam explosion pretreatment at 200 °C for 5 min. Enzymatic hydrolysis conditions: 1 % glucan loading (w/v), 60 FPU/g glucan [1]
Status of Pretreatment Processes
The results of different historical research showed that whatever the substrate and bacterial strain type of pretreatment always affect butanol yield significantly. The agricultural residues and wastes are composed primarily of polysaccharides that contain six and five carbon sugars. Prior to the use of these substrates, these feed stocks must be hydrolyzed using a combination of alkali/acid pretreatment and enzymes [11, 15]. Thus, considerable research effort has been made towards utilization of lignocellulosic hydrolysate for butanol fermentation [16, 37–41]. The comparative analysis of different pretreatment process is given in Table 2. Besides these pretreatment (Table 2), ionic liquid pretreatment [42] and microwave pre-treatment [43] are also used for corn stover.
Among pretreatment processes used for corn stalk, corn straw and corn stover along with their best known results are presented in Table 3. Consequences of past researches showed that sulfuric acid pretreatment has the highest sugar yield followed by aqueous ammonia, steam explosion, NaOH, and hydrothermal pretreatment, particularly corn stover as a substrate. Almost all pretreatment processes listed in Table 3 are used to cellulase as a major enzyme for hydrolysis. The selection of best pretreatment for a particular substrate not only depends on sugar yield but also highly influences by concentration of microbial inhibitors. Microbial inhibitors depend on time and temperature of pretreatment process.
Effect of Severity Factor (SF) on Common Pretreatment Processes
The relationship between time and temperature of pretreatment process is a severity factor (SF). This parameter facilitates comparison of a broad range of yield data by coupling the reaction conditions of time and temperature into a single variable. The severity factor was defined as [23, 46]:
where t is reaction time in minutes, T H is the hydrolysis temperature in °C, and T R is a reference temperature, most often 100 °C.
Overend and Chornet developed the first equation of SF in 1987 as reaction ordinate or severity parameter. It is being used to represent SF since then. Pretreatment conditions can be compared combining both pretreatment time and temperature into a single reaction ordinate using SF.
Steam explosion (SE) is the most extensively studied and commonly applied physicochemical method of biomass pretreatment. An “explosive” action on the fibers is necessary for them to become hydrolysable biomass is usually treated with high pressure saturated steam at temperatures of about 160–240 °C and pressures between 0.7 and 4.8 MPa. On the other hand, the liquid ammonia pretreatment process is usually conducted at a temperature less than 90 °C and the duration could be up to 10–60 days. At higher temperatures (150–190 °C) the effect of ammonia is rapid and the duration of pretreatment is reduced to minutes. Figure 4 highlights the relationship between glucose and inhibitors yield with SF during steam explosion and aqueous ammonia pretrement process of corn stover [1, 2, 4, 5, 44, 47–50]. Glucose yield in both pretreatment processes is decreased significantly after the SF threshold value of 2.58 for aqueous ammonia and 3.79 for steam explosion. The decrease in glucose reflects the formation of HMF and furfural as well as other byproduct of exceeding in SF from threshold value. Aqueous ammonia pretreatment process is considered as inhibitors free treatment process. Thus formation of HMF and furfural is not reported.
Relationship between glucose and inhibitor yields with SF during steam explosion and aqueous ammonia pretreatment. SF was determined using different pretreatment temperature, and time such as (90 °C, 5 min), (40 °C, 480 min), (60 °C, 720 min), (60 °C, 1,440 min), (20 °C, 86,400 min), (170 °C, 10 min) for aqueous ammonia, and similarly, (200 °C, 5 min), (205 °C, 5 min), and (210 °C, 10 min) for steam explosion as reported in the references [1, 2, 4, 5, 42–46]
In the case of hydrothermal pretreatment, total sugar yield is increased below threshold value of SF (3.88) as shown in Fig. 5 [23]. After threshold value of SF total sugar yield is decreased rapidly first and then slow down its declining rate (Fig. 5). However, there is a polynomial relationship of inhibitors having R 2 value 0.997 with SF, which shows an increase in inhibitors with SF.
