Abstract
Immobilized lipase from Candida antarctica (Novozyme 435) was tested for the synthesis of various phenolic acid esters (ethyl and n-butyl cinnamate, ethyl p-coumarate and n-butyl p-methoxycinnamate). The second-order kinetic model was used to mathematically describe the reaction kinetics and to compare present processes quantitatively. It was found that the model agreed well with the experimental data. Further, the effect of alcohol type on the esterification of cinnamic acid was investigated. The immobilized lipase showed more ability to catalyze the synthesis of butyl cinnamate. Therefore, the process was optimized for the synthesis of butyl cinnamate as a function of solvent polarity (logP) and amount of biocatalyst. The highest ester yield of 60.7 % was obtained for the highest enzyme concentration tested (3 % w/w), but the productivity was for 34 % lower than the corresponding value obtained for the enzyme concentration of 1 % (w/w). The synthesized esters were purified, identified, and screened for antioxidant activities. Both DPPH assay and cyclic voltammetry measurement have shown that cinnamic acid esters have better antioxidant properties than cinnamic acid itself.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Phenolic acids, especially cinnamic acid and its derivatives, are inevitable component of our daily diet due to their ubiquitous presence in plant food. These compounds are components of soybeans, cotton seeds, peanuts, and coffee, and they are proved to be one of the reasons for antioxidant potential of oilseeds [1–3]. They are also widely used as antimicrobial preservatives in pharmaceuticals, cosmetics, foods, beverages etc. Cinnamic acid derivatives are present in sunscreens as UVB-absorbing chemicals [4–6]. Consequently, these phenolic acids are very interesting substrates for the production of a variety flavor and fragrance compounds such as ethyl cinnamate or butyl cinnamate. There is growing interest in phenolic acid esters synthesis using free or immobilized lipase in non-aqueous solvents [3, 7, 8]. Although their synthesis can also be chemically catalyzed by acids or bases, enzymatic esterification offers environmental advantages and a reduction in energy consumption. Furthermore, the enzymatic synthesis is strongly preferred when product quality is a main issue, as is the case for food production.
Though primary function of lipases (triacylglycerol acyl-hydrolase, EC 3.1.1.3) is to catalyze the hydrolysis of triglycerides, resulting in free fatty acid, di- and monoacylglycerols, and glycerol, these enzymes can also catalyze formation of ester bond between alcohol and carboxylic acid [9, 10]. Lipase esterifications are performed under mild conditions, without usage of aggressive compounds that could harm human health [11–13]. Esters obtained in lipase-catalyzed synthesis are considered to be natural products of higher quality compared to those that are produced by traditional chemical methods [14, 15]. Nevertheless, the application of lipases to catalyze cinnamic acid esters synthesis reactions has been explored to a far lesser extent than applications involving fats and oils hydrolysis/transesterification or other esters synthesis.
Lipase-catalyzed esterification of phenolic acids has been shown to be possible using two enzymes, lipase B from Candida antarctica and lipase from Rhizomucor miehei. Guyot et al. was the first to report an efficient enzymatic esterification of phenolic acids and fatty alcohols catalyzed by lipase B from C. antarctica [16]. Afterwards, several reports on enzymatic synthesis of aliphatic phenolic acid esters in non-aqueous media including organic solvent and binary solvent systems [3, 7, 8, 17, 18] or even ionic liquids [19] have been published. Most of the research reported in the literature are aimed at the esterification of phenolic acids including dihydrocaffeic acid [7, 8, 17], ferulic acid [7, 17, 18], or caffeic acid [17]; however, there are only a few reports on the esterification of cinnamic acid with aliphatic alcohols that could find numerous applications in cosmetics and foods [20]. Stamatis et al. described enzymatic esterification of cinnamic acid with aliphatic alcohols using both lipase from R. miehei and lipase B from C. antarctica as the catalyst; however, data related to enzyme catalyzed esterification of cinnamic acid and its esters are very incomplete and contradictory, making them an interesting topic for research [3].
