Abstract
Rapid quality deterioration after harvest restricts the shelf life of broccoli, and reduces its commercial value. In this study, the effects of combined edible coating with chitosan and tea polyphenol (CTS + TP) on the post-harvest quality of broccoli were investigated. Results showed that the combined treatment of chitosan and tea polyphenol had better effects on the decay rate, weight loss rate, and respiratory intensity of broccoli than the single chitosan treatment and control. CTS + TP treatment mitigated the severity of broccoli yellowing, which was demonstrated by the higher hue angle, chlorophyll level, and strong red fluorescence emission of the treated samples, as well as delayed yellowing time compared to that observed in controls. Moreover, CTS + TP treatment better protected the sensory quality of the product, such as odor, hardness, and chewiness, and prevented evident changes. The vitamin C level in the treated samples was extremely higher, and the higher antioxidant capacity was also noted. The content of sulforaphane, the typical bioactive substance of broccoli, declined conspicuously in the yellowed broccoli, while the change was noticeably relieved by CTS + TP coating. The significantly upregulated expressions of BoCYP83A1, BoMYB28, and BoMYR in treated samples may have played an important role in the improvement of sulforaphane contents. These findings indicated that the edible coating with CTS + TP alleviated yellowing symptoms, improved the sensory quality, and enhanced the nutraceutical value of broccoli.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Broccoli (Brassica oleracea L. var. italica) is a crop widely cultivated worldwide with high nutraceutical value. This vegetable, as an excellent source of vitamins, antioxidant substances, and bioactive elements, is an integral part of a healthy human diet (Soares et al., 2017). Health-promoting compounds in broccoli have attracted substantial attention; especially sulforaphane (4-methylsulfinylbutyl isothiocyanate) has been extensively studied and proved to be a promising anticancer agent (Alvarez-Jubete et al., 2014; Guo et al., 2018). However, harvested broccoli undergoing vigorous physiological metabolism is prone to yellowing during shelf life at room temperature, accompanied by a rapid loss of nutrients. Accordingly, the exploration of safe and effective technology to solve these problems has always been a research hotspot.
Emerging study revealed that the changes in calyx pigment observed under the anatomical microscope gradually proceed from the base of the flower bud to the top, and eventually manifested as yellowing in phenotype, whereas the chlorophyll level decreased significantly as the degree of yellowing intensified (Fang et al., 2020). In recent years, great progress has been made in the study of mechanisms underlying the chlorophyll metabolism in plants (Pružinská et al., 2007; Sánchez-Vega et al., 2014), and it has been demonstrated that chlorophyll degradation is the main factor leading to broccoli yellowing (Shi et al., 2016). To reduce yellowing and maintain the post-harvest quality of broccoli, various strategies, such as controlled atmosphere, chemical preservative, light illumination, and coating, have been applied (Cai et al., 2019; Eason et al., 2007; Jin et al., 2015; Moreira et al., 2011a). Edible coating can form a protective film on the surface of food, and has emerged as an ideal substitute with several advantages over synthetic materials, including biodegradability, environmental friendliness, and renewability (Cazón et al., 2016; Tharanathan, 2003).
Consumers’ widespread concern about food safety has promoted the development and study of edible coatings. Among them chitosan (CTS), natural polysaccharide derived from chitin, has been approved as “generally recognized safe” by the US Food and Drug Administration (Romanazzi et al., 2015). CTS stands a good chance of replacing chemical preservatives in food preservation, which benefits by its film-forming and antibacterial characteristics (Bautista-Baños et al., 2006; Han et al., 2014). Over the years, CTS coating has been applied to sweet pepper and cucumber (Ghaouth et al., 2010), papaya (Ali et al., 2011), guava (Hong et al., 2012), sweet cherry (Petriccione et al., 2015), and blackberry (Vilaplana et al., 2020), which can improve their quality and extend shelf life. Previous study suggested that the application of CTS coating alone or in combination with mild heat shocks on broccoli showed aptitude for inhibiting florets yellowing and improving sensory quality (Moreira et al., 2011a). Compared with other bio-based food packaging materials, the structural properties of CTS coating enable it to serve as an edible coating, as well as a carrier for bioactive compounds (Möller et al., 2004; Mustafa et al., 2014). The incorporation of functional substances, such as antioxidants and antimicrobials, into CTS coatings is emerging as a viable technology because it enhances the comprehensive performance of films (Ribeiro et al., 2020; Sarabandi & Mahdi Jafari, 2019). Molamohammadi et al. (2020) reported that the CTS edible coating added with salicylic acid showed a positive effect on reducing bacterial growth and extending the shelf life of fresh pistachios. In addition, the efficiency of CTS coating enriched with bioactive compounds in improving the safety of broccoli was tested, and the results showed that these coatings can be used as natural biopreservatives (Alvarez et al., 2013)
Tea polyphenol (TP), a kind of polyphenols extracted from tea, has good prospects of their use as preservatives and natural antioxidants in food industries (Wang et al., 2019). TP has multiple bioactivities such as antioxidant, anti-cancer, and hypolipidemic effects, and the results of toxicity and pharmacological experiments demonstrated that tea extract is safe and non-toxic and has broad-spectrum antimicrobial activity (Yang et al., 2009). Furthermore, research results showed that TP, as antioxidant or preservative, could delay the aging and deterioration of peach fruit, and extend the shelf life of fresh-cut lettuce (Chen et al., 2013; Martín-Diana et al., 2008). The antioxidant mechanism of TP is mainly ascribed to its capacity for removing excess free radicals from the body (Zhao, 2006). Nevertheless, research on its potential use in food preservation is still limited.
Given the beneficial impact of edible coatings on the quality of fruit and vegetables after harvest, little information is currently available about their application in broccoli. Therefore, the present study had the objective to investigate the effects of the edible coating with CTS+TP on the post-harvest quality of broccoli. It is necessary to consider the sensory traits of broccoli, especially the color and odor, with greater impact on the purchase decision of customers. Also, the effects of combined coating on the broccoli nutritional quality by monitoring the changes in the vitamin C, amino acid, and sulforaphane content and in the antioxidant capacity were evaluated. This study provides a technical basis for effectively alleviating broccoli yellowing and improving its quality.
Materials and Methods
Plant material and Treatment
‘Naihan-Youxiu’ broccoli heads were harvested from a local farm in Jinzhou, Liaoning Province, China. The harvested samples were packed in sealed foam boxes to avoid bruises and hold moisture, then placed in a refrigerated truck and immediately transported to our laboratory within 3 h. Absence of disease or other defects, diameter 9–13 cm with uniform size and color broccoli were selected for the study.
