Abstract
To overcome the poor properties of solubility and stability of cinnamic acid, cinnamate derivatives with sugar alcohols were produced using the immobilized Candida antarctica lipase with vinyl cinnamate and D-sorbitol as substrate at 45 °C. Immobilized C. antarctica lipase was found to synthesize 6-O-cinnamoyl-sorbitol and confirmed by HPLC and 1H-NMR and had a preference for vinyl cinnamate over other esters such as allyl-, ethyl-, and isobutyl cinnamate as co-substrate with D-sorbitol. Contrary to D-sorbitol, vinyl cinnamate, and cinnamic acid, the final product 6-O-cinnamoyl-sorbitol was found to have radical scavenging activity. This would be the first report on the biosynthesis of 6-O-cinnamoyl-sorbitol with immobilized enzyme from C. antarctica.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cinnamic acid is a naturally occurring aromatic fatty acid with low toxicity to cells, and it has been used widely for human welfare for a long time. As it could be obtained easily from natural polymeric products such as lignin, which is the second most abundant polymer on the earth [1–3]. Cinnamic acid and its substituted compounds such as p-coumaric acid, caffeic acid, ferulic acid, and sinapic acid are well-known components in flavoring, perfumes, synthetic indigo, pharmaceuticals, and cosmetics [4]. Besides, it has various pharmacological applications as anticarcinogenic, hepatoprotective, anti-Alzheimer, antidiabetic, and antimalarial agents [5]. Cinnamic acid has been used as a precursor in the synthesis of artificial sweetener aspartame and as an additive in the manufacturing of the methyl, ethyl, and benzyl ester-based perfume industry [6]. Literature evidenced that the cinnamic acid derivatives are having many beneficial effects in the human body and have been using in traditional medicine as an antiaging or antioxidants and also it has the ability to promote the longevity and homeostasis of human body [7].
Cinnamic acid is a white crystalline organic compound that is slightly soluble in water but is freely soluble in many organic solvents [8]. In spite of its various applications in medical field, their physical unstability and decomposition on the human skin as a free acid form are limiting their uses in pharmaceutical or cosmetic field [9]. Moreover, substituted cinnamic acid has similar limitations like getting to discolor quickly over pH 7.0 or precipitates into crystals under pH 7.0, thereby hindering its delivery into the skin [10].
In order to overcome these limitations, herein, we demonstrate the structural modification of cinnamic acid and its substituted compounds with soluble carbohydrate through lipase-dependent esterification. The benefits of the enzymatic modification of cinnamic acid lie in the fact of increasing its stability and solubility without losing their biological activities. For that purpose, sorbitol was chosen as a carbohydrate counterpart due to its excellent solubility and stability. Besides, sorbitol is generally regarded as safe (GRAS) molecule and often used in modern cosmetics as a humectant and thickener and also in mouthwash and toothpaste [6]. In addition, it has a high refractive index which favors to make the transparent formulations [11].
In the present study, lipase-catalyzed synthesis of cinnamic acid derivatives with sorbitol is demonstrated. Various cinnamates were investigated for the esterification with sorbitol, and the best cinnamate among the examined was further investigated for the optimum esterification conditions and conservation of its biological activities as well. Our findings will provide a good example of structural modification of cinnamate to increase their stability at the same time without losing their biological activities.
Materials and Methods
Materials
Immobilized lipase B from Candida antarctica (Novozym 435, 2 unit/mg) was purchased from Novozyme. D-sorbitol, vinyl cinnamate, 0.4-nm molecular sieves (8–12 mesh), tert-butanol, 2,2-diphenyl-1-picrylhydrazyl (DPPH), other esters such as allyl-, ethyl-, and isobutyl cinnamates were purchased from Sigma (USA).
Analytical Methods
The chemical structure of 6-O-cinnamoyl-sorbitol was determined by1H NMR spectrometer (Bruker (400 MHz), Germany). 1H NMR data was interpreted in parts per million (δ) downfield from tetramethylsilane. The following abbreviations have been used: s (singlet), d (doublet), t (triplet), q (quartet), m (multiplet), and br (broad). Thin-layer chromatography (TLC) was performed for qualitative analysis of 6-O-cinnamoyl-sorbitol on silica gel 60F plates (Merck, Germany), with ethyl acetate/methanol/water (17:2:1, v/v) as mobile phase [4]. The isolated spots were detected with UV at 254 nm. Cinnamate ester was measured by HPLC using a Waters Symmetric C18 column (250 × 4.6 mm, 5 μm particle size) with a mobile phase as 35 % acetonitrile in 65 % 25 mM sodium acetate buffer (pH 4.8) at a flow rate of 1 mL min−1, and peaks were detected using an HPLC diode array detector at 254 nm (Younglin, Republic of Korea).