Relationship between total sugar and inhibitors during hydrothermal pretreatment; total inhibitors include HMF and furfural, where SF was determined using at a pretreatment temperature of 200 °C and times 5, 15, 25, 35, and 60 min, respectively [23]
Furthermore, Fig. 6 reveals the relationship between SF, NaOH concentration and average sugar yield during sodium hydroxide pretreatment of corn stover [24]. Pretreatment with alkali such as NaOH, KOH, Ca(OH)2, and hydrazine cause swelling of biomass, which increases the internal surface area of the biomass, and decreases both the degree of polymerization, and cellulose crystallinity. Alkaline pretreatment disrupts the lignin structure and breaks the linkage between lignin and other carbohydrate fractions in lignocellulosic biomass, thus making the carbohydrates in the hetero-matrix more accessible. The reactivity of remaining polysaccharides increases as the lignin is removed. However, most of the alkali is consumed. Alkali pretreatment is most effective with low lignin content biomass like agricultural residues but becomes less applicable as lignin content of the biomass increases. The analysis of data that are reported on [24] clearly shows that with increase in NaOH concentration also increased total sugar yield, however, the threshold value of SF slightly increased from 1.83 to 2.15 when NaOH concentration increased from 0.007 g/g corn stover to 0.088 g/g corn stover, respectively. In the both concentration of NaOH, there is a polynomial relationship between total sugar and severity factor with R 2 value greater than 0.9 as shown in Fig. 6. Hence, researcher should always keep SF below its threshold value to optimize total sugar yield during NaOH pretreatment of corn stover.
Relationship between SF, NaOH concentration, and average sugar yield; where SF was calculated using pretreatment temperature and time such as (60 °C, 75 min), (95 °C, 30 min), (95 °C, 75 min), and (95 °C, 120 min) for 0.07 g NaOH/g corn stover as well as (74 °C, 48 min), (74 °C, 102 min), (116 °C, 48 min), and (116 °C, 102 min) for 0.08 g NaOH/g corn stover [24]
Moreover, the effect of SF during sulfuric acid pretreatment of corn stover is presented in Fig. 7 [2]. In contrast to base higher concentration of sulfuric acid has an adverse effect on total sugar yield from corn stover [24, 34, 51]. In addition, the concentration of total inhibitors from sulfuric acid is also greater than NaOH pretreatment as shown in Fig. 7.
Relationship between sulfuric acid concentration, total sugar, and inhibitors with SF. Inhibitors include HMF and furfural. SF was calculated from pretreatment temperature and time such as (190 °C, 5 min), (200 °C, 5 min), (210 °C, 5 min), (190 °C, 10 min), (200 °C, 10 min), and (210 °C, 10 min) for both cases [2]
The comparison among most common pretreatment processes with SF reveals basic chemical solution (high pH) is better than acidic (low pH) chemical solution for the pretreatment of corn stover. Furthermore, proper choice of severity factor along with concentration is a crucial step for maximum sugar yield and reduces microbial inhibitors. So, every researcher should be considered SF during all types of pretreatment.
Inhibitors Concentration for Inhibition of Clostridium Strain(s)
Chemical pretreatments of corn straw as discussed above produce degradation products, which have an inhibitory effect on the Clostridium strain(s), thus reducing acetone-butanol-ethanol (ABE) yield and productivity. Along with the pretreatment and hydrolysis processes of lignocellulosic biomass, salts, acetate, furfural, HMF, levulinic acid, formic acid, and aromatic compounds such as phenolics are formed. Furfural and HMF are produced from pentose and hexose sugars, respectively. Cell multiplication is inhibited by furfural and HMF at higher concentrations, however, its low concentrations can be beneficial to fermentation because yeast of ABE fermentation medium can use them to regenerate NAD+, which ultimately reduces glycerol production. Weak acids such as levulinic acid, formic acid and acetic acid are produced from the degradation of mainly hemicellulose and lignin. Weak acids inhibit fermentation either uncoupling of metabolism or intracellular anionic accumulation. Phenolic compounds, like vanillin, syringaldehyde, and ferulate are produced from the degradation of lignin, which plays a vital role to decrease adenosine triphosphate (ATP). The total soluble phenolic compounds (TPC), furfural, and HMF inhibit cell growth and have remarkable impact on butanol concentration [16, 38, 52].