CTS (food-grade, 90% of deacetylated degree, average molecular weight, 350 kDa) and TP were purchased from Solarbio Biotechnology Co., Ltd (Beijing China). All other solvents and chemicals were of analytic grade and commercially available. CTS solution (1%, w/v) was prepared by dispersing CTS powder in 1% acetic acid (v/v), and TP solution (1%, w/v) was dissolved in distilled water. The two solutions mentioned above were mixed evenly (CTS:TP=1:1) by magnetic stirring to make the composite solution for use. The concentrations of CTS and TP were determined as optimal based on our preliminary experiment.
In the experiment, a total of 270 broccoli was randomly divided into three groups with 90 heads for each group. Treatments were immersing heads for 3 min in 1% CTS solution; control (distilled water with 1% acetic acid); and composite (CTS+TP) solution. Each treatment had three replicates. After drying naturally, all broccoli samples were enclosed in perforated plastic cartons with polyethylene bags, and then placed at 20 ± 2 °C with 60–70% relative humidity for 5 days. Additional 30 broccoli were randomly collected for initial quality and biochemical analysis prior to application of treatments as described below. Phenotypic color, decay rate, weight loss, and respiration rate were recorded every day. At each sampling time point, six uniform broccoli heads from each treatment were randomly selected, and florets were sampled from five points on each broccoli head, mixed evenly, were immediately frozen in liquid nitrogen, and stored at −80 °C until further analysis. Within each replicate of treatment, collected samples were pooled and measured with three technical repeats.
Decay Rate, Weight Loss, and Respiration Rate
At each sampling time, visual evaluation was performed immediately after the sample was removed from the shelf life. Broccoli with visible mold growth was counted as decayed. Decay rate was determined according to the following equation. The microbiological analysis was performed according to Freitas et al. (2015), and mold counts were expressed as log CFU g−1.
The weight loss of broccoli was calculated as the percentage change relative to the initial weight.
with Wi, initial weight; and Wt, weight in day t.
The respiration rate was assayed according to the method of Wu et al. (2010), and respiration was measured as CO2 output. For each treatment, two broccoli samples were sealed in a 10 L plastic container for 4 h. The effluent air samples were connected to a portable O2/CO2 gas composition tester, and the result was expressed as mg CO2 kg−1 h−1 fresh weight. The above experiments were conducted in triplicate; finally the average values were reported.
Phenotypic Color and Texture Profile Analysis (TPA)
Superficial color of broccoli was evaluated using a CR-400 colorimeter, and calibrated with a standard white and black plate (Chroma Meter CR-400, Konica Minolta, Tokyo, Japan). Six broccoli heads were randomly selected from each replicate and the CIELAB coordinates (L*, a*, b*) were measured three times at five different positions on each broccoli head; finally, the average was calculated as the result. The hue angle (h°) was calculated according to \(\mathrm{h}^\circ ={\mathrm{tan}}^{-1}\left(\frac{b*}{a*}\right)\), which varied constantly from 0° to 360°, where 90° manifests yellow and 180° manifests green.
A texture analyzer (TA.XT Plus, Stable Micro Systems Ltd., UK) was used to assay the firmness value. Ten broccoli samples were randomly drawn from each treatment. The pre-test speed, test speed, and post-test speed were 2.0 mm s−1, 1.0 mm s−1, and 1.0 mm s−1, respectively. The time interval between the two compressions was 5 s. The probe test distance = 10.0 mm, and the trigger load = 4.0 g. Textural parameters including hardness and chewiness were obtained from the analyses of typical TPA curves of texture character.
Electronic Nose (E-nose) Detection
The broccoli was cut into small pieces, and some of the samples (30 g) were put into a 1 L beaker and sealed tightly with plastic wrap. After balancing for 15 min, the odor analysis was performed using a PEN3 electronic nose (Airsense Analytics, GmBH, Schwerin, Germany). The headspace inhalation method was adopted to directly insert the injection needle into the beaker for measurement. The e-nose sensor cleaning time was 70 s, auto-zero setting time was 10 s, the sample preparation time was 5 s, the sample testing time was 50 s, and the injection flow rate was 100 mL min−1. Before and after the measurement, the sensor was cleaned and standardized, and the measurement was repeated 6 times for each treatment. The e-nose was equipped with ten sensors, and the specific performance of the sensor array matched with the electronic nose is shown in Table 1.
Chlorophyll Determination and Fluorescence Microscope Imaging
The content of chlorophyll was determined according to the method proposed by Lichtenthaler (1987), with slight modifications. 1.0 g broccoli sample was ground in 0.6 g quartz sand, 0.5 g calcium carbonate powder, and 5 mL of 95% ethanol. Thereafter, an equal volume of extract solution was added to the pre-cooled homogenizer. After 3 min of static extraction in an ice bath, the homogenates were filtered. The obtained filtrate was centrifuged at 12, 000 × g for 5 min at 4 °C, and the supernatant was collected to measure the chlorophyll content. The absorbance was measured at 665, and 649 nm with a spectrophotometer (TU-1810, Puxi Instrument Co., Beijing, China), and 95% ethanol was used as blank reference zero.
The chlorophyll fluorophore of fresh samples was observed by fluorescence microscope (LECIA DM4 B, Leica Microsystems, Germany). Extraction of chlorophyll using the same method as mentioned above, and the supernatant was further concentrated at 500 × g for 25 min in a vacuum centrifugal concentrator (CV200, Beijing JM Technology Co., Ltd, China) for subsequent fluorescence observation. The Leica digital camera (Leica DFC7000 T) was used for fluorescence imaging. The objective lens was 40 times, and the observed fluorescence was excited in the green light region.
Measurement of Total Soluble Solid, Vitamin C, and Amino Acid
Total soluble solid (TSS) was measured by RE40 refractometer (Mettler Toledo, Madrid, Spain). Vitamin C (Vc) was quantified following the method of Gillespie and Ainsworth (2007). The content of amino acid was determined using an Amino Acid Analysis Kit (Aidisheng Biological Technology Co., Ltd, Jiangsu, China). Each measurement was conducted in triplicate.
Measurement of Sulforaphane Content
Extraction Method
The method described by Liang et al. (2006) was followed, with some modifications. 1.0 g broccoli sample was triturated in liquid nitrogen, 4 mL of phosphoric acid buffer (pH= 7) was added for further grinding, and then the mixture was transferred to a tube and swirled for 2 min. Subsequently, the homogenates were kept in a water bath at 37 °C for 4 h followed by the ultrasonic extraction with 20 mL of dichloromethane for 70 min, which had been dehydrated using 2.5 g anhydrous sodium sulfate. Thereafter, the excess heat was released by gently shaking the test tube and loosening its lid. The supernatant was collected by centrifugation at 10, 000 × g at 4 ℃ for 20 min, and then dried on a rotary evaporator at 30 °C. The concentrated residue was dissolved in 1 mL of the mobile phase.