Enzymatic Synthesis of 6-O-cinnamyl-sorbitol
The reactions were initiated by the addition of 1 g of Novozym 435 to 100 mL of 100 % tert-butanol containing 40 mM sorbitol, 100 mM vinyl cinnamate, and 1 g of molecular sieves [12]. The suspension was stirred at 200 rpm for 5 days at 37 °C after the reactions were terminated by filtering off the enzyme. The solvents were then evaporated, and formation of the products was confirmed by TLC. The product was separated by silica gel chromatography with an eluent consisting of chloroform/methanol (7:1, v/v) [13].
DPPH Assay to Determine Free-Radical Scavenging Activity
The free-radical scavenging activity (%RSA) of compounds was measured using a modified DPPH radical method [14]. For analysis, 100 μL of the sample was mixed with 0.2 mM DPPH solution in 100 μL of methanol (final concentration was 0.1 mM, pH 7.8) [15]. Concentration of substrates ranged from 1∼10 to 0∼100 μM [16]. The reduction of DPPH radical was monitored spectrophotometrically using a Sunrise 96-well plate reader (Tecan Group, Switzerland) at 520 nm over a period of 20 min in a darkroom at 30 °C against a blank assay. The percentage of the remaining radical was calculated as the absorbance of the sample at 520 nm, divided by that of the DPPH control at the same time multiplied by 100 (Scavenging Activity(%) = (Ablank − Asample)/Ablank × 100) [17].
Results and Discussion
Synthesis and Analysis of 6-O-cinnamyl-sorbitol
Cinnamic acids, which play an important role in the plant physiological activities, are frequently found in nature in the form of esters with polyhydric alcohols. The use of natural esters of aromatic acids and polyols is restricted by their poor availability, which is a consequence of the difficulty of isolating them from plants and the complexity of their synthesis [18]. In this study, a simple and accessible method to synthesize water soluble analogue of cinnamic acids was developed. Initial enzyme activity with D-sorbitol, vinyl cinnamate, and 6-O-cinnamoyl-sorbitol was determined by TLC with UV irradiation at 254 nm (data not shown) (Fig. 1). HPLC analysis was performed to analyze the reaction, and different compounds 6-O-cinnamoyl-sorbitol (RT = 3.4 min), cinnamic acid (RT = 4.3 min), and vinyl cinnamate (64.6 min) were observed at different time interval, respectively (Fig. S1).
A spot with R f = 0.43 was observed by the conditions explained in the “Materials” section. For the identification of 6-O-cinnamoyl-sorbitol, the reaction was performed in a scale of 100 mL. After the solvent was evaporated off, the product was purified by silica gel chromatography. TLC and HPLC were used to monitor the purification, which resulted in a white powder. The 1H-NMR results obtained were as follows: 1H NMR of major diastereomer (400 MHz, DMSO-d 6 ): 7.60–7.67 (2H, m), 7.59 (1H, d, J = 16.0 Hz), 7.32–7.38 (3H, m), 6.55 (1H, d, J = 16.0 Hz), 4.87 (1H, d, J = 4.8 Hz), 4.42 (1H, d, J = 4.8 Hz), 4.35 (1H, d, J = 6.4 Hz), 4.29 (1H, d, J = 6.0 Hz), 4.27 (1H, d, J = 6.4 Hz), 4.16 (1H, dd, J = 11.4, 3.8 Hz), 4.06 (1H, dd, J = 11.4, 7.4 Hz), 3.75–3.81 (1H, m), 3.61–3.67 (1H, m), 3.47–3.52 (1H, m), 3.37–3.43 (1H, m), 3.34–3.40 (1H, m), and 3.28–3.34 (1H, m).
To determine the effect of the ester group on 6-O-cinnamoyl-sorbitol production, four compounds including vinyl cinnamate, allyl cinnamate, ethyl cinnamate, and isobutyl cinnamate were examined. Vinyl cinnamate resulted in the highest activity with D-sorbitol and isobutyl cinnamate resulted in the second highest (Table 1), and cinnamic acid showed 70.6 % relative activity to vinyl cinnamate for 6-O-cinnamoyl-sorbitol synthesis.
