Abstract
In these studies, butanol (acetone butanol ethanol or ABE) was produced from wheat straw hydrolysate (WSH) in batch cultures using Clostridium beijerinckii P260. In control fermentation 48.9 g L−1 glucose (initial sugar 62.0 g L−1) was used to produce 20.1 g L−1 ABE with a productivity and yield of 0.28 g L−1 h−1 and 0.41, respectively. In a similar experiment where WSH (60.2 g L−1 total sugars obtained from hydrolysis of 86 g L−1 wheat straw) was used, the culture produced 25.0 g L−1 ABE with a productivity and yield of 0.60 g L−1 h−1 and 0.42, respectively. These results are superior to the control experiment and productivity was improved by 214%. When WSH was supplemented with 35 g L−1 glucose, a reactor productivity was improved to 0.63 g L−1 h−1 with a yield of 0.42. In this case, ABE concentration in the broth was 28.2 g L−1. When WSH was supplemented with 60 g L−1 glucose, the resultant medium containing 128.3 g L−1 sugars was successfully fermented (due to product removal) to produce 47.6 g L−1 ABE, and the culture utilized all the sugars (glucose, xylose, arabinose, galactose, and mannose). These results demonstrate that C. beijerinckii P260 has excellent capacity to convert biomass derived sugars to solvents and can produce over 28 g L−1 (in one case 41.7 g L−1 from glucose) ABE from WSH. Medium containing 250 g L−1 glucose resulted in no growth and no ABE production. Mixtures containing WSH + 140 g L−1 glucose (total sugar approximately 200 g L−1) showed poor growth and poor ABE production.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Research has been intensified towards production of alternative fuels such as ethanol and butanol by fermentation in response to increasing price of gasoline and decreasing of foreign oil reserves [1]. Both of these fermentations were used commercially during the early part of the twentieth century. While ethanol fermentation continued to be more successful, butanol fermentation suffered due to development of less costly petrochemically derived butanol from crude oil. Hence most of the plants producing butanol by fermentation ceased operation by the end of 1960s. The only plant that remained operational until the early 1980s was in South Africa. This plant used molasses as a substrate for fermentation. Unfortunately, availability of molasses in large quantities became scarce due to draught, which ultimately forced the last butanol fermentation plant to cease its operation. Butanol fermentation plants were also operational in Soviet Union (now Russia) during 1980s [2]. While commercial production of butanol was discontinued, research toward developing superior butanol producing cultures and process technology continued.
Butanol, a product of acetone butanol ethanol (ABE or AB) fermentation, is an excellent feedstock chemical (in the plastics industry), a food-grade extractant (in the food and flavor industry) and, more importantly, a superior fuel to ethanol [3–5]. Butanol contains 22% oxygen making it an excellent fuel extender and a cleaner burning fuel [1, 3, 6, 7]. It is produced by fermentation using the anaerobic bacterium Clostridium acetobutylicum or C. beijerinckii [8, 9]. Fermentation of these microorganisms is biphasic-involving acidogenesis, the production of acids (acetic and butyric acids), followed by solventogenesis, which is the production of solvents (ABE in a 3:6:1 ratio) from the previously produced acids during fermentation [1, 10]. In the past decades, traditional substrates such as glucose, starch, molasses, and whey permeate have been used to produce butanol. Since substrate cost affects the price of butanol production by fermentation most [11–13], use of economically available nontraditional substrates such as agricultural residues, wastes, and energy crops is being investigated.
The agricultural residues and wastes used for the production of biofuels (including ABE) include rice straw, wheat straw, wood (hardwood), byproducts left over from the corn milling process (corn fiber), annual and perennial crops, and waste paper [14–16]. These substrates (agricultural residues) are composed primarily of polysaccharides that contain six and five carbon sugars. Prior to use of these substrates, these feedstocks must be hydrolyzed using a combination of alkali/acid pretreatment and enzymes. Butanol producing cultures (C. acetobutylicum and/or C. beijerinckii) have an added advantage over natural ethanol producing strains that they can utilize both hexose and pentose sugars released from agricultural residues [9, 16, 17]. However, it should be noted that pretreatment of agricultural residues, such as corn fiber, with acid/alkali results in generation of inhibitors that inhibit fermentation [18, 19]. These inhibitors include salts (neutralization products), acids (glucuronic, coumaric, and ferulic acids), and phenolic compounds [20]. It is possible that generation of these inhibitors is substrate specific. In the current study, we investigated use of wheat straw (WS) as a potential substrate for butanol fermentation employing C. beijerinckii. WS supplemented with glucose was also used to produce ABE, in combination with product recovery, to make ABE production process economically attractive. As a result of recent progress made in this fermentation, biological production of butanol is nearing commercialization [21, 22].