UPLC-ESI–MS Conditions
Ultra-high performance liquid chromatography electrospray ionization tandem mass spectroscopy (UPLC-ESI-MS) was carried out using the ACQUITY UPLC H-Class_XEVO TQD-System (Waters Technologies, Milford, USA). The optimized conditions of the electrospray ionization method were as follows: capillary voltage, 1.08 kV; cone voltage, 20 V; desolvation temperature, 650 °C; desolvation gas flow, 995 L h−1; mode, positive; MS/MS condition, 174>114; and collision energy, 10 eV. Three microliters of the filtrate was then injected into a 1.7 µm ACQUITY UPLC® CSHTM C18 column and a solvent flow rate of 0.3 mL min−1, with the following mobile phases: 0.1% formic acid in water (A) and acetonitrile with 0.1% formic acid (B). The elution gradient was as follows: 0–0.5 min 90% A+10% B; 0.5–3 min 60% A+40% B; 3–3.5 min 60% A; 3.5–4.5 min 90% A; 4.5–6.5 min 90% A+10% B. Quantification was based on the external standard method to calculate the content of sulforaphane. There were three biological replicates per treatment, and the experiments were measured in triplicate.
Measurement of Antioxidant Capacity
The antioxidant capacity was evaluated by ABTS assay, which was performed as described by Conversa et al. (2019) with minor modifications. The method used was based on the ability of the sample to inhibit ABTS·+ with reference to antioxidant standard (Trolox). 1.0 g broccoli sample was homogenized in an ice bath with 10 mL of pre-cooled phosphate buffered saline, and then centrifuged at 10, 000 × g for 10 min at 4 °C. ABTS·+ was prepared by mixing 7 mmol L−1 ABTS stock solution [2,2′-azino-bis (3-ethyl-benzothiazoline-6-sulfonic acid)] with 140 mmol L−1 potassium persulfate. After 14 h in the dark, the above solution was diluted with phosphate buffer (pH 7.4) until absorbance of 0.700 ± 0.020 at 734 nm. For the spectrophotometric measurement, 50 μL of standard or extract supernatant was mixed with 1950 μL of ABTS·+ solution, and the absorbance was determined at 734 nm. The obtained radical ABTS·+ scavenging activity of broccoli sample was converted to the antioxidant capacity by μmol of Trolox equivalent (TE) per gram of fresh weight.
Expression Analysis of Sulforaphane Related Genes
Total RNA was extracted from the collected broccoli buds using the OminiPlant RNA kit (CWBIO, Beijing, China) according to the manufacturer’s instructions. The cDNA synthesis from total RNA was performed using the HiFiScript cDNA Synthesis Kit (CWBIO, Beijing, China). Specific primer sets of target genes (Table 2) were designed by Primer 3 Plus and Primer 5.0 software according to related gene sequences, and were synthesized by GENEWIZ Biotechnology Synthesis Lab (Jiangshu, China). The real-time quantitative PCR was carried out on a QuantStudioTM 6 Flex system (Life Technologies, Thermo Fisher Scientific, Waltham, USA) using UltraSYBR Mixture (CWBIO, Beijing, China) with a total volume of 50 µL. Three independent biological replicates were performed for this experiment. The expression level of β-actin was used as an internal reference and the expression of other genes was computed according to the 2−∆∆Ct method.
Statistical Analysis
All experiments in this study were performed in a completely randomized design and with three repetitions. Data were represented as means with standard deviation, and were statistically analyzed by one-way analysis of variance (ANOVA) using SPSS software (version 19.0, SPSS Inc., Chicago, IL, USA). Means were compared by the least significant difference (LSD) test and differences at P < 0.05 were considered significant. Duncan’s test was used for multiple comparisons between more treatment groups.
Results and Discussion
Effects of Different Treatments on the Decay Rate, Weight Loss, and Respiratory Rate of Broccoli
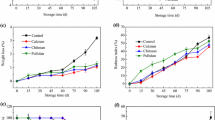
Harvested broccoli is perishable at room temperature, leading to considerable economic losses. In this work, decay symptoms in untreated samples were initially found on the 3rd day, and gradually worsened thereafter, with the decay rate increasing to 88.9% by the 5th day (Fig. 1a). However, broccoli treated with CTS coating consistently showed a lower decay rate than the control. Such a result was aligned with the finding of Vilaplana et al. (2020) that the CTS coating can suppress the decay of blackberries. Notably, the CTS+TP treatment had better inhibition effect on broccoli decay. On the 3rd and 5th day of shelf life, the decay rates of this treatment were one-quarter and two-thirds of the control, respectively, which were 21% and 15% lower than those of single CTS treatment. This effect was due to the potent antimicrobial actions of TP (Chen et al., 2013; Yang et al., 2009). In addition, the mold counts had an overall upward trend with all treatments as the shelf life proceeded (Fig. 1b); the values for the control were significantly higher than those of the other two treatments from the 3rd day (P < 0.05). The application of combined coating with CTS+TP resulted in a distinct reduction in mold counts. In another study made by Falcó et al. (2019), the incorporation of green tea extract into alginate coatings was shown to prominently improve the safety of strawberries and raspberries against pathogens. These further suggest that the application of CTS+TP to broccoli effectively prevented decay.
The vegetable, however, experiences moisture loss after harvest, which is detrimental to the maintenance of products quality (Zhang et al., 2019). Water loss caused by respiration and transpiration is the main cause of weight loss. In the present study, the weight loss of untreated broccoli increased remarkably with the extension of shelf life, and this ascent was mitigated by single CTS treatment, which, by the end of shelf life, had only half the value of the control (Fig. 1c). Broccoli treated with CTS+TP displayed the slowest rise, and significantly lower weight loss rates were observed on days 4 and 5 in comparison with single CTS-treated samples (P < 0.05). Our results are supported by Ghaouth et al. (2010), where the application of CTS coating to sweet peppers and cucumbers resulted in greater weight retention. CTS coating has also been reported to limit rapid water loss of sweet cherries (Petriccione et al., 2015). Thereout, the lesser tendency to lose weight in coated broccoli was probably due to the formed film covering samples that delays the transport of water vapor.
High respiratory rate is one of the main factors resulting in short shelf life of post-harvest broccoli. Overall, the respiratory rate of broccoli in the different treatments showed an initial rise, and then decreased, reaching their peak on day 3 (Fig. 1d). However, the change magnitude of the three treatments was in the order of control > single CTS treatment > combined CTS+TP treatment, in which the peak values of combined treatment and single treatment decreased by 30% and 18%, respectively, compared with the control. The reduction in respiratory rate of the coated samples may be attributed to the barrier properties of CTS to absorb moisture at elevated temperature (Olivas & Barbosa-Cánovas, 2005), which reduces the gas exchange between food and the atmosphere corresponding a lower CO2 transmission rate. Han et al. (2014) obtained similar results on sponge gourd, where the CTS treatment was effective in reducing the respiration rate.
