Abstract
Water extract of steam-exploded corn stalk (SECS) was detoxified and used as feed for acetone–butanol–ethanol (ABE) fermentation using Clostridium beijerinckii. Utilization of water extract improved the total ABE yield (g ABE/g dry SECS). Separated fermentation showed higher fermentability (0.078 g ABE/g dry SECS) over typical fermentation (0.058 g ABE/g dry SECS). Furthermore, the final ABE yields (g ABE/g utilized sugar) from water extract neutralized by Ca(OH)2, NaOH, and Na2SO3 were 0.16, 0.1 and 0.07, respectively, suggesting that Ca(OH)2 had the best detoxification effect.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Acetone–butanol–ethanol (ABE) production from lignocellulosic biomass, such as wheat straw and corn fiber (Keasling and Chou 2008; Ljungdahl 2009), is a promising alternative for the sustainable production of fuels. As a pretreatment method to make lignocellulose more accessible to enzymes (Qureshi et al. 2007), steam explosion is an effective technique for practical cellulose conversion. The major changes to lignocellulosic biomass by steam explosion are often attributed to the removal of hemicelluloses (Mosier et al. 2005). Steam explosion also produces certain fermentation inhibitors (Ramos 2003) that reduce the fermentability of lignocellulosic materials, necessitating detoxification of SECS before enzymatic hydrolysis. Water-washing (Koukiekolo et al. 2005) and neutralization treatment (Marchal et al. 1986) are promising detoxification methods. When water-washing is used to remove inhibitors present in SECS, most hemicellulose-derived pentoses, representing about 20–35% of lignocellulosic biomass (Jesse et al. 2002), dissolve in the water extract. Utilization of xyloses present in water extract elevates the percent conversion of biomass into ABE and avoids environmental pollution. Moreover, neutralization with Ca(OH)2 or NaOH detoxifies pretreated biomass to remove inhibitors and excess acid (Marchal et al. 1986). Although Na2SO3 has been used in delignification of Lantana camara (Kuhad et al. 2010), no method for detoxification pretreatment using Na2SO3 has been reported.
Monosaccharides from lignocellulosic biomass, such as glucose, xylose, galactose and arabinose, can be used for ABE fermentation (Ezeji et al. 2007). In a typical process, hexoses (mainly glucose) from celluloses and pentoses (mainly xylose) from hemicelluloses are mixed in fermentation broth, where hexoses partially inhibit the utilization of xyloses and thus lead to incomplete xylose utilization (Öhgren et al. 2007). Fermenting glucose (in washed SECS enzymatic hydrolysate) and xylose sugars (in water extract) separately may consequently improve the utilization of these sugars.
The aim of this study is to evaluate and compare the fermentability of separated versus mixed sugars. The detoxification effect of three alkalis on water extract is also investigated. To our knowledge, this is the first report to systematically investigate separated fermentation involving water extract and washed SECS enzymatic hydrolysate using Clostridia beijerinckii.
Materials and methods
Biomass preparation and composition analysis
Corn stalk was air-dried overnight at room temperature and chopped into 5–8 mm pieces. The corn stalks were chemically analyzed for holocellulose, lignin, water–ethanol extractives, and ash. The raw material was consecutively extracted with water and ethanol. The extract-free corn stalk dust was processed for chemical analysis following the National Renewable Energy Laboratory analytical methods (Sluiter et al. 2008).
Detoxification process
For separate fermentation, steam-exploded corn stalks (SECS) were washed in tap water (1/10 w/v) and stirred for 30 min at room temperature. Washed biomass was then filtered through double-layered muslin cloth and the cellulosic residue was collected for enzymatic hydrolysis. Three kinds of alkali were used to detoxify water extract. For typical fermentation, suspension fluid of unwashed SECS at 10% (w/v) solid content was mixed with Ca(OH)2 to raise the pH of solution to 6.7 ± 0.05. No sugar loss was detected after neutralization.
Enzymatic hydrolysis
Washed and unwashed SECS were enzymatically hydrolyzed by cellulolytic complex after detoxification. Cellulose enzyme was added at 20 filter paper units (FPU)/g substrate. Fungal β-glucosidase was used to supplement the β-glucosidase activity at 5 IU/g substrate.
Microorganism and fermentation
The freeze-dried cultures of Clostridium beijerinckii ATCC 55025 were rejuvenated and prepared by the method of Zhang (Zhang et al. 2009). Water extract and both washed and unwashed SECS enzymatic hydrolysates were then fermented. Samples were withdrawn at every 12 h and centrifuged at ~10,000×g. The cell free supernatant was used to determine the ABE and residual sugar concentration.
Analytical methods
The composition of raw and pretreated materials was determined according to the National Renewable Energy Laboratory (NREL, Golden, CO) analytical methods for biomass. A CarboPac PA-10 carbohydrate analysis column was used with 7.5 mM NaOH as a mobile-phase (1 ml/min) at 30°C. The concentration of total reducing sugars in cultures was measured according to the 3,5-dinitrosalicyclic acid method (Jesse et al. 2002).
