Abstract
In this study, the immobilized lipase was prepared by fabric membrane adsorption in fermentation broth. The lipase immobilization method in fermentation broth was optimized on broth activity units and pH adjustments. The viscose fermentation broth can be used with a certain percentage of dilution based on the original broth activity units. The fermentation broth can be processed directly without pH adjustment. In addition, the oleic acid ethyl ester production in solvent-free system catalyzed by the immobilized lipase was optimized. The molar ratio of ethanol to oil acid, the enzyme amount, the molecular amount, and the temperature were 1:1, 12% (w/w), 9% (w/w)(based the total amount of reaction mixture), and 30 °C, respectively. Finally, the optimal condition afforded at least 19 reuse numbers with esterification rate above 80% under stepwise addition of ethanol. Due to simple lipase immobilization preparation, acceptable esterification result during long-time batch reactions and lower cost; the whole process was suitable for industrial ethyl oleate production.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
As a traditional chemical method of alternative technologies and green energy-saving emission reduction, enzyme-catalyzed synthesis of synthetic chemicals and fuels becomes an important development direction [1]. Biodiesel are methyl or ethyl esters derived from fatty acid through esterification or transesterification [2, 3]. Enzymatic biodiesel production has been investigated intensively. Lai et al. [4] studied lipase-catalyzed production of biodiesel from rice bran oil. Ruzich and Bassi [5] investigated the enzymatic biodiesel production using ionic liquid as a co-solvent. Recently, the methanolysis of soybean oil to produce a fatty acid methyl ester was catalyzed by lipase-producing filamentous fungi immobilized on biomass support particles [6]. Many studies have also been done on esterification catalyzed by lipase in solvent-free system. Foresti and Ferreira [7] studied the enzymatic synthesis of ethyl oleate by direct esterification of oleic acid and ethanol in solvent-free media. Sandoval et al. [8] characterized the kinetic and thermodynamic performance of batch lipase-catalyzed esterification of oleic acid with ethanol in solvent-free systems. Trubiano et al. [9] studied the role of hydrophobic interactions in ethyl oleate synthesis using Candida rugosa lipase.
In addition, water activity is a key parameter for optimizing the rate of ester synthesis [10]. The reasons for these differences in optimum water activity are discussed in terms of enzyme specificity, substrate solution, and mass transfer effects [11]. Lene et al. [12] reviewed a critical analysis of the current status of research in this area and accentuated the main obstacles to the widespread use of enzymes for commercial biodiesel. They pointed out that the performance and price of the enzymes need further advances to become attractive industrially for biodiesel production.
As one of the ethyl esters, in spite of the role as biodiesel, ethyl oleate was also widely applied in many other fields (including surface-active agents, gas chromatography stationary phase, solvent, lubricant, resin-toughening agent, and anti-agent). In our study, we presented a comprehensive study on the solvent-free esterification of oleic acid and ethanol using lipase from Candida sp. 99–125 as catalyst. The immobilized lipase was prepared by fabric membrane adsorption in fermentation broth. After related optimization in the oleic acid ethyl ester production system, acceptable operational stability of the immobilized lipase was attained. The cost-effective way to produce biodiesel was dependent on the reaction conversion rate and reuse batch [13]. Our results show that the established solvent-free system was suitable to industrialize.
Materials and Methods
Materials
The species of lipase from Candida sp. 99–125 [14] was screened by a series of mutations in our laboratory. Cotton silk fabric membrane (chemical composition of protein, surface area of 0.216 m2 g-1, weight of 149 g m-2) was kindly donated by Beijing CTA New Century Biotechnology, China. Olive oil was purchased from Beijing Fangcao Chemicals Factory. All the other organic solvents and salts, including alcohol, n-hexane, PVA (MW1750), NaOH, were obtained from Beijing Chemical Factory and were of analytical grade.
Methods
Preparation of Immobilized Lipase
The condition of the lipase fermentation in our lab was as described by Tan et al. [14]. The preparation of immobilized lipase can be started when the lipase produced reached a proper activity. The cells were removed by centrifuged at 8,000 rpm for 10 min. The supernatant fermentation broth was used for fabric membrane immobilization. Cotton silk fabric membrane was cut into small pieces of 3 cm × 3 cm2 in the square size. A piece of fabric membrane was adsorbed in 10 mL of fermentation broth and stirred for 2 h. Then the fabric membrane was taken out and dried at room temperature. The adsorption procedure above can be repeated for several times to attain a proper lipase loading amount. To study the effect of broth pH on the preparation of immobilized lipase membrane, the initial fermentation (activity of 4,500 U mL-1, pH 3.8) was diluted with phosphate buffered saline (PBS) buffer (pH 8.0) to 2,000 U mL-1. A little amount of HCl (1 M) or NaOH (3 M) was added to adjust for a certain pH.