Edible Coating Ameliorated Sensory Quality Change of Broccoli
The above results showed that adding TP on the basis of CTS coating substantially retarded the rapid rise of broccoli decay rate, and also reduced the weight loss and respiratory intensity to a certain extent. In the following studies, we focused on comparing the protective effects of CTS and TP combinative treatments on the quality of broccoli.
Color is the primary consideration for intuitively determining broccoli quality. Broccoli without treatment showed yellowing on the 3rd day, and became serious yellowing on the 5th day. However, the effect of CTS+TP coating on retaining the green color was more evident. The treated samples remained their initial green on day 3 and, despite the appearance of yellowing symptoms on day 5, were still obviously better visually than the control. Chromaticity values can objectively reflect color changes, so we further evaluate the surface color of broccoli by measuring the hue angle (h°). The h° values in the different treatments began to decrease rapidly from the 3rd day of shelf life (Fig. 2a), with the largest decline in the control. The values in the CTS+TP treatment were significantly higher than those in the single CTS treatment and the control on the days 3 and 4 (P < 0.05), and their values were 10% higher than those of the control at the end of shelf life. These results suggest that the edible coating with CTS+TP effectively ameliorated the yellowing of broccoli. Additionally, firmness is one of the main quality attributes, affecting the overall acceptance of the product. We observed the firmness in the three treatments decreased progressively along with yellowing (Fig. 2b), with differences becoming obvious from the day the control broccoli turned yellow, and this decline was delayed by coating treatment. However, the slowest decline rate was observed in the CTS+TP-treated broccoli throughout the shelf life. The maintenance of firmness could be attributable to the high antifungal activity of this coating, which reduced infection and other ripening processes (Hong et al., 2012). Moreover, chewiness is also an important parameter to evaluate the sensory properties of food. Broccoli that loses its crisp and refreshing mouthfeel reduces people’s appetite. In the present study, the change trend of chewiness in the coated samples agreed with the results of the controls (Fig. 2c), but their values were evidently higher during the yellowing period. With regard to treated broccoli, the CTS+TP coating was effective in delaying the decline of chewiness in the late shelf life.
Changes in h° value (a), firmness (b), and chewiness (c) of broccoli during shelf life. Data are expressed as means of triplicate samples ± standard deviation. Different letters indicate significant differences among the treatments (P < 0.05). PCA analysis of the e-nose data collected from CTS + TP-treated and control samples at 0 day, 2 days, and 4 days of shelf life (d). Effects of CTS + TP treatment on odor (volatile signature) during broccoli yellowing (e)
The generation of unpleasant odors is also a deterioration phenomenon in post-harvested broccoli, which greatly strangles consumers’ buying desire. Consequently, finding control measures is crucial to limit the incidence of off-flavor. Volatile signatures emitted by broccoli were analyzed, and the data collected from the e-nose were presented using a principal component analysis (PCA) plot. The contribution rates of the first principal component (PC1) and the second principal component (PC2) were 81.6% and 12.1%, respectively (Fig. 2d), and the cumulative contribution rate reached 93.7%, indicating that the two PCs are basically adequate to represent the main information characteristics of the samples. We used the e-nose to monitor odor changes before and after broccoli yellowing, indeed, achieved a clear separation between the both groups of samples. The radar map reflects the characteristics of different types of volatiles, and when control broccoli has turned yellow, there was a conspicuous variation in the values of W5S and W1W (Fig. 2e), suggesting that the two sensors had a high influence on the sensor-output pattern for broccoli odor. Meanwhile, the volatile components of the treated samples were closer to the initial value. Previous research showed that the major contributor to the pungent odor in broccoli during storage was the enzymatic production of volatile sulfur compounds (Di Pentima et al., 1995). Lv et al. (2017) reported that selenium treatment significantly delayed the offensive odor produced by broccoli during storage, which was associated with obvious differences in the composition of volatile compounds between selenium-treated and control broccoli, mainly including alcohols and sulfide compounds. Our results suggest that the change in broccoli odor along with yellowing was mainly owing to variations in volatile oxynitride compounds and sulfur compounds. In brief, the edible coating with CTS+TP can subdue the odor change and postpone the off-odor production of broccoli.
Variations in Chlorophyll Content and Chlorophyll Fluorophore of Broccoli
Post-harvest quality of broccoli, especially the yellowing problem, seriously affects its commodity quality and value. Therefore, it is of great significance to explore the corresponding control technology. Chlorophyll degradation is the principal cause of broccoli yellowing (Fang et al., 2020; Shi et al., 2016). In this study, we monitored the changes in the color of broccoli by measuring chlorophyll content. The total chlorophyll content of broccoli with the different treatments exhibited a gradual downward trend (Fig. 3a), and their values presented remarkable discrepancies from the 3rd day of shelf life (P < 0.05). Notably, the decline range of the CTS+TP-treated samples was consistently smaller than that of the single CTS treatment and the control. When control broccoli changed from green to yellow, its total chlorophyll content decreased by 42%, while that of the combined coated samples declined only 20%, showing the protective effect of CTS+TP coating on chlorophyll in broccoli. The reduction of chlorophyll content in the treated samples is similar with the result of Hong et al. (2012), where the CTS coating was effective in conferring control effect to retain chlorophyll content of guava fruit. Previous research has demonstrated that the CTS treatment can prevent the chlorophyll degradation of broccoli florets (Moreira et al., 2011b), and similar effects of deferring chlorophyll were also observed in cucumbers and bell peppers (Ghaouth et al., 2010). Interestingly, Ali et al. (2011) reported that the delayed color change may be due to the slow rate of respiration, resulting in a modified internal atmosphere of papaya fruit. In our study, the CTS+TP coating formed an excellent semi-permeable film around the broccoli, which retarded senescence to some extent and prevented the degradation of chlorophyll.