Materials and methods
Culture and cell propagation
Clostridium beijerinckii P260 was a generous gift from Professor David Jones (University of Otago, Dunedin, New Zealand). Spores of the culture were stored in distilled water at 4 °C. Spores (0.1 mL) of C. beijerinckii were heat shocked at 75 °C for 2 min followed by transferring to cooked meat medium (CMM; Difco Laboratories, Detroit, MI, USA) [23]. In order to prepare liquid CMM, 2.5 g solid CMM pellets and 0.2–0.4 g glucose were suspended in 20 mL distilled water in a 25 mL screw capped PyrexTM bottle. The mixture was autoclaved at 121 °C for 15 min followed by cooling to 35 °C. The heat-shocked spores were incubated at 35 °C for 16–18 h when it was ready for inoculum development. Following growth, 5–7 mL of the culture was transferred to 100 mL of P2 medium [30 g L−1 glucose (Sigma Chemicals, St. Louis, MO, USA), 1 g L−1 yeast extract (Bacto-Dickinson & Co., Sparks, MD, USA), and 1 mL each of stock solutions (mineral, buffer, and vitamin)], prepared in 125 mL screw capped bottle. Cell growth was allowed at 35 °C for 16–18 h. The composition of P2 medium (vitamin, mineral, and buffer stock solutions) has been given elsewhere [24]. Following culture growth in P2 medium, 5–7 mL of actively growing culture was transferred to 100 mL of wheat straw hydrolysate (WSH) medium. WS was pretreated with dilute acid as described below.
Wheat straw pretreatment and hydrolysis
Wheat straw, obtained from a local farmer, was ground to fine particles (1.27 mm sieve screen) using a hammer mill. WS contains 35–40% cellulose, 20–30% hemicellulose, and relatively low lignin (<20%). Eighty six gram of WS powder was suspended in 1 L dilute sulfuric acid (Sigma Chemicals; 10 mL sulfuric acid in 990 mL distilled water and final volume adjusted to 1 L) in a glass pan (24 × 35 cm) followed by autoclaving at 121 °C for 1 h. Prior to autoclaving the mixture, the pan was covered with aluminum foil. The pan containing the mixture was weighed before and after autoclaving to account for the loss of water. The lost water was replaced by adding distilled water to the mixture. Upon autoclaving, the mixture was cooled to room temperature followed by adjusting pH to 5.0 with 10 M NaOH (Sigma Chemicals). After this, 6 mL each of three enzyme solutions [Celluclast 1.5 L (cellulase; supplier—Sigma Chemicals); Novozyme 188 (β-glucosidase; supplier—Sigma Chemicals); and Viscostar 150 L (xylanase; supplier—Dyadic Corporation, Jupiter, FL, USA)] were added and mixed well. Finally, the mixture was incubated at 45 °C for 72 h with agitation at 80 rpm. After incubation, the WSH was filtered twice (11 μm pore size, 110 mm diameter; supplier—Whatman International Ltd, Maid Stone, England) to remove sediments. Following this, the clear liquid was filter sterilized by passing through a 0.2 μm filter. The sterilized solution was stored in a presterilized screw capped bottle at 4 °C for fermentation studies to be conducted later. The hydrolysate contained approximately 55–65 g L−1 total sugars.