Furthermore, based on discussion and graphical analysis demonstrated in Fig. 4, 5, 6 and 7, microbial inhibitors increased with increasing in SF. The level of toxicity depends on many factors such as type of strain, strain physiological conditions, dissolved oxygen concentration, and pH of the medium. For example, C. beijerinckii BA101 can tolerate more furfural and HMF, whereas C. beijerinckii RT66 can tolerate more phenolic compounds in comparison to other strain(s) [38, 53]. The inhibitory effect of these compounds is higher when they are present together due to a synergistic effect [54]. Besides this, one interesting thing is that butanol itself is toxic to butanol producing Clostridium species. Most of the common Clostridium strain(s) effectiveness decline, when concentration of total solvents (ABE) in the fermentation broth is 20 g/l, of which butanol is only about 13 g/l [9, 10, 13, 38, 55].
Hence, presence of all inhibitors at a time severely affects microbes of fermentation broth in one and other ways. Several methods have been investigated, ranging from over liming [41], ion exchange resins adsorption [16], and peroxidase treatment [53] for the removal of microbial inhibitors. Table 4 reflects widely used Clostridium strain(s), type of inhibitors, and an indication of allowable concentration for ABE fermentation.
Analysis of effect of inhibitors on fermentation is widely studied in ethanol fermentation; however, it is limited explored in ABE fermentation. Every researcher should investigate the mentioned inhibitors to identify allowable concentration as well as its effects. It is obvious from Figs. 4, 5, 6, and 7 that along with type of pretreatment process SF has a major role in the formation of such inhibitors. In each pretreatment process, total sugar is maximum at a particular SF, on the other hand inhibitors formation is polynomial increases in SF. Maximization of sugar is the primary objective of every pretreatment process, however, researchers should look to their corresponding inhibitors in the fermentation broth because they have also a major impact on ABE yield and productivity.
Prospective of Pretreatment Process and Bio-butanol Production
In China, ABE entrepreneurs are increasingly interested in the utilization of lignocellulosic biomass, while research progress has remained in a preliminary stage [57]. In fact, the total solvent concentration in fermentation broth having corn straw/stover as a substrate is usually around 5–27 g/l as gave in Table 5. Additionally, cell growth and solvent production are often seriously inhibited by butanol concentrations of over 12 g/l [57].The contradiction between their low production and high demands gives rise to the necessity of addressing and solving the problems related to the butanol fermentation process. Now, most of the researchers have focused on butanol fermentation, however, mechanism of metabolic shift from acidogenesis to solventogenesis is still unclear. In order to improve butanol production and reduce overall production cost extensive study will be done in substrate pretreatment, the composition of strain, fermentation process and product recoveries.
In addition, the problem associated with the raw material can be eliminated by cheap and abundant lignocellulosic biomass such as corn stalk in agriculture. However, the authors are worried about the solvent concentration of corn stalk, which is one of the lowest solvent substrate among other lignocellulosic substrates.
The problems associated with pretreatment process can be solved by selection of proper SF. Biphasic pretreatment, hydrothermal followed by acidic/alkaline pretreatment with larger particle size (>1 cm) may be another option. Furthermore, biological pretreatment process should be explored in future researches.