Changes in chlorophyll content (a) of broccoli during shelf life. Data are expressed as means of triplicate samples ± standard deviation. Different letters indicate significant differences among the treatments (P < 0.05). Fluorescence image observed with a fluorescence microscope of broccoli samples on the day of harvesting (b) and turning yellow (c-control group; d-CTS + TP treatment group)
The basic skeleton of chlorophyll molecule is a macrocycle (porphyrin ring) connected by four pyrrole rings and four methylene groups (=CH-), in which Mg2+ porphyrin is the chromophore, enabling chlorophyll to emit fluorescence under certain conditions. Moreover, changes in the chlorophyll molecular structure will affect the emission fluorescence and fluorescence intensity of substances (Franck et al., 2002). We observed dense red fluorescence in the fresh samples under a fluorescence microscope (Fig. 3b), suggesting that the chlorophyll level was high at this time. When the broccoli turned yellow from green, the quenching of red fluorescence was noted, displaying sparse and weak red fluorescence in the control samples. Meanwhile, the treated samples exhibited stronger fluorescence (Fig. 3d), and the fluorescence quenching was moderated. In the process of chlorophyll degradation, a cascade of green substances containing complete porphyrin macrocycles can be detected, including chlorophyllide (Childe), pheophorbide a (Pheide a) and pheophytin (Phein, Mg-free chlorophyll). Pružinská et al. (2007) reported that Pheide a oxygenase (PAO) catalyzed the only oxidation step and led to porphyrin ring opening of Pheide a to the primary fluorescent chlorophyll catabolite, which was an initial signal of chlorophyll degradation. It signifies that the weak red fluorescence was observed in controls when broccoli turned yellow, which may be ascribed to the damage of chlorophyll molecular structure (the cracking of the porphyrin macrocyclic or the loss of Mg2+) at this time, causing free chlorophyll to break down more easily, accompanied by the quenching of red fluorescence. This was also evidenced by a precipitous decline in chlorophyll content. According to the results of changes in chlorophyll content and fluorescence images, the effective mitigation of broccoli yellowing by CTS+TP coating might be attributed to the protection of the treatment on chlorophyll.
Variations in TSS, Vc, Amino Acid Contents, and Antioxidant Capacity of Broccoli
In addition to the distinct changes in appearance, the nutrient contents of broccoli are also the main parameters to estimate its post-harvest quality. In the current study, TSS with all treatments reached their peak on the 2nd day (Fig. 4a); obviously higher values were detected in the CTS+TP-treated samples than those in the other treatments afterwards. By the end of shelf life, the values in the samples treated with CTS+TP were about 8% and 15% higher than those in the single CTS treatment and the control, respectively. Among treatments, the highest TSS was recorded in the control during shelf life, and similar results were obtained by Vilaplana et al. (2020) in blackberries treated with CTS. The effect of CTS+TP coating on the TSS was presumably due to slowing down its respiration and metabolic activities, thus delaying the senescence process. Broccoli is rich in Vc and provides a good source of it in the daily diet. Nevertheless, it is known from literature that Vc content of broccoli degraded seriously after harvest (Raseetha et al., 2013). In this study, the content of Vc in the different treatments exhibited a continuous decline along with yellowing (Fig. 4b). When control broccoli was severely yellowed, its Vc content dropped by 70%, at which point the value of CTS+TP-treated broccoli was 62% higher than the control. There were significant differences in Vc content among the three treatments on day 4 (P < 0.05). However, the descent velocity of the CTS+TP-treated samples was consistently smaller than that of the other treatments. Similar results have been reported in sponge gourds coated with CTS, and the slower loss of Vc shown in the treated samples may be a consequence of the modified atmosphere created by the CTS coating (Han et al., 2014). Amino acid is also important substance contributing to the nutraceutical value of broccoli. The content of amino acid in the different treatments increased during the first 3 days of shelf life, and higher rise amplitude was detected in coated samples (Fig. 4c). Analogous results were reported during air-storage of freshly harvested broccoli; the initial increase in amino acid might be associated with an upturn of proteinase activity (Hansen et al., 2001). Subsequently, amino acid content decreased rapidly, but by the end of shelf life, there were no significant differences among the three treatments. We speculated that enhanced catabolism after harvest expedited the protein decomposition and amino acid conversion, but with the extension of shelf life, the internal nutrients gradually lost. In addition, an interesting paper was published by Wang et al. (2013), who discovered that the synergistic effect was noticeable after the incorporation of TP into CTS film, leading to a significant increase in the antioxidant activity of the blended film. The antioxidant capacity, measured with ABTS assay in our study, exhibited a fluctuation change in the control samples, with a quick rise before turning yellow and dramatic decline thereafter (Fig. 4d). Unlike the control, the antioxidant capacity of broccoli in the single CTS treatment increased slightly during the first 2 days, with a greater rise in amplitude thereafter, and decreased at the end of shelf life. Nevertheless, the antioxidant capacity of CTS+TP-treated broccoli increased progressively, although it declined slightly towards the end of shelf life. Compared with the other two treatments, the broccoli with CTS+TP treatment maintained higher antioxidant capacity during the entire shelf life. Our findings manifest that Vc content of broccoli reduced by a large margin during yellowing, along with the decrease of antioxidant capacity, while the CTS+TP coating showed a beneficial effect in retarding this change. They also imply that exogenous TP had a protective effect on endogenous antioxidant compounds (Chen et al., 2013; Zhao, 2006).
Variations in Sulforaphane Content and Related Genes Expression of Broccoli
There is an immense interest in health benefits of broccoli, among which glucosinolates are of great concern health-promoting compounds. Sulforaphane is the hydrolysate of glucosinolates, which is considered the most typical bioactive substance in broccoli with anti-cancer function (Guo et al., 2018). The content of sulforaphane in post-harvested broccoli decreased rapidly at ambient temperature (Jin et al., 2015). In the present study, when control broccoli turned yellow, its sulforaphane content dropped sharply, with a decrease rate of 40.8%; meanwhile, the content of the treated samples was 1.26 times that of the control (Table 3). As the yellowing of broccoli intensified, the sulforaphane level of the treated samples also decreased but was consistently higher, and its value was 1.54 times as high as that in controls by the end of shelf life. Furthermore, Rybarczyk-Plonska et al. (2006) also pointed that the synthesis and content of sulforaphane were affected by exogenous treatments. Likewise, we observed that application of CTS+TP to broccoli sustained a higher sulforaphane level.
Sulforaphane is an isothiocyanate formed by endogenous myrosinase acting on glucosinolates, and the synthesis and hydrolysis of glucosinolates affect the sulforaphane content (Alvarez-Jubete et al., 2014; Soares et al., 2017). Consequently, we further determined the expression levels of genes involved in sulforaphane production when broccoli turned yellow, mainly including glucosinolates biosynthesis genes (CYP79F1, CYP83A1, SUR1, MYB28, UGT74B1) and hydrolysis genes (MYR, ESP). Guo et al. (2016) applied jasmonic acid to broccoli sprouts with accumulation of sulforaphane and found prominent upregulation in CYP83A1 expression. MYB28 transcription factor, a key regulator of glucosinolates biosynthesis, has been reported to play a positive role in the high glucoraphanin content of broccoli (Traka et al., 2013). In the present study, the CTS+TP treatment substantially increased the expression levels of BoCYP83A1 and BoMYB28 by 1.5- and 2.3-fold (Fig. 5). On the other hand, MYR encodes myrosinase which participates in the hydrolysis of glucosinolatesl. Comparable trends in the expression of hydrolyzed genes after the CTS+TP treatment were also observed, and the transcription level of BoMYR was notably upregulated by 1.9-fold. Similarly, MYR expression was significantly upregulated in methyl jasmonate–treated broccoli, thus promoting the production of sulforaphane (Ku et al., 2013). To sum up, it can be inferred that the higher level of sulforaphane maintained by CTS+TP coating is attributed to the activation of glucosinolate-related gene expression. A myrosinase cofactor, the epithiospecifier protein (ESP), is known to catalyze the conversion of glucoraphanin into inactive sulforaphane nitrile, resulting in the loss of sulforaphane (Matusheski et al., 2006). Consistent with our results, exogenous jasmonic acid treatment did not affect the expression of ESP statistically (Guo et al., 2016). However, ESP activity in broccoli sprouts was enhanced by jasmonic acid treatment. Our findings, together with previous reports, further confirm that BoCYP83A1, BoMYB28, and BoMYR are key genes regulating the metabolism of glucosinolates during broccoli yellowing. In the future, considerable research is still needed on maintaining glucosinolates and enhancing sulforaphane production to mitigate the nutritional deterioration in post-harvested broccoli.