Fermentation
Fermentation studies were conducted in 250 mL PyrexTM screw capped bottles containing 100 mL medium and 2.5 L bioreactor (Bioflo 2000, New Brunswick Scientific Co., New Brunswick, NJ, USA) containing 1 L medium. Studies with glucose as the substrate at various levels (60–250 g L−1) were conducted in 250 mL bottles. Various amounts of glucose (to the specified concentration) and 1 g L−1 yeast extract (Bacto-Dickinson & Co.) were sterilized at 121 °C for 15 min followed by cooling to room temperature. Upon cooling 1 mL of each of stock solutions (vitamin, buffer, and mineral) were added to the medium. Then the bottles were placed in an anaerobic jar (BBL GasPakTM, Sparks, Maryland, USA) for 48 h at room temperature. Anaerobic conditions inside the medium were developed using BD GasPakTM EZ (Sigma Chemicals) envelopes with indicator. Prior to placing the bottles in an anaerobic jar, caps were loosened to facilitate exchange of gases between jars (anaerobic gases) and the medium inside bottles. Then the bottles were inoculated with 6 mL of actively growing 16–18 h old culture developed above. During fermentation, 1.5 mL samples were taken for sugar and ABE measurement. The samples were centrifuged at 15,000×g for 3 min to separate sediments, and the clear liquid was stored at −18°C until analyzed for ABE and sugars. Fermentation was conducted at 35°C until culture ceased ABE production.
Wheat straw hydrolysate medium was prepared as follows. One hundred milliliter filter sterilized WSH solution was transferred to 250 mL presterilized screw capped PyrexTM bottle. At this stage, pH of the solution was adjusted to 6.5 with 10 M NaOH solution. To the WSH solution, 2.5 mL of 40 g L−1 sterilized yeast extract (Bacto-Dickinson & Co.) solution, and 1 mL each of stock solutions were added as described above. The bottle was placed in an anaerobic jar for anaerobiosis for 48 h at room temperature as described above. After 48 h the bottles were inoculated with 6 mL of actively growing 16–18 h old culture developed above. During fermentation, samples were taken and prepared for analysis as described above.
For fermentation studies of WSH with added sugar, various amounts of glucose (35–110 g L−1) were added to WSH (in addition to WSH sugars, usually 65 g L−1), followed by filter sterilization. Prior to filter sterilization, pH values of the solutions were adjusted to 6.5 using 10 M NaOH solution. This was followed by adding yeast extract (2.5 mL of 40 g L−1) and stock solutions as above. Anaerobic conditions were achieved as described above followed by inoculation and fermentation.
An experiment on product recovery by gas stripping was performed in a 2.5-L glass bioreactor containing 1 L medium. To 950 mL WSH, 60 g glucose was added followed by filter sterilization using a 0.2 μm filter. Prior to filter sterilization the pH of WSH solution was adjusted to 6.5 using 10 M NaOH solution. The solution was then transferred to a presterilized bioreactor (Bioflo 2000). To this solution, 20 mL of yeast extract [contained 1 g YE (Bacto-Dickinson & Co.)] solution (presterilized) was added. At this time, 10 mL each of stock solutions (30 mL total) were added. The medium so prepared was sparged with oxygen free nitrogen gas for 48 h at a rate of 150 mL min−1 with agitation at 150 rpm. The bioreactor exit gas was cooled to 4 °C to ensure that any moisture or volatiles were returned to the bioreactor. After 48 h the reactor was inoculated with 70 mL inoculum developed above and gas sparging was stopped. In order to maintain anaerobic environment developed inside the reactor, N2 gas sparging was switched to sweeping across the surface of the medium until the culture started producing its own gases (CO2 and H2). Also, agitation was stopped prior to inoculation. Fermentation temperature was maintained at 35 °C. The pH inside the reactor was not controlled as the culture controlled its own pH at approximately 5.2 ± 0.2 during solventogenic stage.
After a period of 34 h, product (ABE) recovery by gas stripping was initiated using fermentation gases (CO2 and H2). Prior to initiation, the environment inside the gas recycles line and condenser were made anaerobic by passing fermentation gases produced in the reactor (through the line and condenser). The gas recycle line was 8 m long (8 mm internal diameter and 13 mm outside diameter) low diffusion Viton tubing (No. 96412-35) obtained from Cole Parmer (Vernon Hills, IL, USA). The gases were recycled at a flow rate of 4 L min−1 using a twin head Masterflex peristaltic pump and 18 size Norprene pump tubing (No. 06402-18) both obtained from Cole Parmer. The condenser was made of glass with 2,000 mL inside volume (condenser length 584 mm, outside diameter 244 mm, cooling coil inside diameter 7 mm, and cooling coil inside volume 141 mL). The cooling machine was obtained from Cole Parmer (Thermo Haake C-35P) and 40% (v/v) ethylene glycol was used as a coolant at 1 °C. A schematic diagram of gas stripping has been given elsewhere [10, 25, 26]. Condensed ABE were recovered from the receiving flask regularly during the experiment.