Focus of this work was to examine prospects for lipase-catalyzed esterification of cinnamic acid and its derivatives with different aliphatic alcohols and thereby to determine optimal requirements for maximal product formation. Lipase B from C. antarctica immobilized on macro porous acrylic resin has been selected for this investigation due to the many benefits of using immobilized enzymes rather than their soluble counterparts including repeated reuse of the biocatalyst, ease of removal from the reaction mixture, good or improved enzyme stability, and properties which permit use in column reactors and continuous processes. Esterifications were carried out in different solvents as well as in solvent-free systems, where excess of alcohol replaced solvent and provided efficient stirring of reaction mixture and proper dispersal of insoluble matters. In addition, the antioxidant activity of the synthesized esters was tested and compared with that of unesterified cinnamic acid and its related known antioxidant such as coumaric acid, thus looking into influence of esterification on antioxidant properties of cinnamic acid.
Materials and Methods
Chemicals
Lipase B from C. antarctica, expressed in Aspergillus oryzae immobilized on macro porous acrylic resin (Novozyme 435) and used in syntheses was obtained from Sigma Aldrich (St. Louis, MO, USA). The enzyme activity is ≥10,000 propyl laurate units (PLU) per gram of solid enzyme, where PLU is defined as the amount of propyl laurate (in gram) formed per minute, catalyzed by 1 unit of enzyme. Cinnamic acid, p-coumaric acid, p-methoxycinnamic acid and alcohols, all of analytical grade, were purchased from Sigma Aldrich, while 3-Å molecular sieves, isooctane and other solvents were purchased from Fluka (StQuentin-Fallavier, Switzerland). 2,2-Diphenyl-1-picrylhydrazyl (DPPH) used for radical scavenging test was also purchased from Sigma Aldrich.
Lipase-Catalyzed Phenolic Acid Esters Production
All esterification reactions were performed batchwise under temperature control at 55 °C and 250 rpm in reaction flasks, using phenolic acids as limiting substrate. Also, the reactions were carried out in the presence of 3-Å molecular sieves (20 mg mL−1) to remove water produced as the by-product and depress water-dependent reversible reactions. Typically, reaction mixture consisted of 0.846 mmol of phenolic acid (cinnamic, p-methoxycinnamic and p-coumaric acid) and 15 equivalents of alcohol (ethanol, butanol) and was filled with isooctane to reach a total volume of 5 mL. Esterification was started by adding immobilized lipase (75 mg) into the reaction flask (100 mL). Samples were taken each 12 h and analyzed for esters content. Control reactions, without enzyme, were performed simultaneously under the same conditions. Each reaction was done in duplicate and standard deviations were less than 5 %.
Kinetic Data Analysis
The reaction under study can be expressed as follows:
where Ac and Al are phenolic acid and alcohol, E and W are products (phenolic acid ester and water) and k 1 and k 2 are reaction rate constants for ester bond formation and decomposition, respectively.
The second-order kinetic model was used to describe time course of the reactions:
where c Ac and c Al are concentrations of phenolic acid and alcohol, respectively, and t is reaction time. The conversion yield, Y, can be then expressed as a function of the initial acid, C 0Ac, and alcohol, C 0Al, substrate concentrations at any reaction time, t, as:
where M is a constant defined as:
In the proposed kinetic model, adjustable parameter is the rate constant, k 1, which was determined by least-squares fits to the experimental data.
Esterification of Cinnamic Acid
The esterification reactions were carried out under the same conditions as described in previous section. Cinnamic acid was esterified with aliphatic alcohols with increasing carbon number (C4–C7). In order to maximize the yield of butyl cinnamate, series of experiments were performed in several reactions, in which type of organic solvent and mass of biocatalyst, were alternatively varied. The effect of solvent polarity on product formation was examined using solvents with different logP values. Further reactions were carried out with solvent that gave the highest reaction yield. The effect of increasing the amount of biocatalyst used in reaction (5–30 mg mL−1) was studied in a system consisting of cinnamic acid, n-butanol and isooctane (concentrations are given in previous section).