Effects of CTS + TP treatment on the expression levels of sulforaphane related genes. Data were recorded after 3 days of shelf life. Each value is the mean ± standard deviation of three replicates. Relative expression values are given compared with the control, which was set as 1. Asterisks indicate significant differences between treated and control samples. *, P < 0.05
Conclusion
Published reports have shown that the CTS coating can be used as a post-harvest treatment to alleviate the quality deterioration of fruit and vegetables; our results demonstrated the combined treatment with CTS and TP had better effects on the decay rate, weight loss, and respiratory intensity of broccoli than the single CTS treatment and control. In the current study, the protective effects of combinative treatment (CTS+TP) on the post-harvest quality of broccoli were mainly investigated. This treatment effectively ameliorated broccoli yellowing by sustaining higher h° value, chlorophyll level, and strong red fluorescence emission. It also retarded evident change in sensory quality including odor, firmness, and chewiness upon yellowing. Broccoli treated with CTS+TP exhibited significantly higher Vc content and antioxidant capacity than the control. Furthermore, the higher level of sulforaphane was maintained during broccoli yellowing by this treatment, which was ascribed to the upregulated expression of related gene (BoCYP83A1, BoMYB28, BoMYR). Taken together, the edible coating with CTS+TP reduced the yellowing symptoms and adverse changes in sensorial and nutraceutical traits, improving the quality deterioration of broccoli.
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Ali, A., Muhammad, M., Sijam, K., & Siddiqui, Y. (2011). Effect of chitosan coatings on the physicochemical characteristics of Eksotika II papaya (Carica papaya L.) fruit during cold storage. Food Chemistry, 124, 620–626. https://doi.org/10.1016/j.foodchem.2010.06.085
Alvarez, M. V., Ponce, A. G., del Moreira, M., & R. (2013). Antimicrobial efficiency of chitosan coating enriched with bioactive compounds to improve the safety of fresh cut broccoli. LWT - Food Science and Technology, 50, 78–87. https://doi.org/10.1016/j.lwt.2012.06.021
Alvarez-Jubete, L., Valverde, J., Kehoe, K., Reilly, K., Rai, D. K., & Barry-Ryan, C. (2014). Development of a novel functional soup rich in bioactive sulforaphane using broccoli (Brassica oleracea L. ssp. italica) florets and byproducts. Food and Bioprocess Technology, 7, 1310–1321. https://doi.org/10.1007/s11947-013-1113-9
Bautista-Baños, S., Hernández-Lauzardo, A. N., Velázquez-del Valle, M. G., Hernández-López, M., Ait Barka, E., Bosquez-Molina, E., & Wilson, C. L. (2006). Chitosan as a potential natural compound to control pre and postharvest diseases of horticultural commodities. Crop Protection, 25, 108–118. https://doi.org/10.1016/j.cropro.2005.03.010
Cai, J. H., Cheng, S. C., Luo, F., Zhao, Y. B., Wei, B. D., Zhou, Q., Zhou, X., & Ji, S. J. (2019). Influence of ethylene on morphology and pigment changes in harvested broccoli. Food and Bioprocess Technology, 12, 883–897. https://doi.org/10.1007/s11947-019-02267-1
Cazón, P., Velazquez, G., Ramírez, J. A., & Vázquez, M. (2016). Polysaccharide-based films and coatings for food packaging: A review. Food Hydrocolloids, 68, 136–148. https://doi.org/10.1016/j.foodhyd.2016.09.009
Chen, J., Zhang, S., & Yang, X. (2013). Control of brown rot on nectarines by tea polyphenol combined with tea saponin. Crop Protection, 45, 29–35. https://doi.org/10.1016/j.cropro.2012.11.006
Conversa, G., Lazzizera, C., Chiaravalle, A. E., Miedico, O., Bonasia, A., La Rotonda, P., & Elia, A. (2019). Selenium fern application and arbuscular mycorrhizal fungi soil inoculation enhance Se content and antioxidant properties of green asparagus (Asparagus officinalis L.) spears. Scientia Horticulturae, 252, 176–191. https://doi.org/10.1016/j.scienta.2019.03.056
Di Pentima, J. H., Rios, J. J., Clemente, A., & Olias, J. M. (1995). Biogenesis of off-odor in broccoli storage under low-oxygen atmosphere. Journal of Agricultural and Food Chemistry, 43, 1310–1313. https://doi.org/10.1021/jf00053a035
Eason, J. R., Ryan, D., Page, B., Watson, L., & Coupe, S. A. (2007). Harvested broccoli (Brassica oleracea) responds to high carbon dioxide and low oxygen atmosphere by inducing stress-response genes. Postharvest Biology and Technology, 43, 358–365. https://doi.org/10.1016/j.postharvbio.2006.10.001
Falcó, I., Flores-Meraz, P. L., Randazzo, W., Sánchez, G., López-Rubio, A., & Fabra, M. J. (2019). Antiviral activity of alginate-oleic acid based coatings incorporating green tea extract on strawberries and raspberries. Food Hydrocolloids, 87, 611–618. https://doi.org/10.1016/j.foodhyd.2018.08.055
Fang, H. X., Luo, F., Li, P. X., Zhou, Q., Zhou, X., Wei, B. D., Cheng, S. C., Zhou, H. S., & Ji, S. J. (2020). Potential of jasmonic acid (JA) in accelerating postharvest yellowing of broccoli by promoting its chlorophyll degradation. Food Chemistry, 309, 125737. https://doi.org/10.1016/j.foodchem.2019.125737
Franck, F., Juneau, P., & Popovic, R. (2002). Resolution of the photosystem I and photosystem II contributions to chlorophyll fluorescence of intact leaves at room temperature. Biochimica et Biophysica Acta (BBA) - Bioenergetics, 1556, 239–246. https://doi.org/10.1016/S0005-2728(02)00366-3
Freitas, P. M., López-Gálvez, F., Tudela, J. A., Gil, M. I., & Allende, A. (2015). Postharvest treatment of table grapes with ultraviolet-C and chitosan coating preserves quality and increases stilbene content. Postharvest Biology and Technology, 105, 51–57. https://doi.org/10.1016/j.postharvbio.2015.03.011