Analyses
Fermentation products (ABE, acetic acid, and butyric acid) were analyzed by gas chromatography (6890N; Agilent Technologies, Wilmington, DE, USA) using a packed column as described previously [10, 25]. Before injection into the GC, the samples were diluted fourfold with distilled water. The GC was equipped with an autosampler and an integrator. Sugars were measured using Surveyor HPLC equipped with an automatic sampler/injector (Thermo Electron Corporation, West Palm Beach, FL, USA). The HPLC column (HPX-87P; Aminex Resin-based) was obtained from BioRad (Hercules, CA, USA). Solvent (milliQ water) flow rate was maintained at 0.6 mL min−1. For sugar analysis, the mixture was centrifuged at 15,000×g for 15 min followed by diluting 20× and injecting into the HPLC. ABE productivity was calculated as total ABE (present in the reactor plus condensate) produced in g L−1 divided by the fermentation time and is expressed as g L−1 h−1. ABE yield was calculated as total ABE produced divided by the total sugar utilized. During the experiment, cell concentration was measured by optical density method. The results presented here have error margins of 5–8%.
Results and discussion
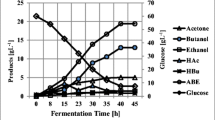
In an attempt to evaluate performance of C. beijerinckii in various reactors, a control batch experiment was run. The culture produced 20.1 g L−1 total ABE in a 72 h period and used 48.9 g L−1 glucose of 62.0 g L−1 present in the beginning of fermentation (Fig. 1). In this run, ABE productivity and yield of 0.28 g L−1 h−1, and 0.41 were obtained, respectively. At the end of fermentation, concentrations of acetone, butanol, and ethanol were 6.7, 12.7, and 0.6 g L−1, respectively. Acetic acid (4.9 g L−1) and butyric acid (1.9 g L−1) were also produced in the system. Production of 4.9 g L−1 acetic acid is relatively high. It should be noted that acetic acid concentrations at 0, 15, 24, 38, 47, 62, and 72 h were 2.5, 3.7, 2.6, 1.8, 2.7, 3.8, and 4.9 g L−1, respectively. These results suggest that the fermentation became acidogenic (acetic acid production) near the end of bioreaction. Considering production of good amount of ABE (20.1 g L−1), it is speculated that acetic acid production did not affect solventogenesis or fermentation negatively.
Since one of the objectives of these studies was to integrate fermentation with product recovery (to use concentrated sugar solutions), fermentations were run with various initial levels of glucose ranging from 60 to 250 g L−1 (Fig. 2a). Integrated fermentations with product recovery can be run at high initial sugar levels to reduce reactor size and various process streams [27, 28]. At 100 g L−1 glucose, the culture produced 23.5 g L−1 ABE and 4.58 g L−1 acids. At 150 and 200 g L−1 glucose levels, the culture produced 22.8 and 14.7 g L−1 ABE, respectively (Fig. 2a). No cell growth was observed at an initial glucose level of 250 g L−1. At these sugar levels, productivities and yields ranged from 0.15 to 0.36 g L−1 h−1 and 0.38–0.45, respectively (Fig. 2b). The concentrations of acetone and butanol that were produced at these sugar levels are presented in Fig. 2c. In most cases, the concentration of ethanol was less than 1.2 g L−1. In one of the experiments (not shown in figure), the culture produced 41.7 g L−1 ABE at an initial glucose level of 200 g L−1 of which acetone, butanol, and ethanol were 21.3, 19.5, and 0.9 g L−1, respectively. For this experiment, fermentation time was 288 h of which 36 h was lag period for visible cell growth.