Analysis and Monitoring of Reaction Mixtures
Ester formation was monitored by HPLC analysis by measuring the ester concentration on a C18 Spherisorb column (particle size was 5 μm; length 250 mm; diameter 4.6 mm). Samples were filtered to remove the immobilized enzyme, evaporated under vacuum and diluted with methanol (15 μL sample/950 μL methanol) before analysis. Reaction products were detected by UV detector at 215 and 280 nm. Elution was performed with methanol/H3PO4 (100/0.1 (v/v)) at flow rate of 1 mL min−1. The reported ester yield in percentage was defined as the amount of ester produced to initial substrate in defect (mole of ester/ mole of cinnamic acid or its derivatives × 100).
Esters Purification
Various cinnamic acid esters were purified according to method which was described elsewhere [3, 21]. The reaction mixture was filtered to remove the immobilized enzyme, washed with 5 % sodium bicarbonate, and dried under vacuum at 20 °C using an Automatic Speed Vac system (Buchi Vacuum pump V-700/710). The residue was extracted with diethyl ether followed by washing with saturated NaHCO3 solution as described elsewhere [3, 21]. In the case of both butyl cinnamate and ethyl cinnamate, the produced esters were further purified by column chromatography (silica gel, 100–200 mesh) using hexane–ethyl acetate (96:4) as the solvent system and thus used in further analyzes.
Highly purified phenolic acid esters obtained were identified by NMR analysis and by using 2,2-dimethyl-2-silapentane-5-sulfonate sodium salt as an internal standard on a Varian Gemini 200 spectrometer (1H NMR at 200 MHz, 13C NMR at 50 MHz).
Antioxidant Activity Measured by DPPH Assay
Radical-scavenging activity, expressed as the percentage of inhibition, was measured by their ability to scavenge DPPH radical. It was monitored by decrease of absorbance at 517 nm, as described elsewhere [22, 23].
Antioxidant Activity Measured by Cyclic Voltammetry
In order to confirm the results obtained by DPPH radical scavenging ability, the antioxidant activity was also estimated from cyclic voltammetry experiments. The experiments were performed in a three-electrode cell, at ambient temperature. Glassy carbon electrode was used as working, Pt wire as counter, while saturated calomel electrode served as reference electrode. Prior to all experiments, working electrode was polished with 0.05-μm alumina suspension on polishing cloths and rinsed with doubly distilled water. The cyclic voltammograms were recorded at scan rate of 50 mV s−1, starting at open circuit potential to potential of 2.0 V (for cinnamic acid) and 1.2 V (for p-coumaric acid and ethyl cinnamate). The experiments were performed using SP-200 (BioLogic Science Instruments) potentiostat/galvanostat.
Results and Discussions
In the present work, the synthesis of aliphatic cinnamic acid esters through lipase-catalyzed reaction is reported. The reaction mechanism for the enzymatic synthesis of cinnamic acid esters from the cinnamic acid and an appropriate side-chains donor (alcohol) has been presented (Scheme 1). Lipase from C. antarctica was the catalyst of choice for this presentation due to its unique selectivity and stability in the organic solvents. Different recent reviews have been published about structure of the lipase B from C. antartica [24, 25]. Like in most lipases’ structures, a catalytic triad was identified consisting of Ser-105, Hys-224 and Asp-187, as presented in Scheme 1 [25, 26]. The scheme clearly shows that two stages occur in this reaction. In the first step of enzyme acylation, the active-site Ser-105 is activated by histidine-224 and aspartate-187 and attacks the carbonyl group of the first substrate, cinnamic acid, forming a tetrahedral intermediate. The acyl enzyme complex is formed when water, as the first product, leaves the active site. The subsequent step (deacetylation of enzyme) starts when alcohol enters the enzyme active site and second tetrahedral intermediate forms. Ester as second product exits enzyme active site, leaving free enzyme which is ready for another catalytic cycle.