Results and discussion
Compositional analysis of raw materials
The dried pieces of raw corn stalks contained holocellulose (55.8%), consisting of cellulose (35%) and hemicellulose (20.8%), lignin (22.5%) composed of acid-soluble lignin (ASL, 0.4%) and acid-insoluble lignin (AIL, 22.1%), water–ethanol extractives (11.2%), and ash (4%). This composition is in the range reported for other stalks like corn stover from Europe and North America (Öhgren et al. 2007). The 55.8% holocellulose content made these corn stalks good potential source material for ABE production.
Compositional analysis of pretreated materials
An orthogonal experiment was performed to find the optimum steam explosion conditions for subsequent enzymatic hydrolysis and fermentation (Table 1). The optimum conditions were determined to be presoaking in 1% (w/w) acid for 30 min and a steam explosion pressure of 1.9 MPa for 5 min.
The cellulose content in SECS increased after pretreatment, from 35 to 45.6%. Total lignin content also increased after pretreatment, from 22.5 to 35.8%, due to the removal of hemicellulose. The hemicellulosic sugars content of SECS decreased from 20.8 to 3.2%, suggesting that most of pentoses were released after pretreatment. In water extract fermentation, hemicellulosic sugars were the primary fermentable sugars used to produce ABE. As discussed above, xylose utilization would be inhibited by glucose so it was necessary to separate glucose and xylose sugars released from biomass before fermentation.
Detoxification of water extract
The sugar consumption rate and ABE yield from water extract fermentation under detoxification by the three alkali treatments are compared in Fig. 1. Cultures neutralized by Na2SO3 showed poor fermentability, with low ABE yields (0.07 g/g) and sugar consumption rates (44.6%) because Na2SO3 could not remove fermentation inhibitors present in cultures. Furthermore, accumulation of sulphite during Na2SO3-mediated detoxification might restrain metabolism by microorganism. The ABE yield and sugar consumption during NaOH-mediated detoxification was also lower than measured in Ca(OH)2-treated cultures.
Consumption rate of sugar and acetone–butanol–ethanol yield from water extract fermentation under detoxification by three kinds of alkali. Water extract was recovered and mixed with Ca(OH)2, NaOH, or Na2SO3 and stirred slowly at 50°C until the pH was 6.7 ± 0.05. Fermentation neutralized by Ca(OH)2 showed the best fermentability with ABE yield of 0.16 g/g (compared with 0.18 g/g of control fermentation). Besides, culture neutralized by Ca(OH)2 also showed a high sugar consumption of 91.7% (compared with 92.7% in control fermentation)
Enzymatic saccharification of washed and unwashed SECS
During the course of enzymatic saccharification, the total sugar concentration increased up to 60 h after which it remained almost constant (Fig. 2). The maximum saccharification rate was achieved between 12 and 24 h, followed by decline, likely due to end product inhibition of enzymes by glucose and cellobiose (Kuhad et al. 1999). Unwashed SECS enzymatic hydrolysate contained more total sugar (39.5 g sugar l−1 vs. 31.1 g sugar l−1) because xylose released during pretreatment was also dissolved in solution. For washed SECS enzymatic hydrolysate, only glucose and other hexose were dissolved in solution.
Total sugar concentration of washed and unwashed steam-exploded corn stalk (SECS) enzymatic hydrolysate (Symbol: open circle: washed SECS; filled square: unwashed SECS). Enzymatic hydrolysis was performed in 0.05 M sodium citrate buffer (pH 4.8) at 50°C. The solid content of the reaction was 10% (w/v). Sealed flasks were fixed on a swing bed and rotated at 160 rpm for 72 h. Samples were taken every 12 h to determine the glucose concentration. All enzymatic hydrolysis experiments were performed in duplicate
Fermentation
Separate fermentations of glucose, xylose, and glucose/xylose (1:1) sugars were performed before the water extract fermentation to determine the ABE production period and to clarify the necessity of separating glucose and xylose sugars. During the course of fermentation, the ABE concentration increased until 60 h and then remained almost constant (Fig. 3), possible due to end product inhibition on the microorganisms. The final ABE concentration (5.48 g ABE l−1) in the mixed glucose/xylose culture was lower than the average ABE concentration obtained in separate glucose and xylose cultures (5.74 g ABE l−1), indicating that glucose partially inhibited xylose utilization in glucose/xylose mixtures.