Lipase Hydrolytic Activity
Lipase activity was determined according to olive oil emulsion method with some modifications as Yu et al. [15] described.
Esterification of Oleic Acid and Ethanol in Solvent-Free System
Oleic acid (2.82 g), a piece of immobilized lipase (shaped in 3 cm × 3 cm2/piece, 0.20 g), and 584 μL ethanol was added in 50 mL screw-capped flask at 30 °C, 190 rpm. At the end of reaction, the catalyzed system was mixed with 20 mL ethanol, using 0.5 M NaOH titration to determine the conversation rate of oleic acid. Blank samples were prepared in parallel almost the same. The only difference was that the substrates were titrated directly after mixing with 20 mL ethanol without lipase catalyzation (at the 0 h of reaction time course). Esterification conversion rate = [(V 0 - V 1) / V 0] × 100%, where V 0 is the volume of NaOH consumed in the initial substrate and V 1 is the volume of NaOH consumed in the remnants after reaction. All the experiments were replicated at least 3 times, and the results presented were the mean values for the replicated data.
Determination of the Stability of Immobilized Lipase in Batch Reaction
Reaction was taken in the 50 mL screw-capped flask by adding 2.82 g oleic acid and two pieces of immobilized lipase membrane (0.39 g), with ethanol added by five-stage stepwise at 30 °C, 190 rpm. At the end of each reaction, the immobilized lipase membrane was rinsed out with 3 mL n-hexane and put into fresh substrate for the next reaction. Other treatment in the catalyzed system was the same as described above in solvent-free esterification (see Section “Esterification of Oleic Acid and Ethanol in Solvent-Free System”).
Results and Discussion
Optimization for Lipase Immobilization
The Influence of Broth Activity Units
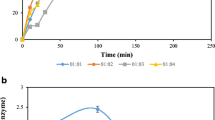
The fermentation broth in different hydrolysis activity units was obtained by proper phosphate buffer dilution (Table 1). The effect of different activity units of fermentation broth on immobilized enzyme preparation was studied in solvent-free system through the reaction process. Figure 1 shows that the higher the hydrolysis activity of the fermentation broth, the faster is the reaction rate. The trend of the time course in 3,000–4,500 U was basically the same. After 3 h, the conversion rate got up to 68–80%, and equilibrium was achieved at 9 h. A conversion rate of about 85% was achieved for all units ranging from 500 to 4,500 U except 20 U (due to very low activity, the requirements of lipase loading for the reaction equilibrium cannot be met).
Esterification process of immobilized lipase prepared from fermentation broth at different activity units. The fermentation broth in different hydrolysis activity units was obtained by proper buffer water diluted. (Reaction conditions: 2.82 g oleic acid, one-stage stepwise addition of 584 μL ethanol, a piece of immobilized lipase in square shape of 3 × 3 cm2, 30 °C, 190 rpm)
The fermentation broth immobilized from 500 U mL-1 got its half-lives at the 5th batch. When higher activity concentrations of 2,500–4,500 U mL-1 were used, the immobilized lipase batch life became longer (Fig. 2). Gradient of immobilized lipase from activity 2,500–4,500 U mL-1 reached the half-life starting from the 10th use. With activity increasing, the difference in reuse times was not so obvious. The batch numbers were extended to additional 1 day with the gradient of activity 2,500–4,500 U mL-1. As a result, the viscous fermentation broth can be used after a certain percentage of appropriate dilution as 0.4:1 (water:fermentation broth, v/v).
Operational stability of immobilized lipase prepared from fermentation broth at different activity units. The fermentation broth in different hydrolysis activity units was obtained by proper buffer water diluted. (Reaction conditions: 2.82 g oleic acid, one-stage stepwise addition of 584 μL ethanol, a piece of immobilized lipase in square shape of 3 × 3 cm2, 30 °C, 190 rpm, 24 h/batch)
The Influence of Broth pH
The immobilized lipase was prepared in different pH conditions, and the catalytic activity of oleic acid esterification was measured in the reaction of 3 h esterification rate as shown in Fig. 3. From the experimental results, pH was significantly effected by immobilization. At pH 2.0 and > 10.0, the immobilized lipase almost failed to show any catalytic activity, while the pH range of 4–7 was beneficial for lipase immobilization. However, the enzyme reaction reached optimum value at the original fermentation broth of pH 3.84. Therefore, the fermentation broth can be processed directly without pH adjustment.
Optimization for Esterification Reaction of Oleic Acid and Ethanol Process in Solvent-Free System
The Influence of Temperature
Enzyme-catalyzed reaction temperature is an important parameter; an appropriate reaction temperature not only favors the reaction rate but also benefits to extending the life of enzymes. However, when the temperature exceeds a certain range, the enzyme activity will be decreased due to enzyme protein denaturation.