Effect of Phenolic Acid Type on Esterification
Esterification reactions of cinnamic acid and its two derivatives, including hydroxylated, p-coumaric, and metoxylated, p-metoxycinnamic acid, were carried out with ethanol and/or butanol in the isooctane in the conditions described before and the progress of reactions was presented in Fig. 1. It appeared that esterifications of cinnamic acid and its derivatives were done successfully but in different esterification yields due to differences in acid structure. In order to mathematically describe the reaction kinetics and to compare present processes quantitatively, the second-order kinetic model was adopted to fit the kinetic data. The magnitude of rate constants is very important for a rational design, optimization, scaling up and control of the esterification process. The nonlinear regression fitting of data to the second-order kinetic model equation resulted in the kinetic parameters given in Table 1. Both experimental and predicted data, which apparently correlated well, are presented in Fig. 1.
Lipase-catalyzed synthesis of phenolic acid esters: (white square) n-butyl cinnamate, (black circle) ethyl cinnamate, (black triangle) n-butyl p-methoxycinnamate, (white diamon) ethyl p-coumarate. Reaction mixture consisted of 0.167 M phenolic acid (cinnamic acid, p-methoxycinnamic acid and p-coumaric acis) and 2.53 M aliphatic alcohol (ethanol and n-butanol). Reactions were carried out at 55 °C and 250 rpm, with 75 mg of lipase from C. antarctica
In the reaction of cinnamic acid with both ethanol and butanol, higher ester yields were achieved than those obtained with cinnamic acid derivatives (Table 1). It appeared that the reaction rate constant for ethyl cinnamate (1.95 h−1 mM−1) was more than four times higher than for ethyl-p-coumarate (0.47 h−1 mM−1). This result was in accordance with literature data and it implicated that presence of the substituents on the aromatic ring affected lipase affinity towards acid [3, 16]. Both cinnamic acid derivatives used in this experiment, p-coumaric and p-methoxycinnamic acid, have electron-donating groups, hydroxyl and methoxy, in para position on the ring. Lower lipase affinity towards these acids is explained by electron effect, where electron donating groups on the ring cause deactivation of electrophilic carbon from carboxylic group which is responsible for nucleophilic attack on the alcohol (Scheme 1) [3, 27]. Also steric effect should not be forgotten, so most authors attribute this low enzyme affinity to combined effect of these factors along with reaction conditions [3, 16, 27].
Apparently, higher esterification yield was also obtained when cinnamic acid was esterified with n-butanol, probably because ethanol in an excess caused denaturation of the enzyme (Table 1). Guyot et al. reported that esterification of cinnamic acid with ethanol was possible in a solvent free system, but ester yield of 2 % was achieved after 7 days, while n-butanol gave the highest reaction yields of 73 % in the same reaction time [16]. However, this study was performed in a solvent-free system where a high excess of ethanol was probably the reason for enzyme inactivation [16]. Current work was done in isooctane and higher ethyl cinnamate yields were obtained (35.2 ± 1.8 %, Table 1). Further optimization should include substrate initial molar ratio optimizations in order to determine relation between ethanol concentration and enzyme inactivation. Later in this work n-butyl cinnamate was used as a model reaction and all optimizations were done in order to obtain higher ester yields.
Enzymatic Synthesis of Aliphatic Cinnamic Acid Esters
Investigation of the influence of an acyl acceptor on lipase-catalyzed synthesis of cinnamic acid esters demonstrated that all of the used alcohols could form esters with cinnamic acid under previously described conditions. Results obtained during this experiment are presented in Table 2.
Apparently, the profile of conversion yield versus alcohol chain length displayed a single maximum for butyl cinnamate (Table 2). However, a series of esterifications with alcohol of increasing chain length predominantly resulted in a decrease in ester yield. The reason for this phenomenon was probably as follows. Namely, since alcohols reacted with the acyl enzyme intermediate formed during the acylation process, the rate of synthesis was determined by the diffusion of alcohol molecules into the active site of the enzyme (Scheme 1). Since smaller alcohols were able to diffuse into the active site more readily than bulky ones, an increase of the chain length led to a decrease of the enzyme activity. However, yields did not seem to be significantly corrupted with the increase in acyl acceptor chain length. The differences were rather small (STD = ±12.6 %). Similar results for esterification of cinnamic acid could be found in the literature indicating that the reaction yields of 30 % was achieved after 48 h with both butanol and octanol [16]. However, in a separate study by Stematis et al., higher reaction rates were observed when the esterification of cinnamic acid was carried out with medium- or long-chain alcohols [3].