Once the problems associated with pretreatment and bio-butanol fermentation are solved, then all agricultural based countries like Nepal, China can effectively launch commercial production of bio-butanol from corn stalk, which ultimately reduces wealth export and help to decrease fossil fuel crisis.
Conclusion
This paper examined consequences of particle size on sugar and inhibitor production; however, most researchers are silenced about it. From the limited available information and preliminary analysis of an ongoing experiment, the authors are believed that 10–15 mm particle sizes may be more effective for efficient pretreatment because the large particle size ultimately reduced expensive milling cost. In addition, pretreatment processes like physical, chemical and both together are widely used for corn straw. Chemical pretreatment, dilute sulfuric acid treatment is among the most explored pretreatment processes and is one of the foremost options for application in industrial scales as well. Proper choice of time and temperature, SF, can be minimized some of the limitation of dilute acid pretreatment. But, acid concentration during pretreatment is highly sensitive with SF, the environmental impacts of the process, especially waste disposal, and preliminary results of an ongoing investigation to hinder the choice of sulfuric acid pretreatment particularly for corn straw. The analysis of past researches also showed that alkaline pretreatment as NaOH and aqueous ammonia treatment will be helpful for pretreatment of corn straw with proper SF. Furthermore, alkaline pretreatment decreased lignin barrier and increased the accessible surface area, which is more applicable for enzymatic hydrolysis. Moreover, research and development are essential to select proper pretreatment for corn straw that will enhance microbial fermentation of bio-butanol. Such researches ultimately transform huge amount of worldwide corn straw into bio-butanol effectively and efficiently.
References
Liu, Z. H., Qin, L., Pang, F., Jin, M. J., Li, B. Z., Kang, Y., & Yuan, Y. J. (2013). Effects of biomass particle size on steam explosion pretreatment performance for improving the enzyme digestibility of corn stover. Industrial Crops and Products, 44, 176–184. doi:10.1016/j.indcrop.2012.11.009.
Bondesson, P. M., Galbe, M., & Zacchi, G. (2013). Ethanol and biogas production after steam pretreatment of corn stover with or without the addition of sulphuric acid. Biotechnology for biofuels, 6(1), 11. doi:10.1186/1754-6834-6-11.
Yang, L., Cao, J., Mao, J., & Jin, Y. (2013). Sodium carbonate–sodium sulfite pretreatment for improving the enzymatic hydrolysis of rice straw. Industrial Crops and Products, 43, 711–717. doi:10.1016/j.indcrop.2012.08.027.
Yoo, C. G., Wang, C., Yu, C., & Kim, T. H. (2013). Enhancement of enzymatic hydrolysis and Klason lignin removal of corn stover using photocatalyst-assisted ammonia pretreatment. Applied biochemistry and biotechnology, 169(5), 1648–1658. doi:10.1007/s12010-012-0002-4.
Zhang, C., Pang, F., Li, B., Xue, S., & Kang, Y. (2013). Recycled aqueous ammonia expansion (RAAE) pretreatment to improve enzymatic digestibility of corn stalks. Bioresource Technology, 138, 314–320. doi:10.1016/j.biortech.2013.03.091.
Fischer, C. R., Klein-Marcuschamer, D., & Stephanopoulos, G. (2008). Selection and optimization of microbial hosts for biofuels production. Metabolic engineering, 10(6), 295–304. doi:10.1016/j.ymben.2008.06.009.
Atsumi, S., Cann, A. F., Connor, M. R., Shen, C. R., Smith, K. M., Brynildsen, M. P., & Liao, J. C. (2008). Metabolic engineering of Escherichia coli for 1-butanol production. Metabolic engineering, 10(6), 305–311. doi:10.1016/j.ymben.2007.08.003.
Cheng, C.-L., Che, P.-Y., Chen, B.-Y., Lee, W.-J., Lin, C.-Y., & Chang, J.-S. (2012). Biobutanol production from agricultural waste by an acclimated mixed bacterial microflora. Applied Energy, 100, 3–9. doi:10.1016/j.apenergy.2012.05.042.