Figure 4 shows the enzymatic reaction rate decreasing gradually with the temperature increasing. This is possibly due to the effect of inhibition synergies between temperature and ethanol on the enzyme. At higher temperature, the role of ethanol on the inactivation of immobilized grows, leading to a decreasing enzyme activity and reaction rate. A comprehensive consideration of the reaction rate, the enzyme operational stability and energy consumption, and the reaction taken at 30 °C was more appropriate.
The Influence of Enzyme Dosage
Figure 5 shows that the esterification rate improved accordingly with the increasing enzyme concentration (which accounted for the mass ratio of substrate). When the enzyme concentration was improved up to 12%, the esterification rate attained the max. figure of 79%.
The Influence of Substrate Molar Ratio
The molar ratio of substrate effects conversion rate and the equilibrium. Usually, the excessive alcohol was used to break the equilibrium on a general acid–alcohol esterification process. In the lipase-catalyzed reaction, excessive alcohol process is more preferred. In the experiment, the ethanol and oleic acid molar ratio of 1:1–5:1 behavior was studied under the conditions of esterification, as shown in Fig. 6. The esterification rate was significantly reduced with an increasing molar ratio. When oleic acid reacted with ethanol in the ratio of 1:2, the conversion rate decreased from 82.0% to 61.9%. The esterification rate indicated that the excess in ethanol produced a significant inhibition to the lipase-catalyzed reaction.
The Influence of Ethanol Stepwise Addition
Stage stepwise addition of ethanol was widely used in enzymatic synthesis. Marcella et al. [16] studied the synthesis of ethyl esters via esterification using Novozym 435 with two-stage stepwise addition of ethanol. Hernández-Martín and Otero [17] used three-stage stepwise addition of ethanol in enzymatic syntheses of biodiesel. In order to minimize the toxicity of the short-chain alcohols to enzyme, the stepwise addition technique of ethanol was carried out in our study. Due to more toxicity presented when compared to that in equimolar ratio, a molar ratio of 1:2 (acid:alcohol) was selected as the model acid-to-alcohol ratio in the reaction. Although the reaction time was ranged from 3 to 24 h, the oleic acid conversion rate was still around 50% (Table 2). The results showed that the esterification rate cannot be improved by extending reaction time. However, through stage stepwise additions of ethanol for 3, 6, and 10 times according to the corresponding reaction time, the conversion rate had obviously improved from 64.5% (3 h) to 78.5% (24 h). As a result, stepwise addition of ethanol played an important role in the controlling of ethanol concentration to enhance esterification degree.
The Influence of Water Content
It is well known that water has a very significant impact in enzymatic esterification system. First, the water produced in the reaction affects the reaction equilibrium [18]. Second, in non-aqueous systems, water content is essential to maintain conformation of the enzyme catalysis site [19]. In some special cases, the addition of relatively high amounts of water showed an unexpected beneficial effect. Foresti et al. [10] studied the role of water in the solvent-free enzymatic esterification and emphasized another effect of water related to the formation of a two-liquid phase system. The generation of two-liquid phase systems was due to the addition of high amounts of water to the mixture of substrates.
To investigate the effect of water content on esterification, we adjusted water content by water initially added on the surface of the immobilized lipase membrane. Many researchers agreed on the existence of optimum water contents generally in the range of 0.2–3% [20, 21]. As similar with most cases, the esterification rate (Fig. 7) in our study reduced obviously with the water content increasing from 0% up to 15% ( w/w). However, after drying up, the activity of the lipase membrane can return to the original level again as catalyzed in the next reaction without water added.
The influence of water initially added on the lipase membrane, water content was determined by the water added, accounting for the percentage of the immobilized lipase membrane. (Reaction conditions: 2.82 g oleic acid, one-stage stepwise addition of 584 μL ethanol, a piece of immobilized lipase in square shape of 3 × 3 cm2, 30 °C,190 rpm, 3 h. And immobilized lipase after dried was reacted in esterification system of no water initially added)
Due to the disadvantage of water added in the reaction system, molecular sieve was used as water adsorbent. By adding water-adsorbent agent, the reaction equilibrium was broken and the reaction rate was significantly improved by about 10% in oleic acid conversion rate (Fig. 8). The reaction tended to equilibrium after 3 hs and the oleic acid conversion rate increased to 89% as compared to that with no adsorbent added of 79% at 24 h. However, no significant effects were found when the dosage increased from 0.3 to 0.9 g during the reaction time course. Thus, 0.3 g of molecular sieve was selected as the optimum dosage for water removal.
The Operational Stability of Immobilized Lipase
Meng et al. [22] found that the optimal condition allowed 30 days with 82.6% conversion in 1.41 g oleic acid using immobilized Y. lipolytica lipase. To verify the feasibility of industrial applications in our study, we investigated operational stability of the immobilized lipase in a solvent-free system. As a result (Fig. 9), in 5 h reaction, under the condition of two pieces of lipase membranes catalyzed and five stages of ethanol added stepwise, the reaction rate was kept stable and was greater than 80% up to 19 reuse numbers.