Next, an experiment was performed with WSH as a substrate. The fermentation was rapid and completed in 42 h (Fig. 3). At 42 h total ABE in the system was 25.0 g L−1 of which acetone, butanol, and ethanol were 11.9, 12.0, and 1.1 g L−1, respectively. At the end of fermentation acetic acid, and butyric acid concentrations were 3.2 and 1.4 g L−1, respectively. Based on a fermentation time of 42 h (when the culture stopped producing ABE), a productivity of 0.60 g L−1 h−1 was obtained which is over 200% of the control experiment (initial sugar concentration in the control experiment was 62.0 g L−1). The WSH fermentation was initiated at a total WSH sugar level of 60.2 g L−1 (Fig. 3b). At 42 h, the residual sugar level was 1.0 g L−1 (glucose), thus consuming 59.2 g L−1 total sugar. Initially, 28.9 g L−1 glucose, 20.1 g L−1 xylose, 5.0 g L−1 arabinose, 3.5 g L−1 galactose, and 2.7 g L−1 mannose were present in the medium. All the five WSH sugars were consumed by the culture. In our previous studies, it has been reported that C. beijerinckii P260 utilizes all the five components of lignocellulosic sugars [9]. In our previous studies on ABE production using glucose and C. beijerinckii BA101, a productivity of 0.34 g L−1 h−1 was obtained [29]. In the present work, a productivity of 0.60 g L−1 h−1 is much higher than reported earlier using glucose [29].
Since it has been demonstrated that C. beijerinckii P260 can grow and produce ABE in 200 g L−1 glucose medium, WSH was supplemented with 140 g L−1 glucose and fermentation was initiated. This was performed to reduce various streams by using a concentrated sugar solution. The culture showed poor growth and produced 0.1 g L−1 acetone, 0.5 g L−1 butanol, and 0.2 g L−1 ethanol, thus totaling to less than 1 g L−1 ABE. The level of acids (acetic acid 5.9 g L−1 and butyric acid 1.2 g L−1) was 7.1 g L−1. This experiment suggested that fermentation of WSH supplemented with 140 g L−1 glucose was not practically viable, perhaps due to combined inhibition caused by high sugar level, acids, and other toxic chemicals that may have been present in WSH. Hydrolysates of agricultural residues contain fermentation inhibitors [20].
In order to find a suitable combination of glucose and WSH, experiments were conducted where WSH was supplemented with various levels of glucose (35–110 g L−1). Fermentation of WSH supplemented with 35 g L−1 glucose resulted in the accumulation of 28.2 g L−1 total ABE, and 1.9 g L−1 acids (Fig. 4a). Fermentation was complete in 45 h resulting in a productivity of 0.63 g L−1 h−1 (Fig. 4b). This productivity is 225% of the control experiment in which only glucose was used as a substrate. During this experiment, ABE yield of 0.42 was achieved (Fig. 4b). The other combinations of glucose and WSH that were used included supplementation of WSH with 60, 85, and 110 g L−1 glucose. At these sugar levels 13.0, 9.9, and 6.4 g L−1 ABE was produced, respectively (Fig. 4a). The levels of acids were 2.6, 3.5, and 5.0 g L−1 in the broth, respectively. At a supplemented sugar level of 110 g L−1 both productivity and yield were low at 0.11 g L−1 h−1 and 0.15, respectively (Fig. 4b). The concentrations of acetone and butanol achieved in these experiments are shown in Fig. 4c. Table 1 shows initial and final levels of various sugars present in these experiments.
Production of ABE from WSH supplemented with various levels of glucose in batch reactors of C. beijerinckii P260. (35 + WSH means WSH supplemented with 35 g L−1 glucose in addition to WS sugars. Likewise 60 + WSH, 85 + WSH, and 110 + WSH). a ABE and acids at various levels of added glucose to WSH; b yield and productivity at various levels of added glucose to WSH; and c amounts of acetone and butanol at various levels of added glucose to WSH
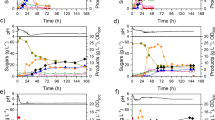