Optimization of Enzyme Reaction
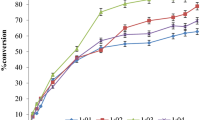
To find the optimal conditions for the enzymatic reaction, the temperature and molar ratio of D-sorbitol to vinyl cinnamate were optimized. Temperature ranges are 30, 37, 45, and 60 °C were examined to determine the best conversion within 4 days (Fig. 2a). Conversion was expected to increase with increasing temperature up to 55 °C, as previously reported for aliphatic esters of cinnamic acid [19–21], and the reaction at 45 °C had showed the best conversion. When the molar ratio of D-sorbitol to vinyl cinnamate was examined, the ratio of 1:8 (D-sorbitol/vinyl cinnamate) was found to give the best conversion (Fig. 2b). Although 1:8 (D-sorbitol/vinyl cinnamate) ratio was observed as the best ratio for 6-O-cinnamoyl-sorbitol production, excess use of vinyl cinnamate was not cost-effective and also resulted in excess of cinnamic acid leftover in the solution as residues, and they were affecting the further separation and purification processes; hence, 1:1 ratio was used for the time-dependent experiments.
To monitor the enzymatic reaction at different time points, reaction was performed in 2 mL volume scale with 0.02 g of enzyme (total 40 unit), 3.64 mg of D-sorbitol (10 mM), and 3.47 mg of vinyl cinnamate (10 mM) in 1:1 ratio at 45 °C for 4 days (Fig. 3). Vinyl cinnamate was consumed completely in 2 days by the formation of either 6-O-cinnamoyl-sorbitol or cinnamic acid. The final 20 % of conversion was achieved after 1 day; then, there was no increase in 6-O-cinnamoyl-sorbitol. 6-O-cinnamoyl-sorbitol amount was calculated by the consumption of vinyl cinnamate and concentration of cinnamic acid. Only 2 mM of 6-O-cinnamoyl-sorbitol production was recorded at the end of 4-day reaction, and 80 % of the vinyl cinnamate was converted into cinnamate. Although cinnamate and sorbitol were still present in the reaction mixture, there was no further conversion processes. For this, there should be several reasons such as loss of enzyme activity after the several days of reaction, reversible esterification with water, product inhibition, sorbitol solubility, and so on, although it was not clearly identified yet. In all these reactions, the low solubility of sorbitol was a problem for poor conversion of 6-O-cinnamoyl-sorbitol, and this study was carried out for long time as technique of partial dissolution, which has already been applied for the production of 6-O-acylglucose ester [22, 23] (Fig. 3). The solubility of 6-O-cinnamoyl-sorbitol was not shown here; however, based on previous reports on sugar (alcohol) esters, we can easily expect 6-O-cinnamoyl-sorbitol can be more soluble than cinnamic acid [24–26]. The application of sugar or sugar alcohol for fatty acids and phenolics greatly enhanced the solubility of sugar fatty acid esters and phenolic esters [24–26].
Examination of Radical Scavenging Activity by DPPH Assay
Considering that both compounds (vinayl cinnamate and sorbitol) are widely applied in the medical and pharmaceutical industries, it is expected that they might have possible applications in the cosmetic field as fragrance material, thickener, and humectants [27]. In addition, there has been a report on the synergetic effect for radical scavenging activity by combining two different molecules to make an ester form [28]. Based on this idea, the radical scavenging activity by DPPH assay was examined (Fig. 4). Substrates, D-sorbitol and vinyl cinnamate, as well as the products, cinnamic acid and 6-O-cinnamoyl-sorbitol, were examined for scavenging activity. When 1∼10 mM of cinnamic acid, sorbitol, vinyl cinnamate, and 6-O-cinnamoyl-sorbitol were examined, only 6-O-cinnamoyl-sorbitol showed 30 % radical scavenging activity. Compared to the ascorbic acid (control), at the range between 0 and 100 μM, it showed 8 % activity, while ascorbic acid showed 60 % at 100 μM. Although the current analysis showed a lower activity than ascorbic acid at 100 μM, but considering that their substrates were not showing any radical scavenging activity, it thus provides great insights and potential for possible applications in cosmetics.