Conclusions
The fermentation broth through proper dilution without pH adjustment can be prepared for fabric membrane immobilization. By the simple immobilized lipase, the conditions of oleic acid ethyl ester production in solvent-free system were optimized. The molar ratio of ethanol to oil acid, the enzyme amount, the molecular sieve amount, the temperature, and the shaking rate were1:1, 12% (w/w), 9% (w/w) (based on the substrate weight), 30 °C, and 190 rpm, respectively. As a result, under the optimum conditions, the activity of the immobilized lipase membrane can be continuously operated for at least 19 reuse numbers with an oil acid conversion rate greater than 80%. The operational stability study also indicated that the fabric lipase membrane from fermentation broth had good stability for catalysis and is in favor of the industrial ethyl oleate production.
References
Enweremadu, C. C., & Mbarawa, M. M. (2009). Renewable & Sustainable Energy Reviews, 13, 2205–2224.
Lien, Y. S., Hsieh, L. S., & Wu, J. C. S. (2010). Industrial and Engineering Chemistry Research, 49(5), 2118–2121.
Hanh, H. D., Dong, N. T., Okitsu, K., Nishimura, R., & Maeda, Y. (2009). Renewable Energy, 34, 780–783.
Lai, C. C., Zullaikah, S., Vali, S. R., & Ju, Y. H. (2005). Journal of Chemical Technology and Biotechnology, 80, 331–337.
Ruzich, N. I., & Bassi, A. S. (2010). Canadian Journal of Chemical Engineering, 88, 277–282.
Araia, S., Nakashimab, K., Taninoc, T., & Oginoa, C. (2010). Enzyme and Microbial Technology, 46, 51–55.
Foresti, M. L., & Ferreira, M. L. (2005). Catalysis Today, 107–108, 23–30.
Sandoval, G., Condoret, J. S., Monsan, P., & Marty, A. (2002). Biotechnology and Bioengineering, 78, 313–320.
Trubiano, G., Borio, D., & Ferreira, M. L. (2004). Biomacromolecules, 5, 1832–1840.
Foresti, M. L., Pedernera, M., Bucalá, V., & Ferreira, M. L. (2007). Enzyme and Microbial Technology, 41, 62–70.
Goldberg, M., Thomas, D., & Legoy, M. D. (1990). European Journal of Biochemistry, 190(3), 603–609.
Fjerbaek, L., Christensen, K. V., & Norddahl, B. (2009). Biotechnology and Bioengineering, 102(5), 1298–1315.
Nordblad, M., & Adlercreutz, P. (2008). Biotechnology and Bioengineering, 99, 1518–1524.
Tan, T. W., Zhang, M., Wang, B. W., Ying, C. H., & Deng, L. (2003). Process Biochemistry, 39(4), 459–465.
Yu, M., Qin, S., & Tan, T. (2007). Process Biochemistry, 42(3), 384–391.
Souza, M. S., Aguieiras, E. C. G., da Silva, M. A. P., & Langone, M. A. P. (2009). Applied Biochemistry and Biotechnology, 154, 74–88.
Hernández-Martín, E., & Otero, C. (2008). Bioresource Technology, 99(2), 277–286.
Petersson, A. E. V., Adlercreutz, P., & Mattiasson, B. (2007). Biotechnology and Bioengineering, 97(2), 235–241.
Yahya, A. R. M., Anderson, W. A., & Moo-Young, M. (1998). Enzyme and Microbial Technology, 23, 438–450.
Rocha, J., Gil, M., & Garcia, F. (1999). Journal of Chemical Technology and Biotechnology, 74, 607–612.
Iso, M., Chen, B., Eguchi, M., Kudo, T., & Shrestha, S. (2001). Journal of Molecular Catalysis B: Enzymatic, 16, 53–58.
Meng, Y. H., Chen, B. Q., Yang, N., Wang, G. L., Li, Y., & Tan, T. W. (2010). Journal of Biobased Materials and Bioenergy, 4, 1–6.
Acknowledgements
This project has been funded by the National High Technology Research and Development Program of China (2006AA020203), the National Nature Science Foundation of China (20876011), the State Key Development Program for Basic Research of China (2007CB714304), and the Natural Science Foundation of Beijing, China (2071002).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Li, WN., Chen, BQ. & Tan, TW. Esterification Synthesis of Ethyl Oleate in Solvent-Free System Catalyzed by Lipase Membrane from Fermentation Broth. Appl Biochem Biotechnol 163, 102–111 (2011). https://doi.org/10.1007/s12010-010-9020-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-010-9020-2