Fermentations of WSH supplemented with glucose resulted in residual sugars (Table 1). This is generally due to accumulation of high concentration of ABE at high substrate concentration, which is toxic to the culture. In order to utilize all the sugars present in the system, ABE should be removed simultaneously [26–28]. Hence, an experiment was performed where WSH was supplemented with 60 g L−1 glucose and fermentation was initiated. After a period of 34 h, as ABE concentration reached 4.4 g L−1, product removal by gas stripping was initiated (Fig. 5a). This figure shows levels of ABE during fermentation. It should be noted that at 42 and 81 h, levels of ABE were lower than before or after these fermentation times. These lower levels of ABE may have been either due to faster removal of ABE by gas stripping or reduced rate of their (ABE) production or a combination of both. Compression of gas recycle tubing during prolonged recycle of gases may also have played a role. Levels of acetic and butyric acid are shown in Fig. 5b. At 17 h, 7.4 g L−1 acids had accumulated in the system, which continued to remain high in the reactor until 81 h at which time it was 7.6 g L−1. At 95 h, total acid concentration reduced to 5.0 g L−1. At the end of fermentation, all the acids were utilized by the culture except 2.6 g L−1. In the beginning of this experiment, 128.3 g L−1 total sugars were present in the system, which was completely utilized by the culture in 131 h (Fig. 5c). The levels of individual sugars are shown in Table 2. In the beginning of the experiment, fermentation pH was 6.37, which dropped to 5.08 at 11 h suggesting that the culture produced acids at a rapid rate. The pH of the culture medium further dropped to 4.97 at 17 h before rising to 5.38 at 34 h. During the remaining fermentation period (34–131 h), pH remained between 5.24 and 5.48 (Table 2). It clearly demonstrated that the culture controlled its own pH. Acids were produced during acidogenic stage and utilized during solventogenic phase. As shown in Table 2, cell growth was slow during initial 42 h (0.60 g L−1), perhaps due to inhibition caused by increased sugar concentration. At 58 h a cell concentration of 1.2 g L−1 was measured. A maximum cell concentration of 2.2 g L−1 was measured at 95 h during this experiment (Table 2). In a non-integrated batch fermentation, a cell concentration of the order of 3–4 g L−1 is achieved [29]. An examination of sugar levels in the fermentation broth and cell concentration confirms that the reduced cell growth was as a result of inhibition caused by increased sugar level.
Production of ABE from WSH supplemented with 60 g L−1 glucose in a batch reactor of C. beijerinckii P260 coupled with simultaneous product recovery by gas stripping. a Products at various fermentation times; b acids at various fermentation times; c total sugar concentration in the reactor at various times
As shown in Table 2, arabinose, galactose, and mannose were utilized completely during the first 34 h of fermentation. It should be noted that an initial glucose level in the medium was 97.5 g L−1, which was consumed by the culture in 111 h. The initial level of xylose was 21.2 g L−1 and the culture took 131 h to utilize this xylose. The reader is advised that xylose utilization was faster when all other sugars were completely utilized. At 111 h, 8.3 g L−1 xylose was present in the broth. During the next 20 h, the culture utilized it completely. During the fermentation, 128.3 g L−1 total sugar was used and 47.6 g L−1 total ABE was produced in 131 h. This conversion process resulted in a productivity of 0.36 g L−1 h−1 and a yield of 0.37. It should be noted that the culture can grow and produce ABE in 200 g L−1 glucose solution. However, when supplemented with WSH, it could not tolerate above 175 g L−1 (110 g L−1 glucose + 65 g L−1 WSH sugars) total sugars. In our previous studies on use of glucose for ABE production, the culture (C. beijerinckii BA101) was not able to be grown in glucose above 160 g L−1 [29]. We also used another strain, C. acetobutylicum P262, that was able to grow and produce ABE in lactose/whey permeate medium containing 227 g L−1 lactose [28]. These results illustrated the differences between strains in their tolerance to high concentration of sugars. The objective of these comparisons is to inform the reader that tolerance to sugar varies from culture to culture. The authors are also aware that the comparisons should be made under identical sugar levels.
During recovery of ABE, it was noticed that acetic and butyric acids were also removed during the stripping process, though at a slow rate. In the condensate, acetic acid and butyric acid concentrations were 0.11–0.17 and 0.06–0.28 g L−1, respectively. In our previous work [10, 25, 27, 30] on ABE removal by gas stripping, acetic and butyric acids were not removed. At this stage we are not clear as why acids were removed in the present studies.