Conclusion
In this study, we attempted to improve the property of cinnamic acid by esterification with sorbitol and tried to explore the synergistic effects of both compounds together in a reaction. The enzyme-catalyzed synthesis of 6-O-cinnamoyl-sorbitol using D-sorbitol and vinyl cinnamate was performed in an organic solvent under mild conditions, resulting the production of 6-O-cinnamoyl-sorbitol, which also possesses the substrate specificities of a cinnamate derivative. The synthesized 6-O-cinnamoyl-sorbitol showed a considerable radical scavenging activity. Although, the optimization of production to improve the yield is still needed, and our study provides novel insights in the functional modification of cinnamic acid, and this would be the first report on the production of 6-O-cinnamoyl-sorbitol in vitro.
References
Liu, L., Hudgins, W. R., Shack, S., Yin, M. Q., & Samid, D. (1995). Cinnamic acid: a natural product with potential use in cancer intervention. International Journal of Cancer, 62, 345–350.
Humphreys, J. M., & Chapple, C. (2002). Rewriting the lignin roadmap. Current Opinion in Plant Biology, 5, 224–229.
Boudet, A. M., Kajita, S., Grima-Pettenati, J., & Goffner, D. (2003). Lignins and lignocellulosics: a better control of synthesis for new and improved uses. Trends in Plant Science, 8, 576–581.
Mi, J., Sun, Z. H., Zhong, M. H., Yang, Y. H., Chen, T., Xiong, G. J., Luo, H., & Qi, X. Q. (2012). Predictive factors of chronic thromboembolic pulmonary hypertension in patients with acute pulmonary thromboembolism. Zhonghua Xin Xue Guan Bing Za Zhi, 40, 497–501.
Lone, R., Shuab, R., & Koul, K. K. (2014). Role of cinnamate and cinnamate derivatives in pharmacology. Global Journal of Pharmacology., 8, 328–335.
Yang, Y. H., Raku, T., Song, E., Park, S. H., Yoo, D., Park, H. Y., Kim, B. G., Kim, H. J., Lee, S. H., Kim, H. S., & Tokiwa, Y. (2012). Lipase catalyzed reaction of L-ascorbic acid with cinnamic acid esters and substituted cinnamic acids. Biotechnology and Bioprocess Engineering, 17, 50–54.
Pandey, K. B., & Rizvi, S. I. (2009). Plant polyphenols as dietary antioxidants in human health and disease. Oxidative Medicine and Cellular Longevity, 2, 270–278.
Bradley, J.-C., Neylon, C., Williams, A., Guha, R., Hooker, B., Lang, A. S., Freisen, B., Bohinski, T., Bulger, D., & Federici, M. (2009). Open notebook science challenge: Solubilities of organic compounds in organic solvents. ed. ONS Books.
East, A., Jaffe, M., & Zhang, Y. (2006). Ultraviolet absorber for cosmetics and polymeric materials. Google Patents.
Graf, E. (1992). Antioxidant potential of ferulic acid. Free Radical Biology and Medicine, 13, 435–448.
Leinen, H.-T., Gregori, D., Pujol, M., & Carbó, M. R. (2003). Water, silica polishing agent and humectant comprised of sorbitol, glycerol and polyethylene glycol; transparent; remains in gel form on toothbrush. Google Patents.
Degn, P., & Zimmermann, W. (2001). Optimization of carbohydrate fatty acid ester synthesis in organic media by a lipase from Candida antarctica. Biotechnology and Bioengineering, 74, 483–491.
Raku, T., & Tokiwa, Y. (2003). Chemoenzymatic synthesis of fucose- or rhamnose-branched polymer. Macromolecular Bioscience, 3, 151–156.
Sabally, K., Karboune, S., St-Louis, R., & Kermasha, S. (2006). Lipase-catalyzed transesterification of trilinolein or trilinolenin with selected phenolic acids. Journal of the American Oil Chemists Society, 83, 101–107.
Xu, B., & Chang, S. (2007). A comparative study on phenolic profiles and antioxidant activities of legumes as affected by extraction solvents. Journal of Food Science, 72, S159–S166.
Choi, J., Oh, J., Hwang, I., Kim, S., Jeon, J., Lee, B., Kim, J., Kim, T., & Cho, K. (2003). Application and high throughput screening of DPPH free radical scavenging activity by using 96-well plate. The Korean Journal of Pesticide Science, 7, 92–99.