Jang, Y., Lee, J. Y., & Lee, J. (2012). Enhanced Butanol Production Obtained by Reinforcing the Direct Butanol-Forming Route in Clostridium acetobutylicum. American society of microbiology, 3(5), 1–9. doi:10.1128/mBio.00314-12.Updated.
Lee, S., Cho, M. O., Park, C. H., Chung, Y., & Kim, J. H. (2008). Continuous Butanol Production Using Suspended and Immobilized Clostridium beijerinckii NCIMB 8052 with Supplementary Butyrate. Energy and Fuel, 22, 3459–3464.
Liu, Z., Ying, Y., Li, F., Ma, C., & Xu, P. (2010). Butanol production by Clostridium beijerinckii ATCC 55025 from wheat bran. Journal of industrial microbiology & biotechnology, 37(5), 495–501. doi:10.1007/s10295-010-0695-8.
Steen, E. J., Chan, R., Prasad, N., Myers, S., Petzold, C. J., Redding, A., & Keasling, J. D. (2008). Metabolic engineering of Saccharomyces cerevisiae for the production of n-butanol. Microbial cell factories, 7, 36. doi:10.1186/1475-2859-7-36.
Peralta-Yahya, P. P., Zhang, F., del Cardayre, S. B., & Keasling, J. D. (2012). Microbial engineering for the production of advanced biofuels. Nature, 488, 320–328.
Richmond, C., Ujor, V., & Ezeji, T. C. (2012). Impact of syringaldehyde on the growth of Clostridium beijerinckii NCIMB 8052 and butanol production. 3. Biotech, 2(2), 159–167. doi:10.1007/s13205-011-0042-4.
Qureshi, N., Saha, B. C., & Cotta, M. (2007). Butanol production from wheat straw hydrolysate using Clostridium beijerinckii. Bioprocess and biosystems engineering, 30(6), 419–427. doi:10.1007/s00449-007-0137-9.
Qureshi, N., Ezeji, T. C., Ebener, J., Dien, B. S., Cotta, M. A., & Blaschek, H. P. (2008). Butanol production by Clostridium beijerinckii. Part I: use of acid and enzyme hydrolyzed corn fiber. Bioresource technology, 99(13), 5915–5922. doi:10.1016/j.biortech.2007.09.087.
Harun, M. Y., Dayang Radiah, A. B., Zainal Abidin, Z., & Yunus, R. (2011). Effect of physical pretreatment on dilute acid hydrolysis of water hyacinth (Eichhornia crassipes). Bioresource technology, 102(8), 5193–5199. doi:10.1016/j.biortech.2011.02.001.
Zhu, J. Y., & Pan, X. J. (2010). Woody biomass pretreatment for cellulosic ethanol production: Technology and energy consumption evaluation. Bioresource technology, 101(13), 4992–5002. doi:10.1016/j.biortech.2009.11.007.
Kumar, P., Barrett, D. M., Delwiche, M. J., & Stroeve, P. (2009). Methods for Pretreatment of Lignocellulosic Biomass for Efficient Hydrolysis and Biofuel Production. Industrial & Engineering Chemistry Research, 48(8), 3713–3729. doi:10.1021/ie801542g.
Taherzadeh, M. J., & Karimi, K. (2007). Acid-based hydrolysis processes for ethanol from lignocellulosic materials: a review. Bioresources, 2, 472–499.
Zheng, Y., Pan, Z., & Zhang, R. (2009). OVerview of Biomass Pretreatment for Cellulosic Ethanol Production. International Journal of Agricultural and Biological Engineering, 2(3), 51–68.
Zheng, Y.-N., Li, L.-Z., Xian, M., Ma, Y.-J., Yang, J.-M., Xu, X., & He, D.-Z. (2009). Problems with the microbial production of butanol. Journal of industrial microbiology & biotechnology, 36(9), 1127–1138. doi:10.1007/s10295-009-0609-9.