The acetone–butanol–ethanol concentration (Con.) in different cultures with the same initial sugar concentration of 40 g sugar l−1 (Symbol: filled square: glucose; filled circle: xylose; open triangle: glucose/xylose (1:1) mixtures). Acetone–butanol–ethanol was measured by a SP6890 gas chromatograph equipped with a flame ionization detector (FID) and 30 m × 0.32 mm glass column (PEG-20 M). Nitrogen was used as the carrier gas
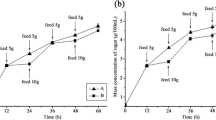
Two series of fermentation were carried out to detect the fermentability of separated and mixed cultures. Groups were both based on SECS with the same dry weight of 15 g. Figure 4 shows the flow diagram of these two fermentation technologies. Compared with typical fermentation, 34.5% more ABE could be produced from the same SECS weight by separated fermentation. As discussed, in typical fermentation, xylose and glucose were mixed together and utilization of xylose was inhibited by glucose, resulting in a reduced ABE yield. In contrast, inhibition of xylose metabolism in presence of glucose was avoided in separated fermentation, thus substantially increasing the ABE yield.
The flow diagram of fermentation. a Separated fermentation. b Typical fermentation in separated fermentation, water extract (detoxified by Ca(OH)2) and washed SECS enzymatic hydrolysate were fermented separately. In typical fermentation, steam-exploded corn stalk hydrolysate was neutralized by Ca(OH)2 directly without water-washing
Conclusions
This article explored a novel technology to produce ABE by Clostridium beijerinckii ATCC55025. Separate fermentation of glucose and xylose produced 34.5% more ABE than fermentation of unwashed SECS enzymatic hydrolysate containing both glucose and xylose. The production of ABE from water extract has the dual advantages of producing more energy and utilizing hemicelluloses. Developing a separated fermentation system with new strains capable of fermenting both hexose and pentose with higher ABE yield would economize ABE production from lignocellulosic biomass.
References
Ezeji T, Qureshi N, Blaschek HP (2007) Butanol production from agricultural residues: impact of degradation products on Clostridium beijerinckii growth and butanol fermentation. Biotechnol Bioeng 97(6):1460–1469
Jesse TW, Ezeji TC, Qureshi N, Blaschek HP (2002) Production of butanol from starch-based waste packing peanuts and agricultural waste. J Ind Microbiol Biotechnol 29(3):117–123
Keasling JD, Chou H (2008) Metabolic engineering delivers next-generation biofuels. Nat Biotechnol 26(3):298–299
Koukiekolo R, Cho HY, Kosugi A, Inui M, Yukawa H, Doi RH (2005) Degradation of corn fiber by Clostridium cellulovorans cellulases and hemicellulases and contribution of scaffolding protein CbpA. Appl Environ Microbiol 71(7):3504–3511
Kuhad RC, Manchanda M, Singh A (1999) Hydrolytic potential of extracellular enzymes from a mutant strain of Fusarium oxysporum. Bioprocess Biosyst Eng 20(2):133–135
Kuhad RC, Gupta R, Khasa YP, Singh A (2010) Bioethanol production from Lantana camara (red sage): pretreatment, saccharification and fermentation. Bioresour Technol 101(21):8348–8354
Ljungdahl LG (2009) A life with acetogens, thermophiles, and cellulolytic anaerobes. Annu Rev Microbiol 63:1–25
Marchal R, Ropars M, Vandecasteele JP (1986) Conversion into acetone and butanol of lignocellulosic substrates pretreated by steam explosion. Biotechnol Lett 8(5):365–370
Mosier N, Wyman C, Dale B, Elander R, Lee YY, Holtzapple M, Ladisch M (2005) Features of promising technologies for pretreatment of lignocellulosic biomass. Bioresour Technol 96(6):673–686
Öhgren K, Bura R, Saddler J, Zacchi G (2007) Effect of hemicellulose and lignin removal on enzymatic hydrolysis of steam pretreated corn stover. Bioresour Technol 98(13):2503–2510
Qureshi N, Saha BC, Cotta MA (2007) Butanol production from wheat straw hydrolysate using Clostridium beijerinckii. Bioprocess Biosyst Eng 30(6):419–427
Ramos LP (2003) The chemistry involved in the steam treatment of lignocellulosic materials. Quím Nova 26:863–871
Sluiter A, Hames B, Ruiz R, Scarlata C, Sluiter J, Templeton D, Crocker D (2008) Determination of Structural Carbohydrates and Lignin in Biomass. vol NREL/TP-510-42618. Laboratory Analytical Procedure (LAP), National Renewable Energy Laboratory
Zhang YD, Ma YJ, Yang FX, Zhang CH (2009) Continuous acetone–butanol–ethanol production by corn stalk immobilized cells. J Ind Microbiol Biotechnol 36(8):1117–1121
Acknowledgments
This work is supported by the Key Science and Technology Program of Shandong Province (No. 2007GG2QT07006 and No. 2008GG20002038) and the Qingdao Key Technology Program (No. 09-1-4-1-nsh).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Mu, X., Sun, W., Liu, C. et al. Improved efficiency of separate hexose and pentose fermentation from steam-exploded corn stalk for butanol production using Clostridium beijerinckii . Biotechnol Lett 33, 1587–1591 (2011). https://doi.org/10.1007/s10529-011-0598-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10529-011-0598-4