Effect of the Solvent Polarity on Butyl Cinnamate Synthesis
Esterification of cinnamic acid with butanol was conducted in variety of solvents in order to determine optimal solvent for this reaction. To quantitatively describe the effect of the organic solvent on the enzyme activity, the logP parameter is the most frequently applied. The logP of a solvent is defined as the logarithm of the partition coefficient of the solvent in a standard mixture of 1-octanol and water [28].
Reaction mixture logP values are different than those for pure solvents and they are calculated according to following equation [28, 29]:
where logP rm, logP sol, logP but represent logP values for reaction mixture, solvent and butanol and x sol and x but present mole fractions of solvent and n-butanol, respectively.
Isooctane was proved to be the best solvent for esterification of cinnamic acid with n-butanol, as can be seen from Table 3. This result agrees with literature where has been noted that solvents with logP > 4 are convenient for biocatalysis since they do not remove essential water layer around the enzyme [29]. Conversions up to 85 % within 5 days were reported for esterification of cinnamic acid with n-butanol, when a-polar solvent n-pentane was used [27].
In solvents with low logP values indicating polar environments, a decrease in solvent polarity caused decrease in product formation and the best esterification yield was achieved when the most polar solvent tert-butanol was used (Table 3). This result was in accordance with literature data, where relatively high reaction yield of 52 % has been obtained in tert-butanol during esterification of cinnamic acid with n-octanol [3]. This result apparently could be due to a good solubility of cinnamic acid in polar solvents.
Effect of the Enzyme Concentration on Esterification Yield
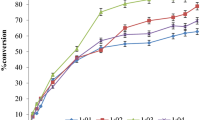
The effect of the enzyme concentration on formation of n-butyl cinnamate was also examined. Results are shown in Fig. 2.
Results presented in Fig. 2 have shown that an increase in enzyme concentration led to increase in esterification yield. Also it can be seen that the highest esterification yield of 60.7 % was reached after 72 h, when the highest enzyme concentration was used (30 mg mL−1, 3 % w/w). Even though it should be noted that slightly less ester yields were achieved with lower enzyme concentrations. For this reason, process productivity was calculated in order to determine optimal enzyme amount needed for successful esterification and the results are also presented in Fig. 2.
It seemed that the highest process productivity was achieved with the lowest enzyme concentration (10 mg mL−1, 1 % w/w). Nevertheless almost the same productivity was achieved with enzyme concentration of 15 mg mL−1, but this enzyme concentration gave much higher ester yields. In general, as to the enzymatic esterification of cinnamic acid, the lipase concentrations required to achieve higher yields of esters are often too high and reaction times relatively too long for industrial application. For example, Stamatis et al. have reported 82 % conversion in 12 days with 150 mg lipase from C. antarctica at 100 mM substrate (enzyme to substrate, E/S ratio was 300 g mol−1) [3]. Buisman et al. have reported 85 % cinnamic acid conversion to butyl cinnamate at 33.5 mM acid concentration with 10 mg of enzyme for 120 h in n-pentane (E/S ratio was 150 g mol−1), by using the same lipase [27]. In this study, it was shown that conversion yield of 30.8 % could be achieved at 170 mM acid concentration, using amounts of enzyme as low as 75 mg and in a relatively short time (72 h) in isooctane (E/S ratio was 88 g mol−1). Namely, the kinetics in our system based on isooctane seems to have a better profile than in polar system since the highest conversion of 60.7 %, with the highest enzyme concentration, was achieved in 72 h, corresponding to the volumetric productivity of 1.4 mmol L−1 h−1.