Saha, B. C., Yoshida, T., Cotta, M. A., & Sonomoto, K. (2013). Hydrothermal pretreatment and enzymatic saccharification of corn stover for efficient ethanol production. Industrial Crops and Products, 44, 367–372. doi:10.1016/j.indcrop.2012.11.025.
Chen, Y., Stevens, M. A., Zhu, Y., Holmes, J., & Xu, H. (2013). Understanding of alkaline pretreatment parameters for corn stover enzymatic saccharification. Biotechnology for biofuels, 6(1), 8. doi:10.1186/1754-6834-6-8.
Lee, H.-J., Seo, Y.-J., & Lee, J.-W. (2013). Characterization of oxalic acid pretreatment on lignocellulosic biomass using oxalic acid recovered by electrodialysis. Bioresource technology, 133, 87–91. doi:10.1016/j.biortech.2013.01.051.
Yu, J., Zhang, J., He, J., Liu, Z., & Yu, Z. (2009). Combinations of mild physical or chemical pretreatment with biological pretreatment for enzymatic hydrolysis of rice hull. Bioresource technology, 100(2), 903–908. doi:10.1016/j.biortech.2008.07.025.
Wang, Y., Spratling, B. M., Zobell, D. R., Wiedmeier, R. D., & Mcallister, T. A. (2004). Effect of alkali pretreatment of wheat straw on the efficacy of exogenous fibrolytic enzymes The online version of this article, along with updated information and services, is located on the World Wide Web at: Effect of alkali pretreatment of wheat st. Journal of Animal Science, 82, 198–208.
Yang, B., & Wyman, C. E. (2008). Pretreatment : the key to unlocking low-cost cellulosic ethanol. Biofuels, Bioproduct and Biorefining, 2, 26–40. doi:10.1002/bbb.
Teghammar, A., Yngvesson, J., Lundin, M., Taherzadeh, M. J., & Horváth, I. S. (2010). Pretreatment of paper tube residuals for improved biogas production. Bioresource technology, 101(4), 1206–1212. doi:10.1016/j.biortech.2009.09.029.
Ezeji, T. C., & Blaschek, H. P. (2010). Butanol from lignocellulosic biomass. Biofuel from agricultural waste and by product, Chapter, 3, 19–36.
Mu, X., Sun, W., Liu, C., & Wang, H. (2011). Improved efficiency of separate hexose and pentose fermentation from steam-exploded corn stalk for butanol production using Clostridium beijerinckii. Biotechnology letters, 33(8), 1587–1591. doi:10.1007/s10529-011-0598-4.
Donghai, S. U., Junshe, S., Ping, L., & Yanping, L. (2006). Effects of Different Pretreatment Modes on the Enzymatic Digestibil- ity of Corn Leaf and Corn Stalk. Chinese Journal of Chemical Engineering, 14(6), 796–801.
Zhu, Z., Sathitsuksanoh, N., Vinzant, T., Schell, D. J., McMillan, J. D., & Zhang, Y.-H. P. (2009). Comparative study of corn stover pretreated by dilute acid and cellulose solvent-based lignocellulose fractionation: Enzymatic hydrolysis, supramolecular structure, and substrate accessibility. Biotechnology and bioengineering, 103(4), 715–724. doi:10.1002/bit.22307.
Um, B.-H., & van Walsum, G. P. (2012). Effect of pretreatment severity on accumulation of major degradation products from dilute acid pretreated corn stover and subsequent inhibition of enzymatic hydrolysis of cellulose. Applied biochemistry and biotechnology, 168(2), 406–420. doi:10.1007/s12010-012-9784-7.
Qin, L., Liu, Z.-H., Li, B.-Z., Dale, B. E., & Yuan, Y.-J. (2012). Mass balance and transformation of corn stover by pretreatment with different dilute organic acids. Bioresource technology, 112, 319–326. doi:10.1016/j.biortech.2012.02.134.