Ghaouth, A., Arul, J., Ponnampalam, R., & Boulet, M. (2010). Use of chitosan coating to reduce water loss and maintain quality of cucumber and bell pepper fruits. Journal of Food Processing and Preservation, 15, 359–368. https://doi.org/10.1111/j.1745-4549.1991.tb00178.x
Gillespie, K. M., & Ainsworth, E. A. (2007). Measurement of reduced, oxidized and total ascorbate content in plants. Nature Protocols, 2, 871–874. https://doi.org/10.1038/nprot.2007.101
Guo, L., Yang, R., & Gu, Z. (2016). Cloning of genes related to aliphatic glucosinolate metabolism and the mechanism of sulforaphane accumulation in broccoli sprouts under jasmonic acid treatment. Journal of the Science of Food and Agriculture, 96, 4329–4336. https://doi.org/10.1002/jsfa.7629
Guo, L., Zhu, Y., & Wang, F. (2018). Calcium sulfate treatment enhances bioactive compounds and antioxidant capacity in broccoli sprouts during growth and storage. Postharvest Biology and Technology, 139, 12–19. https://doi.org/10.1016/j.postharvbio.2018.01.010
Han, C., Zuo, J., Wang, Q., Xu, L., Zhai, B., Wang, Z., Dong, H., & Gao, L. (2014). Effects of chitosan coating on postharvest quality and shelf life of sponge gourd (Luffa cylindrica) during storage. Scientia Horticulturae, 166, 1–8. https://doi.org/10.1016/j.scienta.2013.09.007
Hansen, M. E., Srensen, H., & Cantwell, M. (2001). Changes in acetaldehyde, ethanol and amino acid concentrations in broccoli florets during air and controlled atmosphere storage. Postharvest Biology and Technology, 22, 227–237. https://doi.org/10.1016/S0925-5214(01)00093-X
Hong, K., Xie, J., Zhang, L., Sun, D., & Gong, D. (2012). Effects of chitosan coating on postharvest life and quality of guava (Psidium guajava L.) fruit during cold storage. Scientia Horticulturae, 144, 172–178. https://doi.org/10.1016/j.scienta.2012.07.002
Jin, P., Yao, D., Xu, F., Wang, H., & Zheng, Y. (2015). Effect of light on quality and bioactive compounds in postharvest broccoli florets. Food Chemistry, 172, 705–709. https://doi.org/10.1016/j.foodchem.2014.09.134
Ku, K. M., Choi, J. H., Kim, H. S., Kushad, M. M., Jeffery, E. H., & Juvik, J. A. (2013). Methyl jasmonate and 1-methylcyclopropene treatment effects on quinone reductase inducing activity and post-harvest quality of broccoli. PLoS ONE, 8, e77127. https://doi.org/10.1371/journal.pone.0077127
Liang, H., Yuan, Q. P., Dong, H. R., & Liu, Y. M. (2006). Determination of sulforaphane in broccoli and cabbage by high-performance liquid chromatography. Journal of Food Composition and Analysis, 19(5), 473–476. https://doi.org/10.1016/j.jfca.2005.11.005
Lichtenthaler, H. K. (1987). Chlorophylls and carotenoids: Pigments of photosynthetic biomembranes. Methods in Enzymology, 148, 350–382. https://doi.org/10.1016/0076-6879(87)48036-1
Lv, J., Wu, J., Zuo, J., Fan, L., Shi, J., Gao, L., Li, M., & Wang, Q. (2017). Effect of Se treatment on the volatile compounds in broccoli. Food Chemistry, 216, 225–233. https://doi.org/10.1016/j.foodchem.2016.08.005
Martín-Diana, A. B., Rico, D., & Barry-Ryan, C. (2008). Green tea extract as a natural antioxidant to extend the shelf-life of fresh-cut lettuce. Innovative Food Science and Emerging Technologies, 9, 593–603. https://doi.org/10.1016/j.ifset.2008.04.001
Matusheski, N. V., Swarup, R., Juvik, J. A., Mithen, R., Bennett, M., & Jeffery, E. H. (2006). Epithiospecifier protein from broccoli (Brassica oleracea L. ssp. italica) inhibits formation of the anticancer agent sulforaphane. Journal of Agricultural and Food Chemistry, 54, 2069–2076. https://doi.org/10.1021/jf0525277
Molamohammadi, H., Pakkish, Z., Akhavan, H. R., & Saffari, V. R. (2020). Effect of salicylic acid incorporated chitosan coating on shelf life extension of fresh in-hull pistachio fruit. Food and Bioprocess Technology, 13, 121–131. https://doi.org/10.1007/s11947-019-02383-y
Möller, H., Grelier, S., Pardon, P., & Coma, V. (2004). Antimicrobial and physicochemical properties of chitosan−HPMC-based films. Journal of Agricultural and Food Chemistry, 52, 6585–6591. https://doi.org/10.1021/jf0306690
Moreira, M. R., Ponce, A., Ansorena, R., & Roura, S. I. (2011a). Effectiveness of edible coatings combined with mild heat shocks on microbial spoilage and sensory quality of fresh cut broccoli (Brassica oleracea L.). Journal of Food Science, 76, M367–M374. https://doi.org/10.1111/j.1750-3841.2011.02210.x
Moreira, M. R., Roura, S. I., & Ponce, A. (2011b). Effectiveness of chitosan edible coatings to improve microbiological and sensory quality of fresh cut broccoli. LWT - Food Science and Technology, 44, 2335–2341. https://doi.org/10.1016/j.lwt.2011.04.009
Mustafa, M. A., Ali, A., Manickam, S., & Siddiqui, Y. (2014). Ultrasound-assisted chitosan–surfactant nanostructure assemblies: Towards maintaining postharvest quality of tomatoes. Food and Bioprocess Technology, 7, 2102–2111. https://doi.org/10.1007/s11947-013-1173-x
Olivas, G. I., & Barbosa-Cánovas, G. V. (2005). Edible coatings for fresh-cut fruits. Critical Reviews in Food Science and Nutrition, 45, 657–670. https://doi.org/10.1080/10408690490911837
Petriccione, M., Sanctis, F. D., Pasquariello, M. S., Mastrobuoni, F., Rega, P., Scortichini, M., & Mencarelli, F. (2015). The effect of chitosan coating on the quality and nutraceutical traits of sweet cherry during postharvest life. Food and Bioprocess Technology, 8, 394–408. https://doi.org/10.1007/s11947-014-1411-x
Pružinská, A., Anders, I., Aubry, S., Schenk, N., Tapernoux-Lüthi, E., Müller, T., Kräutler, B., & Hörtensteiner, S. (2007). In vivo participation of red chlorophyll catabolite reductase in chlorophyll breakdown. The Plant Cell, 19, 369–387. https://doi.org/10.1105/tpc.106.044404