Structural Analysis
The result of the 1H NMR (200 MHz, CDCl3) data of ethyl cinnamate was: δ (ppm) 7.69 (1H, J = 15.8 Hz, d), 7.54–7.50 (2H, m), 7.40–7.32 (3H, m), 6.44 (1H J = 15.8 Hz, d), 4.26 (2H, q), 1.33 (3H, t).13C NMR (CDCl3, 50 MHz) δ: 14.2, 60.4, 118.2, 128.0, 128.8, 130.2, 134.4, 144.5, 166.9.
The result of the 1H NMR (200 MHz, CDCl3) data of butyl cinnamate was: δ (ppm) 7.69 (1H, J = 15.8 Hz, d), 7.55–49 (2H, m), 7.40–7.32 (3H, m),6.44 (1H J = 15.8 Hz, d), 4.21 (2H, t), 1.70 (2H quintet), 1.44 (2H, sex), 0.96 (3H, t). The characteristic functional groups were confirmed with 13C NMR (CDCl3, 50 MHz) δ: 13.7, 19.1, 30.7, 64.4, 118.2, 127.0, 128.8, 130.2, 134.4, 144.5, 167.1. Spectral analysis confirmed that compounds were identical with
Ethyl Cinnamate and Butyl Cinnamate
Radical-Scavenging Activity of Cinnamic Acid Aliphatic Esters
Cinnamic acid and its alkyl esters were tested for their radical-scavenging activity against DPPH in order to elucidate the influence of esterification on the antioxidant activity. The results are presented in Fig. 3. It appeared that all-synthesized esters exhibited better antioxidant potential than cinnamic acid itself (4.1 ± 1 %), though it is still very low comparing to other natural phenolic antioxidants, such as gallic acid. Also, the alkyl chain length has been shown to influence the antioxidant activity of esters, as their radical-scavenging effectiveness decreased with the increase of their alkyl chain length, with ethyl cinnamate being the most potent antioxidant. These results are in agreement with an earlier work in which the long chain alkyl cinnamates isolated from Sophora flavescens have been shown to be free radical scavengers [30]. Similarly, Garrido et al. have shown that caffeic acid esters have higher DPPH radical scavenging activities compared to caffeic acid itself [31]. However, this is in conflict with reports concerning the antioxidant activity of esters of other cinnamic acid derivatives. For example, Menezes et al. evaluated radical-scavenging activity of eight long chain alkyl hydroxycinnamyl esters which were chemically prepared indicating that esterification did not significantly modify the radical-scavenging activity of the precursor [32]. Similarly, several tested alkyl ferulate esters showed almost the same scavenging activity against DPPH radical and their activity seemed to be lower than that of ferulic acid [33]. Sabally et al. [7] investigated the radical scavenging activity of dihydrocaffeic acid and its phenolic lipid ester finding that the esterification of dihydrocaffeic acid with linolenyl alcohol resulted in a decrease in the radical scavenging activity of the phenolic acid. Overall, the present study and previous reports appeared to suggest that the effect of alkyl esterification on the antioxidant activity may differ from one phenolic acid to another, suggesting that optimization of the chain length for each type of phenolic acid could be necessary. This can be explained by the fact that phenolic acids have different mechanisms of action, which are mainly determined by their ring substitutions.
Cyclic Voltammetry Results
The aim of this part of research was to investigate whether the esterification of phenolic acid resulted in increase in the antioxidant activity compared with the native phenolic acid. For this purposes, produced ethyl cinnamate was completely purified, characterized by NMR analysis and used as a model cinnamic ester for electrochemical assay.
Figure 4a, b represents the cyclic voltammograms of the standard cinnamic and coumaric acids, respectively. As it can be seen in Fig. 4a, cinnamic was not oxidized since no anodic peaks were observable, which is in agreement with literature [34]. On the other hand, at voltammogram for cumaric acid (Fig. 4b), one characteristic anodic peak at 0.6 V was observed. This peak referred to oxidative process and is typical for antioxidant compound. The absence of the corresponding peaks at cathodic part of cyclic voltammograms referred to irreversible oxidation process. This observation was in good agreement with the proposed mechanism for the oxidation of phenolic compounds with hydroxy group at para position, leading to the generation of an unstable correspondent quinone. The rapid decomposition of the quinone made the process irreversible, which is characterized by the absence of reductive peaks at cathodic part of the voltammograms.