Gao, K., Li, Y., Tian, S., & Yang, X. (2012). Screening and characteristics of a butanol-tolerant strain and butanol production from enzymatic hydrolysate of NaOH-pretreated corn stover. World journal of microbiology & biotechnology, 28(10), 2963–2971. doi:10.1007/s11274-012-1107-1.
Ezeji, T., & Blaschek, H. P. (2008). Fermentation of dried distillers’ grains and solubles (DDGS) hydrolysates to solvents and value-added products by solventogenic clostridia. Bioresource technology, 99(12), 5232–5242. doi:10.1016/j.biortech.2007.09.032.
Ezeji, T., Qureshi, N., & Blaschek, H. P. (2007). Butanol Production From Agricultural Residues : Impact of Degradation Products on Clostridium beijerinckii Growth and Butanol Fermentation. Biotechnology and bioengineering, 97(6), 1460–1469. doi:10.1002/bit.
Lu, X. B., Zhang, Y. M., Yang, J., & Liang, Y. (2007). Enzymatic Hydrolysis of Corn Stover after Pretreatment with Dilute Sulfuric Acid. Chemical Engineering & Technology, 30(7), 938–944. doi:10.1002/ceat.200700035.
Qureshi, N., Saha, B. C., Dien, B., Hector, R. E., & Cotta, M. A. (2010). Production of butanol (a biofuel) from agricultural residues: Part I – Use of barley straw hydrolysate☆. Biomass and Bioenergy, 34(4), 559–565. doi:10.1016/j.biombioe.2009.12.024.
Qureshi, N., Saha, B. C., Hector, R. E., Dien, B., Hughes, S., Liu, S., & Cotta, M. A. (2010). Production of butanol (a biofuel) from agricultural residues: Part II – Use of corn stover and switchgrass hydrolysates☆. Biomass and Bioenergy, 34(4), 566–571. doi:10.1016/j.biombioe.2009.12.023.
Sun, L., Li, C., Xue, Z., Simmons, B., & Singh, S. (2013). Unveiling high-resolution, tissue specific dynamic changes in corn stover during ionic liquid pretreatment. RSC Advances, 3(6), 2017. doi:10.1039/c2ra20706k.
Chundawat, S. P. S., Venkatesh, B., & Dale, B. E. (2007). Effect of Particle Size Based Separation of Milled Corn Stover on AFEX Pretreatment and Enzymatic Digestibility, 96(2), 219–231. doi:10.1002/bit.
Kim, T. H., & Lee, Y. Y. (2005). Pretreatment of Corn Stover by Soaking AND. Applied Biochemistry And Biotechnology, 121, 1119–1132.
Avci, A., Saha, B. C., Dien, B. S., Kennedy, G. J., & Cotta, M. (2013). Response surface optimization of corn stover pretreatment using dilute phosphoric acid for enzymatic hydrolysis and ethanol production. Bioresource technology, 130, 603–612. doi:10.1016/j.biortech.2012.12.104.
Lloyd, T., & Wyman, C. E. (2005). Combined sugar yields for dilute sulfuric acid pretreatment of corn stover followed by enzymatic hydrolysis of the remaining solids. Bioresource technology, 96(18), 1967–1977. doi:10.1016/j.biortech.2005.01.011.
Kim, T. H., & Lee, Y. Y. (2005). Pretreatment and fractionation of corn stover by ammonia recycle percolation process. Bioresource technology, 96(18), 2007–2013. doi:10.1016/j.biortech.2005.01.015.
Liu, Z.-H., Qin, L., Jin, M.-J., Pang, F., Li, B.-Z., Kang, Y., & Yuan, Y.-J. (2013). Evaluation of storage methods for the conversion of corn stover biomass to sugars based on steam explosion pretreatment. Bioresource technology, 132, 5–15. doi:10.1016/j.biortech.2013.01.016.