Sanna, D., Delogu, G., Mulas, M., Schirra, M., & Fadda, A. (2011). Determination of free radical scavenging activity of plant extracts through DPPH assay: an EPR and UV–Vis study. Food Analytical Methods, 5, 759–766.
Artamonov, A., Burkovskaya, L., Nigmatullina, F., & Dzhiembaev, B. Z. (1997). Synthesis of monoesters of D-sorbitol and aromatic acids. Chemistry of Natural Compounds, 33, 571–573.
Jakovetic, S. M., Jugovic, B. Z., Gvozdenovic, M. M., Bezbradica, D. I., Antov, M. G., Mijin, D. Z., & Knezevic-Jugovic, Z. D. (2013). Synthesis of aliphatic esters of cinnamic acid as potential lipophilic antioxidants catalyzed by lipase B from Candida antarctica. Applied Biochemistry and Biotechnology, 170, 1560–1573.
Song, Q. X., & Wei, D. Z. (2002). Study of Vitamin C ester synthesis by immobilized lipase from Candida sp. Journal of Molecular Catalysis B-Enzymatic, 18, 261–266.
Poojari, Y., Beemat, J. S., & Clarson, S. J. (2013). Enzymatic synthesis of poly(epsilon-caprolactone): thermal properties, recovery, and reuse of lipase B from Candida antarctica immobilized on macroporous acrylic resin particles. Polymer Bulletin, 70, 1543–1552.
Arcos, J. A., Bernabe, M., & Otero, C. (1998). Quantitative enzymatic production of 6-O-acylglucose esters. Biotechnology and Bioengineering, 57, 505–509.
Tarahomjoo, S., & Alemzadeh, I. (2003). Surfactant production by an enzymatic method. Enzyme and Microbial Technology, 33, 33–37.
Arcos, J., Bernabe, M., & Otero, C. (1998). Quantitative enzymatic production of 1, 6-diacyl fructofuranoses. Enzyme and Microbial Technology, 22, 27–35.
Degn, P., & Zimmermann, W. (2001). Optimization of carbohydrate fatty acid ester synthesis in organic media by a lipase from Candida antarctica. Biotechnology and Bioengineering, 74, 483–491.
Otto, R. T., Bornscheuer, U. T., Scheib, H., Pleiss, J., Syldatk, C., & Schmid, R. D. (1998). Lipase-catalyzed esterification of unusual substrates: synthesis of glucuronic acid and ascorbic acid (vitamin C) esters. Biotechnology Letters, 20, 1091–1094.
Letizia, C., Cocchiara, J., Lapczynski, A., Lalko, J., & Api, A. (2005). Fragrance material review on cinnamic acid. Food and Chemical Toxicology, 43, 925–943.
Choo, W. S., & Birch, E. (2009). Radical scavenging activity of lipophilized products from lipase-catalyzed transesterification of triolein with cinnamic and ferulic acids. Lipids, 44, 145–152.
Acknowledgments
The authors follow the Instructions for Authors to make sure their manuscript complies to the ethical rules applicable for this journal. The study was partially supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (NRF-2013R1A1A2A10004690), by KOPRI (PE14030), and by the R& D Program of MOTIE/KEIT (10048350) and “Cooperative Research Program for Agriculture Science & Technology Development (Project title: Isolation and identification of rhizobacteria for indoor VOCs removal, Project No. 010205022014)” Rural Development Administration, Republic of Korea. In addition, this research was supported by the 2014 KU Brain Pool of Konkuk University.
Author information
Authors and Affiliations
Corresponding author
Additional information
Jung-Ho Kim and Shashi Kant Bhatia contributed equally to this work.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Fig. S1
HPLC chromatogram of each compound with their retention time, i.e., RT = 3.4 for 6-O-cinnamoyl-sorbitol, 4.3 for cinnamic acid, and 64.6 min for vinyl cinnamate (DOC 76 kb).
Rights and permissions
About this article
Cite this article
Kim, JH., Bhatia, S.K., Yoo, D. et al. Lipase-Catalyzed Production of 6-O-cinnamoyl-sorbitol from D-sorbitol and Cinnamic Acid Esters. Appl Biochem Biotechnol 176, 244–252 (2015). https://doi.org/10.1007/s12010-015-1570-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-015-1570-x