Conclusions
In conclusion, the control batch fermentation used 48.9 g L−1 glucose to produce 20.1 g L−1 ABE with a productivity and yield of 0.28 g L−1 h−1 and 0.41, respectively. In a similar experiment where WSH was used, the culture produced 25 g L−1 ABE with productivity and yield of 0.60 g L−1 h−1 and 0.42, respectively (Table 3). These results are superior to the control experiment and productivity was improved by 214% of the control experiment. This suggested that WSH can be used effectively by this strain to produce ABE. It appears that the cost of WS sugars (cost of WS plus hydrolysis) would be much less than glucose after processing. When WSH was supplemented with 35 g L−1 glucose, reactor productivity was improved to 0.63 g L−1 h−1 with a yield of 0.42. In this case, ABE concentration in the broth was 28.2 g L−1. When WSH was supplemented with 60 g L−1 glucose, the resultant medium containing 128.3 g L−1 sugars was successfully fermented to ABE to produce 47.6 g L−1 ABE, and the culture utilized all the sugars (glucose, xylose, arabinose, galactose, and mannose). Production of 47.6 g L−1 ABE has been possible as a result of product removal by gas stripping. This amount of ABE is the sum of recovered products (ABE) plus residual left in the fermentor (1 L broth) at the end of fermentation. There is no possibility to reach a concentration of 47.6 g L−1 ABE in the broth (without recovery). It should be noticed that xylose utilization rate was much lower than glucose and other sugars. Hence, it is concluded that WSH can be successfully fermented to butanol (ABE) without WSH treatment with resins to remove inhibitors as was the case with corn fiber hydrolysate [18, 19]. It should also be noted that C. beijerinckii P260 is a good strain that can produce over 28 g L−1 (in one case 41.7 g L−1 from glucose) ABE from WSH. Complete utilization of 128.3 g L−1 sugars was possible when product was removed simultaneously. It should be noted that in some of the processes, listed in Table 3, there was a considerable amount of residual sugar due to butanol toxicity. These processes would not be useful unless one of the product recovery techniques is applied to recover ABE.
References
Ezeji TC, Qureshi N, Karcher P, Blaschek HP (2006) Production of butanol from corn. In: Minteer S (ed) Alcoholic fuels. Taylor and Francis Group, LLC, CRC Press, Boca Raton, pp 99–122
Zverlov VV, Berezina O, Velikodvorskaya GA, Schwarz WH (2006) Bacterial acetone butanol production by industrial fermentation in Soviet Union: use of hydrolyzed agricultural waste fro biorefining. Appl Microbiol Biotechnol 71:587–597
Formanek J, Mackie R, Blaschek HP (1997) Enhanced butanol production by Clostridium beijerinckii BA101 grown in semi-defined P2 medium containing 6 percent maltodextrin or glucose. Appl Environ Microbiol 63:2306–2310
Parekh M, Formanek J, Blaschek HP (1998) Development of cost-effective glucose-corn steep medium for the production of butanol by Clostridium beijerinckii. J Ind Microbiol Biotechnol 21:187–191
Parekh M, Formanek J, Blaschek HP (1999) Pilot-scale production of butanol by Clostridium beijerinckii BA101 using low-cost fermentation medium based on corn steep water. Appl Microbiol Biotechnol 51:152–157
Ezeji TC, Qureshi N, Blaschek HP (2005) Industrially relevant fermentation. In: Durre P (ed) Handbook on Clostridia. Taylor & Francis Group, CRC Press, Boca Raton, pp 797–812
Ladisch MR (1991) Fermentation derived butanol and scenarios for its uses in energy-related applications. Enzyme Microbial Technol 13:280–283
Durre P (1998) New insights and novel developments in clostridial acetone/butanol/isopropanol fermentation. Appl Microbiol Biotechnol 49:639–648
Qureshi N, Li X-L, Hughes S, Saha BC, Cotta MA (2006) Butanol production from corn fiber xylan using Clostridium acetobutlycum. Biotechnol Prog 22:673–680
Ezeji TC, Qureshi N, Blaschek HP (2004) Acetone–butanol–ethanol production from concentrated substrate: reduction in substrate inhibition by fed-batch technique and product inhibition by gas stripping. Appl Micriobiol Biotechnol 63:653–658
Qureshi N, Maddox IS (1992) Application of novel technology to the ABE fermentation process: an economic analysis. Appl Biochem Biotechnol 34:441–448
Qureshi N, Blaschek HP (2000) Economics of butanol fermentation using hyper-butanol producing Clostridium beijerinckii BA101. Trans Inst Chem Eng (Trans IChemE): (Chem Eng Res Design) 78:139–144