It seems that the esterification of cinnamic acid provoked increase of its antioxidant activity (Fig. 4c). As seen in Fig. 4c, it was possible to observe oxidation process in the cyclic voltammogram of ethyl cinnamate with a broad anodic peak at E a 0.7 V. This suggested an oxidation process at higher potential comparing to p-coumaric acid, indicating that the ester was less easily oxidized. In contrast, our preliminary experiment has shown its better antioxidant activity compared to cinnamic acid. Experiments are now in progress to investigate the antioxidant activity of other cinnamic acid aliphatic esters.
Generally, esterification of cinnamic acid appeared to increase its antioxidant activity as well as its radical-scavenging ability. Furthermore, the esterification of this compound with aliphatic alcohols through the addition of aliphatic side chain groups can be used as a tool to alter its solubility in fat- and oil-based food systems which is one of major drawback limiting its use in food applications.
Conclusion
The esterification activity of the commercial immobilized lipase CALB towards cinnamic acids and its derivative has been studied. Using cinnamic acid as substrate, the reaction rate constants (1.95 h−1 mM−1 for ethanol and 3.07 h−1 mM−1 for butanol) were more than four and nine times higher compared to those obtained with p-coumaric (0.47 h−1 mM−1) and p-methoxycinnamic acids (0.32 h−1 mM−1), respectively. The alcohol carbon chain lengths, the nature of organic solvent, and the biocatalyst amount have been parameters to consider in the optimization of the esterification process. Isooctane seemed to be the best solvent for this reaction even though solubility of cinnamic acid in this polar solvent is very low. It was proved that lesser amounts of enzyme can be used giving slightly lower ester yields. Esterification of cinnamic acid appeared to result in increasing radical scavenging ability. The effect of esterification of cinnamic acid was also confirmed by electrochemical method using ethyl cinnamate which appeared to enhance the antioxidant activity. These findings should stimulate the application of such lipase-catalyzed reactions for the preparation of food acceptable esters of cinnamic acid as potential lipophilic antioxidants.
References
Pratt, D. E., & Hudson, B. J. F. (1990). In B. J. F. Hudson (Ed.), Food antioxidants (pp. 171–192). London: Elsevier Science.
Larson, R. A. (1988). Phytochemistry, 27, 969–978.
Stamatis, H., Sereti, V., & Kolisis, F. N. (1999). Journal of the American Oil Chemists’ Society, 76, 1505–1510.
Shaath, N. A. (1997). In N. J. Lowe, N. A. Shaath, & M. A. Pathak (Eds.), Sunscreens: development, evaluation, and regulatory aspects (2nd ed., pp. 3–33). New York: Marcel Dekker, Inc.
Compton, D. L., Laszlo, J. A., & Berhow, M. A. (2000). Journal of the American Oil Chemists’ Society, 77, 513–519.
Patil, D., Dev, B., & Nag, A. (2011). Journal of Molecular Catalysis B: Enzymatic, 73, 5–8.
Sabally, K., Karboune, S., Yeboah, F., & Kermasha, S. (2005). Applied Biochemistry and Biotechnology, 127, 17–27.
Feddern, V., Yang, Z., Xu, X., Badiale-Furlong, E., & de Souza-Soares, L. A. (2011). Industrial and Engineering Chemistry Research, 50, 7183–7190.
Singh, A., & Mukhopadhyay, M. (2012). Applied Biochemistry and Biotechnology, 166, 486–520.
Kapoor, M., & Gupta, M. N. (2012). Process Biochemistry, 47, 555–569.
Yadav, G. D., & Lathi, P. S. (2004). Journal of Molecular Catalysis B: Enzymatic, 27, 109–115.
Li, W.-N., Chen, B.-Q., & Tan, T.-W. (2011). Applied Biochemistry and Biotechnology, 163, 102–111.