Raseetha, S., Leong, S. Y., Burritt, D. J., & Oey, I. (2013). Understanding the degradation of ascorbic acid and glutathione in relation to the levels of oxidative stress biomarkers in broccoli (Brassica oleracea L. italica cv. Bellstar) during storage and mechanical processing. Food Chemistry, 138, 1360–1369. https://doi.org/10.1016/j.foodchem.2012.09.126
Ribeiro, A. M., Estevinho, B. N., & Rocha, F. (2020). Preparation and incorporation of functional ingredients in edible films and coatings. Food and Bioprocess Technology, 14, 209–231. https://doi.org/10.1007/s11947-020-02528-4
Romanazzi, G., Feliziani, E., Baños, S. B., & Sivakumar, D. (2015). Shelf life extension of fresh fruit and vegetables by chitosan treatment. Critical Reviews in Food Science and Nutrition, 57, 579–601. https://doi.org/10.1080/10408398.2014.900474
Rybarczyk-Plonska, A., Hagen, S. F., Borge, G. I. A., Bengtsson, G. B., Hansen, M. K., & Wold, A.-B. (2016). Glucosinolates in broccoli (Brassica oleracea L. var. italica) as affected by postharvest temperature and radiation treatments. Postharvest Biology and Technology, 116, 16–25. https://doi.org/10.1016/j.postharvbio.2015.12.010
Sánchez-Vega, R., Elez-Martínez, P., & Martín-Belloso, O. (2014). Effects of high-intensity pulsed electric fields processing parameters on the chlorophyll content and its degradation compounds in broccoli juice. Food and Bioprocess Technology, 7, 1137–1148. https://doi.org/10.1007/s11947-013-1152-2
Sarabandi, K., & Mahdi Jafari, S. (2019). Effect of chitosan coating on the properties of nanoliposomes loaded with flaxseed-peptide fractions: stability during spray-drying. Food Chemistry, 310, 125951.1–125951.10. https://doi.org/10.1016/j.foodchem.2019.125951
Shi, J., Gao, L., Zuo, J., Wang, Q., Wang, Q., & Fan, L. (2016). Exogenous sodium nitroprusside treatment of broccoli florets extends shelf life, enhances antioxidant enzyme activity, and inhibits chlorophyll-degradation. Postharvest Biology and Technology, 116, 98–104. https://doi.org/10.1016/j.postharvbio.2016.01.007
Soares, A., Carrascosa, C., & Raposo, A. (2017). Influence of different cooking methods on the concentration of glucosinolates and vitamin C in broccoli. Food and Bioprocess Technology, 10, 1387–1411. https://doi.org/10.1007/s11947-017-1930-3
Tharanathan, R. N. (2003). Biodegradable films and composite coatings: Past, present and future. Trends in Food Science and Technology, 14, 71–78. https://doi.org/10.1016/S0924-2244(02)00280-7
Traka, M. H., Saha, S., Huseby, S., Kopriva, S., Walley, P. G., Barker, G. C., Moore, J., Mero, G., Bosch, F., Constant, H., Kelly, L., Schepers, H., Boddupalli, S., & Mithen, R. F. (2013). Genetic regulation of glucoraphanin accumulation in Beneforté® broccoli. New Phytologist, 198, 1085–1095. https://doi.org/10.1111/nph.12232
Vilaplana, R., Guerrero, K., Guevara, J., & Valencia-Chamorro, S. (2020). Chitosan coatings to control soft mold on fresh blackberries (Rubus glaucus Benth.) during postharvest period. Scientia Horticulturae, 262, 109049. https://doi.org/10.1016/j.scienta.2019.109049
Wang, L., Yan, D., Men, H., Jin, T., & Jiang, Z. (2013). Preparation and characterization of active films based on chitosan incorporated tea polyphenols. Food Hydrocolloids, 32, 35–41. https://doi.org/10.1016/j.foodhyd.2012.11.034
Wang, Y., Wang, J. C., Zhu, G. P., Han, P. I., & Liu, Y. W. (2019). Research progress of application of tea polyphenols degradable composite films in food preservation. Packaging Engineering, 40, 97–103. https://doi.org/10.19554/j.cnki.1001-3563.2019.13.014
Wu, H., Wang, D., Shi, J., Xue, S., & Gao, M. (2010). Effect of the complex of zinc (II) and cerium (IV) with chitosan on the preservation quality and degradation of organophosphorus pesticides in Chinese Jujube (Zizyphus jujuba Mill. cv. Dongzao). Journal of Agricultural and Food Chemistry, 58, 5757–5762. https://doi.org/10.1021/jf100537k
Yang, C. S., Lambert, J. D., & Sang, S. (2009). Antioxidative and anti-carcinogenic activities of tea polyphenols. Archives of Toxicology, 83, 11–21. https://doi.org/10.1007/s00204-008-0372-0
Zhang, X. T., Zhang, M., Devahastin, S., & Guo, Z. (2019). Effect of combined ultrasonication and modified atmosphere packaging on storage quality of pakchoi (Brassica chinensis L.). Food and Bioprocess Technology, 12, 1573–1583. https://doi.org/10.1007/s11947-019-02316-9
Zhao, B. (2006). The health effects of tea polyphenols and their antioxidant mechanism. Journal of Clinical Biochemistry and Nutrition, 38, 59–68. https://doi.org/10.3164/jcbn.38.59
Funding
This work was supported by the National Key R&D Program of China [grant number 2016YFD0400103].
Author information
Authors and Affiliations
Contributions
Huixin Fang: investigation, methodology, data curation, formal analysis, writing—original draft, visualization. Qian Zhou: conceptualization, validation, resources. Qingxi Yang: conceptualization, methodology. Xin Zhou: investigation, supervision. Shunchang Cheng: resources, conceptualization. Baodong Wei: resources, software. Jiangkuo Li: resources. Shujuan Ji: conceptualization, writing—review and editing, visualization, supervision, project administration, funding acquisition.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Fang, H., Zhou, Q., Yang, Q. et al. Influence of Combined Edible Coating with Chitosan and Tea Polyphenol on the Quality Deterioration and Health-promoting Compounds in Harvested Broccoli. Food Bioprocess Technol 15, 407–420 (2022). https://doi.org/10.1007/s11947-021-02751-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11947-021-02751-7