Teymouri, F., Laureano-Perez, L., Alizadeh, H., & Dale, B. E. (2005). Optimization of the ammonia fiber explosion (AFEX) treatment parameters for enzymatic hydrolysis of corn stover. Bioresource technology, 96(18), 2014–2018. doi:10.1016/j.biortech.2005.01.016.
Kim, T. H., & Lee, Y. Y. (2007). Pretreatment of Corn Stover by Soaking in Aqueous Ammonia at Moderate Temperatures. Applied Biochemistry And Biotechnology, 136(7), 81–92.
Vancov, T., & McIntosh, S. (2012). Mild acid pretreatment and enzyme saccharification of Sorghum bicolor straw. Applied Energy, 92, 421–428. doi:10.1016/j.apenergy.2011.11.053.
Guo, T., Tang, Y., Zhang, Q.-Y., Du, T.-F., Liang, D.-F., Jiang, M., & Ouyang, P.-K. (2012). Clostridium beijerinckii mutant with high inhibitor tolerance obtained by low-energy ion implantation. Journal of industrial microbiology & biotechnology, 39(3), 401–407. doi:10.1007/s10295-011-1017-5.
Wang, L., & Chen, H. (2011). Increased fermentability of enzymatically hydrolyzed steam-exploded corn stover for butanol production by removal of fermentation inhibitors. Process Biochemistry, 46(2), 604–607. doi:10.1016/j.procbio.2010.09.027.
Mussatto, S. I., & Roberto, I. C. (2004). Alternatives for detoxification of diluted-acid lignocellulosic hydrolyzates for use in fermentative processes: a review. Bioresource technology, 93(1), 1–10. doi:10.1016/j.biortech.2003.10.005.
Mariano, A. P., Filho, R. M., & Ezeji, T. C. (2012). Energy requirements during butanol production and in situ recovery by cyclic vacuum. Renewable Energy, 47, 183–187. doi:10.1016/j.renene.2012.04.041.
Maddox, I. S., Steiner, E., Hirsch, S., Wessner, S., Gutierrez, N., Gapes, J. R., & Schuster, K. C. (2000). The cause of “acid-crash” and “acidogenic fermentations” during the batch acetone-butanol-ethanol (ABE-) fermentation process. Journal of molecular microbiology and biotechnology, 95(1).
Ni, Y., & Sun, Z. (2009). Recent progress on industrial fermentative production of acetone-butanol-ethanol by Clostridium acetobutylicum in China. Applied microbiology and biotechnology, 83(3), 415–423. doi:10.1007/s00253-009-2003-y.
Parekh, S. R., Parekh, R. S., & Wayman, M. (1988). Ethanol and butanol production by fermentation of enzymatically saccharified SO2-prehydrolysed lignocellulosics. Enzyme and Microbial Technology, 10(11), 660–668.
Zhang, Y., Ma, Y., Yang, F., & Zhang, C. (2009). Continuous acetone-butanol-ethanol production by corn stalk immobilized cells. Journal of industrial microbiology & biotechnology, 36(8), 1117–1121. doi:10.1007/s10295-009-0582-3.
Lin, Y., Wang, J., Wang, X., & Sun, X. (2011). Optimization of butanol production from corn straw hydrolysate by Clostridium acetobutylicum using response surface method. Chinese Science Bulletin, 56(14), 1422–1428. doi:10.1007/s11434-010-4186-0.
Acknowledgments
The authors gratefully acknowledge the National Natural Science Foundation of China (Grant No. 51178136) and the State Key Laboratory of Urban Water Resource and Environment, Harbin Institute of Technology (Grant No.HCK201206) for valuable financial support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Baral, N.R., Li, J. & Jha, A.K. Perspective and Prospective of Pretreatment of Corn Straw for Butanol Production. Appl Biochem Biotechnol 172, 840–853 (2014). https://doi.org/10.1007/s12010-013-0548-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-013-0548-9