Milašinović N, Knežević-Jugović Z, Jakovljević Ž, Filipović J, Kalagasidis Krušić M (2012) Chem Eng J 181–182, 614–623
Bezbradica, D., Mijin, D., Šiler-Marinković, S., & Knežević, Z. (2007). Journal of Molecular Catalysis B: Enzymatic, 45, 97–101.
Martins, A. B., Graebin, N. G., Lorenzoni, A. S. G., Fernandez-Lafuente, R., Ayub, M. A. Z., & Rodrigues, R. C. (2011). Process Biochemistry, 46, 2311–2316.
Guyot, B., Bosquette, B., Pina, M., & Graille, J. (1997). Biotechnology Letters, 19, 529–532.
Yang, Z., Guo, Z., & Xu, X. (2012). Food Chemistry, 132, 1311–1315.
Lee, G.-S., Widjaja, A., & Ju, Y.-H. (2006). Biotechnology Letters, 28, 581–585.
Katsoura, M. H., Polydera, A. C., Tsironis, L. D., Petraki, M. P., Rajačić, S. K., Tselepis, A. D., et al. (2009). New Biotechnology, 26, 83–91.
Belsito, D., Bickers, D., Bruze, M., Calow, P., Greim, H., Hanifin, J. M., et al. (2007). Food and Chemical Toxicology, 45, S1–S23.
Borneman, W. S., Hartley, R. D., Morrison, W. H., Akin, D. E., & Ljungdahl, L. G. (1990). Applied Microbiology and Biotechnology, 33, 345–351.
Blois, M. S. (1958). Nature, 181, 1199–1200.
Gorjanović, S. Z., Novaković, M. M., Potkonjak, N. I., & Sužnjević, D. Z. (2010). Journal of Agricultural and Food Chemistry, 58, 4626–4631.
Basso, A., Braiuca, P., Cantone, S., Ebert, C., Linda, P., Spizzo, P., et al. (2007). Advanced Synthesis and Catalysis, 349, 877–886.
Uppenberg, J., Hansen, M. T., Patkar, S., & Jones, T. A. (1994). Structure, 2, 293–308.
Magnusson A (2005) Ph. D. Thesis, Royal Institute of Technology, Stockholm, Sweden.
Buisman, G. J. H., van Helteren, C. T. W., Kramer, G. F. H., Veldsink, J. W., Derksen, J. T. P., & Cuperus, F. P. (1998). Biotechnology Letters, 20, 131–136.
Laane, C., Boeren, S., Vos, K., & Veeger, C. (1987). Biotechnology and Bioengineering, 30, 81–87.
Fu, B., & Vasudevan, P. T. (2010). Energy & Fuels, 24, 4646–4651.
Jung, H., Kang, S., Hyun, S., & Choi, J. (2005). Archives of Pharmacal Research, 28, 534–540.
Garrido, J., Gaspar, A., Garrido, E. M., Miri, R., Tavakkoli, M., Pourali, S., et al. (2011). Biochimie, 94, 961–967.
Menezes, J. C. J. M. D. S., Kamat, S. P., Cavaleiro, J. A. S., Gaspar, A., Garrido, J., & Borges, F. (2011). European Journal of Medicinal Chemistry, 46, 773–777.
Kikuzaki, H., Hisamoto, M., Hirose, K., Akiyama, K., & Taniguchi, H. (2002). Journal of Agricultural and Food Chemistry, 50, 2161–2168.
Simić, A., Manojlović, D., Šegan, D., & Todorović, M. (2007). Molecules, 12, 2327–2340.
Acknowledgments
This work was supported by grant numbers E!6750 and III 46010 from the Ministry of Education, Science and Technological Development, Republic of Serbia.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jakovetić, S.M., Jugović, B.Z., Gvozdenović, M.M. et al. Synthesis of Aliphatic Esters of Cinnamic Acid as Potential Lipophilic Antioxidants Catalyzed by Lipase B from Candida antarctica . Appl Biochem Biotechnol 170, 1560–1573 (2013). https://doi.org/10.1007/s12010-013-0294-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-013-0294-z