Qureshi N, Blaschek HP (2001) Evaluation of recent advances in butanol fermentation, upstream, and downstream processing. Bioproc Biosys Eng 24:219-226
Tabka MG, Herpoel-Gimbert I, Monod F, Asther M, Sigoillot JC (2006) Enzymatic saccharification of wheat straw for bioethanol production by a combined cellulose xylanase and feruloyl esterase treatment. Enzyme Microb Technol 39:897–902
Esteghlalian A, Hashimoto AG, Fenske JJ, Penner AH (1997) Modeling and optimization of dilute-sulfuric-acid pretreatment of corn stover, poplar, and switchgrass. Biores Technol 59:129–136
Qureshi N, Blaschek HP (2005) Butanol production from agricultural biomass. In: Shetty K, Pometto A, Paliyath G (eds) Food biotechnology. Taylor & Francis Group plc, Boca Raton, pp 525–551
Jones DT, Woods DR (1986) Acetone–butanol fermentation revisited. Microbiol Rev 50:484–524
Ebener J, Qureshi N, Blaschek HP (2003) Corn fiber hydrolysis and fermentation to butanol using Clostridium beijerinckii BA101. In: 25th Biotechnology Symposium for Fuels and Chemicals, Breckenridge, May 4–7
Qureshi N, Ebener J, Ezeji TC, Dien B, Cotta MA, Blaschek HP (2007) Butanol production by Clostridium beijerinckii BA101. Part I: Use of acid and enzyme hydrolysed corn fiber. Biores Technol (Submitted)
Ezeji TC, Qureshi N, Blaschek HP (2007) Butanol production from agricultural residues: Impact of degradation products on Clostridium beijerinckii growth and butanol fermentation. Biotechnol Bioeng (in press)
Feedstuffs (2006) BP, Dupont to develop biofuels, June 26, p 20
Industrial Bioprocessing (2006) Dupont and BP will produce butanol for motor fuel, July issue, 28(7)
Ennis BM, Maddox IS (1985) Use of Clostridium acetobutylicum P262 for production of solvents from whey permeate. Biotechnol Lett 7:601–606
Qureshi N, Blaschek HP (1999) Butanol recovery from model solution/fermentation broth by pervaporation: evaluation of membrane performance. Biomass Bioenergy 17:175–184
Ezeji TC, Qureshi N, Blaschek HP (2003) Production of butanol by Clostridium beijerinckii BA101 and in-situ recovery by gas stripping. World J Microbiol Biotechnol 19:595–603
Qureshi N, Li X, Hughes SR, Saha BC, Cotta MA (2006) Production of acetone butanol ethanol from corn fiber xylan using Clostridium acetobutylicum. Biotechnol Prog 22:673–680
Maddox IS, Qureshi N, Roberts-Thomson K (1995) Production of acetone–butanol–ethanol from concentrated substrates using Clostridium acetobutylicum in an integrated fermentation-product removal process. Process Biochem 30:209–215
Qureshi N, Maddox IS (2005) Reduction in butanol inhibition by perstraction: utilization of concentrated lactose/whey permeate by Clostridium acetobutylicum to enhance butanol fermentation economics. Official J Eur Fed Chem Eng (formerly Trans IChemE; Chem Eng Res Design): Food Bioproducts Processing C 83(C1):43–52
Qureshi N, Blaschek HP (1999) Production of acetone–butanol–ethanol (ABE) by hyper-butanol producing mutant strain of Clostridium beijerinckii BA101 and recovery by pervaporation. Biotechnol Prog 15:594–602
Qureshi N, Maddox IS, Friedl A (1992) Application of continuous substrate feeding to the ABE fermentation: relief of product inhibition using extraction, perstraction, stripping and pervaporation. Biotechnol Prog 8:382–390
Acknowledgments
N. Qureshi would like to thank Professor David Jones (University of Otago, Dunedin, New Zealand) for his generous gift of C. beijerinckii P260. N. Qureshi would also like to thank Marsha Ebener for reading and formatting this manuscript. Help from John Michael Henderson, Mark Maroon, and Gregory Kennedy for preparing WSH and conducting some of the experiments is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Additional information
Mention of trade names or commercial products in this article is solely for the purpose of providing scientific information and does not imply recommendation or endorsement by the United States Department of Agriculture.
Rights and permissions
About this article
Cite this article
Qureshi, N., Saha, B.C. & Cotta, M.A. Butanol production from wheat straw hydrolysate using Clostridium beijerinckii . Bioprocess Biosyst Eng 30, 419–427 (2007). https://doi.org/10.1007/s00449-007-0137-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00449-007-0137-9